A Mixture of Artemisia argyi and Saururus chinensis Improves PM2.5-Induced Cognitive Dysfunction by Regulating Oxidative Stress and Inflammatory Response in the Lung and Brain
Abstract
1. Introduction
2. Results and Discussion
2.1. Main Compounds Analysis in AASC Using UPLC/Q-TOF-MS/MS
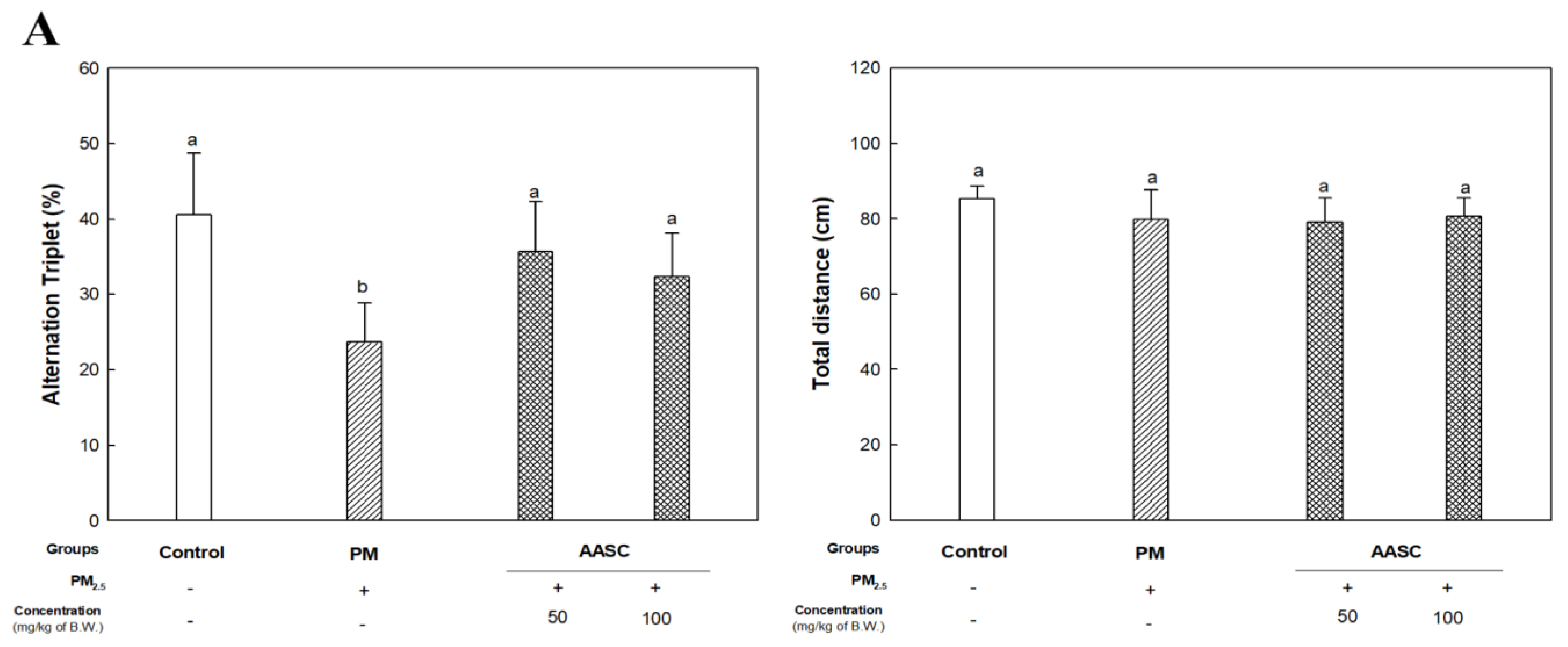
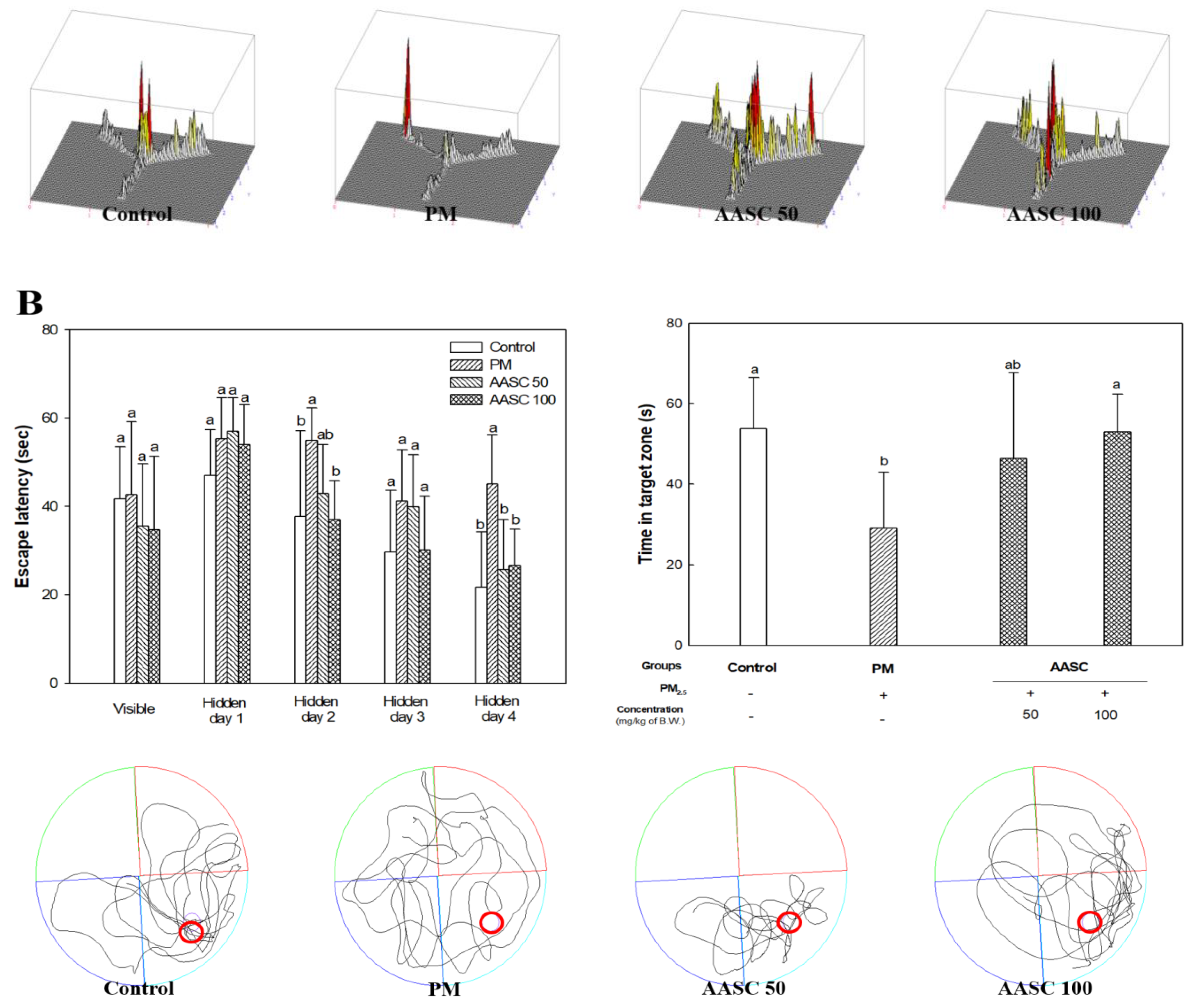
2.2. Evaluation of Cognitive Function with Behavioral Tests
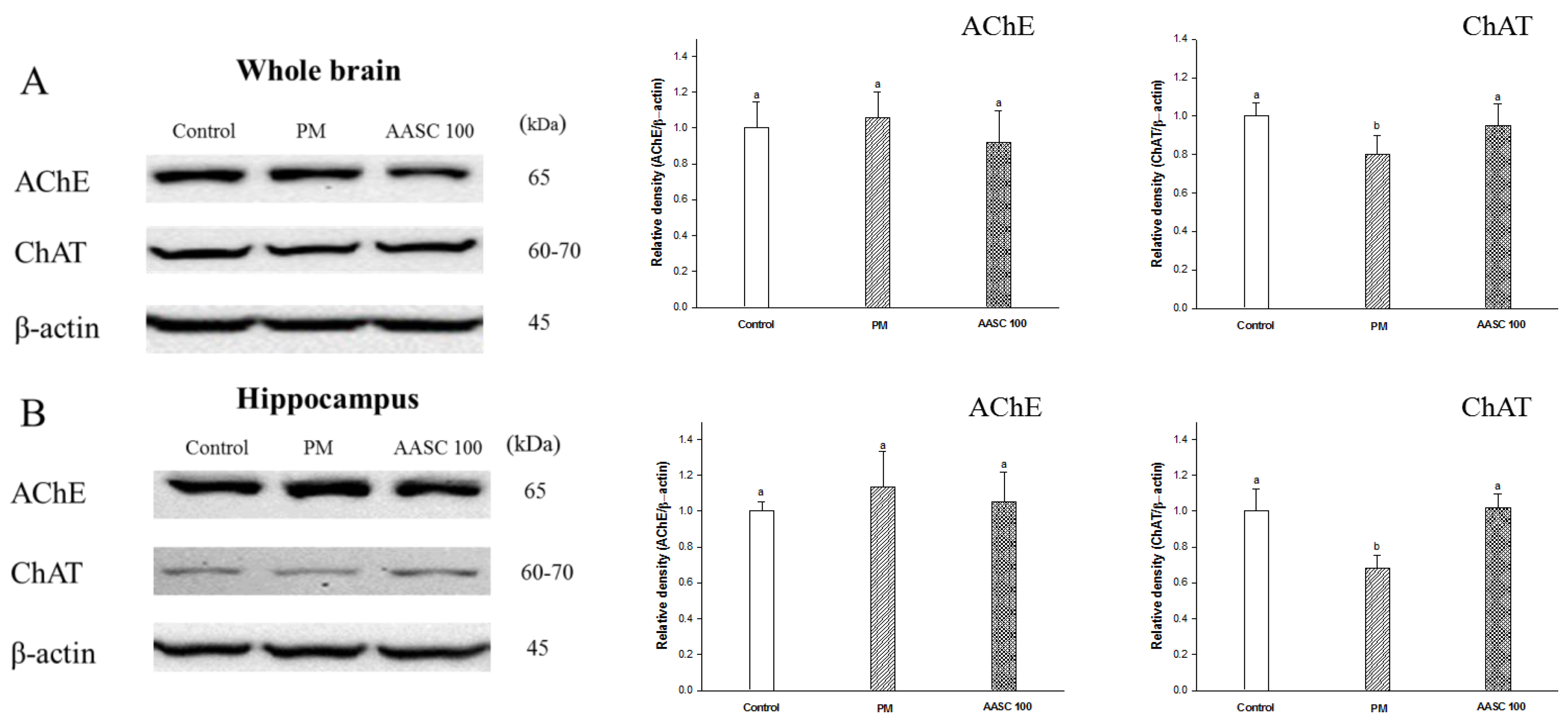
2.3. Analysis of the Cholinergic System in Brain Tissue
2.4. Measurement of Oxidative Damage in the Brain, Lung Tissues, and Serum
2.5. Measurement of Mitochondrial Function in the Brain and Lung Tissues
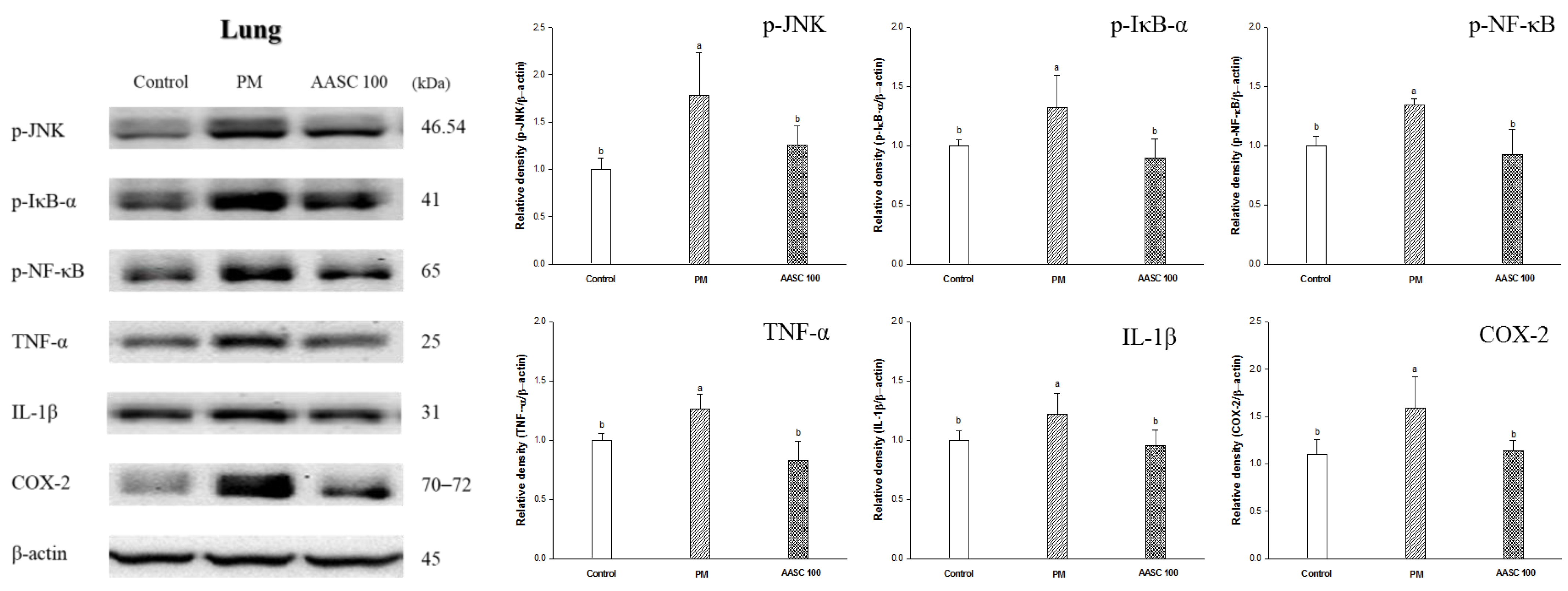
2.6. Protein Expression in Lung Tissue
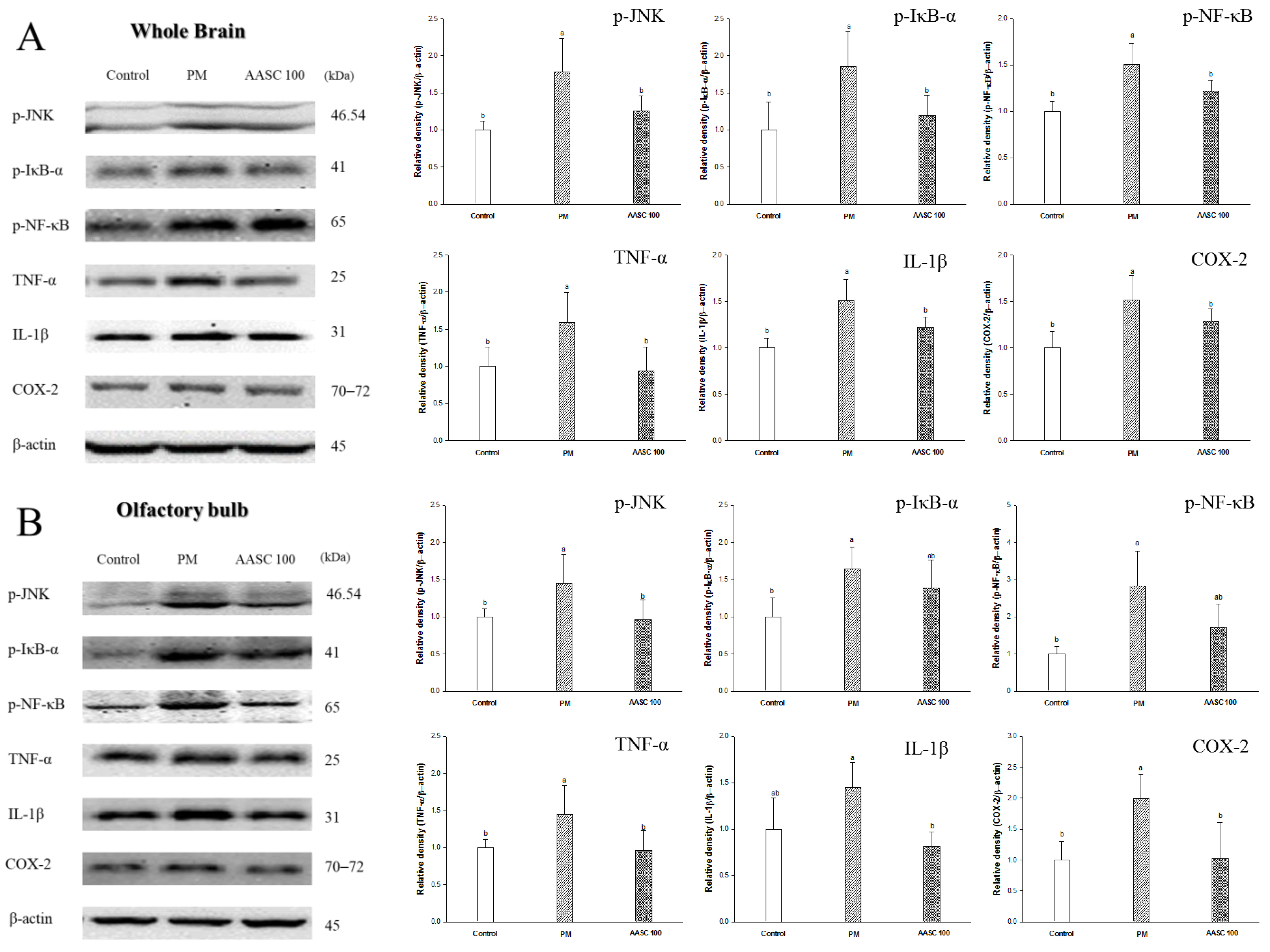
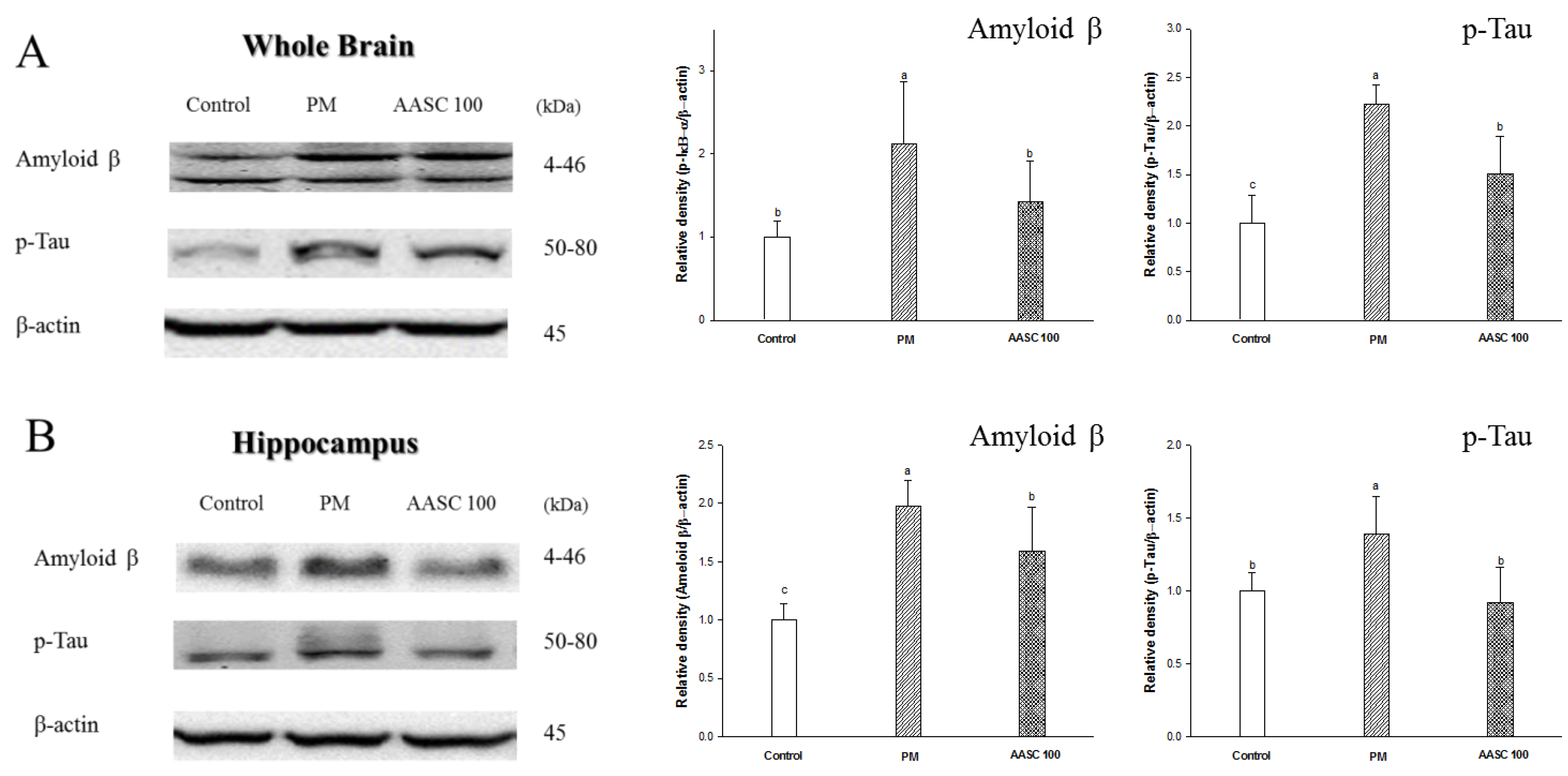
2.7. Protein Expression in Brain Tissue
3. Materials and Methods
3.1. Sample Preparation
3.1.1. Sample Extraction
3.1.2. Main Compounds Analysis Using UPLC/Q-TOF-MS/MS
3.2. Animal Experimental Design
3.3. Behavioral Tests
3.3.1. Y-Maze Test
3.3.2. Morris Water Maze Test
3.4. Cholinergic System
3.4.1. Tissue Pretreatment
3.4.2. ACh Content
3.4.3. AChE Activity
3.5. Serum Biomarkers Analysis
3.5.1. Serum LDH Levels
3.5.2. Serum FRAP Activity
3.6. Oxidative Stress and Mitochondrial Function
3.6.1. Tissue Pretreatment
3.6.2. Antioxidant System
3.6.3. Mitochondria Function
3.7. Western Blot Analysis
3.7.1. Protein Extraction
3.7.2. Western Blotting
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.N.; Balde, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Solt, A.C.; Henríquez-Roldán, C.; Torres-Jardón, R.; Nuse, B.; Herritt, L.; Villarreal-Calderón, R.; Osnaya, N.; Stone, I.; Garcia, R. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid β-42 and α-synuclein in children and young adults. Toxicol. Pathol. 2008, 36, 289–310. [Google Scholar] [CrossRef] [PubMed]
- Ajmani, G.S.; Suh, H.H.; Pinto, J.M. Effects of Ambient Air Pollution Exposure on Olfaction: A Review. Environ. Health Perspect. 2006, 124, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Underwood, E. The polluted brain. Science 2017, 355, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Xu, Y.; Shi, M.; Lian, Y. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69. [Google Scholar] [PubMed]
- Alaklabi, A.; Arif, I.A.; Ahamed, A.; Kumar, R.S.; Idhayadhulla, A. Evaluation of antioxidant and anticancer activities of chemical constituents of the Saururus chinensis root extracts. Saudi J. Biol. Sci. 2018, 25, 1387–1392. [Google Scholar] [CrossRef]
- Song, X.; Wen, X.; He, J.; Zhao, H.; Li, S.; Wang, M. Phytochemical components and biological activities of Artemisia Argyi. J. Funct. Food 2019, 52, 648–662. [Google Scholar] [CrossRef]
- Kim, Y.B.; Cho, H.J.; Yi, Y.S. Anti-inflammatory role of Artemisia argyi methanol extract by targeting the caspase-11 non-canonical inflammasome in macrophages. J. Ethnopharmacol. 2023, 307, 116231. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, L.; Huang, H.; Li, Q.; Wang, X.; Sun, Q.; Wang, Q.; Li, N. In vitro antioxidant analysis of flavonoids extracted from Artemisia argyi stem and their anti-inflammatory activity in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. 2023, 407, 135198. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Xin, Z.; Ma, S.; Liu, W.; Zhang, B.; Ran, L.; Yi, L.; Ren, D. Comprehensive characterization and identification of antioxidants in Folium Artemisiae Argyi using high-resolution tandem mass spectrometry. J. Chromatogr. B 2017, 1063, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.; Lee, S.; Hong, J.; Park, B.; Kim, D.; Kim, C. Anti-Inflammatory Effect of 4, 5-Dicaffeoylquinic Acid on RAW264. 7 Cells and a Rat Model of Inflammation. Nutrients 2021, 13, 3537. [Google Scholar] [CrossRef]
- Hong, S.; Joo, T.; Jhoo, J. Antioxidant and anti-inflammatory activities of 3, 5-dicaffeoylquinic acid isolated from Ligularia fischeri leaves. Food Sci. Biotechnol. 2015, 24, 257–263. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Food 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Derlindati, E.; Dall’Asta, M.; Ardigò, D.; Brighenti, F.; Zavaroni, I.; Crozier, A.; Del Rio, D. Quercetin-3-O-glucuronide affects the gene expression profile of M1 and M2a human macrophages exhibiting anti-inflammatory effects. Food Funct. 2012, 3, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Ferruzzi, M.G.; Janle, E.M.; Wang, J.; Gong, B.; Chen, T.Y.; Lobo, J.; Cooper, B.; Wu, Q.L.; Talcott, S.T.; et al. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013, 27, 769–781. [Google Scholar] [CrossRef]
- Baral, S.; Pariyar, R.; Kim, J.; Lee, H.S.; Seo, J. Quercetin-3-O-glucuronide promotes the proliferation and migration of neural stem cells. Neurobiol. Aging 2017, 52, 39–52. [Google Scholar] [CrossRef]
- Cacciottolo, M.; Wang, X.; Driscoll, I.; Woodward, N.; Saffari, A.; Reyes, J.; Serre, M.L.; Vizuete, W.; Sioutas, C.; Morgan, T.E. Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl. Psychiatr. 2017, 7, e1022. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Xu, J.; Hu, Q.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Park, S.K.; Guo, T.J.; Ha, J.S.; Lee, D.S.; Kim, J.M.; Lee, U.; Kim, D.O.; Heo, H.J. Reversal of trimethyltin-induced learning and memory deficits by 3, 5-dicaffeoylquinic acid. Oxidative Med. Cell. Longev. 2016, 2016, 6981595. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Park, R.Y.; Jeon, H.J.; Kwon, Y.S.; Chun, W. Neuroprotective effects of 3, 5-dicaffeoylquinic acid on hydrogen peroxide-induced cell death in SH-SY5Y cells. Phytother. Res. 2005, 19, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Haam, J.; Yakel, J.L. Cholinergic modulation of the hippocampal region and memory function. J. Neurochem. 2017, 142, 111–121. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.; Guimaraes, I.; Silva, F.; Ribeiro, F. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Kolisnyk, B.; Al-Onaizi, M.A.; Hirata, P.H.; Guzman, M.S.; Nikolova, S.; Barbash, S.; Soreq, H.; Bartha, R.; Prado, M.A.; Prado, V.F. Forebrain deletion of the vesicular acetylcholine transporter results in deficits in executive function, metabolic, and RNA splicing abnormalities in the prefrontal cortex. J. Neurosci. 2013, 33, 14908–14920. [Google Scholar] [CrossRef]
- Hahad, O.; Lelieveld, J.; Birklein, F.; Lieb, K.; Daiber, A.; Münzel, T. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int. J. Mol. Sci. 2020, 21, 4306. [Google Scholar] [CrossRef]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef]
- Jung, M.; Park, M. Acetylcholinesterase inhibition by flavonoids from Agrimonia pilosa. Molecules 2007, 12, 2130–2139. [Google Scholar] [CrossRef]
- Elgoyhen, A.B.; Johnson, D.S.; Boulter, J.; Vetter, D.E.; Heinemann, S. α9: An acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 1994, 79, 705–715. [Google Scholar] [CrossRef]
- González-Castañeda, R.E.; Sánchez-González, V.J.; Flores-Soto, M.; Vázquez-Camacho, G.; Macías-Islas, M.A.; Ortiz, G.G. Neural restrictive silencer factor and choline acetyltransferase expression in cerebral tissue of Alzheimer’s disease patients: A pilot study. Genet. Mol. Biol. 2013, 36, 025–036. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Abdennour, L.; Zeghal, C.; Dème, M.; Puybasset, L. Interaction cerveau-poumon. Ann. Fr. Anesth. Réanim. 2012, 31, e101–e107. [Google Scholar] [CrossRef] [PubMed]
- Koutsoukou, A.; Katsiari, M.; Orfanos, S.E.; Kotanidou, A.; Daganou, M.; Kyriakopoulou, M.; Koulouris, N.G.; Rovina, N. Respiratory mechanics in brain injury: A review. World J. Crit. Care. Med. 2016, 5, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Baniahmad, B.; Safaeian, L.; Vaseghi, G.; Rabbani, M.; Mohammadi, B. Cardioprotective effect of vanillic acid against doxorubicin-induced cardiotoxicity in rat. Res. Pharm. Sci. 2020, 15, 87–96. [Google Scholar]
- Jovanović, P.; Žorić, L.; Stefanović, I.; Džunić, B.; Djordjević-Jocić, J.; Radenković, M.; Jovanović, M. Lactate dehydrogenase and oxidative stress activity in primary open-angle glaucoma aqueous humour. Bosn. J. Basic Med. Sci. 2010, 10, 8–88. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Edmondson, D.E. Hydrogen peroxide produced by mitochondrial monoamine oxidase catalysis: Biological implications. Curr. Pharm. Des. 2014, 20, 155–160. [Google Scholar] [CrossRef]
- Yoshino, S.; Hara, A.; Sakakibara, H.; Kawabata, K.; Tokumura, A.; Ishisaka, A.; Ishisaka, A.; Yoshichika, K.; Terao, J. Effect of quercetin and glucuronide metabolites on the monoamine oxidase-A reaction in mouse brain mitochondria. Nutrition 2011, 27, 847–852. [Google Scholar] [CrossRef]
- Lim, D.W.; Park, J.; Jung, J.; Kim, S.H.; Um, M.Y.; Yoon, M.; Kim, Y.T.; Han, D.; Lee, C.; Lee, J. Dicaffeoylquinic acids alleviate memory loss via reduction of oxidative stress in stress-hormone-induced depressive mice. Pharmacol. Res. 2020, 161, 105252. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxidative Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Carvalho, C.; Zhu, X.; Smith, M.A.; Perry, G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta-Mol. Basis Dis. 2010, 1802, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; Roy, J.; Fung, M.L.; Heng, B.C.; Zhang, C.; Lim, L.W. Relationships between mitochondrial dysfunction and neurotransmission failure in Alzheimer’s disease. Aging Dis. 2020, 11, 1291–1316. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Rashid, F.; Bragg, J.; Ibdah, J.A. Role of the JNK signal transduction pathway in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 200–202. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-κB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.; Lee, C.; Kim, Y.T. 3, 5-Dicaffeoylquinic acid attenuates microglial activation-mediated inflammatory pain by enhancing autophagy through the suppression of MCP3/JAK2/STAT3 signaling. Biomed. Pharmacother. 2022, 153, 113549. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neuro. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Reale, M.; Costantini, E. Cholinergic modulation of the immune system in neuroinflammatory diseases. Diseases 2021, 9, 29. [Google Scholar] [CrossRef]
- Gallowitsch-Puerta, M.; Pavlov, V.A. Neuro-immune interactions via the cholinergic anti-inflammatory pathway. Life Sci. 2007, 80, 2325–2329. [Google Scholar] [CrossRef]
- Alasmari, F.; Alshammari, M.A.; Alasmari, A.F.; Alanazi, W.A.; Alhazzani, K. Neuroinflammatory cytokines induce amyloid beta neurotoxicity through modulating amyloid precursor protein levels/metabolism. Biomed Res. Int. 2018, 2018, 3087475. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhao, B.; Ratka, A. Oxidative stress and β-amyloid protein in Alzheimer’s disease. Neuromol. Med. 2011, 13, 223–250. [Google Scholar] [CrossRef]
- Morishima, Y.; Gotoh, Y.; Zieg, J.; Barrett, T.; Takano, H.; Flavell, R.; Davis, R.J.; Shirasaki, Y.; Greenberg, M.E. Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J. Neurosci. 2001, 21, 7551–7560. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Bastianetto, S.; Mennicken, F.; Ma, W.; Kar, S. Amyloid β peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience 2002, 115, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, E.S.; Finegan, K.G.; Wang, X.; Robinson, A.C.; Guo, C.; Kayahara, M.; Tournier, C. The regulation of Bax by c-Jun N-terminal protein kinase (JNK) is a prerequisite to the mitochondrial-induced apoptotic pathway. FEBS Lett. 2006, 580, 1320–1326. [Google Scholar] [CrossRef]
- Vyas, Y.; Montgomery, J.M.; Cheyne, J.E. Hippocampal deficits in amyloid-β-related rodent models of Alzheimer’s disease. Front. Neurosci. 2020, 14, 226. [Google Scholar] [CrossRef]
- Ha, G.J.; Lee, D.S.; Seung, T.W.; Park, C.H.; Park, S.K.; Jin, D.E.; Kim, N.K.; Shin, H.Y.; Heo, H.J. Anti-amnesic and neuroprotective effects of Artemisia argyi H.(Seomae mugwort) extracts. Korean J. Food Sci. Technol. 2015, 47, 380–387. [Google Scholar] [CrossRef]
- Van Der Borght, K.; Havekes, R.; Boß, T.; Eggen, B.J.L.; Van Der Zee, E.A. Exercise improves memory acquisition and retrieval in the Y-maze task: Relationship with hippocampal neurogenesis. Behav. Neurosci. 2007, 121, 324–334. [Google Scholar] [CrossRef]
- Vincent, A. Acetylcholine receptor antibody as a diagnostic test for myasthenia gravis: Results in 153 validated cases and 2967 diagnostic assays. J. Neurol. Neurosurg. Psychiatr. 2012, 83, 237–238. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, N.; Mamcarz, M.; Zhu, Y.; Buzzeo, R.; Tan, J.; Arendash, G.W.; Bradshaw, P.C. Mitochondrial amyloid-β levels are associated with the extent of mitochondrial dysfunction in different brain regions and the degree of cognitive impairment in Alzheimer’s transgenic mice. J. Alzheimer’s Dis. 2010, 20, S535–S550. [Google Scholar] [CrossRef] [PubMed]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef] [PubMed]


| No. | RT (min) | Parent Ion (m/z) | MS2 Fragments (m/z) | Proposed Compound |
|---|---|---|---|---|
| 1 | 0.77 | 192.05 | 191 | Quinic acid |
| 2 | 2.83 | 354.08 | 191, 135 | Chlorogenic acid |
| 3 | 2.97 | 564.14 | 563, 473, 443, 383, 353 | Isoschaftoside |
| 4 | 3.12 | 610.15 | 609, 301, 300 | Rutin |
| 5 | 3.21 | 478.07 | 301, 151 | Quercetin-3-gucuronide |
| 6 | 3.29 | 516.12 | 353, 191, 179,173, 135 | 3,4-dicaffeoylquinic acid |
| 7 | 3.35 | 516.12 | 191, 179 | 3,5-dicaffeoylquinic acid |
| 8 | 3.4 | 516.12 | 191, 179, 135, 353 | 4,5-dicaffeoylquinic acid |
| Control | PM | AASC 50 * | AASC 100 * | |
|---|---|---|---|---|
| ACh contents (mmole/mg of protein) | 2.18 ± 0.17 a | 1.68 ± 0.08 c | 1.95 ± 0.10 b | 1.96 ± 0.10 b |
| AChE activity (% of control) | 100.0 ± 7.0 b | 108.5 ± 4.1 a | 89.2 ± 5.3 c | 89.7 ± 2.7 c |
| Brain | Lung | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | PM | AASC 50 * | AASC 100 * | Control | PM | AASC 50 * | AASC 100 * | |
| MDA contents (nmole/mg of protein) | 3.50 ± 0.48 bc | 4.32 ± 0.46 a | 3.79 ± 0.15 ab | 3.04 ± 0.07 c | 1.34 ± 0.04 b | 1.47 ± 0.05 a | 1.30 ± 0.10 b | 1.31 ± 0.08 b |
| SOD contents (U/mg of protein) | 4.10 ± 0.31 a | 3.36 ± 0.16 b | 3.86 ± 0.20 a | 3.73 ± 0.15 a | 5.14 ± 0.41 a | 4.17 ± 0.17 b | 4.67 ± 0.39 ab | 4.63 ± 0.31 ab |
| Reduced Glutathione (% of control) | 100.0 ± 8.7 a | 74.9 ± 12.3 b | 85.2 ± 8.9 ab | 107.1 ± 14.4 a | 100.0 ± 24.0 a | 61.0 ± 12.1 b | 77.8 ± 8.4 ab | 74.8 ± 7.2 ab |
| Control | PM | AASC 50 * | AASC 100 * | |
|---|---|---|---|---|
| FRAP activity (Absorbance at 593 nm) | 0.43 ± 0.02 a | 0.28 ± 0.01 c | 0.31 ± 0.01 c | 0.35 ± 0.03 b |
| LDH contents (U/L) | 187.3 ± 23.8 ab | 256.0 ± 42.8 a | 146.3 ± 33.3 b | 186.0 ± 34.4 ab |
| Brain | Lung | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | PM | AASC 50 * | AASC 100 * | Control | PM | AASC 50 * | AASC 100 * | |
| ROS contents (% of control) | 100.0 ± 6.4 b | 133.7 ± 16.9 a | 135.74 ± 15.6 a | 71.1 ± 4.6 c | 100.0 ± 26.5 b | 136.4 ± 25.5 a | 112.6 ± 32.6 ab | 107.6 ± 20.3 ab |
| MMP (% of control) | 100.0 ± 14.4 b | 73.6 ± 12.2 c | 101.5 ± 8.8 b | 123.2 ± 6.8 a | 100.0 ± 14.4 bc | 74.1 ± 26.4 c | 118.5 ± 31.9 ab | 149.7 ± 5.9 a |
| ATP contents (nmole/mg) | 318.5 ± 67.0 a | 90.1 ± 16.0 b | 79.5 ± 3.0 b | 421.0 ± 146.2 a | 306.8 ± 21.4 a | 170.2 ± 53.9 b | 241.1 ± 56.4 ab | 310.0 ± 63.2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.-Y.; Kim, J.-M.; Park, S.-K.; Lee, H.-L.; Heo, H.-J. A Mixture of Artemisia argyi and Saururus chinensis Improves PM2.5-Induced Cognitive Dysfunction by Regulating Oxidative Stress and Inflammatory Response in the Lung and Brain. Plants 2023, 12, 1230. https://doi.org/10.3390/plants12061230
Kang J-Y, Kim J-M, Park S-K, Lee H-L, Heo H-J. A Mixture of Artemisia argyi and Saururus chinensis Improves PM2.5-Induced Cognitive Dysfunction by Regulating Oxidative Stress and Inflammatory Response in the Lung and Brain. Plants. 2023; 12(6):1230. https://doi.org/10.3390/plants12061230
Chicago/Turabian StyleKang, Jin-Yong, Jong-Min Kim, Seon-Kyeong Park, Hyo-Lim Lee, and Ho-Jin Heo. 2023. "A Mixture of Artemisia argyi and Saururus chinensis Improves PM2.5-Induced Cognitive Dysfunction by Regulating Oxidative Stress and Inflammatory Response in the Lung and Brain" Plants 12, no. 6: 1230. https://doi.org/10.3390/plants12061230
APA StyleKang, J.-Y., Kim, J.-M., Park, S.-K., Lee, H.-L., & Heo, H.-J. (2023). A Mixture of Artemisia argyi and Saururus chinensis Improves PM2.5-Induced Cognitive Dysfunction by Regulating Oxidative Stress and Inflammatory Response in the Lung and Brain. Plants, 12(6), 1230. https://doi.org/10.3390/plants12061230





