Protective Effect of the Phycobiliproteins from Arthrospira maxima on Indomethacin-Induced Gastric Ulcer in a Rat Model
Abstract
1. Introduction
2. Results
2.1. Evaluation of Phycobiliprotein Content and Purity of PhyEx
2.2. Antiulcerogenic Activity of the Aqueous Extract
2.3. Histopathology
2.4. Antioxidant Enzymes and Lipoperoxidation
3. Discussion
4. Materials and Methods
4.1. Preparation of the Aqueous Extract Rich in Phycobiliprotein
4.2. Animals and Housing
4.3. Drugs and Chemicals
4.4. Antiulcerogenic Activity of the Aqueous Extract
4.5. Enzymatic Activity
4.5.1. Stomach Tissue Preparation
4.5.2. Glutathione Peroxidase Activity
4.5.3. Superoxide Dismutase Activity
4.5.4. Catalase Activity
4.6. Total Proteins
4.7. Lipoperoxidation Assessment
4.8. Histopathological Examination
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, T.F.; Gnanapandithan, K.; Singh, K. Peptic Ulcer Disease; StatPearls Publish: Tampa, FL, USA, 2022; p. 30521213. [Google Scholar]
- Prabhu, V.; Shivani, A. An overview of history, pathogenesis and treatment of perforated peptic ulcer disease with evaluation of prognostic scoring in adults. Ann. Med. Health Sci. Res. 2014, 4, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Yu, J.; Choi, H.H.; Jeon, B.S.; Kim, H.K.; Kim, S.W.; Kim, S.S.; Park, Y.G.; Chae, H.S. The association between peptic ulcer diseases and mental health problems: A population-based study: A STROBE compliant article. Medicine 2017, 96, e7828. [Google Scholar] [CrossRef]
- Lanas, A.; Chan, F.K.L. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef]
- Najm, W.I. Peptic ulcer disease. Prim. Care 2011, 38, 383–394. [Google Scholar] [CrossRef]
- Axon, A. Helicobacter pylori and public health. Helicobacter 2014, 1, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Vomero, N.D.; Colpo, E. Nutritional care in peptic ulcer. Arq. Bras Cir. Dig. 2014, 27, 298–302. [Google Scholar] [CrossRef]
- Ren, J.; Jin, X.; Li, J.; Li, R.; Gao, Y.; Zhang, J.; Wang, X.; Wang, G. The global burden of peptic ulcer disease in 204 countries and territories from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Int. J. Epidemiol. 2022, 51, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Kangevari, M.; Ahmadi, N.; Fattahi, N.; Rezaei, N.; Malekpour, M.R.; Ghamari, S.H.; Moghaddam, S.S.; Azadnajafabad, S.; Esfahani, Z.; Kolahi, A.A.; et al. Quality of care of peptic ulcer disease worldwide: A systematic analysis for the global burden of disease study 1990-2019. PLoS ONE 2022, 17, e0271284. [Google Scholar] [CrossRef]
- Taş, İ.; Ülger, B.V.; Önder, A.; Kapan, M.; Bozdağ, Z. Risk factors influencing morbidity and mortality in perforated peptic ulcer disease. Ulus. Cerrahi Derg. 2014, 31, 20–25. [Google Scholar] [CrossRef]
- Lanas, Á.; Carrera-Lasfuentes, P.; Arguedas, Y.; García, S.; Bujanda, L.; Calvet, X.; Ponce, J.; Perez-Aísa, Á.; Castro, M.; Muñoz, M.; et al. Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants. Clin. Gastroenterol. Hepatol. 2015, 13, 906–912.e2. [Google Scholar] [CrossRef]
- Xie, X.; Ren, K.; Zhou, Z.; Dang, C.; Zhang, H. The global, regional and national burden of peptic ulcer disease from 1990 to 2019: A population-based study. BMC Gastroenterol. 2022, 22, 58. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; Reddy, K.M.; Marsicano, E. Peptic Ulcer Disease and Helicobacter pylori infection. Mo. Med. 2018, 115, 219–224. [Google Scholar]
- Laine, L.; Takeuchi, K.; Tarnawski, A. Gastric mucosal defense and cytoprotection: Bench to bedside. Gastroenterology 2008, 135, 41–60. [Google Scholar] [CrossRef]
- Azhari, H.; Underwood, F.; King, J.; Coward, S.; Shah, S.; Chan, C.; Ho, G.; Ng, S.; Kaplan, G. The global incidence of peptic ulcer disease and its complications at the turn of the 21st century: A systematic review. Am. J. Gastroenterol. 2018, 113, S684–S685. [Google Scholar] [CrossRef]
- Takeuchi, K.; Amagase, K. Roles of cyclooxygenase, prostaglandin e2 and ep receptors in mucosal protection and ulcer healing in the gastrointestinal tract. Curr. Pharm. Des. 2018, 24, 2002–2011. [Google Scholar] [CrossRef]
- Peng, H.; Chen, F.E. Recent advances in asymmetric total synthesis of prostaglandins. Org. Biomol. Chem. 2017, 15, 6281–6301. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, R.; Cuzzolin, L.; Arceri, A.; Fanos, V. Urinary prostaglandin E2 in the newborn and infant. Prostaglandins Other Lipid Mediat. 2007, 84, 1–13. [Google Scholar] [CrossRef]
- Fujii, S.; Suzuki, K.; Kawamoto, A.; Ishibashi, F.; Nakata, T.; Murano, T.; Ito, G.; Shimizu, H.; Mizutani, T.; Oshima, S.; et al. PGE2 is a direct and robust mediator of anion/fluid secretion by human intestinal epithelial cells. Sci. Rep. 2016, 6, 36795. [Google Scholar] [CrossRef]
- Kirkby, N.S.; Chan, M.V.; Zaiss, A.K.; Garcia-Vaz, E.; Jiao, J.; Berglund, L.M.; Verdu, E.F.; Ahmetaj-Shala, B.; Wallace, J.L.; Herschman, H.R.; et al. Systematic study of constitutive cyclooxygenase-2 expression: Role of NF-κB and NFAT transcriptional pathways. Proc. Natl. Acad. Sci. USA 2016, 113, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Matsui, H.; Hirayama, A.; Indo, H.P.; Majima, H.J.; Hyodo, I. Reactive oxygen species induced by non-steroidal anti-inflammatory drugs enhance the effects of photodynamic therapy in gastric cancer cells. J. Clin. Biochem. Nutr. 2016, 58, 180–185. [Google Scholar] [CrossRef]
- Zhang, M.; Xia, F.; Xia, S.; Zhou, W.; Zhang, Y.; Han, X.; Zhao, K.; Feng, L.; Dong, R.; Tian, D.; et al. NSAID-Associated Small Intestinal Injury: An Overview From Animal Model Development to Pathogenesis, Treatment, and Prevention. Front. Pharmacol. 2022, 13, 818877. [Google Scholar] [CrossRef]
- Papalia, T.; Sidari, R.; Panuccio, M.R. Impact of Different Storage Methods on Bioactive Compounds in Arthrospira platensis Biomass. Molecules 2019, 24, 2810. [Google Scholar] [CrossRef] [PubMed]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, Immunomodulating, and Microbial-Modulating Activities of the Sustainable and Ecofriendly Spirulina. Oxid. Med. Cell. Longev. 2017, 2017, 3247528. [Google Scholar] [CrossRef] [PubMed]
- Ragab, F.M.; Alagawany, M.; Ezzat Abd El-Hack, M.; Dhama, K. Nutritional and Healthical Aspects of Spirulina (Arthrospira) for Poultry, Animals and Human. Int. J. Pharmacol. 2016, 12, 36–51. [Google Scholar] [CrossRef]
- Sánchez García, I.I.; Medina Jaritz, N.B.; Olvera Ramírez, R. Antioxidant Effect of Phycobiliproteins of the Cyanobacteria Arthrospira maxima on Growth of Saccharomyces cerevisiae under Oxidative Stress. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 233–239. [Google Scholar] [CrossRef]
- Kamran, S.; Karimian, H.; Salehen, N.; Khalajhedayati, A.; Abdul Razak, B.; Abdul Majid, N. Acute Toxicity and Gastroprotective Effect of 2-pentadecanone in Ethanol-induced Gastric Mucosal Ulceration in Rats. Int. J. Pharmacol. 2019, 15, 944–952. [Google Scholar] [CrossRef]
- Pan-utai, W.; Iamtham, S. Extraction, purification and antioxidant activity of phycobiliprotein from Arthrospira platensis. Process Biochem. 2019, 82, 189–198. [Google Scholar] [CrossRef]
- Chandra, R.; Parra, R.; Iqbal, H.M. Phycobiliproteins: A Novel Green Tool from Marine Origin Blue-Green Algae and Red Algae. Protein Pept. Lett. 2017, 24, 118–125. [Google Scholar] [CrossRef]
- Sonani, R.R.; Rastogi, R.P.; Madamwar, D. Antioxidant Potential of Phycobiliproteins: Role in Anti-Aging Research. Biochem. Anal. Biochem. 2015, 4, 1000172. [Google Scholar] [CrossRef]
- Guzmán-Gómez, O.; García-Rodríguez, R.V.; Quevedo-Corona, L.; Pérez-Pastén-Borja, R.; Rivero-Ramírez, N.L.; Ríos-Castro, E.; Pérez-Gutiérrez, S.; Pérez-Ramos, J.; Chamorro-Cevallos, G.A. Amelioration of Ethanol-Induced Gastric Ulcers in Rats Pretreated with Phycobiliproteins of Arthrospira (Spirulina) Maxima. Nutrients 2018, 10, 763. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, F.; Yuan, X.; Yang, T.; Liang, X.; Wang, Y.; Tu, H.; Chang, J.; Nan, K.; Wei, Y. Reactive Oxygen Species Are Involved in the Development of Gastric Cancer and Gastric Cancer-Related Depression through ABL1-Mediated Inflammation Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 5813985. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Chen, H.; Wang, Y.; Lin, G.; Li, C.; Tan, L.; Su, Z.; Lai, X.; Xie, J.; Zeng, H. Protective effect of coptisine free base on indomethacin-induced gastric ulcers in rats: Characterization of potential molecular mechanisms. Life Sci. 2018, 193, 47–56. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Fokou, P.V.T.; Sharopov, F.; Martorell, M.; Ademiluyi, A.O.; Rajkovic, J.; Salehi, B.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Antiulcer Agents: From Plant Extracts to Phytochemicals in Healing Promotion. Molecules 2018, 23, 1751. [Google Scholar] [CrossRef]
- Park, J.U.; Kang, J.H.; Rahman, M.A.A.; Hussain, A.; Cho, J.S.; Lee, Y.I. Gastroprotective Effects of Plants Extracts on Gastric Mucosal Injury in Experimental Sprague-Dawley Rats. BioMed Res. Int. 2019, 2019, 8759708. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, S.; Arian, A.A.; Farzaei, M.H. Gastroprotective effect of aqueous stem bark extract of Ziziphus jujuba L. against HCl/Ethanol-induced gastric mucosal injury in rats. J. Tradit. Chin. Med. 2015, 35, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.M.; El-Sammad, N.M.; Hassan, S.K.; Madboli, A.E.N.A.; Hashim, A.N.; Moustafa, E.S.; Bakry, S.M.; Elsayed, E.A. Antiulcerogenic effect of Cuphea ignea extract against ethanol-induced gastric ulcer in rats. BMC Complement. Altern. Med. 2019, 19, 345. [Google Scholar] [CrossRef]
- Mahmoud, Y.I.; Abd El-Ghffar, E.A. Spirulina ameliorates aspirin-induced gastric ulcer in albino mice by alleviating oxidative stress and inflammation. Biomed. Pharmacother. 2019, 109, 314–321. [Google Scholar] [CrossRef]
- Rito-Palomares, M.; Nuez, L.; Amador, D. Practical Application of Aqueous Two-Phase Systems for the Development of a Prototype Process for c-Phycocyanin Recovery from Spirulina Maxima. J. Chem. Technol. Biotechnol. 2001, 76, 1273–1280. [Google Scholar] [CrossRef]
- Dagnino-Leone, J.; Figueroa, C.P.; Castañeda, M.L.; Youlton, A.D.; Vallejos-Almirall, A.; Agurto-Muñoz, A.; Pavón Pérez, J.; Agurto-Muñoz, C. Phycobiliproteins: Structural aspects, functional characteristics, and biotechnological perspectives. Comput. Struct. Biotechnol. J. 2022, 20, 1506–1527. [Google Scholar] [CrossRef]
- Sonani, R.R.; Singh, N.K.; Kumar, J.; Thakar, D.; Madamwar, D. Concurrent purification and antioxidant activity of phycobiliproteins from Lyngbya sp. A09DM: An antioxidant and anti-aging potential of phycoerythrin in Caenorhabditis elegans. Process. Biochem. 2014, 49, 1757–1766. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Yin, H.; Pan, X.; Song, Z.; Wang, S.; Yang, L.; Sun, G. Protective effect of wheat peptides against indomethacin-induced oxidative stress in IEC-6 cells. Nutrients 2014, 6, 564–574. [Google Scholar] [CrossRef]
- Kalinina, E.V.; Gavriliuk, L.A.; Pokrovsky, V.S. Oxidative Stress and Redox-Dependent Signaling in Prostate Cancer. Biochemistry 2022, 87, 413–424. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Nishizawa, T.; Tsugawa, H.; Mogami, S.; Hibi, T. Roles of oxidative stress in stomach disorders. J. Clin. Biochem. Nutr. 2012, 50, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Blas-Valdivia, V.; Rojas-Franco, P.; Serrano-Contreras, J.I.; Sfriso, A.A.; Garcia-Hernandez, C.; Franco-Colín, M.; Cano-Europa, E. C-phycoerythrin from Phormidium persicinum Prevents Acute Kidney Injury by Attenuating Oxidative and Endoplasmic Reticulum Stress. Mar. Drugs 2021, 19, 589. [Google Scholar] [CrossRef] [PubMed]
- Soni, B.; Visavadiya, N.P.; Dalwadi, N.; Madamwar, D.; Winder, C.; Khalil, C. Purified c-phycoerythrin: Safety studies in rats and protective role against permanganate-mediated fibroblast-DNA damage. J. Appl. Toxicol. 2010, 30, 542–550. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Yin, Q.; Liu, G.; Liu, H.; Huang, Y.; Li, B. Phycocyanin: A Potential Drug for Cancer Treatment. J. Cancer 2017, 8, 3416–3429. [Google Scholar] [CrossRef] [PubMed]
- Madhyastha, H.K.; Radha, K.S.; Nakajima, Y.; Omura, S.; Maruyama, M. uPA dependent and independent mechanisms of wound healing by C-phycocyanin. J. Cell. Mol. Med. 2008, 12, 2691–2703. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, T.; Konturek, P.C.; Konturek, S.J.; Brzozowska, I.; Pawlik, T. Role of prostaglandins in gastroprotection and gastric adaptation. J. Physiol. Pharmacol. 2005, 56, 33–55. [Google Scholar]
- Wallace, J.L. COX-2: A pivotal enzyme in mucosal protection and resolution of inflammation. Sci. World J. 2006, 6, 577–588. [Google Scholar] [CrossRef]
- Halter, F.; Tarnawski, A.S.; Schmassmann, A.; Peskar, B.M. Cyclooxygenase 2-implications on maintenance of gastric mucosal integrity and ulcer healing: Controversial issues and perspectives. Gut 2007, 49, 443–453. [Google Scholar] [CrossRef]
- Reddy, C.M.; Bhat, V.B.; Kiranmai, G.; Reddy, M.N.; Reddanna, P.; Madyastha, K.M. Selective inhibition of cyclooxygenase-2 by C-phycocyanin, a biliprotein from Spirulina platensis. Biochem. Biophys. Res. Commun. 2000, 277, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.; De, R.; Debsharma, S.; Bindu, S.; Maity, P.; Sarkar, S.; Jyoti, S.; Azhar-Siddiqui, A.; Banerjee, C.; Nag, S.; et al. Indomethacin impairs mitochondrial dynamics by activating the PKCζ-p38-DRP1 pathway and inducing apoptosis in gastric cancer and normal mucosal cells. J. Biol. Chem. 2019, 294, 8238–8258. [Google Scholar] [CrossRef] [PubMed]
- Chanudom, L.; Tangpong, J. Anti-Inflammation Property of Syzygium cumini (L.) Skeels on Indomethacin-Induced Acute Gastric Ulceration. Gastroenterol. Res. Pract. 2015, 2015, 343642. [Google Scholar] [CrossRef]
- Alsherbiny, M.A.; Abd-Elsalam, W.H.; El-Badawy, S.H.; Taher, E.; Fares, M.; Torres, A.; Chang, D.; Guang-Li, C. Ameliorative and protective effects of ginger and its main constituents against natural, chemical and radiation-induced toxicities: A comprehensive review. Food Chem. Toxicol. 2019, 123, 72–97. [Google Scholar] [CrossRef]
- Santin, J.R.; Lemos, M.; Klein-Júnior, L.C.; Machado, I.D.; Costa, P.; de Oliveira, A.P.; Tilia, C.; de Souza, J.P.; de Sousa, J.P.; Bastos, J.K.; et al. Gastroprotective activity of essential oil of the Syzygium aromaticum and its major component eugenol in different animal models. Naunyn. Schmiedebergs Arch. Pharmacol. 2011, 383, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Oluwabunmi, I.J.; Abiola, T. Gastroprotective effect of methanolic extract of Gomphrena celosioides on indomethacin induced gastric ulcer in Wistar albino rats. Int. J. Appl. Basic Med. Res. 2015, 5, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.A.; da Silva-Reis, L.; Nogueira-Mendes, A.; de Barros-Fernandes, H.; Días-Rufino-Arcanjo, D.; Fortes-Rodrigues Júnior, A.; Martins Sousa, J.; Medeiros-Barreto, H.; de Araújo-Santos, J.; Anteveli-Osajima, J.; et al. Antiulcerogenic and Antibacterial Effects of Chitosan Derivatives on Experimental Gastric Ulcers in Rats. Evid. Based Complement. Altern. Med. 2022, 2022, 4743041. [Google Scholar] [CrossRef]
- Arun, M.; Asha, V.V. Gastroprotective effect of Dodonaea viscosa on various experimental ulcer models. J. Ethnopharmacol. 2008, 118, 460–465. [Google Scholar] [CrossRef]
- Alirezaei, M.; Dezfoulian, O.; Neamati, S.; Rashidipour, M.; Tanideh, N.; Kheradmand, A. Oleuropein prevents ethanol-induced gastric ulcers via elevation of antioxidant enzyme activities in rats. J. Physiol. Biochem. 2012, 68, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Adinortey, M.B.; Ansah, C.; Galyuon, I.; Nyarko, A. In Vivo Models Used for Evaluation of Potential Antigastroduodenal Ulcer Agents. Ulcers 2013, 2013, 796405. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [CrossRef]
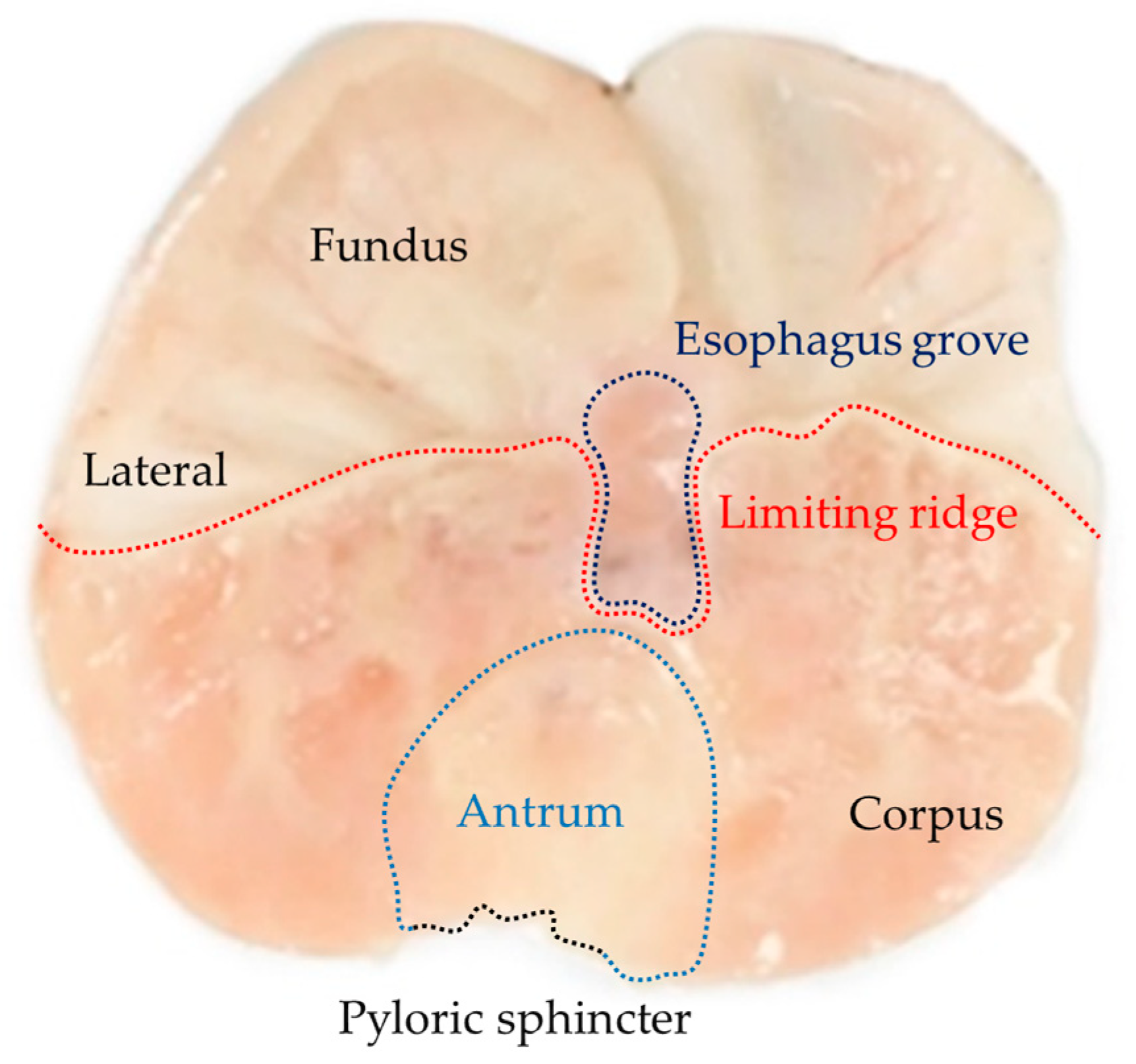
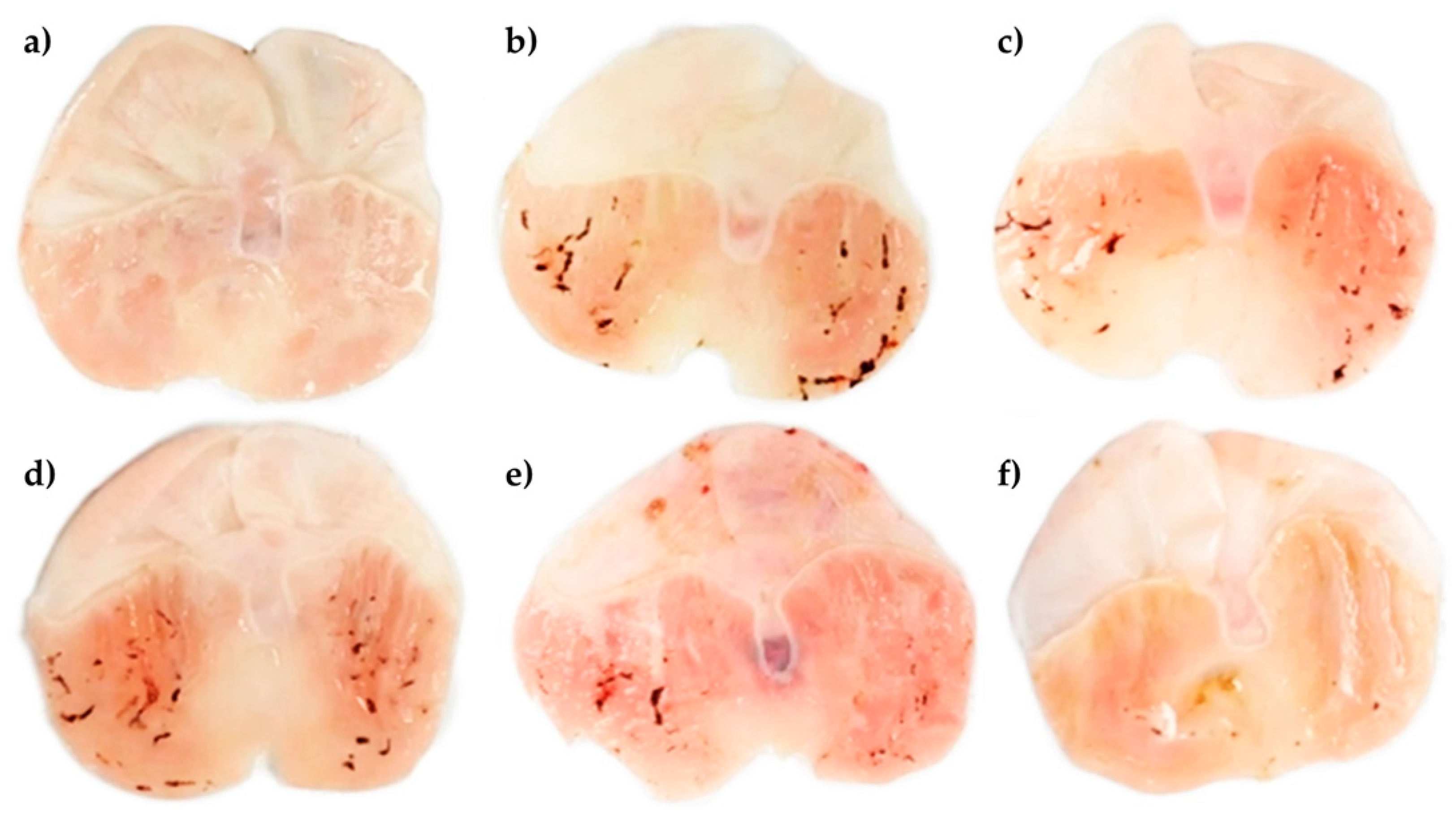
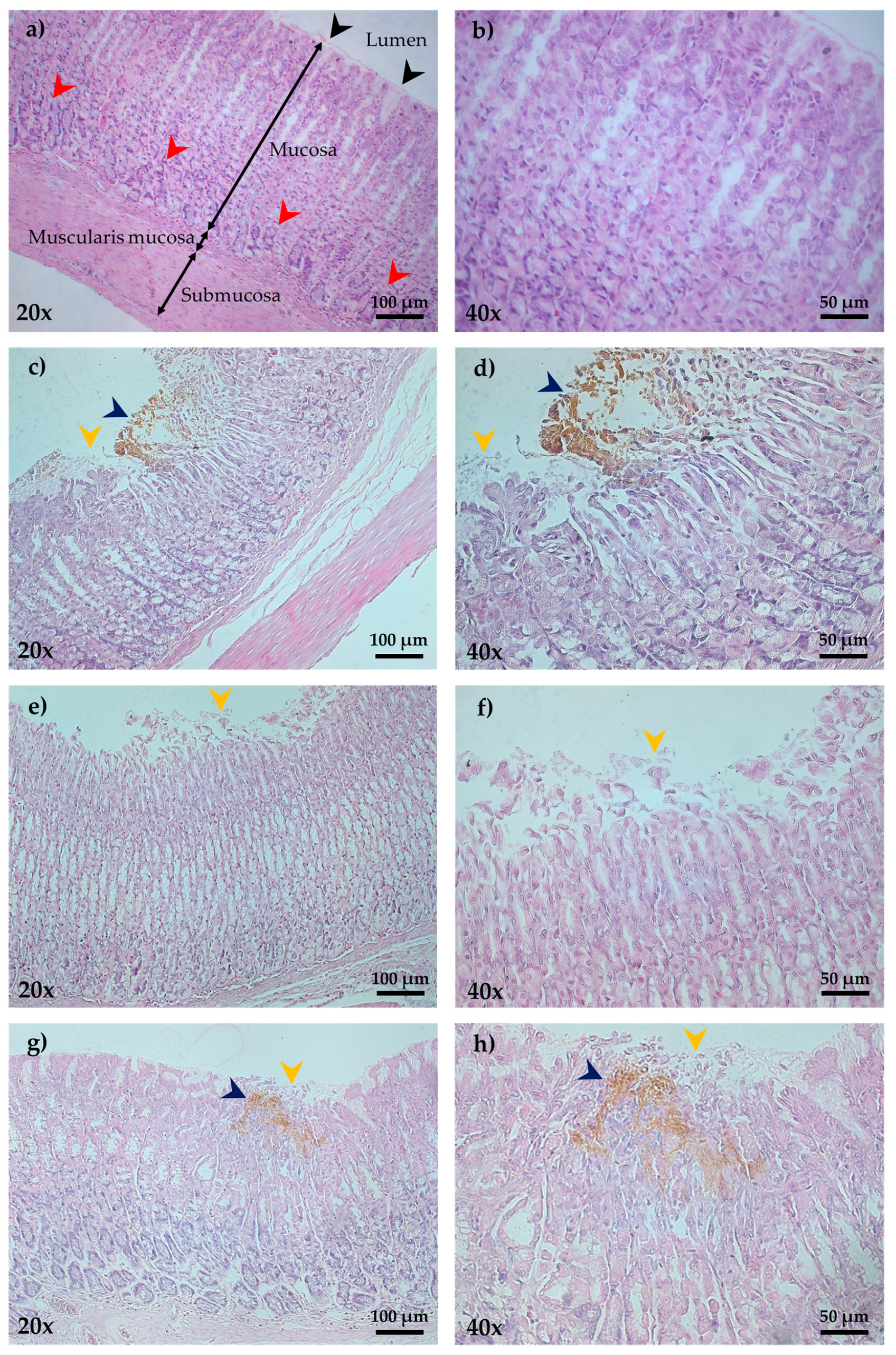
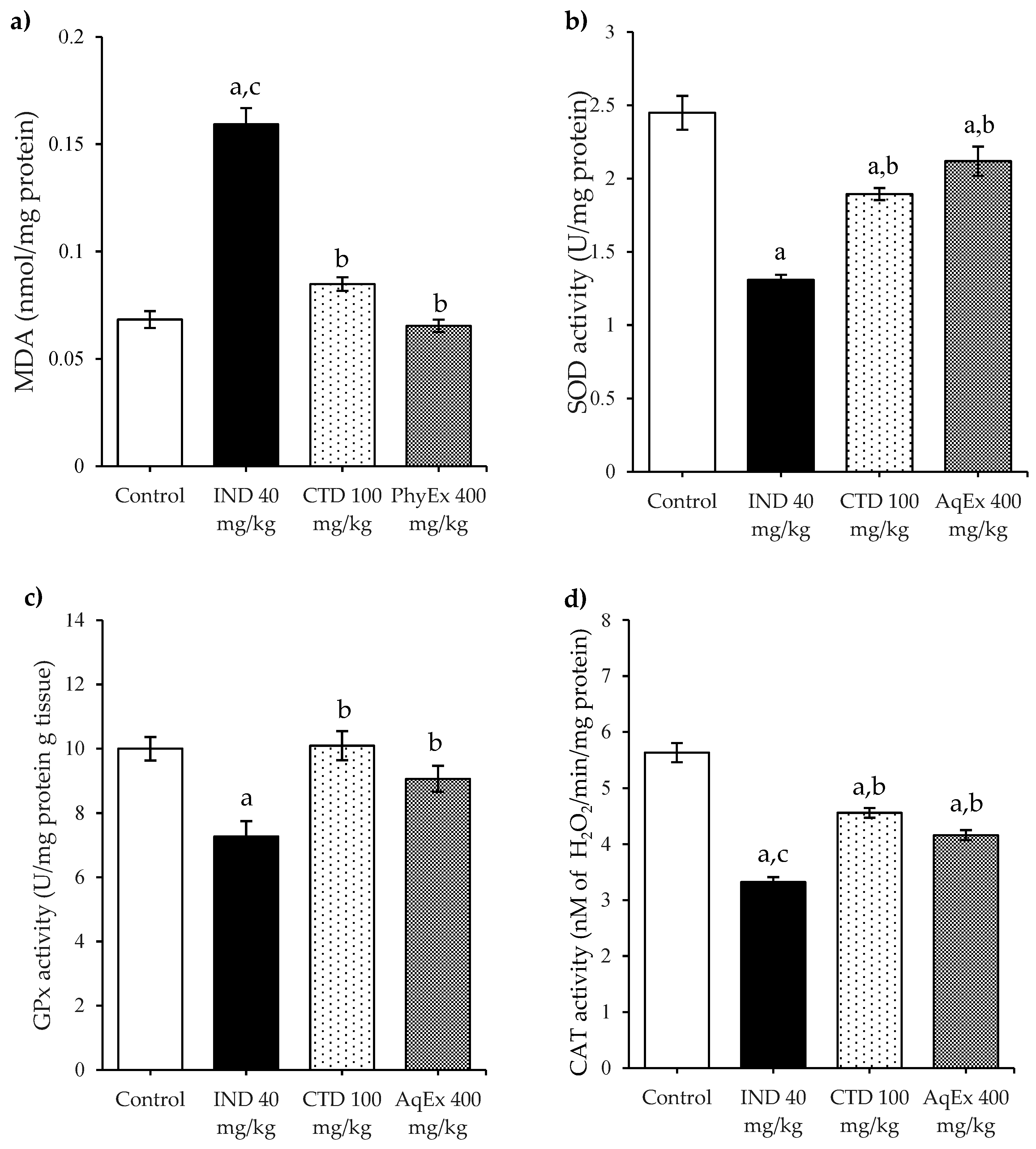

| Groups | Treatments (mg/kg) | Ulcer Index (mm2) | Protection Percentage (%) |
|---|---|---|---|
| I | Control (vehicle) | 0.0 ± 0.00 | 0.0 |
| II | Vehicle + IND 40 | 2.81 ± 0.33 | 0.0 |
| III | CTD 100 + IND 40 | 0.15 ± 0.01 | 94.59 a |
| IV | PhyEx 100 + IND 40 | 2.07 ± 0.22 | 26.47 a |
| V | PhyEx 200 + IND 40 | 2.15 ± 0.23 | 23.57 a |
| VI | PhyEx 400 + IND 40 | 0.92 ± 0.17 | 67.19 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-Gómez, O.; García-Rodríguez, R.V.; Pérez-Gutierrez, S.; Rivero-Ramírez, N.L.; García-Martínez, Y.; Pablo-Pérez, S.S.; Pérez-Pastén-Borja, R.; Cristóbal-Luna, J.M.; Chamorro-Cevallos, G. Protective Effect of the Phycobiliproteins from Arthrospira maxima on Indomethacin-Induced Gastric Ulcer in a Rat Model. Plants 2023, 12, 1586. https://doi.org/10.3390/plants12081586
Guzmán-Gómez O, García-Rodríguez RV, Pérez-Gutierrez S, Rivero-Ramírez NL, García-Martínez Y, Pablo-Pérez SS, Pérez-Pastén-Borja R, Cristóbal-Luna JM, Chamorro-Cevallos G. Protective Effect of the Phycobiliproteins from Arthrospira maxima on Indomethacin-Induced Gastric Ulcer in a Rat Model. Plants. 2023; 12(8):1586. https://doi.org/10.3390/plants12081586
Chicago/Turabian StyleGuzmán-Gómez, Oscar, Rosa Virginia García-Rodríguez, Salud Pérez-Gutierrez, Nora Lilia Rivero-Ramírez, Yuliana García-Martínez, Saudy Saret Pablo-Pérez, Ricardo Pérez-Pastén-Borja, José Melesio Cristóbal-Luna, and Germán Chamorro-Cevallos. 2023. "Protective Effect of the Phycobiliproteins from Arthrospira maxima on Indomethacin-Induced Gastric Ulcer in a Rat Model" Plants 12, no. 8: 1586. https://doi.org/10.3390/plants12081586
APA StyleGuzmán-Gómez, O., García-Rodríguez, R. V., Pérez-Gutierrez, S., Rivero-Ramírez, N. L., García-Martínez, Y., Pablo-Pérez, S. S., Pérez-Pastén-Borja, R., Cristóbal-Luna, J. M., & Chamorro-Cevallos, G. (2023). Protective Effect of the Phycobiliproteins from Arthrospira maxima on Indomethacin-Induced Gastric Ulcer in a Rat Model. Plants, 12(8), 1586. https://doi.org/10.3390/plants12081586









