Exogenous Calcium Reinforces Photosynthetic Pigment Content and Osmolyte, Enzymatic, and Non-Enzymatic Antioxidants Abundance and Alleviates Salt Stress in Bread Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. Measurements
2.2.1. Biochemical Analysis
2.2.2. Estimation of Enzyme Activities
2.2.3. Measurement of Non-Enzymatic Antioxidants
2.3. Statistical Analysis
3. Results
3.1. Changes in Photosynthetic Pigments and Endogenous IAA
3.2. Changes in Osmoprotectants and Total Carbohydrates
3.3. Changes in Hydrogen Peroxide, Lipid Peroxidation, and Malondialdehyde
3.4. Changes in Antioxidant Enzymes
3.5. Changes in Non-Enzymatic Antioxidants
3.6. Changes in the Activity of Free Radical Scavenging and Enzymatic Scavenging
3.7. Changes in Plant Growth
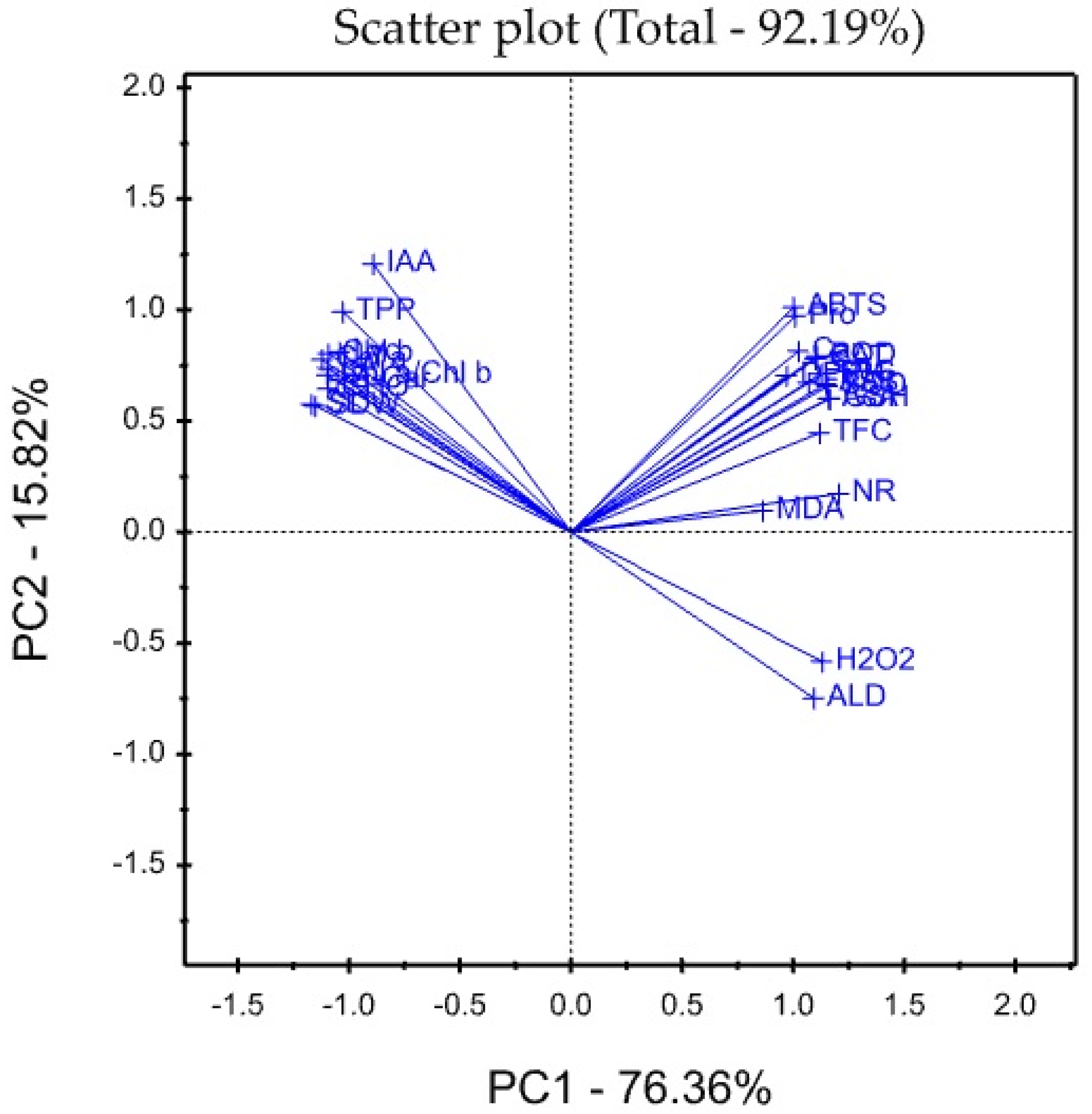
3.8. Treatment x Traits (TT) Biplot
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, Y.; Jahan, N.; Chen, G.; Ren, D.; Guo, L. Sensing of abiotic stress and ionic stress responses in plants. Int. J. Mol. Sci. 2018, 19, 3298. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, D.; Yang, T.; Huang, F.; Fu, S.; Li, L. Changes in soil labile and recalcitrant carbon pools after land-use change in a semi-arid agro-pastoral ecotone in Central Asia. Ecol. Indic. 2020, 110, 105925. [Google Scholar] [CrossRef]
- Sadak, M.S.; Sekara, A.; Al-Ashkar, I.; Habib-ur-Rahman, M.; Skalicky, M.; Brestic, M.; Kumar, A.; El Sabagh, A.; Abdelhamid, M.T. Exogenous aspartic acid alleviates salt stress-induced decline in growth by enhancing antioxidants and compatible solutes while reducing reactive oxygen species in wheat. Front. Plant Sci. 2022, 13, 987641. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Lamers, J.; Van Der Meer, T.; Testerink, C. How plants sense and respond to stressful environments. Plant Physiol. 2020, 182, 1624–1635. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Xu, T.; Niu, J.; Jiang, Z. Sensing mechanisms: Calcium signaling mediated abiotic stress in plants. Front. Plant Sci. 2022, 13, 925863. [Google Scholar] [CrossRef]
- Sadak, M.S.; Ahmed, M. Physiological role of cyanobacteria and glycine betaine on wheat plant grown under salinity stress. Int. J. Pharm. Tech Res. 2016, 9, 78–92. [Google Scholar]
- Aldon, D.; Mbengue, M.; Mazars, C.; Galaud, J.-P. Calcium signalling in plant biotic interactions. Int. J. Mol. Sci. 2018, 19, 665. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Shomali, A.; Azad, N.; Hassani, B.; Lastochkina, O.; Li, T. Calcium signaling and salt tolerance are diversely entwined in plants. Plant Signal. Behav. 2019, 14, 1665455. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Ben Amor, N.; Megdiche, W.; Jiménez, A.; Sevilla, F.; Abdelly, C. The effect of calcium on the antioxidant systems in the halophyte Cakile maritima under salt stress. Acta Physiol. Plant. 2010, 32, 453–461. [Google Scholar] [CrossRef]
- Tobe, K.; Li, X.; Omasa, K. Effects of five different salts on seed germination and seedling growth of Haloxylon ammodendron (Chenopodiaceae). Seed Sci. Res. 2004, 14, 345–353. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Gusmiaty, M.; Payangan, R. Production of IAA (Indole Acetic Acid) of the rhizosphere fungus in the Suren community forest stand. IOP Conf. Ser. Earth Environ. Sci. 2019, 343, 012058. [Google Scholar] [CrossRef]
- Kalsoom, U.; Bennett, I.; Boyce, M.C. A review of extraction and analysis: Methods for studying osmoregulants in plants. J. Chromatogr. Sep. Tech. 2016, 7, 315. [Google Scholar]
- Tamayo, P.R.; Bonjoch, N.P. Free proline quantification. In Handbook of Plant Ecophysiology Techniques; Springer: Berlin/Heidelberg, Germany, 2001; pp. 365–382. [Google Scholar]
- Verslues, P.E. Quantification of water stress-induced osmotic adjustment and proline accumulation for Arabidopsis thaliana molecular genetic studies. In Plant Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2010; pp. 301–315. [Google Scholar]
- Chow, P.S.; Landhäusser, S.M. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X. Plant Physiology Experimental Guide; Higher Education Press: Beijing, China, 2006; Volume 24, pp. 55–56. [Google Scholar]
- Bergmeyer, H.U.; Bernt, E. UV-assay with pyruvate and NADH. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1974; pp. 574–579. [Google Scholar]
- Jaworski, E.G. Nitrate reductase assay in intact plant tissues. Biochem. Biophys. Res. Commun. 1971, 43, 1274–1279. [Google Scholar] [CrossRef]
- Helrich, K. AOAC Official Methods of Analysis. Vitamin C (Ascorbic Acid); Association of Official Analytical Chemists: Arlington, VA, USA, 1990; Volume 2, pp. 1058–1059. [Google Scholar]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Diaz, D.H.; Martin, G.C. Peach Seed Dormancy in Relation to Endogenous Inhibitors and Applied Growth Substances1. J. Am. Soc. Hortic. Sci. 1972, 97, 651–654. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Nagata, M.; Yamashita, I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Gyamfi, M.A.; Yonamine, M.; Aniya, Y. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. Gen. Pharmacol. Vasc. Syst. 1999, 32, 661–667. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.-F.; Tsimidou, M.; Zhang, H.-Y. Estimation of scavenging activity of phenolic compounds using the ABTS•+ assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef] [PubMed]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Levene, H. Robust tests for equality of variances. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Stanford University Press: Palo Alto, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Nassar, R.M.; Kamel, H.A.; Ghoniem, A.E.; Alarcón, J.J.; Sekara, A.; Ulrichs, C.; Abdelhamid, M.T. Physiological and anatomical mechanisms in wheat to cope with salt stress induced by seawater. Plants 2020, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Mounzer, O.; Alarcón, J.; Abdelhamid, M.; Howladar, S. Growth, heavy metal status and yield of salt-stressed wheat (Triticum aestivum L.) plants as affected by the integrated application of bio-, organic and inorganic nitrogen-fertilizers. J. Appl. Bot. Food Qual. 2016, 89. [Google Scholar] [CrossRef]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R.; Shoukat, A.; Hussan, M.U.; Sarwar, M.I. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.G.; Abdelhamid, M.T.; Schmidhalter, U. Potassium fertiliser enhances the salt-tolerance of common bean (Phaseolus vulgaris L.). J. Hortic. Sci. Biotechnol. 2014, 89, 185–192. [Google Scholar] [CrossRef]
- Rehman, H.; Alharby, H.F.; Bamagoos, A.A.; Abdelhamid, M.T.; Rady, M.M. Sequenced application of glutathione as an antioxidant with an organic biostimulant improves physiological and metabolic adaptation to salinity in wheat. Plant Physiol. Biochem. 2021, 158, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Oraki, H.; Aghaalikhana, M. Effect of water deficit stress on proline contents, soluble sugars, chlorophyll and grain yield of sunflower (Helianthus annuus L.) hybrids. Afr. J. Biotechnol. 2012, 11, 164–168. [Google Scholar]
- Mazars, C.; Thuleau, P.; Lamotte, O.; Bourque, S. Cross-talk between ROS and calcium in regulation of nuclear activities. Mol. Plant 2010, 3, 706–718. [Google Scholar] [CrossRef]
- Silva, E.N.d.; Ribeiro, R.V.; Ferreira-Silva, S.L.; Viégas, R.A.; Silveira, J.A.G. Salt stress induced damages on the photosynthesis of physic nut young plants. Sci. Agric. 2011, 68, 62–68. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Alam, M.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. BioMed Res. Int. 2014, 2014, 757219. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, Z.; Zhou, S.; Ou, L.; Dai, X.; Ma, Y.; Zhang, Z.; Chen, W.; Li, X.; Liang, C. Exogenous Ca2+ alleviates waterlogging-caused damages to pepper. Photosynthetica 2016, 54, 620–629. [Google Scholar] [CrossRef]
- Li, Z.; Tan, X.; Lu, K.; Liu, Z.; Wu, L. The effect of CaCl2 on calcium content, photosynthesis, and chlorophyll fluorescence of tung tree seedlings under drought conditions. Photosynthetica 2017, 55, 553–560. [Google Scholar] [CrossRef]
- Sadak, M.S.; Talaat, I.M. Attenuation of negative effects of saline stress in wheat plant by chitosan and calcium carbonate. Bull. Natl. Res. Cent. 2021, 45, 136 . [Google Scholar] [CrossRef]
- Arshi, A.; Abdin, M.Z.; Iqbal, M. Effect of CaCl2 on growth performance, photosynthetic efficiency and nitrogen assimilation of Cichorium intybus L. grown under NaCl stress. Acta Physiol. Plant. 2006, 28, 137–147. [Google Scholar] [CrossRef]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front. Plant Sci. 2016, 7, 609. [Google Scholar] [CrossRef]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Ragaey, M.M.; Sadak, M.S.; Dawood, M.F.; Mousa, N.H.; Hanafy, R.S.; Latef, A.A.H.A. Role of Signaling Molecules Sodium Nitroprusside and Arginine in Alleviating Salt-Induced Oxidative Stress in Wheat. Plants 2022, 11, 1786. [Google Scholar] [CrossRef]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Bhardwaj, R.D.; Grewal, S.K. Mitigation of salinity-induced oxidative damage in wheat (Triticum aestivum L.) seedlings by exogenous application of phenolic acids. Acta Physiol. Plant. 2017, 39, 221. [Google Scholar] [CrossRef]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Naeem, M. Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide 2012, 27, 210–218. [Google Scholar] [CrossRef] [PubMed]
- El-Bassiouny, H.; Sadak, M.S. Impact of foliar application of ascorbic acid and α-tocopherol on antioxidant activity and some biochemical aspects of flax cultivars under salinity stress. Acta Biológica Colomb. 2015, 20, 209–222. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B.; Mohanty, P. Investigations on the antioxidative defence responses to NaCl stress in a mangrove, Bruguiera parviflora: Differential regulations of isoforms of some antioxidative enzymes. Plant Growth Regul. 2004, 42, 213–226. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Plieth, C.; Vollbehr, S. Calcium promotes activity and confers heat stability on plant peroxidases. Plant Signal. Behav. 2012, 7, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Debouba, M.; Maâroufi-Dghimi, H.; Suzuki, A.; Ghorbel, M.H.; Gouia, H. Changes in growth and activity of enzymes involved in nitrate reduction and ammonium assimilation in tomato seedlings in response to NaCl stress. Ann. Bot. 2007, 99, 1143–1151. [Google Scholar] [CrossRef]
- Guo, Y.; Ren, G.; Zhang, K.; Li, Z.; Miao, Y.; Guo, H. Leaf senescence: Progression, regulation, and application. Mol. Hortic. 2021, 1, 5. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Abd El-Hameid, A.R.; Sadak, M.S. Impact of glutathione on enhancing sunflower growth and biochemical aspects and yield to alleviate salinity stress. Biocatal. Agric. Biotechnol. 2020, 29, 101744. [Google Scholar] [CrossRef]
- Hu, W.; Sarengaowa; Guan, Y.; Feng, K. Biosynthesis of phenolic compounds and antioxidant activity in fresh-cut fruits and vegetables. Front. Microbiol. 2022, 13, 906069. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Geetha, S.; Ram, M.S.; Mongia, S.; Singh, V.; Ilavazhagan, G.; Sawhney, R. Evaluation of antioxidant activity of leaf extract of Seabuckthorn (Hippophae rhamnoides L.) on chromium (VI) induced oxidative stress in albino rats. J. Ethnopharmacol. 2003, 87, 247–251. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Zhou, T.; Chen, Z.; Gu, Z.; Yang, R. Role of Ca2+ in phenolic compound metabolism of barley (Hordeum vulgare L.) sprouts under NaCl stress. J. Sci. Food Agric. 2019, 99, 5176–5186. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G. Carotenoid biosynthesis in microorganisms and plants. Eur. J. Biochem. 1994, 223, 7–24. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Cooney, R.V.; Bertram, J.S. Carotenoids enhance gap junctional communication and inhibit lipid peroxidation in C3H/10T1/2 cells: Relationship to their cancer chemopreventive action. Carcinogenesis 1991, 12, 2109–2114. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Klapaki, G. Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci. Hortic. 2000, 86, 247–260. [Google Scholar] [CrossRef]
- Yu, L.; Haley, S.; Perret, J.; Harris, M. Antioxidant properties of hard winter wheat extracts. Food Chem. 2002, 78, 457–461. [Google Scholar] [CrossRef]
- Allah, M.; El-Bassiouny, H.M.S.; Bakry, B.A.; Sadak, M.S. Effect of Arbuscular mycorrhiza and glutamic acid on growth, yield, some chemical composition and nutritional quality of wheat plant grown in newly reclaimed sandy soil. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1038–1054. [Google Scholar]
- Awad, N.; Turky, A.; Abdelhamid, M.; Attia, M. Ameliorate of environmental salt stress on the growth of Zea mays L. plants by exopolysaccharides producing bacteria. J. Appl. Sci. Res. 2012, 8, 2033–2044. [Google Scholar]
- El-Awadi, M.E.; Sadak, M.S.; El-Rorkiek, K.G.A.; Dawood, M. Physiological Response of Two Wheat Cultivars Grown under Sandy Soil Conditions to Aspartic Acid Application. J. Appl. Sci. 2019, 19, 811–817. [Google Scholar]
- Suryaman, M.; Saepudin, A.; Natawijaya, D.; Zumani, D. Salt Stess on Soybean (Glycine max L Merr): Improving Salt Stress Tolerance through Seed Priming. Int. J. Sci. Technol. Res. 2012, 6, 278–283. [Google Scholar]
- Alharbi, B.M.; Elhakem, A.H.; Alnusairi, G.S.; Soliman, M.H.; Hakeem, K.R.; Hasan, M.M.; Abdelhamid, M.T. Exogenous application of melatonin alleviates salt stress-induced decline in growth and photosynthesis in Glycine max (L.) seedlings by improving mineral uptake, antioxidant and glyoxalase system. Plant Soil Environ. 2021, 67, 208–220. [Google Scholar] [CrossRef]
- Mohamed, H.I.; El-Sayed, A.A.; Rady, M.M.; Caruso, G.; Sekara, A.; Abdelhamid, M.T. Coupling effects of phosphorus fertilization source and rate on growth and ion accumulation of common bean under salinity stress. PeerJ 2021, 9, e11463. [Google Scholar] [CrossRef]
- Youssef, M.H.; Raafat, A.; El-Yazied, A.A.; Selim, S.; Azab, E.; Khojah, E.; El Nahhas, N.; Ibrahim, M.F. Exogenous application of alpha-Lipoic acid mitigates salt-induced oxidative damage in sorghum plants through regulation growth, leaf pigments, ionic homeostasis, antioxidant enzymes, and expression of salt stress responsive genes. Plants 2021, 10, 2519. [Google Scholar] [CrossRef]
- Tuteja, N. Integrated calcium signaling in plants. In Signaling in Plants; Springer: Berlin/Heidelberg, Germany, 2009; pp. 29–49. [Google Scholar]
- Lauchli, A. Calcium, salinity and the plasma membrane. In Calcium in Plant Growth and Development; Leonard, R.T., Hepler, P.K., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 1990; Volume 4, pp. 26–35. [Google Scholar]
- Shao, H.-B.; Song, W.-Y.; Chu, L.-Y. Advances of calcium signals involved in plant anti-drought. Comptes Rendus Biol. 2008, 331, 587–596. [Google Scholar] [CrossRef]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef]
- Halfter, U.; Ishitani, M.; Zhu, J.-K. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl. Acad. Sci. USA 2000, 97, 3735–3740. [Google Scholar] [CrossRef]

| Treatment | Ca (mM) | Chl a | Chl b | Chl a/Chl b | Car | TPP | IAA |
|---|---|---|---|---|---|---|---|
| (mg g−1 FW) | (μg g−1 FW) | ||||||
| NaCl (mM) | |||||||
| 0.0 | 2.02 † a | 0.95 a | 2.13 a | 0.38 c | 3.34 a | 9.40 a | |
| 30 | 1.65 b | 0.84 b | 1.96 b | 0.50 b | 2.99 b | 6.44 b | |
| 60 | 1.25 c | 0.69 c | 1.81 c | 0.52 a | 2.46 c | 4.74 c | |
| Ca (mM) | |||||||
| 0.0 | 1.45 c | 0.76 c | 1.90 c | 0.42 c | 2.63 c | 4.68 c | |
| 2.5 | 1.67 b | 0.84 b | 1.97 b | 0.46 b | 2.98 b | 6.93 b | |
| 5.0 | 1.79 a | 0.88 a | 2.03 a | 0.51 a | 3.17 a | 8.98 a | |
| NaCl (mM) | Ca (mM) | ||||||
| 0 | 0.0 | 1.80 c | 0.86 c | 2.08 a | 0.35 e | 3.01 d | 6.50 d |
| 2.5 | 2.07 b | 0.96 b | 2.15 a | 0.36 e | 3.39 b | 9.28 b | |
| 5.0 | 2.18 a | 1.01 a | 2.15 a | 0.41 d | 3.61 a | 12.41 a | |
| 30 | 0.0 | 1.49 e | 0.79 d | 1.88 b | 0.42 d | 2.70 e | 4.34 f |
| 2.5 | 1.67 d | 0.87 c | 1.92 b | 0.51 b | 3.05 d | 6.55 d | |
| 5.0 | 1.78 c | 0.86 c | 2.08 a | 0.56 a | 3.21 c | 8.44 c | |
| 60 | 0.0 | 1.07 h | 0.62 g | 1.73 c | 0.49 c | 2.18 g | 3.18 g |
| 2.5 | 1.28 g | 0.69 f | 1.85 b | 0.52 b | 2.50 f | 4.95 e | |
| 5.0 | 1.39 f | 0.75 e | 1.85 b | 0.55 a | 2.69 e | 6.09 d | |
| Treatment | Ca (mM) | FAA | Pro | TSS | CHO |
|---|---|---|---|---|---|
| (mg g−1 FW) | (%) | ||||
| NaCl (mM) | |||||
| 0.0 | 235 † c | 34.3 c | 44.6 c | 45.6 a | |
| 30 | 265 b | 43.6 b | 63.7 b | 44.1 b | |
| 60 | 325 a | 59.0 a | 82.9 a | 42.1 c | |
| Ca (mM) | |||||
| 0.0 | 253 c | 37.2 c | 55.6 c | 43.0 c | |
| 2.5 | 281 b | 45.6 b | 64.0 b | 44.8 a | |
| 5.0 | 291 a | 54.3 a | 71.7 a | 44.0 b | |
| NaCl (mM) | Ca (mM) | ||||
| 0 | 0.0 | 225 i | 29.2 i | 41.7 g | 44.3 d |
| 2.5 | 237 h | 34.1 h | 42.8 g | 46.8 a | |
| 5.0 | 241 g | 39.7 f | 49.4 f | 45.8 b | |
| 30 | 0.0 | 254 f | 35.9 g | 51.9 e | 43.2 e |
| 2.5 | 274 d | 43.9 e | 66.3 d | 44.9 c | |
| 5.0 | 268 e | 51.1 c | 72.9 c | 44.1 d | |
| 60 | 0.0 | 279 c | 46.4 d | 73.2 c | 41.4 g |
| 2.5 | 330 b | 58.7 b | 82.9 b | 42.7 ef | |
| 5.0 | 365 a | 72.0 a | 92.7 a | 42.2 f |
| Treatment | Ca (mM) | H2O2 | MDA | ALD |
|---|---|---|---|---|
| (nmol g g−1 FW) | ||||
| NaCl (mM) | ||||
| 0.0 | 0.222 † c | 0.456 b | 0.220 c | |
| 30 | 0.296 b | 0.556 a | 0.292 b | |
| 60 | 0.324 a | 0.585 a | 0.342 a | |
| Ca (mM) | ||||
| 0.0 | 0.297 a | 0.517 a | 0.314 a | |
| 2.5 | 0.280 b | 0.558 a | 0.275 b | |
| 5.0 | 0.264 c | 0.522 a | 0.264 c | |
| NaCl (mM) | Ca (mM) | |||
| 0 | 0.0 | 0.231 e | 0.449 b | 0.235 e |
| 2.5 | 0.223 e | 0.434 b | 0.213 f | |
| 5.0 | 0.212 e | 0.487 ab | 0.212 f | |
| 30 | 0.0 | 0.320 b | 0.605 ab | 0.317 b |
| 2.5 | 0.296 c | 0.552 ab | 0.289 c | |
| 5.0 | 0.273 d | 0.512 ab | 0.270 d | |
| 60 | 0.0 | 0.342 a | 0.499 ab | 0.390 a |
| 2.5 | 0.321 ab | 0.688 a | 0.324 b | |
| 5.0 | 0.308 bc | 0.568 ab | 0.311 b | |
| Treatment | NaCl (mM) | SOD | POD | CAT | NR |
|---|---|---|---|---|---|
| (U/min/g FW) | (nM NO2 g−1 FW) | ||||
| NaCl (mM) | |||||
| 0.0 | 31.5 † c | 62.1 c | 38.3 c | 323 c | |
| 30 | 47.0 b | 78.5 b | 54.4 b | 378 b | |
| 60 | 57.6 a | 93.3 a | 65.4 a | 466 a | |
| Ca (mM) | |||||
| 0.0 | 39.6 c | 69.9 c | 46.1 c | 380 c | |
| 2.5 | 46.2 b | 77.9 b | 51.3 b | 390 b | |
| 5.0 | 50.2 a | 86.2 a | 60.7 a | 397 a | |
| NaCl (mM) | Ca (mM) | ||||
| 0 | 0.0 | 27.9 h | 57.9 i | 33.7 h | 324 g |
| 2.5 | 30.4 g | 62.6 h | 38.0 g | 330 g | |
| 5.0 | 36.0 f | 65.9 g | 43.0 f | 316 h | |
| 30 | 0.0 | 39.9 e | 67.8 f | 47.4 e | 363 f |
| 2.5 | 49.3 d | 77.9 e | 52.9 d | 377 e | |
| 5.0 | 51.9 c | 89.9 c | 62.8 b | 393 d | |
| 60 | 0.0 | 51.1 c | 84.1 d | 57.1 c | 454 c |
| 2.5 | 58.9 b | 93.1 b | 62.9 b | 463 b | |
| 5.0 | 62.8 a | 102.9 a | 76.3 a | 481 a | |
| Treatment | Ca (mM) | AsA | GSH | TPC | TFC | β-Car | Lyc |
|---|---|---|---|---|---|---|---|
| (µmol ASA/100 g DW) | (µmol GSH/100 g DW) | (mg/100 g DW) | |||||
| NaCl (mM) | |||||||
| 0.0 | 238 † c | 137 c | 48.9 c | 11.8 c | 0.405 a | 0.362 a | |
| 30 | 292 b | 162 b | 77.2 b | 16.5 b | 0.374 b | 0.326 b | |
| 60 | 334 a | 185 a | 89.0 a | 21.4 a | 0.356 c | 0.300 c | |
| Ca (mM) | |||||||
| 0.0 | 269 c | 152 c | 62.1 c | 14.6 c | 0.363 c | 0.308 c | |
| 2.5 | 290 b | 161 b | 72.4 b | 18.6 a | 0.394 a | 0.347 a | |
| 5.0 | 305 a | 170 a | 80.6 a | 16.6 b | 0.379 b | 0.334 b | |
| NaCl (mM) | Ca (mM) | ||||||
| 0 | 0.0 | 219 i | 130 i | 42.9 g | 11.2 f | 0.383 cd | 0.336 c |
| 2.5 | 240 h | 137 h | 48.7 f | 12.3 ef | 0.426 a | 0.376 a | |
| 5.0 | 256 g | 144 g | 55.0 e | 12.0 f | 0.407 ab | 0.375 a | |
| 30 | 0.0 | 268 f | 149 f | 64.4 d | 13.9 e | 0.362 def | 0.307 e |
| 2.5 | 294 e | 163 e | 79.2 c | 19.6 c | 0.389 bc | 0.347 b | |
| 5.0 | 314 d | 173 d | 88.1 b | 15.9 d | 0.372 cde | 0.323 d | |
| 60 | 0.0 | 321 c | 177 c | 79.0 c | 18.5 c | 0.345 f | 0.281 f |
| 2.5 | 335 b | 184 b | 89.2 b | 24.0 a | 0.366 def | 0.317 d | |
| 5.0 | 347 a | 194 a | 98.7 a | 21.8 b | 0.358 ef | 0.303 e | |
| Treatment | Ca (mM) | DPPH (%) | ABTS (%) |
|---|---|---|---|
| NaCl (mM) | |||
| 0.0 | 57.8 † c | 36.0 c | |
| 30 | 64.5 b | 44.6 b | |
| 60 | 70.3 a | 48.9 a | |
| Ca (mM) | |||
| 0.0 | 59.7 c | 38.5 c | |
| 2.5 | 68.4 a | 42.6 b | |
| 5.0 | 64.6 b | 48.3 a | |
| NaCl (mM) | Ca (mM) | ||
| 0 | 0.0 | 51.4 f | 33.1 h |
| 2.5 | 64.1 d | 35.1 g | |
| 5.0 | 58.0 e | 39.7 f | |
| 30 | 0.0 | 59.8 e | 38.8 f |
| 2.5 | 68.0 bc | 45.2 d | |
| 5.0 | 65.8 d | 49.8 b | |
| 60 | 0.0 | 67.9 c | 43.8 e |
| 2.5 | 73.2 a | 47.4 c | |
| 5.0 | 70.0 b | 55.4 a |
| Treatment | Ca (mM) | SL | LA | SDW |
|---|---|---|---|---|
| (cm) | (cm2 Plant−1) | (g Plant−1) | ||
| NaCl (mM) | ||||
| 0.0 | 50.5 † a | 182 a | 1.98 a | |
| 30 | 43.8 b | 112 b | 1.17 b | |
| 60 | 36.5 c | 88 c | 0.76 c | |
| Ca (mM) | ||||
| 0.0 | 41.4 c | 105 c | 1.10 c | |
| 2.5 | 43.7 b | 129 b | 1.29 b | |
| 5.0 | 45.8 a | 148 a | 1.51 a | |
| NaCl (mM | Ca (mM) | |||
| 0 | 0.0 | 47.6 bc | 145 c | 1.62 c |
| 0 | 2.5 | 50.7 ab | 187 b | 1.93 b |
| 0 | 5.0 | 53.3 a | 214 a | 2.38 a |
| 30 | 0.0 | 42.2 de | 95 de | 1.05 e |
| 30 | 2.5 | 43.8 cd | 110 d | 1.17 de |
| 30 | 5.0 | 45.5 cd | 131 c | 1.28 d |
| 30 | 0.0 | 34.3 g | 76 f | 0.64 g |
| 30 | 2.5 | 36.5 fg | 89 ef | 0.76 fg |
| 30 | 5.0 | 38.7 of | 99 de | 0.87 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadak, M.S.; Hanafy, R.S.; Elkady, F.M.A.M.; Mogazy, A.M.; Abdelhamid, M.T. Exogenous Calcium Reinforces Photosynthetic Pigment Content and Osmolyte, Enzymatic, and Non-Enzymatic Antioxidants Abundance and Alleviates Salt Stress in Bread Wheat. Plants 2023, 12, 1532. https://doi.org/10.3390/plants12071532
Sadak MS, Hanafy RS, Elkady FMAM, Mogazy AM, Abdelhamid MT. Exogenous Calcium Reinforces Photosynthetic Pigment Content and Osmolyte, Enzymatic, and Non-Enzymatic Antioxidants Abundance and Alleviates Salt Stress in Bread Wheat. Plants. 2023; 12(7):1532. https://doi.org/10.3390/plants12071532
Chicago/Turabian StyleSadak, Mervat Sh, Rania S. Hanafy, Fatma M. A. M. Elkady, Asmaa M. Mogazy, and Magdi T. Abdelhamid. 2023. "Exogenous Calcium Reinforces Photosynthetic Pigment Content and Osmolyte, Enzymatic, and Non-Enzymatic Antioxidants Abundance and Alleviates Salt Stress in Bread Wheat" Plants 12, no. 7: 1532. https://doi.org/10.3390/plants12071532
APA StyleSadak, M. S., Hanafy, R. S., Elkady, F. M. A. M., Mogazy, A. M., & Abdelhamid, M. T. (2023). Exogenous Calcium Reinforces Photosynthetic Pigment Content and Osmolyte, Enzymatic, and Non-Enzymatic Antioxidants Abundance and Alleviates Salt Stress in Bread Wheat. Plants, 12(7), 1532. https://doi.org/10.3390/plants12071532









