Jambu Flower Extract (Acmella oleracea) Increases the Antioxidant Potential of Beer with a Reduced Alcohol Content
Abstract
1. Introduction
2. Results
2.1. A. oleracea Extract Obtained by Supercritical Extraction
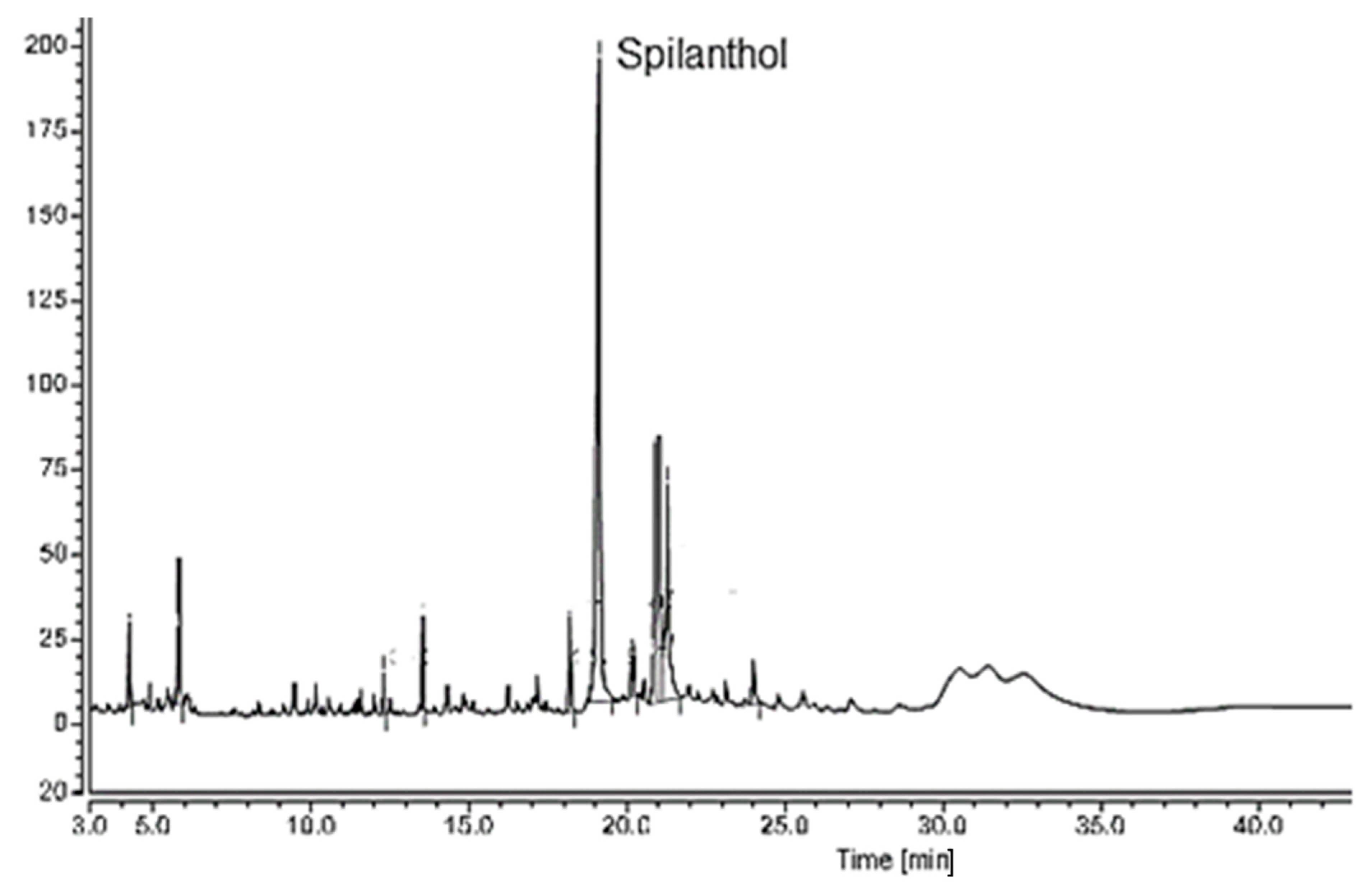
2.1.1. Gas Chromatography with Flame Ionization Detector (GC-FID)
2.1.2. FTIR Analysis
2.2. Physicochemical Parameters of Beers
2.3. Total Phenolics Content (TPC)
2.4. Antioxidant Activity: ABTS, DPPH, and ORAC
2.5. Evaluation of Antioxidant Activities after Six Months of Storage
3. Materials and Methods
3.1. Material
3.2. Pre-Processing of Malt Bagasse and A. olaracea Flowers
3.3. Obtaining the Extract of A. oleracea Flowers Obtained by Supercritical Extraction (SE) and Preparation of the Alcoholic Concentrated Extract (ACE)
3.4. Spilanthol Concentration in A. oleracea Extract
3.4.1. Thin Layer Chromatography (TLC)
3.4.2. Gas Chromatography with Flame Ionization Detector (GC-FID)
3.4.3. Fourier Transform Infrared Analysis (FTIR)
3.5. Lager Beer Production
3.5.1. Mash
3.5.2. Filtration and Washing
3.5.3. Addition of Hops, Whirlpool, and Cooling
3.5.4. Fermentation and Maturation
3.5.5. Addition of the Alcoholic Concentrated Extract (ACE)
3.5.6. Bottling and Priming
3.6. Physicochemical Parameters of Beers
3.6.1. OG and FG
3.6.2. Color
3.6.3. Bitterness (IBU)
3.6.4. Quantifying Ethanol
3.6.5. Total Phenolic Compounds (TPC)
3.7. Antioxidant Activity (Aa)
3.7.1. DPPH Radical Scavenging Activity
3.7.2. ABTS Radical Cation Scavenging Activity
3.7.3. ORAC Method
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koren, D.; Kun, S.; Vecseri, B.H.; Kun-Farkas, G. Study of antioxidant activity during the malting and brewing process. J. Food Sci. Technol. 2019, 56, 3801–3809. [Google Scholar] [CrossRef]
- Martínez, A.; Vegara, S.; Herranz-López, M.; Martí, N.; Valero, M.; Micol, V.; Saura, D. Kinetic changes of polyphenols, anthocyanins and antioxidant capacity in forced aged hibiscus ale beer. J. Inst. Brew. 2017, 123, 58–65. [Google Scholar] [CrossRef]
- Quifer-Rada, P.; Vallverdú-Queralt, A.; Martínez-Huélamo, M.; Chiva-Blanch, G.; Jáuregui, O.; Estruch, R.; Lamuela-Raventós, R. A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC–ESI-LTQ-Orbitrap-MS). Food Chem. 2014, 169, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-H.; Kim, I.-D.; Dhungana, S.K.; Do, H.-M.; Shin, D.-H. Persimmon fruit enhanced quality characteristics and antioxidant potential of beer. Food Sci. Biotechnol. 2018, 27, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Ducruet, J.; Rébénaque, P.; Diserens, S.; Kosińska-Cagnazzo, A.; Héritier, I.; Andlauer, W. Amber ale beer enriched with goji berries—The effect on bioactive compound content and sensorial properties. Food Chem. 2017, 226, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, P.A.; Vidal, J.; Ávila, M.I.; Labbe, M.; Cohen, S.; Salazar, F.N. Effect of the Addition of Propolis Extract on Bioactive Compounds and Antioxidant Activity of Craft Beer. J. Chem. 2017, 2017, 6716053. [Google Scholar] [CrossRef]
- Djordjevic, S.; Popovic, D.; Despotovic, S.; Veljovic, M.; Atanackovic, M.; Cvejic, J.; Nedovic, V.; Leskosek-Cukalovic, I. Extracts of medicinal plants as functional beer additives. Chem. Ind. Chem. Eng. Q. 2016, 22, 301–308. [Google Scholar] [CrossRef]
- Abeysiri, G.; Dharmadasa, R.; Abeysinghe, D.; Samarasinghe, K. Screening of phytochemical, physico-chemical and bioactivity of different parts of Spilantes acmella Murr. (Asteraceae), a natural remedy for toothache. Ind. Crop. Prod. 2013, 50, 852–856. [Google Scholar] [CrossRef]
- Wongsawatkul, O.; Prachayasittikul, S.; Isarankura-Na-Ayudhya, C.; Satayavivad, J.; Ruchirawat, S.; Prachayasittikul, V. Vasorelaxant and Antioxidant Activities of Spilanthes acmella Murr. Int. J. Mol. Sci. 2008, 9, 2724–2744. [Google Scholar] [CrossRef]
- Dubey, S.; Maity, S.; Singh, M.; Saraf, S.A.; Saha, S. Phytochemistry, Pharmacology and Toxicology of Spilanthes acmella: A Review. Adv. Pharmacol. Sci. 2013, 2013, 423750. [Google Scholar] [CrossRef]
- Dias, A.; Santos, P.; Seabra, I.; Júnior, R.; Braga, M.; de Sousa, H. Spilanthol from Spilanthes acmella flowers, leaves and stems obtained by selective supercritical carbon dioxide extraction. J. Supercrit. Fluids 2012, 61, 62–70. [Google Scholar] [CrossRef]
- Molina-Torres, J.; Salazar-Cabrera, C.J.; Armenta-Salinas, C.; Ramírez-Chávez, E. Fungistatic and Bacteriostatic Activities of Alkamides from Heliopsis longipes Roots: Affinin and Reduced Amides. J. Agric. Food Chem. 2004 52, 4700–4704. [CrossRef]
- Barbosa, A.F.; Pereira, C.D.S.S.; Mendes, M.F.; Junior, R.N.D.C.; De Carvalho, M.G.; Maia, J.G.S.; Sabaa-Srur, A.U.O. Spilanthol Content in the Extract Obtained by Supercritical CO2 at Different Storage Times of Acmella Oleracea L. J. Food Process. Eng. 2016, 40, e12441. [Google Scholar] [CrossRef]
- Dias, A.; da Silva, A.; Botelho, J.; Júnior, R.; de Sousa, H.; Braga, M. Temperature and density effects of the scCO2extraction of spilanthol from Spilanthes acmella flowers. J. Supercrit. Fluids 2017, 121, 32–40. [Google Scholar] [CrossRef]
- Venturine Filho, W.G. Bebidas alcóolica: Ciência e Tecnologia; Editora Blucher: Sao Paulo, Brazil, 2016; Volume 1. [Google Scholar]
- Aquilani, B.; Laureti, T.; Poponi, S.; Secondi, L. Beer choice and consumption determinants when craft beers are tasted: An exploratory study of consumer preferences. Food Qual. Prefer. 2015, 41, 214–224. [Google Scholar] [CrossRef]
- Blanco, V.S.D.F.; Michalak, B.; Zelioli, A.M.; Oliveira, A.D.S.S.D.; Rodrigues, M.V.N.; Ferreira, A.G.; Garcia, V.L.; Cabral, F.A.; Kiss, A.K.; Rodrigues, R.A.F. Isolation of spilanthol from Acmella oleracea based on Green Chemistry and evaluation of its in vitro anti-inflammatory activity. J. Supercrit. Fluids 2018, 140, 372–379. [Google Scholar] [CrossRef]
- Nakatani, N.; Nagashima, M. Pungent Alkamides from Spilanthes acmella L. var. oleracea Clarke. Biosci. Biotechnol. Biochem. 1992, 56, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Bhardwaj, S.; Lata, S. Phyto-mediated green synthesis of silver nanoparticles using Acmella oleracea leaf extract: Antioxidant and catalytic activity. Pharmacogn. Mag. 2022, 18, 22. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Mathias, T.R.D.S.; Alexandre, V.M.F.; Cammarota, M.C.; de Mello, P.P.M.; Sérvulo, E.F.C. Characterization and determination of brewer’s solid wastes composition. J. Inst. Brew. 2015, 121, 400–404. [Google Scholar] [CrossRef]
- Saraiva, B.R.; Agustinho, B.C.; Vital, A.C.P.; Staub, L.; Pintro, P.T.M. Effect of brewing waste (malt bagasse) addition on the physicochemical properties of hamburgers. J. Food Process. Preserv. 2019, 43, e14135. [Google Scholar] [CrossRef]
- Moura-Nunes, N.; Brito, T.C.; da Fonseca, N.D.; de Aguiar, P.F.; Monteiro, M.; Perrone, D.; Torres, A.G. Phenolic compounds of Brazilian beers from different types and styles and application of chemometrics for modeling antioxidant capacity. Food Chem. 2016, 199, 105–113. [Google Scholar] [CrossRef]
- Costa, R.H.K. Produção de Cerveja Com Baixo Teor Alcoólico (Dissertação de Mestrado em Biotecnologia Indus-Trial). Ph.D. Thesis, Escola de Engenharia de Lorena da Universidade de São Paulo, Sao Paulo, Brazil, 2016. [Google Scholar]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulaspora delbrueckii in the brewing process: A new approach to enhance bioflavour and to reduce ethanol content. Food Microbiol. 2016, 56, 45–51. [Google Scholar] [CrossRef]
- Bellut, K.; Krogerus, K.; Arendt, E.K. Lachancea fermentati Strains Isolated From Kombucha: Fundamental Insights, and Practical Application in Low Alcohol Beer Brewing. Front. Microbiol. 2020, 11, 764. [Google Scholar] [CrossRef]
- da Silva, R.N.P.; Dias, J.F.; Koblitz, M.G.B. Beers: Relationship between styles; phenolic compounds and antioxidant capacity. Res. Soc. Dev. 2021, 10, e42210313471. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, W.; Lu, J.; Zhao, M. Phenolic profiles and antioxidant activities of commercial beers. Food Chem. 2010, 119, 1150–1158. [Google Scholar] [CrossRef]
- Humia, B.V.; Santos, K.S.; Schneider, J.K.; Leal, I.L.; Barreto, G.D.A.; Batista, T.; Machado, B.A.S.; Druzian, J.I.; Krause, L.C.; Mendonça, M.D.C.; et al. Physicochemical and sensory profile of Beauregard sweet potato beer. Food Chem. 2020, 312, 126087. [Google Scholar] [CrossRef]
- Boonen, J.; Baert, B.; Burvenich, C.; Blondeel, P.; De Saeger, S.; De Spiegeleer, B. LC–MS profiling of N-alkylamides in Spilanthes acmella extract and the transmucosal behaviour of its main bio-active spilanthol. J. Pharm. Biomed. Anal. 2010, 53, 243–249. [Google Scholar] [CrossRef]
- Sharma, R.; Karunambigai, A.; Gupta, S.; Arumugam, N. Evaluation of biologically active secondary metabolites isolated from the toothache plant Acmella ciliata (Asteraceae). Adv. Tradit. Med. 2021, 22, 713–722. [Google Scholar] [CrossRef]
- Thygesen, L.; Thulin, J.; Mortensen, A.; Skibsted, L.H.; Molgaard, P. Antioxidant activity of cichoric acid and alkamides from Echinacea purpurea, alone and in combination. Food Chem. 2007, 101, 74–81. [Google Scholar] [CrossRef]
- Tafulo, P.A.R.; Queirós, R.; Delerue-Matos, C.; Sales, M.G.F. Control and comparison of the antioxidant capacity of beers. Food Res. Int. 2010, 43, 1702–1709. [Google Scholar] [CrossRef]
- Li, H.; Zhao, M.; Cui, C.; Sun, W.; Zhao, H. Antioxidant activity and typical ageing compounds: Their evolutions and relationships during the storage of lager beers. Int. J. Food Sci. Technol. 2016, 51, 2026–2033. [Google Scholar] [CrossRef]
- Saison, D.; De Schutter, D.P.; Vanbeneden, N.; Daenen, L.; Delvaux, F.; Delvaux, F.R. Decrease of aged beer aroma by the reducing activity of brewing yeast. J. Agric. Food Chem. 2010, 58, 3107–3115. [Google Scholar] [CrossRef] [PubMed]
- Berner, T.; Arneborg, N. The role of lager beer yeast in oxidative stability of model beer. Lett. Appl. Microbiol. 2012, 54, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.C.; Carvalho, G.A.; Picanço, M.C.; Morais, E.G.; Pereira, R.M. Bioactivity of compounds from Acmella oleracea against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) and selectivity to two non-target species. Pest Manag. Sci. 2011, 68, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Hildebert, W.; Bladt, S.; Zgainski, E.M. Plant Drug Analysis: A Thin Layer Chromatography Atlas; Springer: Berlin/Heidelberg, Germany, 1984; ISBN 978-3-662-02398-3. [Google Scholar]
- ASBC Methods of Analysis. Beer 10. Spectrophotometric Color Method Approved 1958, rev; American Society of Brewing Chemists: St. Paul, MN, USA. 2015. Available online: http:\\methods.asbcnet.org (accessed on 22 September 2021).
- ASBC Method of Analysis. American Society of Brewing Chemists. Beer Bitterness. Beer-23A; American Society of Brewing Chemists: St Paul, MN, USA. 2011. Available online: http:\\methods.asbcnet.org (accessed on 22 September 2021).
- Cowx, M.; Silva, F.B.G.; Yeung, K. Government Deficits and Corporate Tax Avoidance. SSRN 2022, 4060416. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid rea-gent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Silva, E.M.; Souza, J.N.S.; Rogez, H.; Rees, J.F.; Larondelle, Y. Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem. 2007, 101, 1012–1018. [Google Scholar] [CrossRef]

| Parameters | Beers (50/45% Malt/Bagasse) | |||
|---|---|---|---|---|
| PM | BM | BM5 | BM7.5 | |
| Original Gravity (OG) | 1.038 ± 0.016 | 1.028 ± 0.017 | 1.028 ± 0.016 | 1.028 ± 0.017 |
| Final Gravity (OF) | 1.004 ± 0.017 | 1.009 ± 0.017 | 1.009 ± 0.014 | 1.009 ± 0.017 |
| pH | 4.40 ± 0.002 | 4.10 ± 0.002 | 4.18 ± 0.003 | 4.25 ± 0.002 |
| Color (EBC) | 3.25 ± 0.4 | 2.20 ± 0.3 | 2.20 ± 0.5 | 2.20 ± 0.4 |
| Bitterness (IBU) | 31.29 ± 0.9 | 29.50 ± 0.7 | 29.50 ± 0.5 | 29.50 ± 0.8 |
| Alcohol content (%) | 4.20 ± 0.015 | 2.50 ± 0.016 | 2.50 ± 0.012 | 2.50 ± 0.012 |
| Analysis | Control Beer BM | Beers Added with ACE ml/L | |
|---|---|---|---|
| BM5 | BM7.5 | ||
| TPC0 (mg GAE/L) | 203.34 ± 1.52 | 226.89 ± 2.63 * | 229.11 ± 3.62 * |
| TPC6 (mg GAE/L) | - | 205.96 ± 2.55 | 207.57 ± 3.75 |
| ABTS0 (µmol TE/L) | 771.40 ± 0.01 | 860.30 ± 0.02 * | 944.8 ± 0.02 * |
| ABTS6 (µmol TE/L) | - | 781.40 ± 0.02 | 83,700 ± 0.09 |
| DPPH0 (µmol TE/L) | 265.90 ± 0.03 | 290.60 ± 0.05 * | 335.60 ± 0.01 * |
| DPPH6 (µmol TE/L) | - | 272.60 ± 0.06 | 317.60 ± 0.03 |
| ORAC0 (µmol TE/L) | 3810.84 ± 121.84 | 5087.21 ± 127.00 * | 5396.25 ± 141.00 * |
| ORAC6 (µmol TE/L) | - | 4551.11 ± 217.13 | 4676.67 ± 167.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, S.P.; Fernandes, J.A.L.; Santos, A.S.; Ferreira, N.R. Jambu Flower Extract (Acmella oleracea) Increases the Antioxidant Potential of Beer with a Reduced Alcohol Content. Plants 2023, 12, 1581. https://doi.org/10.3390/plants12081581
da Silva SP, Fernandes JAL, Santos AS, Ferreira NR. Jambu Flower Extract (Acmella oleracea) Increases the Antioxidant Potential of Beer with a Reduced Alcohol Content. Plants. 2023; 12(8):1581. https://doi.org/10.3390/plants12081581
Chicago/Turabian Styleda Silva, Suelem Paixão, José Augusto Lacerda Fernandes, Alberdan Silva Santos, and Nelson Rosa Ferreira. 2023. "Jambu Flower Extract (Acmella oleracea) Increases the Antioxidant Potential of Beer with a Reduced Alcohol Content" Plants 12, no. 8: 1581. https://doi.org/10.3390/plants12081581
APA Styleda Silva, S. P., Fernandes, J. A. L., Santos, A. S., & Ferreira, N. R. (2023). Jambu Flower Extract (Acmella oleracea) Increases the Antioxidant Potential of Beer with a Reduced Alcohol Content. Plants, 12(8), 1581. https://doi.org/10.3390/plants12081581








