Chemical Characterization and Biological Evaluation of Epilobium parviflorum Extracts in an In Vitro Model of Human Malignant Melanoma
Abstract
1. Introduction
2. Results
2.1. Standardisation of UPLC/MS Conditions and Method Validation
2.2. Linearity, Accuracy, and Precision of the Methodology
2.3. Chemical Characterisation of the Isolated E. parviflorum Extracts
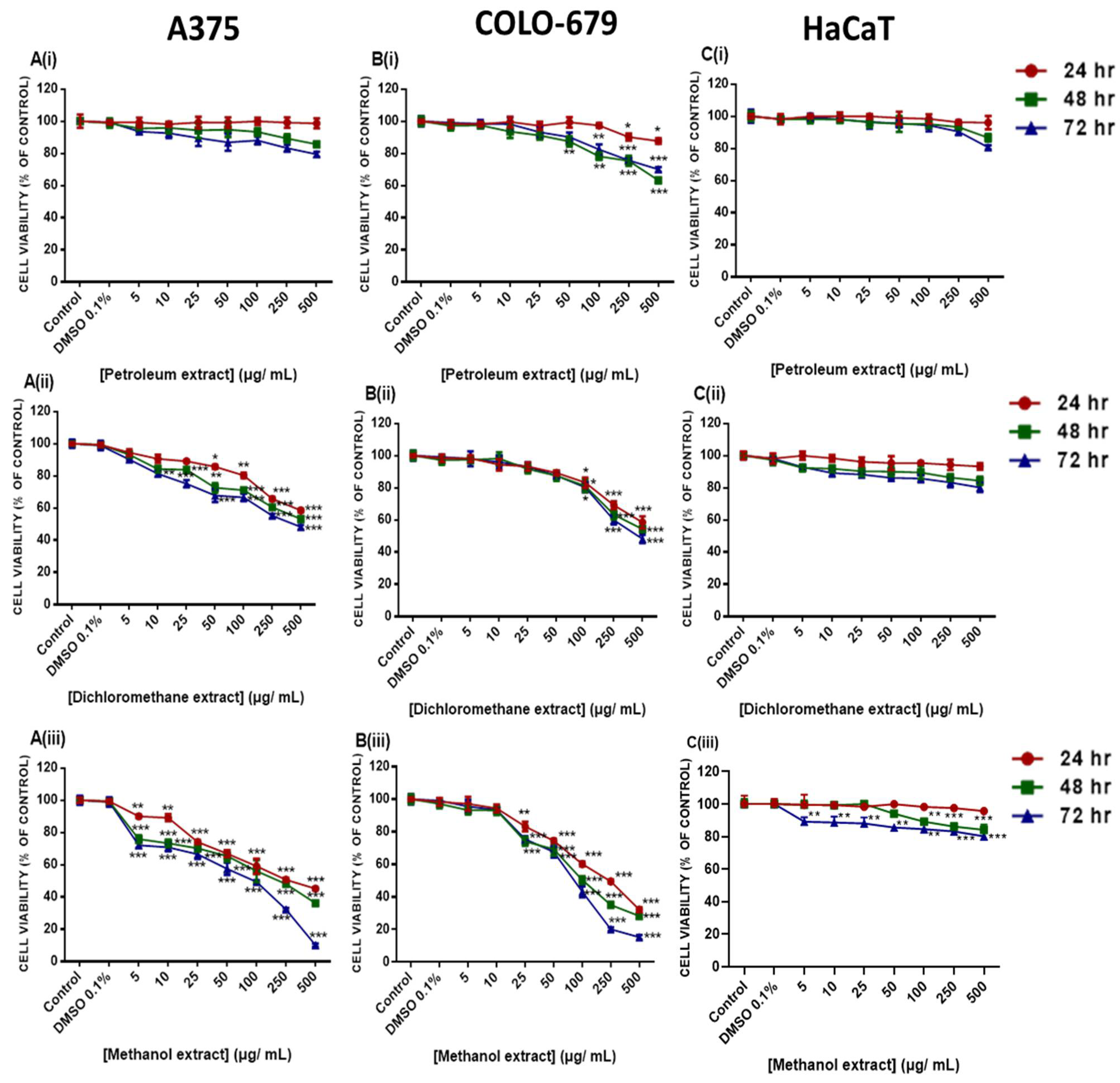
2.4. Cytotoxic Profile of Various E. parviflorum Extracts
2.5. Modulation of Apoptotic Gene Expression by Methanolic Extract of E. parviflorum
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plant Material and Extract Preparation
4.3. Determination of total Phenol, Flavonoid, Condensed Tannin, Monoterpenoid, Soluble Sugar, Protein, and Pigment Contents
4.4. Preparation of Standards and Samples
4.5. Liquid Chromatography Conditions
4.6. Cell Culture
4.7. Determination of Cell Viability
4.8. Determination of Gene Expression
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karakaya, S.; Süntar, I.; Yakinci, O.F.; Sytar, O.; Ceribasi, S.; Dursunoglu, B.; Ozbek, H.; Guvenalp, Z. In vivo bioactivity assessment on Epilobium species: A particular focus on Epilobium angustifolium and its components on enzymes connected with the healing process. J. Ethnopharmacol. 2020, 262, 113207. [Google Scholar] [CrossRef]
- Granica, S.; Piwowarski, J.P.; Czerwińska, M.E.; Kiss, A.K. Phytochemistry, pharmacology and traditional uses of different Epilobium species (Onagraceae): A review. J. Ethnopharmacol. 2014, 156, 316–346. [Google Scholar] [CrossRef]
- Dreger, M.; Adamczak, A.; Seidler-Łożykowska, K.; Wielgus, K. Pharmacological properties of fireweed (Epilobium angustifolium L.) and bioavailability of ellagitannins. A review. Herba Pol. 2020, 66, 52–64. [Google Scholar] [CrossRef]
- Nowak, A.; Zielonka-Brzezicka, J.; Perużyńska, M.; Klimowicz, A. Epilobium angustifolium L. as a Potential Herbal Component of Topical Products for Skin Care and Treatment—A Review. Molecules 2022, 27, 35–36. [Google Scholar] [CrossRef]
- McColl, J. Willowherb (Epilobium angustifolium L.): Biology, chemistry, bioactivity and uses. Agro Food Ind. Hi-Tech 2002, 13, 18–22. [Google Scholar]
- Jung, S.Y.; Kim, G.-D.; Choi, D.W.; Shin, D.-U.; Eom, J.-E.; Kim, S.Y.; Chai, O.H.; Kim, H.-J.; Lee, S.-Y.; Shin, H.S. Epilobium pyrricholophum Extract Suppresses Porcine Pancreatic Elastase and Cigarette Smoke Extract-Induced Inflammatory response in a Chronic Obstructive Pulmonary Disease Model. Foods 2021, 10, 2929. [Google Scholar] [CrossRef]
- Bajer, T.; Šilha, D.; Ventura, K.; Bajerová, P. Composition and antimicrobial activity of the essential oil, distilled aromatic water and herbal infusion from Epilobium parviflorum Schreb. Ind. Crop. Prod. 2017, 100, 95–105. [Google Scholar] [CrossRef]
- Merighi, S.; Travagli, A.; Tedeschi, P.; Marchetti, N.; Gessi, S. Antioxidant and Antiinflammatory Effects of Epilobium parviflorum, Melilotus officinalis and Cardiospermum halicacabum Plant Extracts in Macrophage and Microglial Cells. Cells 2021, 10, 2691. [Google Scholar] [CrossRef] [PubMed]
- Șachir, E.E.; Pușcașu, C.G.; Caraiane, A.; Raftu, G.; Badea, F.C.; Mociu, M.; Albu, C.M.; Sachelarie, L.; Hurjui, L.L.; Bartok-Nicolae, C. Studies Regarding the Antibacterial Effect of Plant Extracts Obtained from Epilobium parviflorum Schreb. Appl. Sci. 2022, 12, 27–51. [Google Scholar] [CrossRef]
- Stolarczyk, M.; Granica, S.; Naruszewicz, M.; Kiss, A.K. Extracts from Epilobium sp. herbs inhibit proliferation, PSA secretion and induce apoptosis in prostate cancer cells (LNCaP). Planta Med. 2012, 78, 1044–1054. [Google Scholar] [CrossRef]
- Stolarczyk, M.; Naruszewicz, M.; Kiss, A.K. Extracts from Epilobium sp. herbs induce apoptosis in human hormone-dependent prostate cancer cells by activating the mitochondrial pathway. J. Pharm. Pharmacol. 2013, 65, 1044–1054. [Google Scholar] [CrossRef]
- Kia, B.H.; Noureini, S.K.; Kakhki, M.R.V. The Extracts of Epilobium Parviflorum Inhibit MCF-7 Breast Cancer Cells. IJT 2021, 15, 65–72. [Google Scholar] [CrossRef]
- Kowalik, K.; Polak-Berecka, M.; Prendecka-Wróbel, M.; Pigoń-Zając, D.; Niedźwiedź, I.; Szwajgier, D.; Baranowska-Wójcik, E.; Waśko, A. Biological Activity of an Epilobium angustifolium L. (Fireweed) Infusion after In Vitro Digestion. Molecules 2022, 27, 1006. [Google Scholar] [CrossRef]
- Akbudak, M.A.; Sut, T.; Eruygur, N.; Akinci, E. Antiproliferative Effect of Epilobium parviflorum Extracts on Colorectal Cancer Cell Line HT-29. bioRxiv 2020, 12, 1–17. [Google Scholar] [CrossRef]
- Granica, S.; Bazylko, A.; Kiss, A.K. Determination of macrocyclic ellagitannin oenothein B in plant materials by HPLC-DAD-MS: Method development and validation. Phytochem. Anal. 2012, 23, 582–587. [Google Scholar] [CrossRef]
- Remmel, I.; Vares, L.; Toom, L.; Matto, V.; Raal, A. Phenolic Compounds in Five Epilobium Species Collected from Estonia. Nat. Prod. Commun. 2012, 7, 1323–1324. [Google Scholar] [CrossRef]
- Hevesi, T.B.; Houghton, P.J.; Habtemariam, S.; Kéry, A. Antioxidant and antiinflammatory effect of Epilobium parviflorum Schreb. Phytother. Res. 2009, 23, 719–724. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Jakiw, L.; Khlebnikov, A.I.; Blaskovich, C.L.; Jutila, M.A.; Quinn, M.T. Immunomodulatory activity of oenothein B isolated from Epilobium angustifolium. J. Immunol. 2009, 183, 6754–6766. [Google Scholar] [CrossRef] [PubMed]
- Ramstead, A.G.; Schepetkin, I.A.; Quinn, M.T.; Jutila, M.A. Oenothein B, a Cyclic Dimeric Ellagitannin Isolated from Epilobium angustifolium, Enhances IFNγ Production by Lymphocytes. PLoS ONE 2012, 7, e50546. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yoshimura, M.; Amakura, Y. Chemical and biological significance of oenothein B and related ellagitannin oligomers with macrocyclic structure. Molecules 2018, 23, 552. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Santarcangelo, C.; Masselli, R.; Buonomo, G.; Nicotra, G.; Insolia, V.; D’Avino, M.; Caruso, G.; Buonomo, A.R.; Sacchi, R.; et al. Epilobium angustifolium L. extract with high content in oenothein B on benign prostatic hyperplasia: A monocentric, randomized, double-blind, placebo-controlled clinical trial. Biomed. Pharmacother. 2021, 138, 111414. [Google Scholar] [CrossRef] [PubMed]
- Harron, D.W.G. Technical Requirements for Registration of Pharmaceuticals for Human Use: The ICH Process. In The Textbook of Pharmaceutical Chemistry, 7th ed.; Griffin, J.P., Posner, J., Barker, G.R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 447–460. [Google Scholar]
- Slacanin, I.; Marston, A.; Hostettmann, K.; Delabays, N.; Darbellay, C. Isolation and determination of flavonol glycosides from epilobium species. J. Chromatogr. A 1991, 557, 391–398. [Google Scholar] [CrossRef]
- Tita, B.; Abdel-Haq, H.; Vitalone, A.; Mazzanti, G.; Saso, L. Analgesic properties of Epilobium angustifolium, evaluated by the hot plate test and the writhing test. Il Farm. 2001, 56, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Hevesi, T.B.; Blazics, B.; Kéry, Á. Polyphenol composition and antioxidant capacity of Epilobium species. J. Pharm. Biomed. Anal. 2009, 49, 26–31. [Google Scholar] [CrossRef]
- Mohammadi, B.M.; Falahati-Anbaran, M.; Rohloff, J. Comparative Analyses of Phytochemical Variation Within and Between Congeneric Species of Willow Herb, Epilobium hirsutum and E. parviflorum: Contribution of Environmental Factors. Front. Plant Sci. 2020, 11, 595190. [Google Scholar] [CrossRef]
- Nowak, A.; Zagórska-Dziok, M.; Ossowicz-Rupniewska, P.; Makuch, E.; Duchnik, W.; Kucharski, Ł.; Adamiak-Giera, U.; Prowans, P.; Czapla, N.; Bargiel, P.; et al. Epilobium angustifolium L. Extracts as Valuable Ingredients in Cosmetic and Dermatological Products. Molecules 2021, 26, 3456. [Google Scholar] [CrossRef]
- Kiss, A.; Kowalski, J.; Melzig, M.F. Effect of Epilobium angustifolium L. extracts and polyphenols on cell proliferation and neutral endopeptidase activity in selected cell lines. Pharmazie 2006, 61, 66–69. [Google Scholar]
- Kiss, A.; Kowalski, J.; Melzig, M.F. Induction of neutral endopeptidase activity in PC-3 cells by an aqueous extract of Epilobium angustifolium L. and oenothein B. Phytomedicine 2006, 13, 284–289. [Google Scholar] [CrossRef]
- Stolarczyk, M.; Piwowarski, J.P.; Granica, S.; Stefańska, J.; Naruszewicz, M.; Kiss, A.K. Extracts from Epilobium sp. herbs, their components and gut microbiota metabolites of Epilobium ellagitannins, urolithins, inhibit hormone-dependent prostate cancer cells-(LNCaP) proliferation and PSA secretion. Phytother. Res. 2013, 27, 1842–1848. [Google Scholar] [CrossRef]
- Rafiq, R.A.; Quadri, A.; Nazir, L.A.; Peerzada, K.; Ganai, B.A.; Tasduq, S.A. A Potent Inhibitor of Phosphoinositide 3-Kinase (PI3K) and Mitogen Activated Protein (MAP) Kinase Signalling, Quercetin (3, 3’, 4’, 5, 7-Pentahydroxyflavone) Promotes Cell Death in Ultraviolet (UV)-B-Irradiated B16F10 Melanoma Cells. PLoS ONE 2015, 10, e0131253. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Peng, M.-J.; Zhang, X.; Chen, Y.; Yang, Y.-Y.; Zhai, X.-X.; Liu, G.; Cao, Y. Oenothein B boosts antioxidant capacity and supports metabolic pathways that regulate antioxidant defense in Caenorhabditis elegans. Food Funct. 2020, 11, 9157–9167. [Google Scholar] [CrossRef]
- Sakagami, H.; Jiang, Y.; Kusama, K.; Atsumi, T.; Ueha, T.; Toguchi, M.; Iwakura, I.; Satoh, K.; Ito, H.; Hatano, T.; et al. Cytotoxic activity of hydrolyzable tannins against human oral tumor cell lines—A possible mechanism. Phytomedicine 2000, 7, 39–47. [Google Scholar] [CrossRef]
- Martins, J.; Costa, E.; Pires Serrano, S.H.; Santos, S.; Gil, E. Redox Behavior of the Ellagitannin Oenothein B and Ellagic Acid at a Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2015, 10, 4552–4561. [Google Scholar]
- Moilanen, J.; Karonen, M.; Tähtinen, P.; Jacquet, R.; Quideau, S.; Salminen, J.P. Biological activity of ellagitannins: Effects as anti-oxidants, pro-oxidants and metal chelators. Phytochemistry 2016, 125, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Yuzugulen, J.; Noshadi, B.; Shukur, K.; Sahin, M.F.; Gulcan, H.O. The metabolites of ellagitannin metabolism urolithins display various biological activities. EMU J. Pharm. Sci. 2019, 2, 102–110. [Google Scholar]
- Alfei, S.; Marengo, B.; Zuccari, G. Oxidative stress, antioxidant capabilities, and bioavailability: Ellagic acid or urolithins? Antioxidants 2020, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Alzayady, K.J.; Chandrasekhar, R.; Yule, D.I. Fragmented inositol 1,4,5-trisphosphate receptors retain tetrameric architecture and form functional Ca2+ release channels. J. Biol. Chem. 2013, 288, 11122–11134. [Google Scholar] [CrossRef]
- Berridge, M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Zaidi, S.F.; Cui, Z.G.; Zhou, D.; Saeed, S.A.; Inadera, H. Potential proapoptotic phytochemical agents for the treatment and prevention of colorectal cancer. Oncol. Lett. 2019, 18, 487–498. [Google Scholar] [CrossRef]
- Rahman, A.; Hannan, A.; Dash, R.; Rahman, H.; Islam, R.; Uddin, J.; Sohag, A.A.M.; Rahman, H.; Rhim, H. Phytochemicals as a Complement to Cancer Chemotherapy: Pharmacological Modulation of the Autophagy-Apoptosis Pathway. Front. Pharmacol. 2021, 12, 639628. [Google Scholar] [CrossRef]
- Sayik, A.; Yusufoğlu, A.S.; Açik, L.; Türker, G.; Aydin, B.; Arslan, L. DNA- Binding, Biological Activities, and Chemical Composition of Wild Growing Epilobium angustifolium L. Extracts from Canakkale, Turkey. J. Turk. Chem. Soc. 2017, 4, 811–840. [Google Scholar] [CrossRef]
- Vitalone, A.; McColl, J.; Thome, D.; Costa, L. Characterization of the Effect of Epilobium Extracts on Human Cell Proliferation. Pharmacology 2003, 69, 79–87. [Google Scholar] [CrossRef]
- Xiaodong, P.; Junsong, X.; Guijiang, W.; Yumeng, Z.; Feng, L.; Zhaocheng, X.; Lan, L.; Xueli, W.; Guangfu, P.; Yan, J.; et al. Oenothein B inhibits human non-small cell lung cancer A549 cell proliferation by ROS-mediated PI3K/Akt/NF-κB signaling pathway. Chem. Biol. Interact. 2019, 298, 112–120. [Google Scholar] [CrossRef]
- Kyriakou, S.; Tragkola, V.; Alghol, H.; Anestopoulos, I.; Amery, T.; Stewart, K.; Winyard, P.G.; Trafalis, D.T.; Franco, R.; Pappa, A.; et al. Evaluation of Bioactive Properties of Lipophilic Fractions of Edible and Non-Edible Parts of Nasturtium officinale (Watercress) in a Model of Human Malignant Melanoma Cells. Pharmaceuticals 2022, 15, 141. [Google Scholar] [CrossRef]
- Shay, P.E.; Trofymow, J.; Constabel, C.P. An improved butanol-HCl assay for quantification of water-soluble, acetone: Methanol-soluble, and insoluble proanthocyanidins (condensed tannins). Plant Methods 2017, 13, 63. [Google Scholar] [CrossRef]
- Ghorai, N.; Chakraborty, S.; Guchhait, S.; Saha, S.; Biswas, S. Estimation of total Terpenoids concentration in plant tissues using a monoterpene, Linalool as standard reagent. Protoc. Exch. 2012, 1–6. [Google Scholar] [CrossRef]
- Zeb, A. Phenolic profile and antioxidant potential of wild watercress (Nasturtium officinale L.). Springerplus 2015, 4, 714–721. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, Q.; Yang, Y. Comprehensive Investigation of Moringa oleifera from Different Regions by Simultaneous Determination of 11 Polyphenols Using UPLC-ESI-MS/MS. Molecules 2020, 25, 676–690. [Google Scholar] [CrossRef]
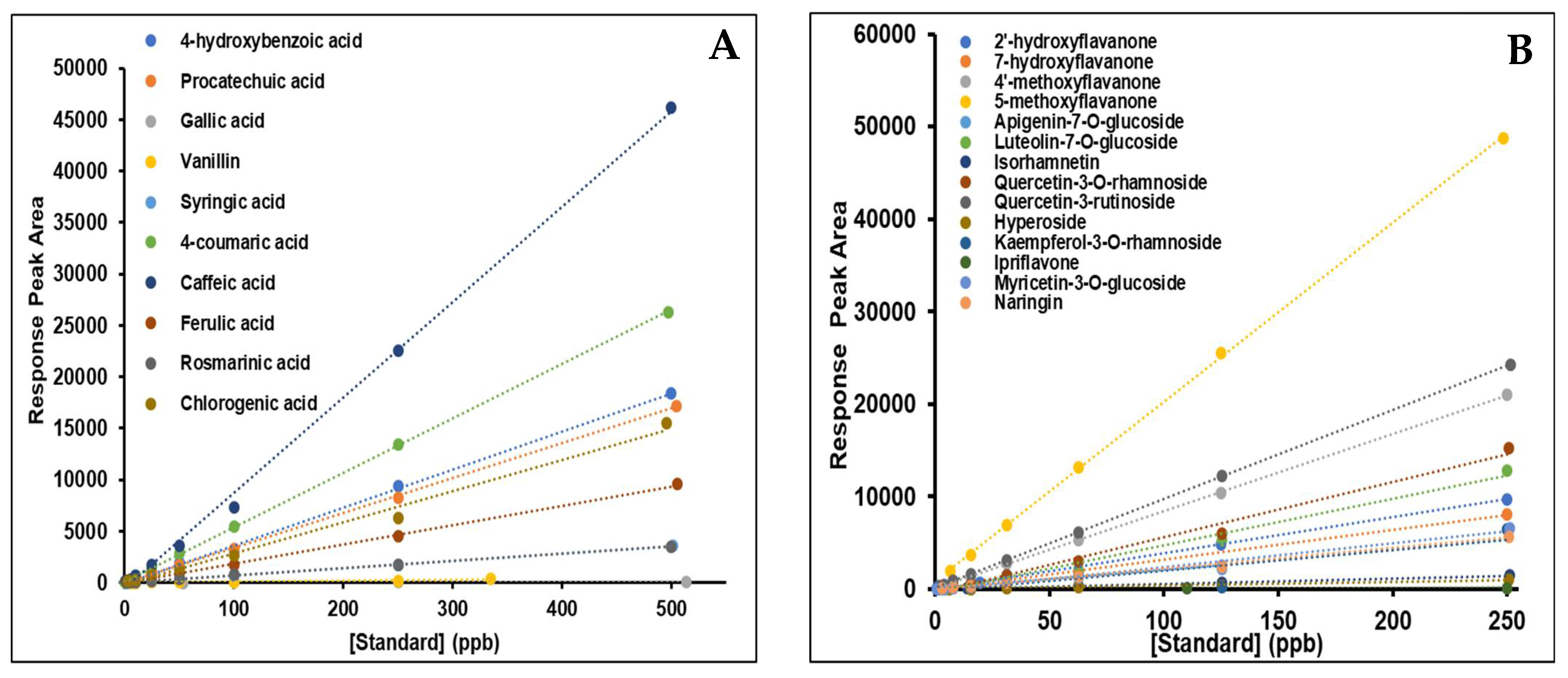

| Compound | Linear Range (ppb) | LOD (ppb) | LOQ (ppb) | Calibration Equation a | Correlation Coefficient (r2) | % RSD | % REC d | |
|---|---|---|---|---|---|---|---|---|
| (Intra-day) b | (Inter-day) c | |||||||
| POLYPHENOLIC COMPOUNDS | ||||||||
| 4-hydroxybenzoic acid | 3.01–499.50 | 3.01 | 14.20 | y = 36.87x − 62.07 | 0.9991 | 1.15 | 2.21 | 98.8 |
| Protocatechuic acid | 0.66–504.50 | 0.66 | 14.70 | y = 34.24x − 69.4 | 0.9995 | 1.25 | 2.65 | 86.3 |
| Gallic acid | 53.20–513.20 | 53.20 | 105.20 | y = 0.67x − 1.5 | 0.9996 | 0.46 | 0.21 | 99.9 |
| Vanillin | 2.87–335.00 | 2.87 | 5.62 | y = 0.67x − 0.1 | 0.9999 | 0.98 | 0.95 | 100.4 |
| Syringic acid | 2.01–501.60 | 2.01 | 2.86 | y = 7.28x − 2.7 | 0.9996 | 1.36 | 1.01 | 96.6 |
| p-coumaric acid | 0.65–497.30 | 0.65 | 1.55 | y = 52.84x + 36.9 | 0.9997 | 1.70 | 1.94 | 93.2 |
| Caffeic acid | 1.21–500 | 1.21 | 1.25 | y = 92.95x − 344.4 | 0.9995 | 1.01 | 2.21 | 100.1 |
| Ferulic acid | 2.10–505.60 | 2.10 | 12.17 | y = 19.02x − 68.4 | 0.9992 | 0.70 | 2.45 | 102.6 |
| Rosmarinic acid | 2.32–499.50 | 2.32 | 2.56 | y = 7.03x + 12.34 | 0.9996 | 1.30 | 3.02 | 86.9 |
| Chlorogenic acid | 3.48–495.60 | 3.48 | 4.76 | y = 25.02x + 60.3 | 0.9991 | 1.35 | 1.98 | 87.4 |
| Ellagic acid | 5.53–499.10 | 5.53 | 75.60 | y = 2.18x + 7.4 | 0.9995 | 1.32 | 3.05 | 89.9 |
| 2′-hydroxyflavanone | 19.50–250.00 | 19.50 | 20.12 | y = 38.69x + 22.5 | 0.9998 | 2.70 | 4.32 | 99.5 |
| 7-hydroxyflavanone | 1.97–249.90 | 1.97 | 2.21 | y = 51.17x − 73.6 | 1.0000 | 2.63 | 1.42 | 98.9 |
| 4′-methoxyflavanone | 2.21–250.00 | 2.21 | 3.89 | y = 83.54x + 60.3 | 0.9999 | 2.89 | 1.87 | 93.6 |
| 5-methoxyflavanone | 6.47–248.50 | 6.47 | 8.52 | y = 195.14x − 493.9 | 0.9992 | 3.21 | 2.69 | 94.7 |
| Apigenin-7-O-glucoside | 1.87–125.30 | 1.87 | 4.42 | y = 6.17x + 3.8 | 0.9998 | 3.48 | 2.54 | 95.8 |
| Luteolin-7-O-glucoside | 2.21–250.10 | 2.21 | 2.22 | y = 51.52x − 89.9 | 0.9998 | 3.64 | 3.22 | 89.2 |
| Isorhamnetin | 14.01–251.1 | 14.01 | 2.31 | y = 6.08x − 15.4 | 0.9992 | 2.48 | 1.18 | 100.1 |
| Quercetin-3-O-rhamnoside | 1.02–250.60 | 1.02 | 4.21 | y = 60.83x − 38.6 | 0.9999 | 2.21 | 3.01 | 99.8 |
| Quercetin-3-O-rutinoside | 1.40–251.30 | 1.40 | 4.32 | y = 97.74x + 109.7 | 0.9999 | 1.35 | 1.89 | 87.4 |
| Hyperoside | 6.32–249.90 | 6.32 | 3.21 | y = 3.97x + 0.5 | 0.9998 | 2.14 | 1.37 | 96.3 |
| Myricetin-3-galactoside | 0.85–251.20 | 0.85 | 2.12 | y = 26.38x − 31.8 | 0.9997 | 1.78 | 1.65 | 100.2 |
| Kaempferol-3-O-rutinoside | 0.76–250.00 | 0.76 | 1.21 | y = 25.73x + 73.7 | 0.9997 | 1.36 | 2.21 | 91.2 |
| Ipriflavone | 109.90–250.00 | 109.90 | 13.21 | y = 0.62x + 2.2 | 0.9994 | 1.69 | 1.11 | 93.6 |
| Naringin | 3.01–250.60 | 3.01 | 1.21 | y = 22.88x − 43.3 | 0.9997 | 2.22 | 4.02 | 95.4 |
| Various Extracts of E. parviflorum | ||||
|---|---|---|---|---|
| Phytochemical | Petroleum | Dichloromethane | Methanol | Expression Units |
| 4-hydroxybenzoic acid | N.D. | 18.20 ± 0.32 a | 18.40 ± 0.64 a | μg/g of dry extract |
| Protocatechuic acid | 1.92 ± 1.01 b | 20.42 ± 1.30 a | 21.95 ± 0.58 a | |
| Gallic acid | 13.36 ± 1.53 a | 38.93 ± 1.41 b | 101.25 ± 1.12 c | |
| Vanillic acid | 3.20 ± 0.01 a | 5.51 ± 0.002 b | 5.86 ± 1.00 c | |
| Syringic acid | 0.26 ± 0.001 a | 0.36 ± 0.01 a | 4.57 ± 0.69 b | |
| p-coumaric acid | N.D. | 12.30 ± 0.59 a | 15.28 ± 0.74 b | |
| Caffeic acid | 0.21 ± 0.001 a | 39.22 ± 1.21 b | 45.68 ± 1.14 c | |
| Ferulic acid | N.D. | N.D. | 4.48 ± 0.04 | |
| Rosmarinic acid | N.D. | N.D. | 6.31 ± 0.11 | |
| Chlorogenic acid | N.D. | N.D. | 7.24 ± 0.01 | |
| Ellagic acid | N.D. | 2.15 ± 0.10 a | 596.10 ± 1.44 b | |
| Total phenols | 60.21 ± 2.25 b | 201.12 ± 13.61 c | 1253.32 ± 19.12 a | μg of gallic acid eq./g of dry extract |
| 2′-hydroxyflavanone | N.D. | 14.05 ± 0.02 b | 4.68 ± 0.17 a | μg/g of dry extract |
| 7-hydroxyflavanone | N.D. | 26.12 ± 1.1 a | 34.59 ± 0.02 b | |
| 4′-methoxyflavanone | 1.12 ± 0.01 a | 2.01 ± 0.02 b | 34.42 ± 0.03 c | |
| 5-methoxyflavanone | N.D. | N.D. | 48.63 ± 0.03 | |
| Apigenin-7-O-glucoside | N.D. | N.D. | 64.10 ± 0.09 | |
| Luteolin-7-O-glucoside | 1.75 ± 0.68 a | 7.23 ± 0.21 b | 19.92 ± 1.00 c | |
| Isorhamnetin | 3.46 ± 0.13 a | 4.35 ± 0.15 b | 50.50 ± 0.13 c | |
| Quercetin-3-O-rhamnoside | N.D. | 0.95 ± 0.01 a | 1.65 ± 0.01 b | |
| Quercetin-3-O-rutinoside | N.D. | N.D. | 62.24 ± 0.02 | |
| Hyperoside | N.D. | 2.21 ± 0.01 a | 6.64 ± 0.02 b | |
| Myricetin-3-galactoside | 1.21 ± 0.63 a | 1.01 ± 0.001 a | 61.10 ± 0.12 b | |
| Kaempferol-3-O-rutinoside | N.D. | 1.11 ± 0.001 a | 32.30 ± 0.10 b | |
| Ipriflavone | 1.35 ± 0.03 a | 27.82 ± 0.12 b | 40.10 ± 0.67 c | |
| Naringin | 1.89 ± 0.11 a | 1.92 ± 0.01 a | 8.43 ± 0.06 b | |
| Total flavonoids | 91.24 ± 1.11 a | 311.68 ± 2.21 b | 642.15 ± 10.35 c | μg of rutin eq./g of dry extract |
| Condensed tannins | 41.78 ± 2.45 a | 59.54 ± 2.14 b | 224.21 ± 1.36 c | μg of catechin eq./g of dry extract |
| Total monoterpenoids | 10.21 ± 0.48 a | 52.23 ± 0.89 c | 21.11 ± 0.94 b | μg of linalool eq./g of dry extract |
| Total soluble protein | 7.48 ± 2.23 a | 49.65 ± 3.64 b | 287.36 ± 10.32 c | mg of BSA eq./g of dry extract |
| Total soluble sugar | 0.42 ± 0.02 a | 1.36 ± 0.02 b | 4.98 ± 0.56 c | nmols of mannose eq./g of dry extract |
| Chlorophyll-a | 36.89 ± 2.23 a | 49.54 ± 2.47 b | 149.36 ± 7.23 c | μg of pigment/g of dry extract |
| Chlorophyll-b | 10.26 ± 0.41 a | 39.14 ± 1.12 b | 245.98 ± 4.11 c | |
| β-Carotene | 57.87 ± 0.01 b | 4.45 ± 0.41 c | 0.24 ± 0.36 a | |
| Lycopene | 66.48 ± 0.27 c | 12.56 ± 1.56 b | 3.42 ± 1.48 a | |
| Time (hr) | Petroleum | Dichloromethane | Methanol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A375 | COLO-679 | HaCaT | A375 | COLO-679 | HaCaT | A375 | COLO-679 | HaCaT | |
| EC50 (μg/mL) | |||||||||
| 24 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 232.7 ± 3.0 | 133.9 ± 3.2 | N.D. |
| 48 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 182.8 ± 4.2 | 73.7 ± 1.2 | N.D. |
| 72 | N.D. | N.D. | N.D. | 470.6 ± 5.7 | 222.8 ± 7.5 | N.D. | 63.3 ± 1.2 | 68.9 ± 1.3 | N.D. |
| Genes | Cell Lines | |
|---|---|---|
| A375 | COLO-679 | |
| Intrinsic Apoptotic Pathway | ||
| CASPASE 3 | 1.942 ± 0.124 | 1.996 ± 0.051 |
| BAX | 2.162 ± 0.262 | 10.510 ± 0.112 |
| BCL2L1 | 4.506 ± 0.034 | 0.320 ± 0.243 |
| BCL2L11 | N.S. | N.S. |
| CAPSASE 9 | 1.732 ± 1.215 | 2.751 ± 0.427 |
| APAF-1 | 1.565 ± 0.102 | 0.889 ± 0.076 |
| BAK | 2.920 ± 0.513 | 1.915 ± 0.063 |
| BID | N.S. | N.S. |
| CYCS | 3.259 ± 0.034 | 1.166 ± 0.139 |
| BAD | 3.087 ± 0.019 | 3.154 ± 0.114 |
| CASPASE 7 | N.S. | N.S. |
| CASPASE 6 | N.S. | N.S. |
| CASPASE 2 | N.S. | N.S. |
| DIABLO | N.S. | N.S. |
| XIAP | 0.268 ± 0.072 | 0.828 ± 0.142 |
| BCL2 | N.S. | N.S. |
| MCL1 | N.S. | N.S. |
| PMAIP1 | N.S. | N.S. |
| EXTRINSIC APOPTOTIC PATHWAY | ||
| TNFRSF1A | 1.975 ± 0.491 | 0.618 ± 0.120 |
| FASL | 0.247 ± 0.646 | 1.107 ± 0.082 |
| CASPASE 8 | 12.064 ± 0.257 | 2.531 ± 0.126 |
| FAS | 0.431 ± 0.050 | 0.610 ± 0.055 |
| CASPASE 10 | N.S. | N.S. |
| TRADD | 0.135 ± 0.039 | N.S. |
| TRAF1 | N.S. | N.S. |
| TNF | N.S. | N.S. |
| TNFRSF1B | 0.162 ± 0.134 | 0.684 ± 0.222 |
| FAIM1 | 0.300 ± 0.121 | 0.552 ± 0.029 |
| FADD | 0.1020 ± 0.093 | 0.469 ± 0.073 |
| C-FLAR | N.S. | N.S. |
| TNFRSF10 | N.S. | N.S. |
| TNFRSF10C | 0.459 ± 0.027 | 0.074 ± 0.120 |
| TNFRSF10A | 0.194 ± 0.232 | 0.384 ± 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriakou, S.; Tragkola, V.; Paraskevaidis, I.; Plioukas, M.; Trafalis, D.T.; Franco, R.; Pappa, A.; Panayiotidis, M.I. Chemical Characterization and Biological Evaluation of Epilobium parviflorum Extracts in an In Vitro Model of Human Malignant Melanoma. Plants 2023, 12, 1590. https://doi.org/10.3390/plants12081590
Kyriakou S, Tragkola V, Paraskevaidis I, Plioukas M, Trafalis DT, Franco R, Pappa A, Panayiotidis MI. Chemical Characterization and Biological Evaluation of Epilobium parviflorum Extracts in an In Vitro Model of Human Malignant Melanoma. Plants. 2023; 12(8):1590. https://doi.org/10.3390/plants12081590
Chicago/Turabian StyleKyriakou, Sotiris, Venetia Tragkola, Ioannis Paraskevaidis, Mihalis Plioukas, Dimitrios T. Trafalis, Rodrigo Franco, Aglaia Pappa, and Mihalis I. Panayiotidis. 2023. "Chemical Characterization and Biological Evaluation of Epilobium parviflorum Extracts in an In Vitro Model of Human Malignant Melanoma" Plants 12, no. 8: 1590. https://doi.org/10.3390/plants12081590
APA StyleKyriakou, S., Tragkola, V., Paraskevaidis, I., Plioukas, M., Trafalis, D. T., Franco, R., Pappa, A., & Panayiotidis, M. I. (2023). Chemical Characterization and Biological Evaluation of Epilobium parviflorum Extracts in an In Vitro Model of Human Malignant Melanoma. Plants, 12(8), 1590. https://doi.org/10.3390/plants12081590








