What Hides in the Heights? The Case of the Iberian Endemism Bromus picoeuropeanus
Abstract
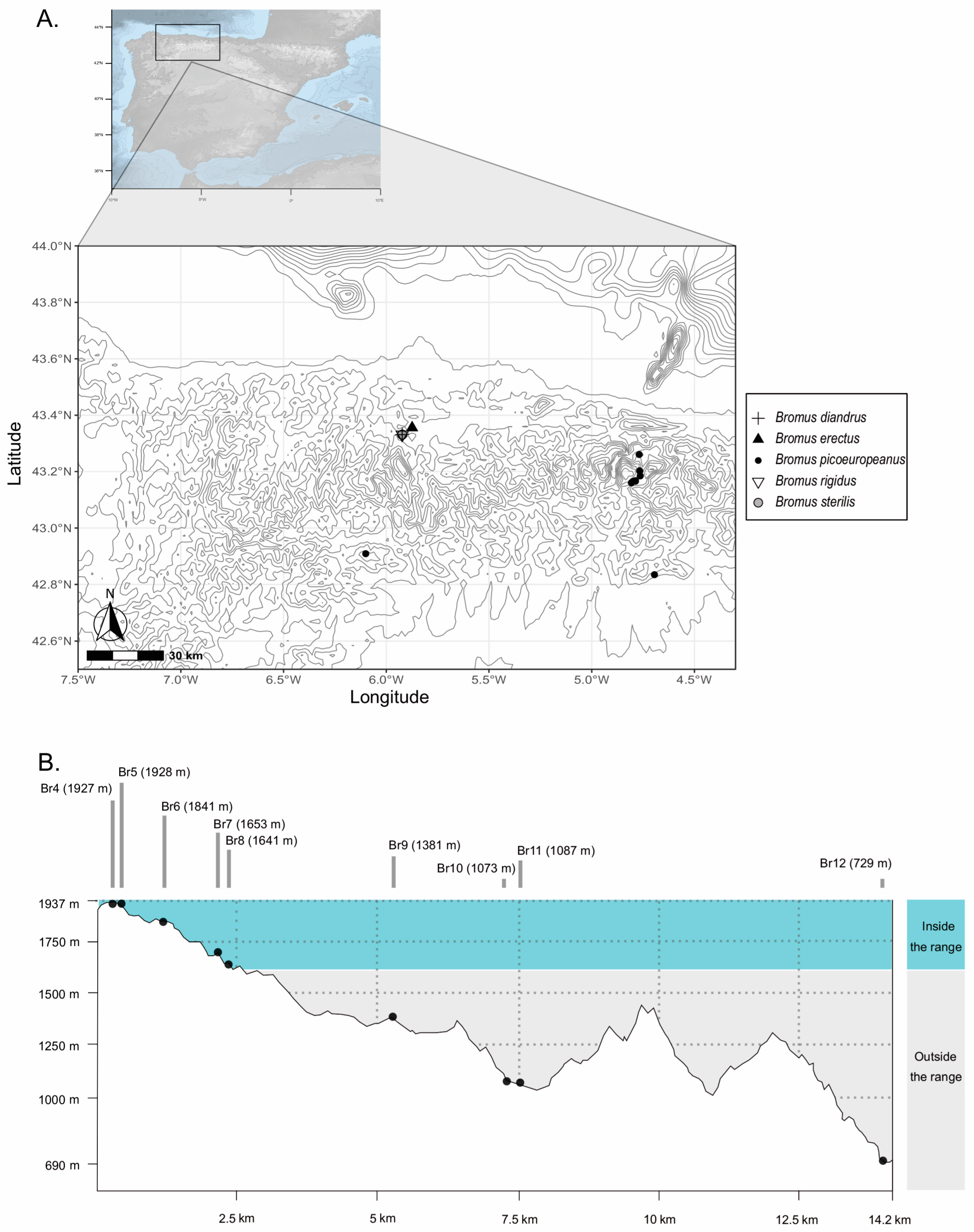
1. Introduction
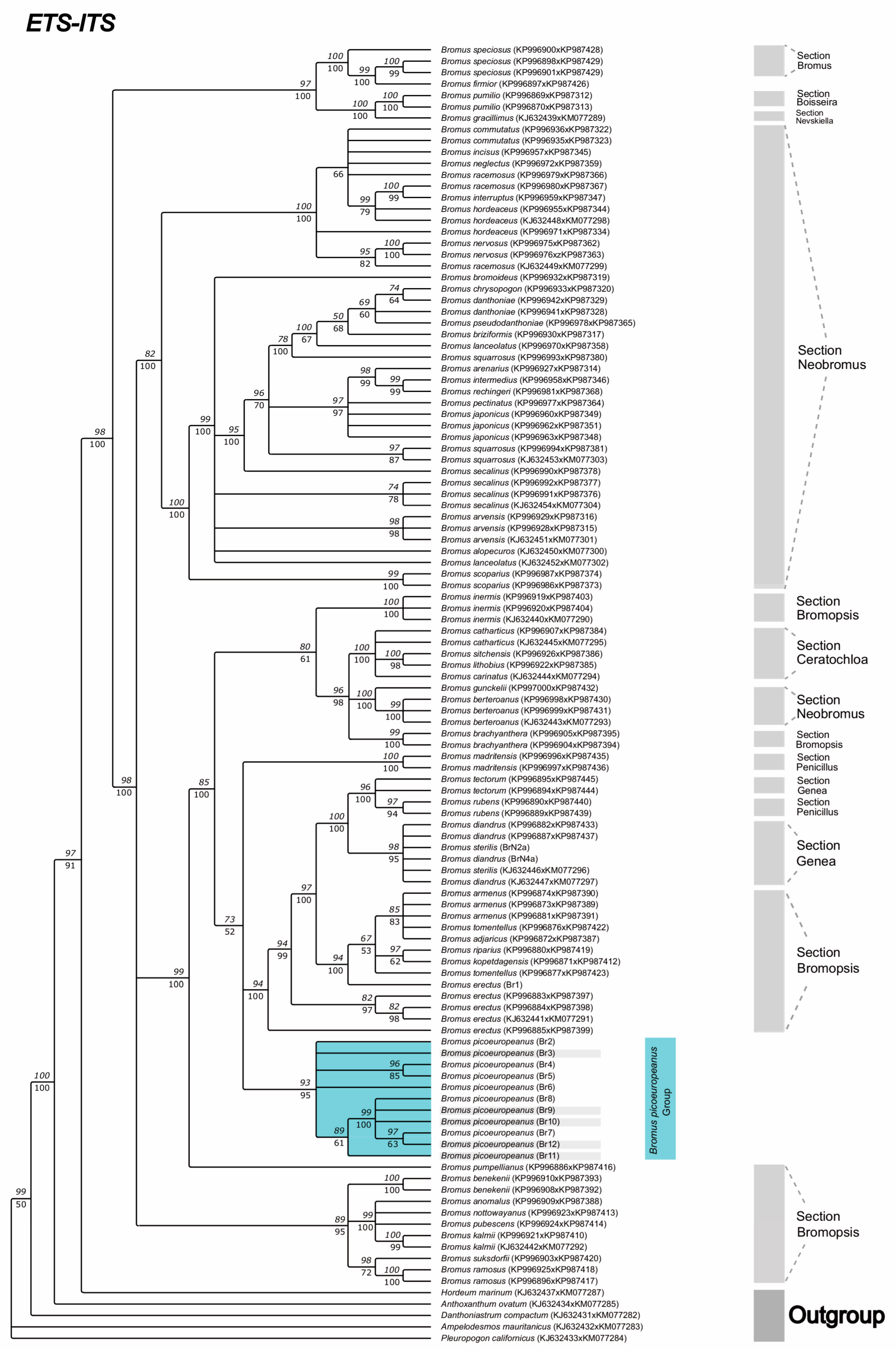
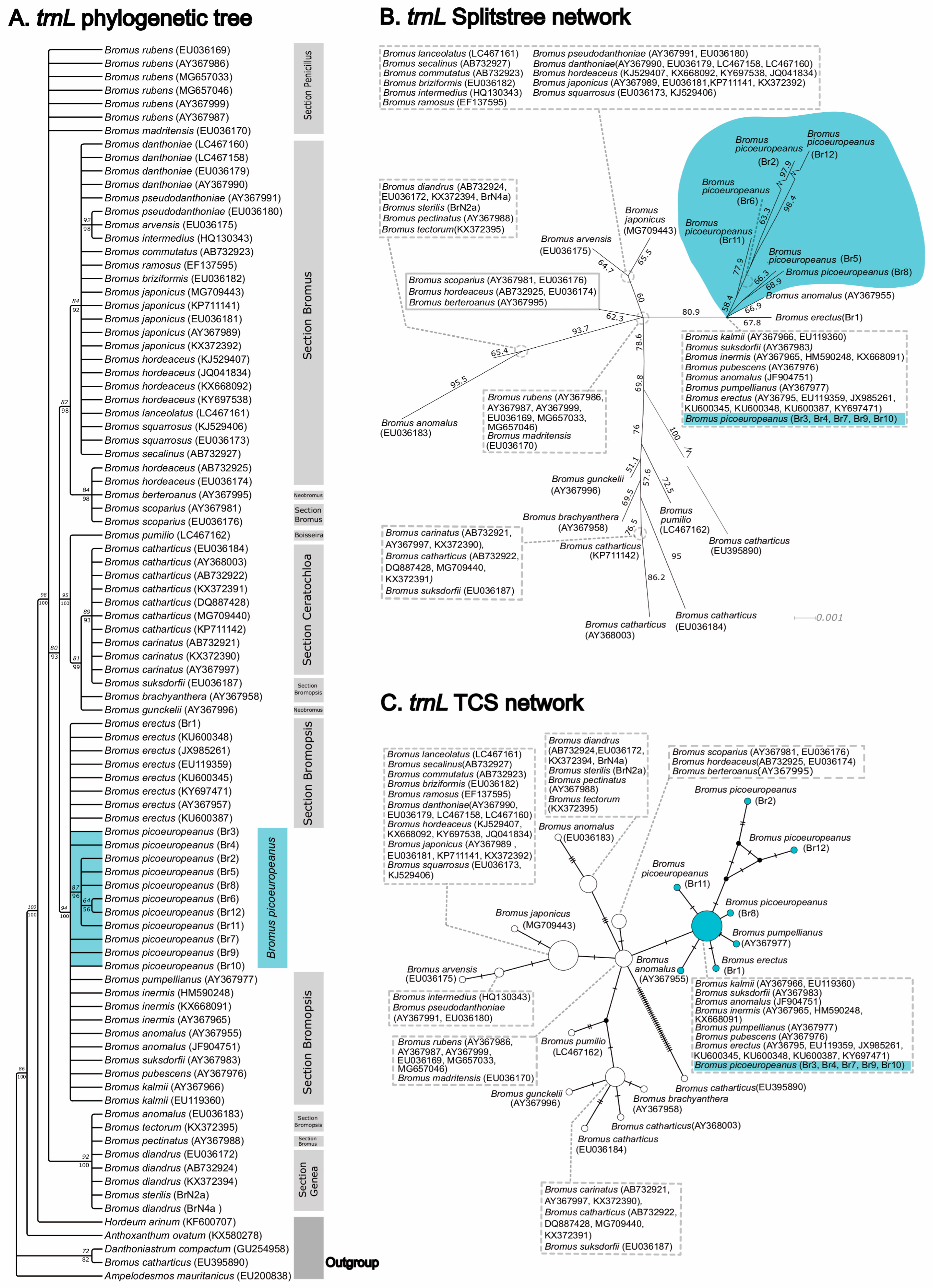
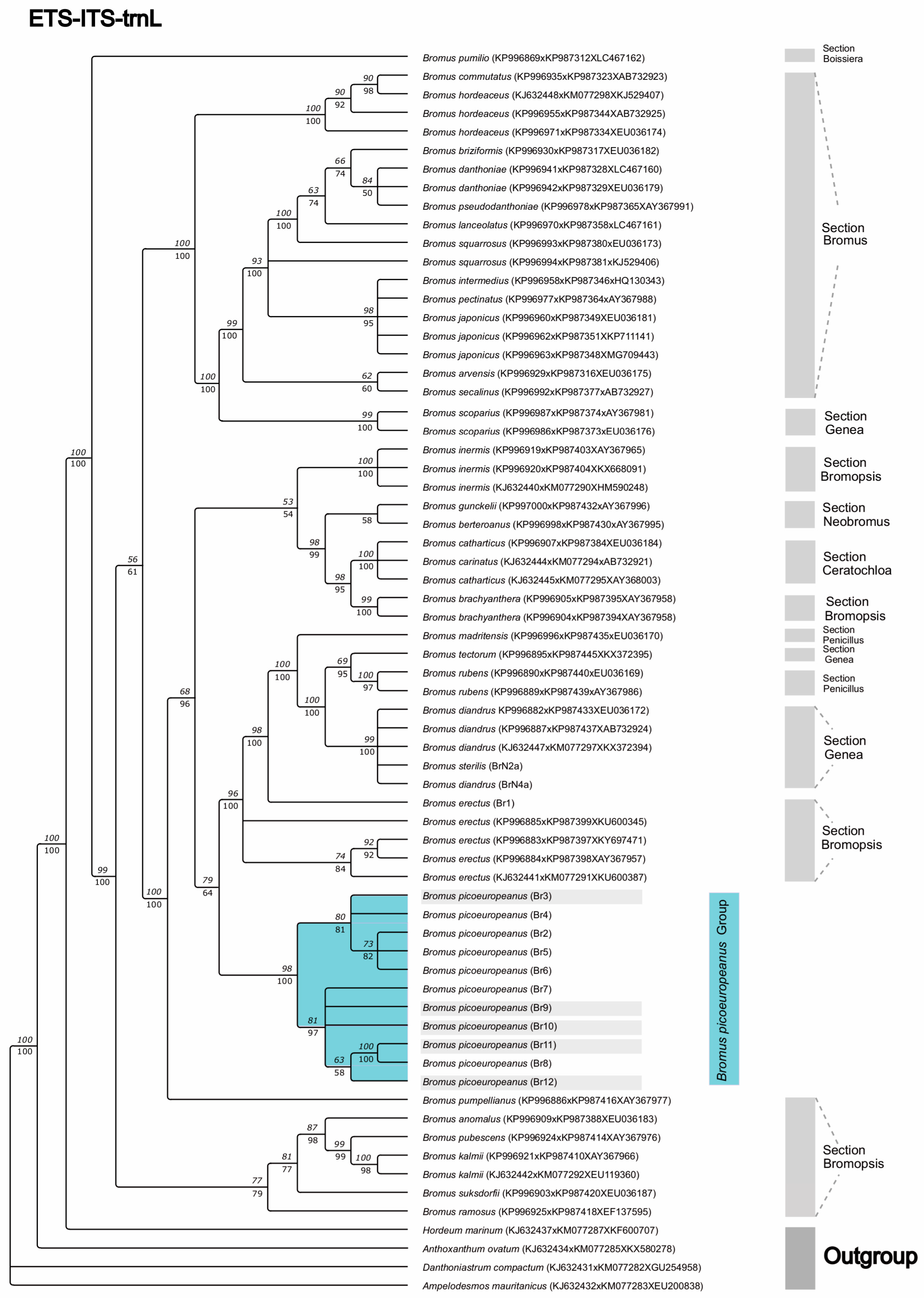
2. Results
3. Discussion
4. Materials and Methods
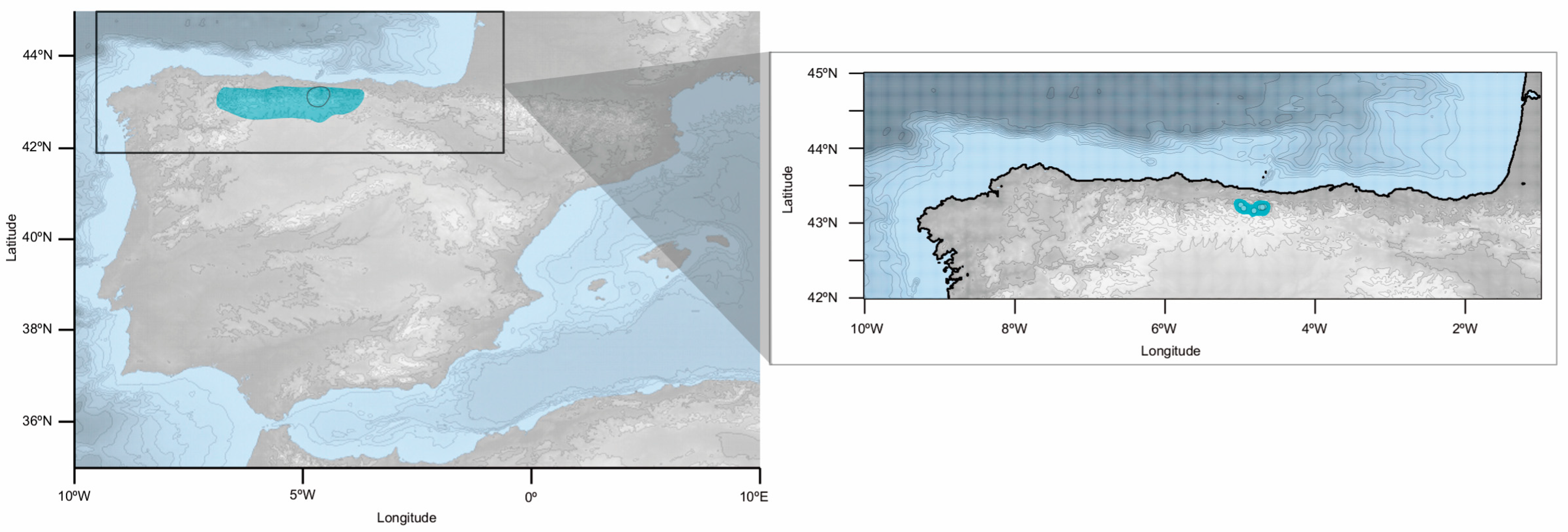
4.1. Plant Material
4.2. DNA Extraction, Amplification and Sequencing
4.3. Phylogenetic Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, E.O. The biological diversity crisis: A challenge to Science. Issues Sci. Technol. 1985, 2, 20–29. [Google Scholar]
- Rosser, A.M.; Mainka, S.A. Overexploitation and Species Extinctions. Conserv. Biol. 2002, 16, 584–586. [Google Scholar] [CrossRef]
- Peh, K.S.-H. Crop failure signals biodiversity crisis. Nature 2011, 473, 284. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Ravenc, P.H.; Roberts, M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, A.M.; Govaerts, R.; Ficinski, S.Z.; Nic Lughadha, E.; Vorontsova, M.S. Global dataset shows geography and life form predict modern plant extinction and rediscovery. Nat. Ecol. Evol. 2019, 3, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, J.J.; Hui, C.; Castillo, M.L.; Iriondo, J.M.; Keet, J.H.; Khapugin, A.A.; Médail, F.; Rejmánek, M.; Theron, G.; Yannelli, F.A.; et al. Recent Anthropogenic plant extinctions differ in biodiversity hotspots and coldspots. Curr. Biol. 2019, 29, 2912–2918. [Google Scholar] [CrossRef]
- Ohlemüller, R.; Anderson, B.J.; Araújo, M.B.; Butchart, S.H.; Kudrna, O.; Ridgely, R.S.; Thomas, C.D. The coincidence of climatic and species rarity: High risk to small-range species from climate change. Biol. Lett. 2008, 4, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Yang, K.; Liang, S.; Guo, X. The altitudinal dependence of recent rapid warming over the Tibetan Plateau. Clim. Chang. 2009, 97, 321–327. [Google Scholar] [CrossRef]
- Pepin, N.; Bradley, R.S.; Diaz, H.F.; Baraer, M.; Caceres, E.B.; Forsythe, N.; Fowler, H.; Greenwood, G.; Hashmi, M.Z.; Liu, X.D.; et al. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Chang. 2015, 5, 424–430. [Google Scholar] [CrossRef]
- Liang, Q.; Xu, X.; Mao, K.; Wang, M.; Wang, K.; Xi, Z.; Liu, J. Shifts in plant distributions in response to climate warming in a biodiversity hotspot, the Hengduan Mountains. J. Biogeogr. 2018, 45, 1334–1344. [Google Scholar] [CrossRef]
- Dinrböck, T.; Essl, F.; Rabitsch, W. Disproportional risk for habitat loss of high-altitude endemic species under climate change. Glob. Change Biol. 2011, 17, 990–996. [Google Scholar] [CrossRef]
- Rumpf, S.B.; Hülber, K.; Klonner, G.; Moser, D.; Schütz, M.; Wessely, J.; Willnera, W.; Zimmermannc, N.E.; Dullinger, S. Range dynamics of mountain plants decrease with elevation. Proc. Natl. Acad. Sci. USA 2018, 115, 1848–1853. [Google Scholar] [CrossRef] [PubMed]
- Acedo, C.; Llamas, F. Variation of micromorphological characters of lemma and palea in the genus Bromus (Poaceae). Ann. Bot. Fenn. 2001, 38, 1–14. [Google Scholar]
- Saarela, J.M.; Peterson, P.M.; Keane, R.M.; Cayouette, J.; Graham, S.W. Molecular phylogenetics of Bromus (Poaceae: Pooideae) based on chloroplast and nuclear DNA sequence data. Aliso 2007, 23, 450–467. [Google Scholar] [CrossRef]
- Simon, B.K. GrassWorld—Interactive key and information system of world grasses. Kew Bull. 2007, 62, 475–484. [Google Scholar]
- Saarela, J.M. Taxonomy of Bromus (Poaceae: Pooideae: Bromeae) sections Bromopsis, Bromus and Genea in British Columbia, Canada. J. Bot. Res. Inst. Tex. 2008, 2, 323–372. [Google Scholar]
- Williams, W.M.; Stewart, A.V.; Williamson, M.L. Bromus. In Wild Crop Relatives: Genomic and Breeding Resources: Millets and Grasses; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 15–30. [Google Scholar] [CrossRef]
- Llamas, F.; Acedo, C. Typification of eight current and seven related names and a new section in the genus Bromus (Bromeae, Pooideae, Poaceae). PhytoKeys 2019, 121, 53–72. [Google Scholar] [CrossRef]
- Gutiérrez, H.F.; Pensiero, J.F. Sinopsis de las especies argentinas del género Bromus (Poaceae). Darwiniana 1998, 35, 75–114. Available online: https://www.jstor.org/stable/23223250?seq=1#metadata_info_tab_contents (accessed on 16 March 2023).
- Massa, A.N.; Jensen, K.B.; Larson, S.R.; Hole, D.J. Morphological variation in Bromus sect. Ceratochloa germplasm of Patagonia. Can. J. Bot. 2004, 82, 136–144. [Google Scholar] [CrossRef]
- Pillay, M. DNA Systematic Studies in the Genus Bromus L. (Poaceae). Ph.D. Dissertation, Virginia Tech, Blacksburg, VA, USA, 1991. [Google Scholar]
- Smith, P. Taxonomy and nomenclature of the bromegrasses (Bromus L. s. l.). Notes R. Bot. Gard. Edinb. 1970, 30, 361–375. [Google Scholar]
- Stebbins, G.L. Chromosomes and evolution in the genus Bromus (Gramineae). Bot. Jahr. 1981, 102, 358–379. [Google Scholar]
- Tzelev, N.N. Tribe 4 Bromeae Dum. USSR. Poaceae USSR; Academy of Science Press: Leningrad, Russia, 1976; pp. 530–608. [Google Scholar]
- Armstrong, K.C. Chromosome Evolution of Bromus. In Chromosome Engineering in Plants: Genetics, Breeding, Evolution, Part B; Tsuchiya, T., Gupta, P.K., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 363–378. [Google Scholar]
- Kosina, R. Patterns of flower microstructural variation within the genus Bromus. Acta Soc. Bot. Pol. 1999, 68, 221–226. [Google Scholar] [CrossRef]
- Schulz-Schaeffer, J. Cytological investigations in the genus Bromus. III. The cytotaxonomic significance of the satellite chromosom. J. Hered. 1960, 51, 269–277. [Google Scholar] [CrossRef]
- Armstrong, K.C. Genome relationships in Bromus erectus, B. pumpellianu ssp. dicksonii and B. pumpellianus. Can. J. Genet. Cytol. 1975, 17, 391–394. [Google Scholar] [CrossRef]
- Acedo, C.; Llamas, F. A new species of perennial Bromus (Bromeae, Poaceae) from the Iberian Peninsula. PhytoKeys 2019, 121, 1–12. [Google Scholar] [CrossRef]
- Kahler, A.L.; Krzakowa, M.; Allard, R.W. Isozyme phenotypes in five species of Bromus sect. Genea. Bot. Jahrb. Syst. Pflanzengesch. Pflanzengeogr. 1981, 102, 401–409. [Google Scholar]
- Ainouche, M.L.; Bayer, R.J. On the origins of the tetraploid Bromus species (section Bromus, Poaceae): Insights from internal transcribed spacer sequences of nuclear ribosomal DNA. Genome 1997, 40, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandez, Y.S.N.; Somers, D.J.; Coulman, B.E. Estimating the genetic relationship of hybrid bromegrass to smooth bromegrass and meadow bromegrass using RAPD markers. Plant Breed. 2001, 120, 149–153. [Google Scholar] [CrossRef]
- Mayor, M.; Diaz, T.E. La flora de los pastizales. In La Flora Asturias; Ayalga Ediciones: Gijón, Spain, 1977; pp. 320–421. [Google Scholar]
- Anthos. Sistema de Información Sobre las Plantas de España Real Jardín Botánico, CSIC-Fund. Biodiversidad. 2011. Available online: http://www.anthos.es/ (accessed on 19 March 2020).
- Fernández Prieto, J.A.; Cires Rodríguez, E.; Bueno Sánchez, A.; Vázquez, V.M.; Nava Fernández, H.S. Catálogo de Plantas Vasculares del Principado de Asturias; Ayuntamiento de Gijón, Jardín Botánico Atlántico: Gijón, Spain, 2014. [Google Scholar]
- Meusel, H.; Jäger, E.J.; Weinert, E. Vergleichende Chorologie der Zentraleuropaischen Flora; Gustav Fischer Verlag: Jena, Spain, 1965. [Google Scholar]
- Eijsink, J.G.H.M.; Ellenbroek, G.; Holzner, W.; Werger, M.J.A. Dry and Semi-Dry Grasslands in the Weinviertel, Lower Austria. Vegetatio 1978, 36, 129–148. [Google Scholar] [CrossRef]
- Bačič, T.; Jogan, N. Multivariate morphometric study of the Bromus erectus group (Poaceae-Bromeae) in Slovenia. Phyton 2001, 41, 295–311. [Google Scholar]
- Wen, J.; Plunkett, G.M.; Mitchell, A.D.; Wagstaff, S.J. The evolution of Araliaceae: A phylogenetic analysis based on ITS sequences of nuclear ribosomal DNA. Syst. Bot. 2001, 26, 144–167. [Google Scholar] [CrossRef]
- Sutkowska, A.; Pasierbiński, A.; Warzecha, T.; Mandal, A.; Mitka, J. Refugial pattern of Bromus erectus in Central Europe based on ISSR fingerprinting. Acta Biol. Crac. Ser. Bot. 2013, 55, 107–119. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2023. Available online: http://www.plantsoftheworldonline.org/ (accessed on 28 February 2023).
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Poldini, L. Osservazioni sul Bromus erectus Huds. s.l. nel Triestino. Giorn. Bot. Ital. 1966, 73, 214–216. [Google Scholar]
- Bačič, M. Taxonomy and Chorology of Bromus erectus agg. in Slovenia. Ph.D. Dissertation, University of Ljubljana, Ljubljana, Slovenia, 1999. [Google Scholar]
- Tuna, M.; Vogel, K.P.; Arumuganathan, K. Cytogenetic and nuclear DNA content characterization of diploid Bromus erectus and Bromus variegatus. Crop Sci. 2006, 46, 637–641. [Google Scholar] [CrossRef]
- Sutkowska, A.; Wilk, L.; Mitka, J.; Joachimiak, A. DNA polymorphism in Bromus erectus and Bromus inermis from Poland’s territory. Zesz. Probl. Postep. Nauk. Rol. 2002, 488, 187–195. [Google Scholar]
- Pante, E.; Simon-Bouhet, B. marmap: A package for importing, plotting and analyzing bathymetric and topographic data in R. PLoS ONE 2013, 8, e73051. [Google Scholar] [CrossRef] [PubMed]
- Inkscape Project. Inkscape 2023. Available online: https://inkscape.org (accessed on 23 October 2022).
- Kramp, K.; Xu, X.; Mao, K.; Wang, M.; Wang, K.; Xi, Z.; Liu, J. Multiple glacial refugia and complex postglacial range shifts of the obligatory woodland plant Polygonatum verticillatum (Convallariaceae). Plant Biol. 2009, 11, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Hudson, W. Flora anglica: Exhibens Plantas Per Regnum Angliae Sponte Crescentes, Distributas Secundum Systema Sexuale: Cum Differentiis Specierum, Synonymis Autorum, Nominibus Incolarum, Solo Locorum, Tempore Florendi, Officinalibus Pharmacopaeorum; Inter-Documentation Company: Zug, Switzerland, 1762. [Google Scholar]
- Nasiri, A.; Kazempour-Osaloo, S.; Hamzehee, B.; Bull, R.D.; Saarela, J.M. A phylogenetic analysis of Bromus (Poaceae: Pooideae: Bromeae) based on nuclear ribosomal and plastid data, with a focus on Bromus sect. Bromus. PeerJ 2022, 10, e13884. [Google Scholar] [CrossRef]
- Fortune, P.M.; Pourtau, N.; Viron, N.; Ainouche, M.L. Molecular phylogeny and reticulate origins of the polyploid Bromus species from section Genea (Poaceae). Am. J. Bot. 2008, 95, 454–464. [Google Scholar] [CrossRef]
- Sheidai, M. Comparative cytogenetic study of some grass genera of the subfamily Pooideae in Iran Systematic study of selected Caryophyllaceae elements of Iran View project. Pol. Bot. J. 2008, 53, 15–28. [Google Scholar]
- Rico, E.; Acedo, C. 83. Bromus L. In Flora Iberica Vol. XIX. Gramineae; Castroviejo, S., Ed.; Real Jardín Botánico, CSIC: Madrid, Spain, 2021; pp. 995–1046. [Google Scholar]
- Sangoony, H.; Vahabi, M.; Tarkesh, M.; Soltani, S. Range Shifft of Bromus tomentellus Boiss. as a reaction to Climate Change in central Zagros, Iran. Appl. Ecol. Environ. Res. 2016, 14, 85–100. [Google Scholar] [CrossRef]
- Byars, S.G.; Parsons, Y.; Hoffmann, A.A. Effect of altitude on the genetic structure of an Alpine grass, Poa hiemata. Ann. Bot. 2009, 103, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M. 44. Bromus L. In Flora Europaea Vol. 5. Alismataceae to Orchidaceae (Monocotyledones); Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1980; pp. 182–190. [Google Scholar]
- Sun, Y.; Skinner, D.Z.; Liang, G.H.; Hulbert, S.H. Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of nuclear ribosomal DNA. Theor. Appl. Genet. 1994, 89, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Bull, R.D.; Acedo, C.; Gillespie, L.J. Design of plant-specific PCR primers for the ETS region with enhanced specificity for tribe Bromeae and their application to other grasses (Poaceae). Botany 2014, 92, 693–699. [Google Scholar] [CrossRef]
- Starr, J.R.; Harris, S.A.; Simpson, D.A. Potential of the 5′ and 3′ ends of the intergenic spacer (IGS) of rDNA in the Cyperaceae: New sequences for lower-level phylogenies in sedges with an example from Uncinia pers. Int. J. Plant Sci. 2003, 164, 213–227. [Google Scholar] [CrossRef]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Soreng, R.J.; Gillespie, L.J.; Koba, H.; Boudko, E.; Bull, R.D. Molecular and morphological evidence for a new grass genus, Dupontiopsis (Poaceae tribe Poeae subtribe Poinae s. l.), endemic to alpine Japan, and implications for the reticulate origin of Dupontia and Arctophila within Poinae s. l. J. Syst. Evol. 2015, 53, 138–162. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef]
- Geneious v.1.3. 2019. Available online: https://www.geneious.com (accessed on 23 October 2022).
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. JModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Zharkikh, A. Estimation of evolutionary distances between nucleotide sequences. J. Mol. Evol. 1994, 39, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Tavaré, S. Some probabilistic and statistical problems in the analysis of DNA sequences. Lect. Math. Life Sci. 1986, 17, 57–86. [Google Scholar]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucl. Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast Approximation for Phylogenetic Bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Chernomor, O.; von Haeseler, A.; Minh, B.Q. Terrace aware data structure for phylogenomic inference from supermatrices. Syst. Biol. 2016, 65, 997–1008. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Huson, D.H.; Bryant, D.; CBOL Plant Working Group. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.; Snell, Q.; Walkerm, P.; Posada, D.; Crandallet, K. TCS: Estimating Gene Genealogies. In Proceedings of the 16th International Parallel and Distributed Processing Symposium, Ft. Lauderdale, FL, USA, 15–19 April 2002; IEEE Computer Society: Washington, DC, USA; p. 0184. [Google Scholar]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Hsiao, C.; Chatterton, N.J.; Asay, K.H.; Jensen, K.B. Phylogenetic relationships of the monogenomic species of the wheat tribe, Triticeae (Poaceae), inferred from nuclear rDNA (internal transcribed spacer) sequences. Genome 1995, 38, 211–223. [Google Scholar] [CrossRef]
- Cialdella, A.M.; Giussani, L.M.; Aagesen, L.; Zuloaga, F.O.; Morrone, O. A phylogeny of Piptochaetium (Poaceae: Pooideae: Stipeae) and related genera based on a combined analysis including trnL-F, rpl16, and morphology. Syst. Bot. 2007, 32, 545–559. [Google Scholar] [CrossRef]
- Barkworth, M.E.; Arriaga, M.O.; Smith, J.F.; Jacobs, S.W.; Valdés-Reyna, J.; Bushman, B.S. Molecules and morphology in South American stipeae (Poaceae). Syst. Bot. 2008, 33, 719–731. [Google Scholar] [CrossRef]
- Schardl, C.L.; Craven, K.D.; Speakman, S.; Stromberg, A.; Lindstrom, A.; Yoshida, R. A novel test for host-symbiont codivergence indicates ancient origin of fungal endophytes in grasses. Syst. Biol. 2008, 57, 483–498. [Google Scholar] [CrossRef]
- Essi, L.; Longhi-Wagner, H.M.; de Souza-Chies, T.T. Phylogenetic analysis of the Briza Complex (Poaceae). Mol. Phylogenet. Evol. 2008, 47, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Bouchenak-Khelladi, Y.; Verboom, A.G.; Hodkinson, T.R.; Salamin, N.; Francois, O.; Chonghaile, N.G.; Savolainen, V. The origins and diversification of C4 grasses and savanna-adapted ungulates. Glob. Chang. Biol. 2009, 15, 2397–2417. [Google Scholar] [CrossRef]
- Romaschenko, K.; Peterson, P.M.; Soreng, R.J.; Garcia-Jacas, N.; Susanna, A. Phylogenetics of Stipeae 511 phylogenetics of Stipeae (Poaceae: Pooideae) based on plastid and nuclear DNA sequences. In Diversity, phylogeny, and evolution in the monocotyledons; Seberg, O., Petersen, G., Barfod, A.S., Davis, J.I., Eds.; Aarhus University Press: Aarhus, Denmark, 2010; pp. 511–538. [Google Scholar]
- Refulio-Rodriguez, N.F.; Columbus, J.T.; Gillespie, L.J.; Peterson, P.M.; Soreng, R.J. Molecular phylogeny of Dissanthelium (Poaceae: Poçoideae) and its taxonomic implications. Syst. Bot. 2012, 37, 122–133. [Google Scholar] [CrossRef]
- Wallinger, C.; Juen, A.; Staudacher, K.; Schallhart, N.; Mitterrutzner, E.; Steiner, E.M.; Thalinger, B.; Traugott, M. Rapid plant identification using species- and group-specific primers targeting chloroplast DNA. PLoS ONE 2012, 7, e29473. [Google Scholar] [CrossRef] [PubMed]
- Hiiesalu, I.; Oepik, M.; Metsis, M.; Lilje, L.; Davison, J.; Vasar, M.; Moora, M.; Zobel, M.; Wilson, S.D.; Paertel, M. Plant species richness belowground: Higher richness and new patterns revealed by next-generation sequencing. Mol. Ecol. 2012, 21, 2004–2016. [Google Scholar] [CrossRef] [PubMed]
- Mason-Gamer, R.J. Phylogeny of a genomically diverse group of Elymus (Poaceae) allopolyploids reveals multiple levels of reticulation. PLoS ONE 2013, 8, e78449. [Google Scholar] [CrossRef]
- Matsushima, R.; Yamashita, J.; Kariyama, S.; Enomoto, T.; Sakamoto, W. A Phylogenetic re-evaluation of morphological variations of starch grains among Poaceae species. J. Appl. Glycosci. 2013, 60, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Escudero, M.; Sahuquillo, E.; Minaya, M.Á.; Catalán, P. Are diversification rates and chromosome evolution in the temperate grasses (Pooideae) associated with major environmental changes in the Oligocene-Miocene? PeerJ 2017, e3815. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.C.; Gao, H.Y.; Wei, Y.N.; Zhang, J.H.; Chen, X.Y.; Li, H.Q. Evaluating sampling strategy for DNA barcoding study of coastal and inland halo-tolerant Poaceae and Chenopodiaceae: A case study for increased sample size. PLoS ONE 2017, 12, e0185311. [Google Scholar] [CrossRef] [PubMed]
- Chumová, Z.; Záveská, E.; Mandáková, T.; Krak, K.; Trávníček, P. The Mediterranean: The cradle of Anthoxanthum (Poaceae) diploid diversity. Ann. Bot. 2017, 120, 285–302. [Google Scholar] [CrossRef]
- Holá, E.; Kocková, J.; Těšitel, J. DNA barcoding as a tool for identification of host association of root-hemiparasitic plants. Folia Geobot. 2017, 52, 227–238. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Wójcik, J.M.; Taberlet, P.; Kamiński, T.; Miquel, C.; Valentini, A.; Craine, J.M.; Coissac, E. Foraging plasticity allows a large herbivore to persist in a sheltering forest habitat: DNA metabarcoding diet analysis of the European bison. For. Ecol. Manag. 2019, 449, 117474. [Google Scholar] [CrossRef]
- Pourmoshir, Z.; Amirahmadi, A.; Naderi, R. An overview of the phylogenetic relationships of Bromus pumilio (Poaceae) and allies based on nrDNA ITS and trnL-F sequences. Iran J. Bot. 2019, 25, 1–10. [Google Scholar] [CrossRef]


| ETS | ITS | trnL | Nuclear | Combined | |
|---|---|---|---|---|---|
| Bromus analyzed taxa | 51 | 50 | 34 | 51 | 34 |
| Number of sequences | 119 | 150 | 87 | 109 | 63 |
| Range of length of sequences (pb) | 160–497 | 512–540 | 425–465 | 691–1034 | 1139–1490 |
| Alignment length (pb) | 529 | 568 | 508 | 1088 | 1678 |
| C + G (%) | 52.7 | 57.8 | 28.7 | 55.6 | 46.8 |
| Conserved sites | 300 | 377 | 413 | 684 | 1196 |
| Variable sites | 214 | 169 | 80 | 375 | 337 |
| Parsimonious-informative sites | 150 | 123 | 22 | 272 | 191 |
| Code | Location | Coordinates | Altitude (m a. s. l.) | Voucher | Collector (Identifier) | Morphological Identification | GenBank Accession n° |
|---|---|---|---|---|---|---|---|
| Br1 | Faculty of Biology, Oviedo (Asturias) | 43°21′19.90″ N, 5°52′26.85″ W | 312 | FCO40796 | JAFP and HSN (HSN) | B. erectus | ETS: OQ557418 ITS: OQ544412 trnL: OQ557422 |
| Br2 | Riolago de Babia, Babia, León (Castilla y León) | 42°54′32.39″ N, 6°5′59.88″ W | 1693 | FCO40799 | JAFP and HSN (HSN) | B. picoeuropeanus | ETS: OQ557413 ITS: OQ544413 trnL: OQ557428 |
| Br3 | Peña Grande, Villaverde de la Peña, Palencia (Castilla y León) | 42°50′44.36″ N, 4°41′40.59″ W | 1625 | FCO40800 | JAFP and HSN (HSN) | B. picoeuropeanus | ETS: OQ557408 ITS: OQ544414 trnL: OQ557423 |
| Br4 | Picos de Europa National Park (Cantabria) | 43°9′38.21″ N, 4°48′23.65″ W | 1927 | FCO40801 | JAFP and HSN (HSN) | B. picoeuropeanus | ETS: OQ557412 ITS: OQ544418 trnL: OQ557424 |
| Br5 | Picos de Europa National Park (Cantabria) | 43°9′37.32″ N, 4°48′24.64″ W | 1928 | FCO40802 | JAFP and HSN (HSN) | B. picoeuropeanus | ETS: OQ557416 ITS: OQ544411 trnL: OQ557429 |
| Br6 | Picos de Europa National Park (Cantabria) | 43°9′56.55″ N, 4°47′57.18″ W | 1841 | FCO40803 | JAFP and HSN (HSN) | B. picoeuropeanus | ETS: OQ557414 ITS: OQ544409 trnL: OQ557430 |
| Br7 | Picos de Europa National Park (Cantabria) | 43°10′3.82″ N, 4°47′17.22″ W | 1653 | FCO40804 | JAFP and HSN (HSN) | B. picoeuropeanus | ETS: OQ557411 ITS: OQ544406 trnL: OQ557425 |
| Br8 | Picos de Europa National Park (Cantabria) | 43°9′57.50″ N, 4°47′13.05″ W | 1641 | FCO40805 | JAFP and HSN (HSN) | B. picoeuropeanus | ETS: OQ557417 ITS: OQ544407 trnL: OQ557427 |
| Br9 | Picos de Europa National Park (Cantabria) | 43°11′5.31″ N, 4°45′50.11″ W | 1381 | FCO40806 | JAFP and HSN (HSN) | B. picoeuropeanus | ETS: OQ557409 ITS: OQ544410 trnL: OQ557431 |
| Br10 | Picos de Europa National Park (Asturias) | 43°12′11.19″ N, 4°46′1.71″ W | 1073 | FCO40807 | JAFP and HSN (HSN) | B. picoeuropeanus | ETS: OQ557415 ITS: OQ544408 trnL: OQ557426 |
| Br11 | Picos de Europa National Park (Asturias) | 43°12′7.17″ N, 4°46′0.00″ W | 1087 | FCO40808 | JAFP and HSN (HSN) | B. picoeuropeanus | ETS: OQ557407 ITS: OQ544419 trnL: OQ557432 |
| Br12 | Picos de Europa National Park (Asturias) | 43°15′38.29″ N, 4°46′8.46″ W | 729 | FCO40809 | JAFP and HSN (HSN) | B. picoeuropeanus | ETS: OQ557410 ITS: OQ544415 trnL: OQ557433 |
| BrN2a | Las Caldas, Oviedo (Asturias) | 43°19′52.13″ N, 5°55′20.338″ W | 99 | FCO40811 | HSN (HSN) | B. sterilis | ETS: OQ557420 ITS: OQ544416 trnL: OQ557434 |
| BrN3a | Las Caldas, Oviedo (Asturias) | 43°19′52.13″ N, 5°55′20.338″ W | 99 | FCO40813 | HSN (HSN) | B. rigidus | ETS: OQ557421 ITS: n.d. trnL: n.d. |
| BrN4a | Las Caldas, Oviedo (Asturias) | 43°19′52.13″ N, 5°55′20.338″ W | 99 | FCO40814 | HSN (HSN) | B. diandrus | ETS: OQ557419 ITS: OQ544417 trnL: OQ557435 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Toral, C.; Nava, H.S.; Fernández Prieto, J.A.; Cires, E. What Hides in the Heights? The Case of the Iberian Endemism Bromus picoeuropeanus. Plants 2023, 12, 1531. https://doi.org/10.3390/plants12071531
González-Toral C, Nava HS, Fernández Prieto JA, Cires E. What Hides in the Heights? The Case of the Iberian Endemism Bromus picoeuropeanus. Plants. 2023; 12(7):1531. https://doi.org/10.3390/plants12071531
Chicago/Turabian StyleGonzález-Toral, Claudia, Herminio S. Nava, José Antonio Fernández Prieto, and Eduardo Cires. 2023. "What Hides in the Heights? The Case of the Iberian Endemism Bromus picoeuropeanus" Plants 12, no. 7: 1531. https://doi.org/10.3390/plants12071531
APA StyleGonzález-Toral, C., Nava, H. S., Fernández Prieto, J. A., & Cires, E. (2023). What Hides in the Heights? The Case of the Iberian Endemism Bromus picoeuropeanus. Plants, 12(7), 1531. https://doi.org/10.3390/plants12071531






