Chlorophylls as Natural Bioactive Compounds Existing in Food By-Products: A Critical Review
Abstract
1. Introduction
2. Chemical Composition and Different Types of Chlorophylls
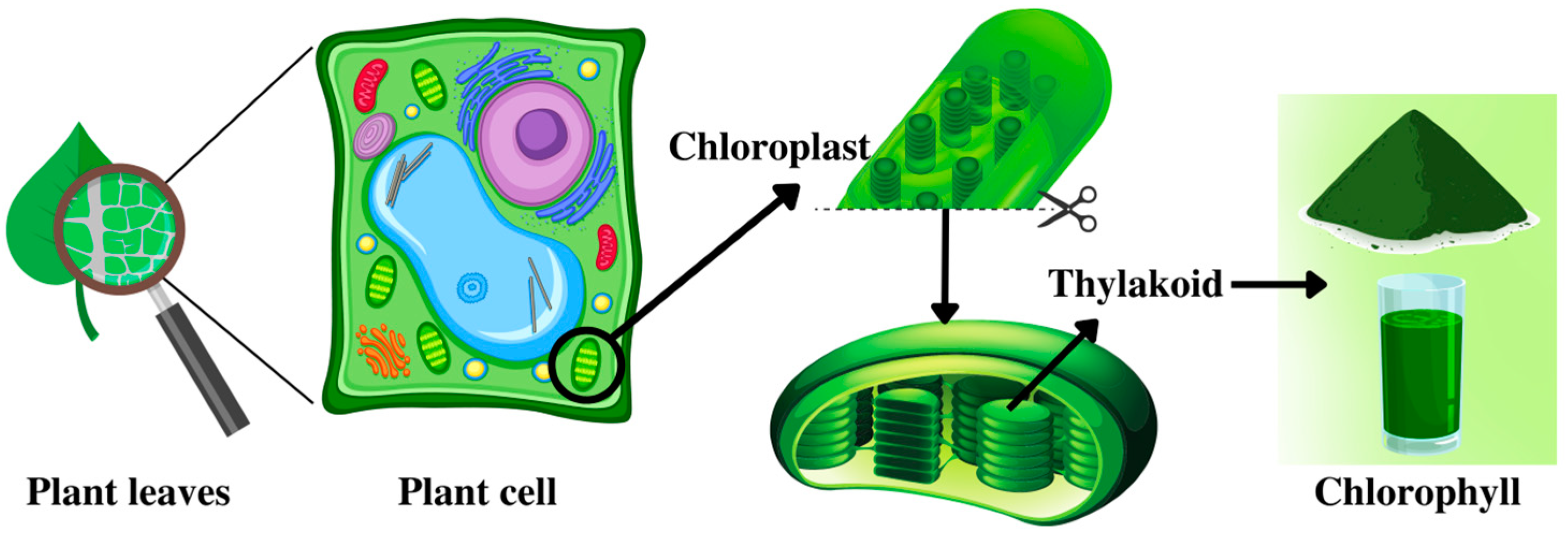
3. The Role and Location of Chlorophylls in Plants
4. Extraction of Chlorophyll
5. Effect of Different Treatments on Chlorophylls
6. Nutritional Properties and Health Benefits of Chlorophylls
7. Application of Recovered Chlorophylls in Functional Foods
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Valorisation of food agro-industrial by-products: From the past to the present and perspectives. J. Environ. Manag. 2021, 299, 113571. [Google Scholar] [CrossRef] [PubMed]
- Pareek, S.; Sagar, N.A.; Sharma, S.; Kumar, V.; Agarwal, T.; González-Aguilar, G.A.; Yahia, E.M. Chlorophylls: Chemistry and biological functions. In Fruit and Vegetable Phytochemicals; John Wiley & Sons, Ltd.: Chichester, UK, 2017; Volume 1, pp. 269–284. ISBN 9781119158042. [Google Scholar]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Artés-Hernández, F. By-products revalorization with non-thermal treatments to enhance phytochemical compounds of fruit and vegetables derived products: A review. Foods 2022, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.V.; Jagus, R.J.; Agüero, M.V. Evaluation and characterization of nutritional, microbiological and sensory properties of beet greens. Acta Sci. Nutr. Health 2017, 1, 37–45. [Google Scholar]
- Ebrahimi, P.; Lante, A. Environmentally friendly techniques for the recovery of polyphenols from food by-products and their impact on polyphenol oxidase: A critical review. Appl. Sci. 2022, 12, 1923. [Google Scholar] [CrossRef]
- Kashaninejad, M.; Sanz, M.T.; Blanco, B.; Beltrán, S.; Niknam, S.M. Freeze dried extract from olive leaves: Valorisation, extraction kinetics and extract characterization. Food Bioprod. Process. 2020, 124, 196–207. [Google Scholar] [CrossRef]
- Bengardino, M.B.; Fernandez, M.V.; Nutter, J.; Jagus, R.J.; Agüero, M.V. Recovery of bioactive compounds from beet leaves through simultaneous extraction: Modelling and process optimization. Food Bioprod. Process. 2019, 118, 227–236. [Google Scholar] [CrossRef]
- Yeasmen, N.; Orsat, V. Green extraction and characterization of leaves phenolic compounds: A comprehensive review. In Critical Reviews in Food Science and Nutrition; Taylor & Francis: Abingdon, UK, 2021; pp. 1–39. [Google Scholar] [CrossRef]
- Goyeneche, R.; Di Scala, K.; Ramirez, C.L.; Fanovich, M.A. Recovery of bioactive compounds from beetroot leaves by supercritical CO2 extraction as a promising bioresource. J. Supercrit. Fluids 2020, 155, 104658. [Google Scholar] [CrossRef]
- Tinello, F.; Lante, A. Valorisation of ginger and turmeric peels as source of natural antioxidants. Plant Foods Hum. Nutr. 2019, 74, 443–445. [Google Scholar] [CrossRef]
- Cisneros-Yupanqui, M.; Chalova, V.I.; Kalaydzhiev, H.R.; Mihaylova, D.; Krastanov, A.I.; Lante, A. Preliminary characterisation of wastes generated from the rapeseed and sunflower protein isolation process and their valorisation in delaying oil oxidation. Food Bioprocess Technol. 2021, 14, 1962–1971. [Google Scholar] [CrossRef]
- Lante, A.; Tinello, F.; Mihaylova, D. Valorization of onion extracts as anti-browning agents. Food Sci. Appl. Biotechnol. 2020, 3, 16–21. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Lante, A.; Mihaylova, D. Comparison of green technologies for valorizing sugar beet (Beta vulgaris L.) leaves. Food Sci. Appl. Biotechnol. 2022, 5, 119–130. [Google Scholar] [CrossRef]
- Derrien, M.; Aghabararnejad, M.; Gosselin, A.; Desjardins, Y.; Angers, P.; Boumghar, Y. Optimization of supercritical carbon dioxide extraction of lutein and chlorophyll from spinach by-products using response surface methodology. LWT 2018, 93, 79–87. [Google Scholar] [CrossRef]
- Leite, A.C.; Ferreira, A.M.; Morais, E.S.; Khan, I.; Freire, M.G.; Coutinho, J.A.P. Cloud point extraction of chlorophylls from spinach leaves using aqueous solutions of nonionic surfactants. ACS Sustain. Chem. Eng. 2018, 6, 590–599. [Google Scholar] [CrossRef]
- Sarkar, S.; Manna, M.S.; Bhowmick, T.K.; Gayen, K. Extraction of chlorophylls and carotenoids from dry and wet biomass of isolated Chlorella Thermophila: Optimization of process parameters and modelling by artificial neural network. Process. Biochem. 2020, 96, 58–72. [Google Scholar] [CrossRef]
- Das, P.; Nayak, P.K.; Kesavan, R. krishnan Ultrasound assisted extraction of food colorants: Principle, mechanism, extraction technique and applications: A review on recent progress. Food Chem. Adv. 2022, 1, 100144. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Nieto, G.; Martínez-Zamora, L.; Ros, G.; Kamiloglu, S.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Fernández-López, J.; Viuda-Martos, M.; et al. Novel approaches for the recovery of natural pigments with potential health effects. J. Agric. Food Chem. 2021, 70, 6864–6883. [Google Scholar] [CrossRef]
- Nguyen, N.H.K.; Diem An, N.T.; Anh, P.K.; Truc, T.T. Microwave-assisted extraction of chlorophyll and polyphenol with antioxidant activity from Pandanus amaryllifolius Roxb. in Vietnam. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1166, 012039. [Google Scholar] [CrossRef]
- Hannachi, S.; Signore, A.; Adnan, M.; Mechi, L. Single and associated effects of drought and heat stresses on physiological, biochemical and antioxidant machinery of four eggplant cultivars. Plants 2022, 11, 2404. [Google Scholar] [CrossRef]
- Scheepers, J.C.; Malan, S.F.; Du Preez, J.L.; Van Dyk, S. The high performance liquid chromatography (HPLC) analysis of ultraviolet (UV) irradiated chlorophyll a and secondary plant compounds. Afr. J. Biotechnol. 2011, 10, 16976–16985. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef]
- Kong, W.; Liu, N.; Zhang, J.; Yang, Q.; Hua, S.; Song, H.; Xia, C. Optimization of ultrasound-assisted extraction parameters of chlorophyll from Chlorella vulgaris residue after lipid separation using response surface methodology. J. Food Sci. Technol. 2014, 51, 2006–2013. [Google Scholar] [CrossRef]
- Ahmadi, A.; Shahidi, S.-A.; Safari, R.; Motamedzadegan, A.; Ghorbani-HasanSaraei, A. Evaluation of stability and antibacterial properties of extracted chlorophyll from alfalfa (Medicago sativa L.). Food Chem. Toxicol. 2022, 163, 112980. [Google Scholar] [CrossRef] [PubMed]
- Tavanandi, H.A.; Raghavarao, K.S.M.S. Recovery of chlorophylls from spent biomass of Arthrospira platensis obtained after extraction of phycobiliproteins. Bioresour. Technol. 2019, 271, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Nikolov, Z. Process for selective extraction of pigments and functional proteins from Chlorella vulgaris. Algal Res. 2018, 35, 185–193. [Google Scholar] [CrossRef]
- Derrien, M.; Badr, A.; Gosselin, A.; Desjardins, Y.; Angers, P. Optimization of a green process for the extraction of lutein and chlorophyll from spinach by-products using response surface methodology (RSM). LWT 2017, 79, 170–177. [Google Scholar] [CrossRef]
- Carbone, K.; Amoriello, T.; Iadecola, R. Exploitation of kiwi juice pomace for the recovery of natural antioxidants through microwave-assisted extraction. Agriculture 2020, 10, 435. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Leite, A.C.; Coutinho, J.A.P.; Freire, M.G. Chlorophylls extraction from spinach leaves using aqueous solutions of surface-active ionic liquids. Sustain. Chem. 2021, 2, 764–777. [Google Scholar] [CrossRef]
- Lee, J.; Kwak, M.; Chang, Y.K.; Kim, D. Green solvent-based extraction of chlorophyll a from Nannochloropsis sp. Using 2,3-butanediol. Sep. Purif. Technol. 2021, 276, 119248. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Dziki, D. Recent trends in pretreatment of food before freeze-drying. Processes 2020, 8, 1661. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Monteiro, F.; Passos, C.P.; Silva, A.M.S.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Blanching impact on pigments, glucosinolates, and phenolics of dehydrated broccoli by-products. Food Res. Int. 2020, 132, 109055. [Google Scholar] [CrossRef] [PubMed]
- Kaewsuksaeng, S.; Urano, Y.; Aiamla-or, S.; Shigyo, M.; Yamauchi, N. Effect of UV-B irradiation on chlorophyll-degrading enzyme activities and postharvest quality in stored lime (Citrus latifolia Tan.) fruit. Postharvest Biol. Technol. 2011, 61, 124–130. [Google Scholar] [CrossRef]
- Phaisan, S.; Yusakul, G.; Sakdamas, A.; Taluengjit, N.; Sakamoto, S.; Putalun, W. A green and effective method using oils to remove chlorophyll from chromolaena odorata (L.) R.M. King & H. Rob. Songklanakarin J. Sci. Technol. 2020, 42, 1084–1090. [Google Scholar]
- Nguyen, T.T.; Uthairatanakij, A.; Srilaong, V.; Laohakunjit, N.; Kato, M.; Jitareerat, P. Impact of electron beam irradiation on the chlorophyll degradation and antioxidant capacity of mango fruit. Appl. Biol. Chem. 2021, 64, 19. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Li, R.X.; Jiang, Q.S.; Bai, R.; Duan, D. Changes in the chlorophyll content of grape leaves could provide a physiological index for responses and adaptation to UV-C radiation. Nord. J. Bot. 2019, 37, 1–11. [Google Scholar] [CrossRef]
- Han, C.; Zhen, W.; Chen, Q.; Fu, M. UV-C irradiation inhibits surface discoloration and delays quality degradation of fresh-cut stem lettuce. LWT 2021, 147, 111533. [Google Scholar] [CrossRef]
- Duarte-Sierra, A.; Munzoor Hasan, S.M.; Angers, P.; Arul, J. UV-B radiation hormesis in broccoli florets: Glucosinolates and hydroxy-cinnamates are enhanced by UV-B in florets during storage. Postharvest Biol. Technol. 2020, 168, 111278. [Google Scholar] [CrossRef]
- Sari, L.K.; Setha, S.; Naradisorn, M. Effect of UV-C irradiation on postharvest quality of ‘Phulae’ pineapple. Sci. Hortic. 2016, 213, 314–320. [Google Scholar] [CrossRef]
- Xie, C.; Tang, J.; Xiao, J.; Geng, X.; Guo, L. Purple light-emitting diode (LED) lights controls chlorophyll degradation and enhances nutraceutical quality of postharvest broccoli florets. Sci. Hortic. 2022, 294, 110768. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Li, L.; Aghdam, M.S.; Wei, X.; Liu, J.; Xu, Y.; Luo, Z. Elevated CO 2 delayed the chlorophyll degradation and anthocyanin accumulation in postharvest strawberry fruit. Food Chem. 2019, 285, 163–170. [Google Scholar] [CrossRef]
- Rehman, S.; Lukins, P.B. Picosecond time-gated microscopy of UV-damaged plant tissue. Opt. Express 2002, 10, 370–375. [Google Scholar] [CrossRef]
- Jovanić, B.R.; Radenković, B.; Despotović-Zrakić, M.; Bogdanović, Z.; Barać, D. Effect of UV-B radiation on chlorophyll fluorescence, photosynthetic activity and relative chlorophyll content of five different corn hybrids. J. Photochem. Photobiol. 2022, 10, 100115. [Google Scholar] [CrossRef]
- Cazzaniga, S.; Bressan, M.; Carbonera, D.; Agostini, A.; Dall’Osto, L. Differential roles of carotenes and xanthophylls in photosystem I photoprotection. Biochemistry 2016, 55, 3636–3649. [Google Scholar] [CrossRef]
- Gonçalves, J.F.D.C.; Marenco, R.A.; Vieira, G. Concentration of photosynthetic pigments and chlorophyll fluorescence of mahogany and tonka bean under two light environments. Rev. Bras. Fisiol. Veg. 2001, 13, 149–157. [Google Scholar] [CrossRef]
- Murch, S.J.; Erland, L.A.E. A systematic review of melatonin in plants: An example of evolution of literature. Front. Plant Sci. 2021, 12, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Chairat, B.; Nutthachai, P.; Varit, S. Effect of UV-C treatment on chlorophyll degradation, antioxidant enzyme activities and senescence in Chinese kale (Brassica oleracea var. alboglabra). Int. Food Res. J. 2013, 20, 623. [Google Scholar]
- Araque, L.C.O.; Rodoni, L.M.; Darré, M.; Ortiz, C.M.; Civello, P.M.; Vicente, A.R. Cyclic low dose UV-C treatments retain strawberry fruit quality more effectively than conventional pre-storage single high fluence applications. LWT 2018, 92, 304–311. [Google Scholar] [CrossRef]
- Aiamla-or, S.; Kaewsuksaeng, S.; Shigyo, M.; Yamauchi, N. Impact of UV-B irradiation on chlorophyll degradation and chlorophyll-degrading enzyme activities in stored broccoli (Brassica oleracea L. Italica Group) florets. Food Chem. 2010, 120, 645–651. [Google Scholar] [CrossRef]
- Loi, M.; Liuzzi, V.C.; Fanelli, F.; De Leonardis, S.; Maria Creanza, T.; Ancona, N.; Paciolla, C.; Mulè, G. Effect of different light-emitting diode (LED) irradiation on the shelf life and phytonutrient content of broccoli (Brassica oleracea L. var. italica). Food Chem. 2019, 283, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Y.; Zheng, D.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Effects of supplementary blue and UV-A LED lights on morphology and phytochemicals of Brassicaceae baby-leaves. Molecules 2020, 25, 5678. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB light, and methyl jasmonate, alone or combined, redirect the biosynthesis of glucosinolates, phenolics, carotenoids, and chlorophylls in broccoli sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Vicente, A.R.; Civello, P.M.; Chaves, A.R.; Martínez, G.A. UV-C treatment delays postharvest senescence in broccoli florets. Postharvest Biol. Technol. 2006, 39, 204–210. [Google Scholar] [CrossRef]
- Srilaong, V.; Aiamla-or, S.; Soontornwat, A.; Shigyo, M.; Yamauchi, N. UV-B irradiation retards chlorophyll degradation in lime (Citrus latifolia Tan.) fruit. Postharvest Biol. Technol. 2011, 59, 110–112. [Google Scholar] [CrossRef]
- Yasuda, M.; Oda, K.; Ueda, T.; Tabata, M. Physico-chemical chlorophyll-a species in aqueous alcohol solutions determine the rate of its discoloration under UV light. Food Chem. 2019, 277, 463–470. [Google Scholar] [CrossRef]
- Pongsri, R.; Aiamla-or, S.; Srilaong, V.; Uthairatanakij, A.; Jitareerat, P. Impact of electron-beam irradiation combined with shellac coating on the suppression of chlorophyll degradation and water loss of lime fruit during storage. Postharvest Biol. Technol. 2021, 172, 111364. [Google Scholar] [CrossRef]
- Wang, E.; Wink, M. Chlorophyll enhances oxidative stress tolerance in Caenorhabditis elegans and extends its lifespan. PeerJ 2016, 2016, e1879. [Google Scholar] [CrossRef]
- Al Mijan, M.; Sim, W.J.; Lim, T.G. Physiological effects of green-colored food-derived bioactive compounds on cancer. Appl. Sci. 2021, 11, 11288. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Y.; Hu, X.; Liao, X.; Zhang, Y. Chlorophyll supplementation in early life prevents diet-induced obesity and modulates gut microbiota in mice. Mol. Nutr. Food Res. 2019, 63, 1801219. [Google Scholar] [CrossRef]
- Wunderlich, A.L.M.; Azevedo, S.C.S.F.; Yamada, L.A.; Bataglini, C.; Previate, C.; Campanholi, K.S.S.; Pereira, P.C.S.; Caetano, W.; Kaplum, V.; Nakamura, C.V.; et al. Chlorophyll treatment combined with photostimulation increases glycolysis and decreases oxidative stress in the liver of type 1 diabetic rats. Braz. J. Med. Biol. Res. 2020, 53, e8389. [Google Scholar] [CrossRef] [PubMed]
- Alsuhaibani, A.M.; ALkehayez, N.M.; Alshawi, A.H.; Al-Faris, N.A. Effects of chlorophyll on body functioning and blood glucose levels. Asian J. Clin. Nutr. 2017, 9, 64–70. [Google Scholar] [CrossRef]
- Carvalho, A.M.S.; Heimfarth, L.; Pereira, E.W.M.; Oliveira, F.S.; Menezes, I.R.A.; Coutinho, H.D.M.; Picot, L.; Antoniolli, A.R.; Quintans, J.S.S.; Quintans-Júnior, L.J. Phytol, a Chlorophyll Component, Produces Antihyperalgesic, Anti-inflammatory, and Antiarthritic Effects: Possible NFκB Pathway Involvement and Reduced Levels of the Proinflammatory Cytokines TNF-α and IL-6. J. Nat. Prod. 2020, 83, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, M.S. Nutrition and cancer: A review of the evidence for an anti-cancer diet. Nutr. J. 2004, 3, 19. [Google Scholar] [CrossRef]
- Nabi, B.G.; Mukhtar, K.; Ahmed, W.; Manzoor, M.F.; Ranjha, M.M.A.N.; Kieliszek, M.; Bhat, Z.F.; Aadil, R.M. Natural pigments: Anthocyanins, carotenoids, chlorophylls, and betalains as colorants in food products. Food Biosci. 2023, 52, 102403. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Mazzocchi, C.; Esti, M. Natural colorants from vegetable food waste: Recovery, regulatory aspects, and stability—A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2715–2737. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Ferreira, I.C.F.R. Wastes and by-products: Upcoming sources of carotenoids for biotechnological purposes and health-related applications. Trends Food Sci. Technol. 2017, 62, 33–48. [Google Scholar] [CrossRef]
- Martirosyan, D.; von Brugger, J.; Bialow, S. Functional food science: Differences and similarities with food science. Funct. Foods Health Dis. 2021, 11, 408–430. [Google Scholar] [CrossRef]
- Zen, C.K.; Tiepo, C.B.V.; da Silva, R.V.; Reinehr, C.O.; Gutkoski, L.C.; Oro, T.; Colla, L.M. Development of functional pasta with microencapsulated Spirulina: Technological and sensorial effects. J. Sci. Food Agric. 2020, 100, 2018–2026. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Ramon-Mascarell, F.; Pallarés, N.; Ferrer, E.; Berrada, H.; Phimolsiripol, Y.; Barba, F.J. Extraction of antioxidant compounds and pigments from spirulina (Arthrospira platensis) assisted by pulsed electric fields and the binary mixture of organic solvents and water. Appl. Sci. 2021, 11, 7629. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Elbatanony, M.M.; El-Feky, A.M.; Hemdan, B.A.; Azab El-Liethy, M. Assessment of the antimicrobial activity of the lipoidal and pigment extracts of Punica granatum L. leaves. Acta Ecol. Sin. 2019, 39, 89–94. [Google Scholar] [CrossRef]
- Pothiraj, C.; Balaji, P.; Shanthi, R.; Gobinath, M.; Suresh Babu, R.; Munirah, A.A.D.; Ashraf, A.H.; Ramesh Kumar, K.; Veeramanikandan, V.; Arumugam, R. Evaluating antimicrobial activities of Acanthus ilicifolius L. and Heliotropium curassavicum L against bacterial pathogens: An in-vitro study. J. Infect. Public Health 2021, 14, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Dziedziński, M.; Kobus-Cisowska, J.; Szymanowska-Powałowska, D.; Stuper-Szablewska, K.; Baranowska, M. Polyphenols composition, antioxidant and antimicrobial properties of Pinus sylvestris L. shoots extracts depending on different drying methods. Emir. J. Food Agric. 2020, 32, 229–237. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Lante, A. Polyphenols: A comprehensive review of their nutritional properties. Open Biotechnol. J. 2021, 15, 164–172. [Google Scholar] [CrossRef]
- Klopsch, R.; Baldermann, S.; Voss, A.; Rohn, S.; Schreiner, M.; Neugart, S. Narrow-banded UVB affects the stability of secondary plant metabolites in kale (Brassica oleracea var. sabellica) and pea (Pisum sativum) leaves being added to lentil flour fortified bread: A novel approach for producing functional foods. Foods 2019, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Sanna, D.; Fadda, A. Waste from food and agro-food industries as pigment sources: Recovery techniques, stability and food applications. Nutraceuticals 2022, 2, 365–383. [Google Scholar] [CrossRef]
- Jayasinghe, P.S.; Pahalawattaarachchi, V.; Ranaweera, K.K.D.S. Seaweed extract as a natural food coloring agent in jelly desserts on chemical, microbial and sensory quality. Acad. Agric. J. 2016, 1, 65–69. [Google Scholar]
- Liu, M.H.; Li, Y.F.; Chen, B.H. Preparation of chlorophyll nanoemulsion from pomelo leaves and its inhibition effect on melanoma cells A375. Plants 2021, 10, 1664. [Google Scholar] [CrossRef]
- Paskeviciute, E.; Zudyte, B.; Luksiene, Z. Innovative nonthermal technologies: Chlorophyllin and visible light significantly reduce microbial load on Basil. Food Technol. Biotechnol. 2019, 57, 126–132. [Google Scholar] [CrossRef]


| Material | Extraction Method | Solvent | Temp (°C) | Extraction Time (min) | Solid-to-Liquid Ratio (g:mL) | TCC 1 (mg/g) | Ref. |
|---|---|---|---|---|---|---|---|
| Chlorella vulgaris residue | UAE 2 | EtOH 79.4% | 61.4 | 78.7 | 50:10 | 31.1 ± 1.56 | [25] |
| Alfalfa (Medicago sativa L.) leaves | UAE | EtOH 96% | 35 | 60 | 1:10 | 1.74 | [26] |
| Biomass of Chlorella thermophila isolate | High-speed homogenizer | EtOH 96% | 58 | 6 | 1:1 | 60.41 | [17] |
| Spinach leaves | CE 3 | Aqueous Solutions of Nonionic Surfactants | 41 | 30 | 7:1000 | 0.94 ± 0.03 | [16] |
| Biomass of Arthrospira platensis | CE | EtOH 100% | 27 | 720 | 1:5 | 5.75 | [27] |
| Pandan leaf | MAE 4 | Acetone 100% | - | 2 | 1:30 | 0.42 | [21] |
| Biomass of Chlorella vulgaris | CE | EtOH 95% | 22–25 | 30 | 1:5 | 15.4 | [28] |
| Spinach by-products | CE | Acetone 100% | 25 | 20 | 05:10 | 1.13 | [29] |
| Kiwi Juice Pomace | MAE | EtOH 50% | 75 | 15 | 1:15 | 0.06 | [30] |
| Materials | Treatment | Condition | Effects | Result | Ref. |
|---|---|---|---|---|---|
| Mango | Electron beam | Treatment with 0.5 kGy electron beam | Decreased pheophytinase and peroxidase activity | Decrease in the degradation of chlorophylls | [38] |
| Grape leaves | UV-C | 245 nm, 15 W, 10 min, distance: 12.5 cm | Increased the reactive oxygen species | Decrease in the chlorophyll content | [39] |
| Fresh-cut stem lettuce | UV-C | 254 nm, intensity: 16.6 W m−2, irradiation: 8 kJm−2, distance: 20 cm | Reduced the activity of chlorophyllase and Mg-dechelatase | Decrease in the chlorophyll degradation | [40] |
| Broccoli florets | UV-B | 310 nm, intensity: 20.4 Wm−2, irradiation: 1.2 kJm−2 | Reduced the activities of chlorophyllase and pheophytinase | Delay in the yellowing of the broccoli florets | [41] |
| Pineapple | UV-C | Irradiation: 26.4 kJm−2 | Increased total phenolic content and antioxidant activity (DPPH and FRAP | Increase in the maintenance of the color characteristics | [42] |
| Broccoli florets | Purple LED | The light intensity was approximately 40 µmols−1m−2 | Downregulated the expression of the genes related to the chlorophyll degradation | Increase in the stability of chlorophyll | [43] |
| Strawberry | Elevated CO2 | Treatment with air containing 20% CO2 | Inhibited chlorophyllase and Mg-dechelatase activity | Delay in the degradation of chlorophylls | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimi, P.; Shokramraji, Z.; Tavakkoli, S.; Mihaylova, D.; Lante, A. Chlorophylls as Natural Bioactive Compounds Existing in Food By-Products: A Critical Review. Plants 2023, 12, 1533. https://doi.org/10.3390/plants12071533
Ebrahimi P, Shokramraji Z, Tavakkoli S, Mihaylova D, Lante A. Chlorophylls as Natural Bioactive Compounds Existing in Food By-Products: A Critical Review. Plants. 2023; 12(7):1533. https://doi.org/10.3390/plants12071533
Chicago/Turabian StyleEbrahimi, Peyman, Zahra Shokramraji, Setareh Tavakkoli, Dasha Mihaylova, and Anna Lante. 2023. "Chlorophylls as Natural Bioactive Compounds Existing in Food By-Products: A Critical Review" Plants 12, no. 7: 1533. https://doi.org/10.3390/plants12071533
APA StyleEbrahimi, P., Shokramraji, Z., Tavakkoli, S., Mihaylova, D., & Lante, A. (2023). Chlorophylls as Natural Bioactive Compounds Existing in Food By-Products: A Critical Review. Plants, 12(7), 1533. https://doi.org/10.3390/plants12071533









