Spontaneous Primary Succession and Vascular Plant Recovery in the Iberian Gypsum Quarries: Insights for Ecological Restoration in an EU Priority Habitat
Abstract
1. Introduction
- Depict the spontaneous successional pattern that takes place in gypsum quarries in southeastern Spain once their exploitation has ceased, and verify that the successional stages experienced a trend towards greater richness and floristic diversity throughout a chronosequence of 13 years. Therefore, the successional SAR curves were expected to converge towards those of the undisturbed gypsum scrublands.
- In the same way, it was hypothesized that the restored plots (active restoration) would achieve levels of richness, floristic composition, species abundance and diversity similar to those of the unaltered reference scrubland in a few years and it was assumed that these levels would be higher than those of the successional plots even for the final year of monitoring, 2021. According to this hypothesis, the SAR curves of these plots should be closer to those of the reference scrub than the successional ones.
- Verify whether the same pattern of spontaneous succession as described above is widespread in gypsum quarries throughout the Iberian Peninsula.
- Draw, from the previous analyses, the implications that both spontaneous succession and active restoration have regarding restoration strategies in an EU priority habitat located in a hotspot of global diversity, especially in that which concerns the gypsicolous vegetation.
2. Results
2.1. Permanent Successional Plots
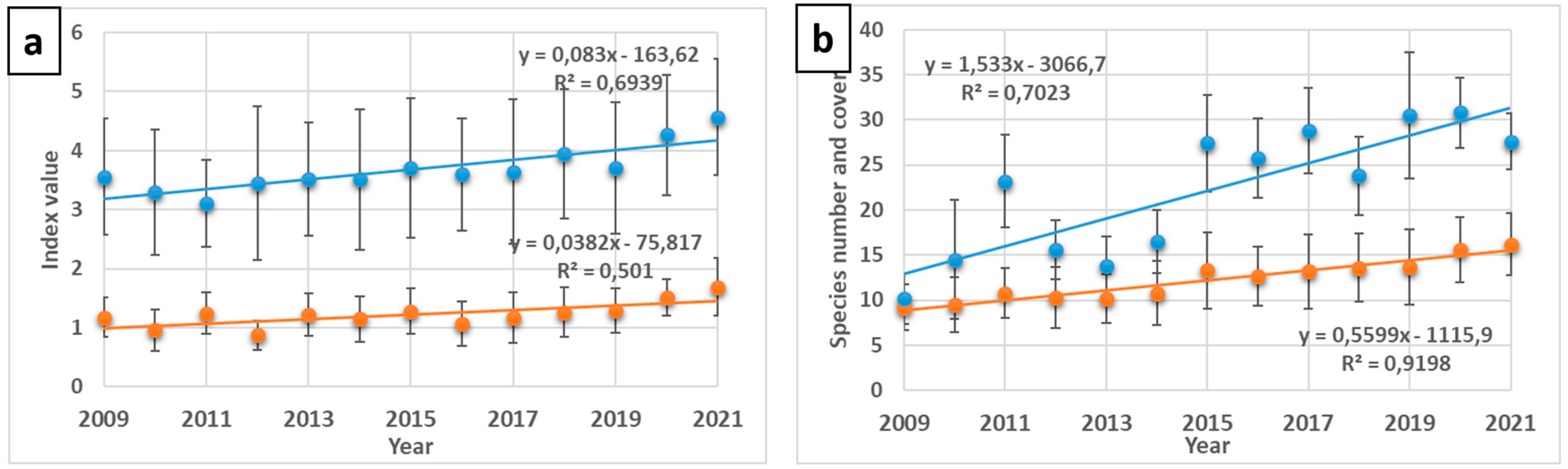
2.1.1. Floristic Changes along the Plots in the Chronosequence (Table S1)
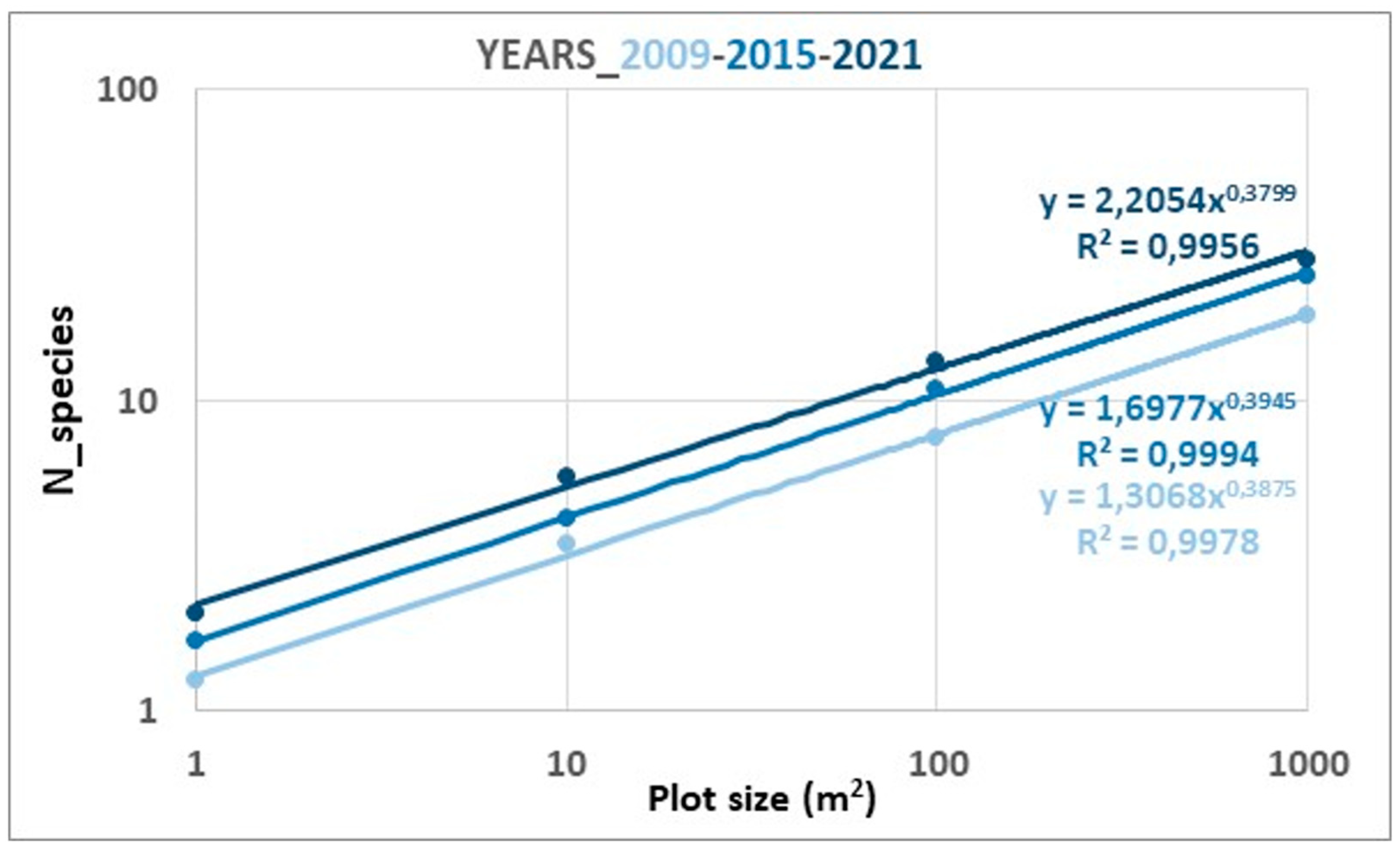
2.1.2. Diversity and SAR Curves in Successional Plots
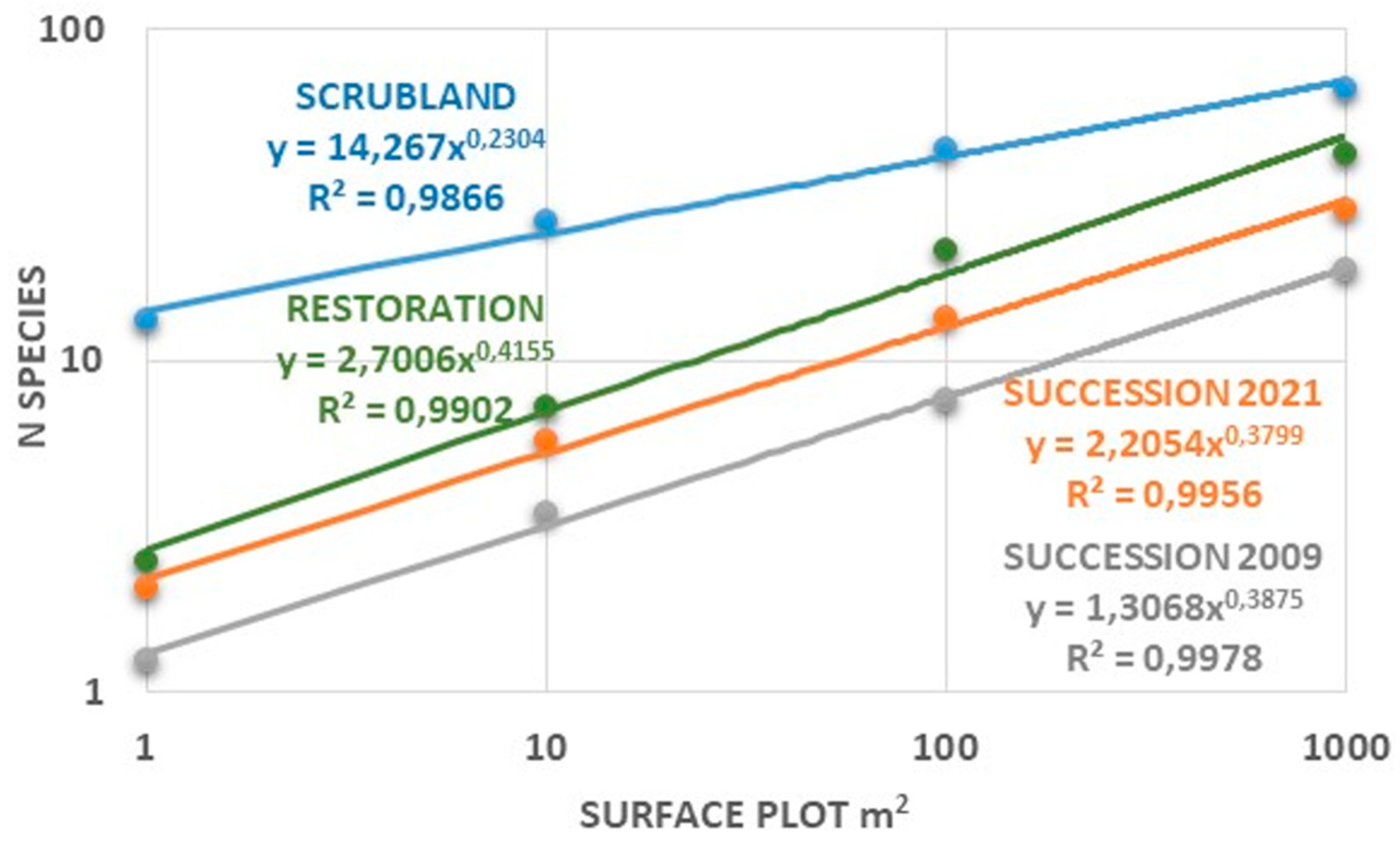
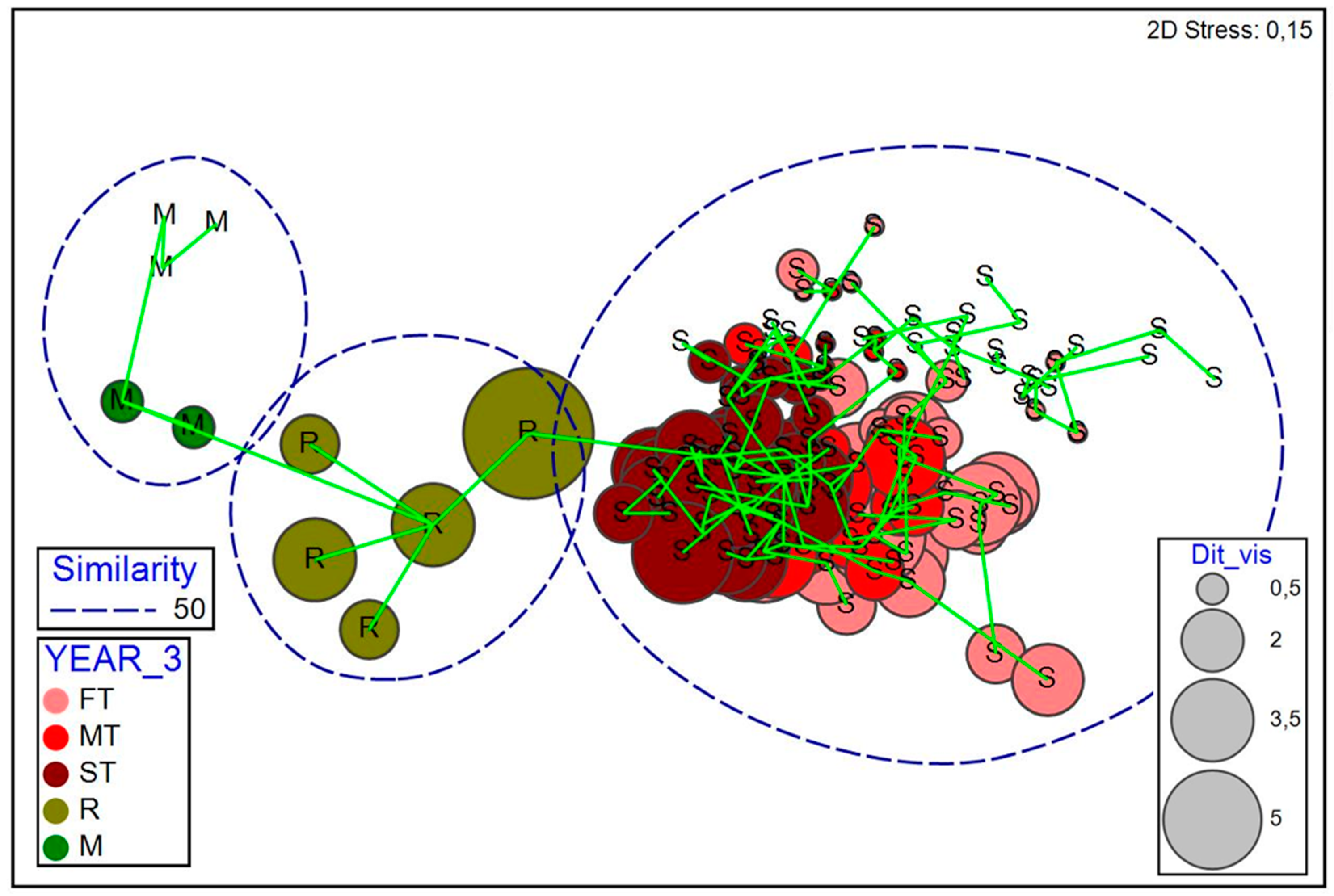
2.2. Restored and Reference Scrubland Plots: Insights for Ecological Restoration
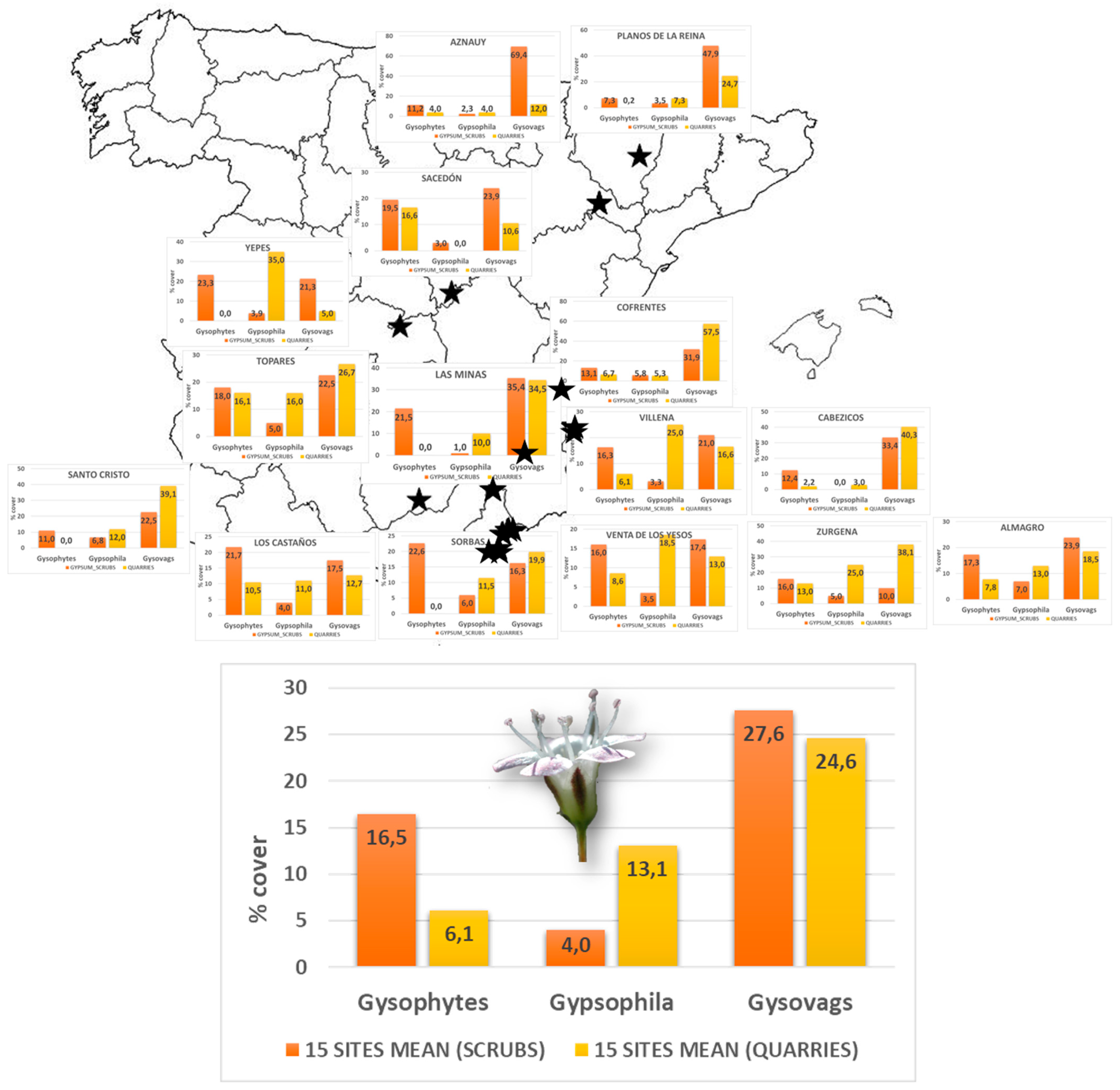
2.3. Succession Patterns in Other Gypsum Quarries Distributed throughout the Iberian Peninsula
3. Discussion
3.1. Successional Plots
3.2. Insights for Ecological Restoration
3.3. Succession Patterns in Gypsum Quarries in the Iberian Peninsula
4. Materials and Methods
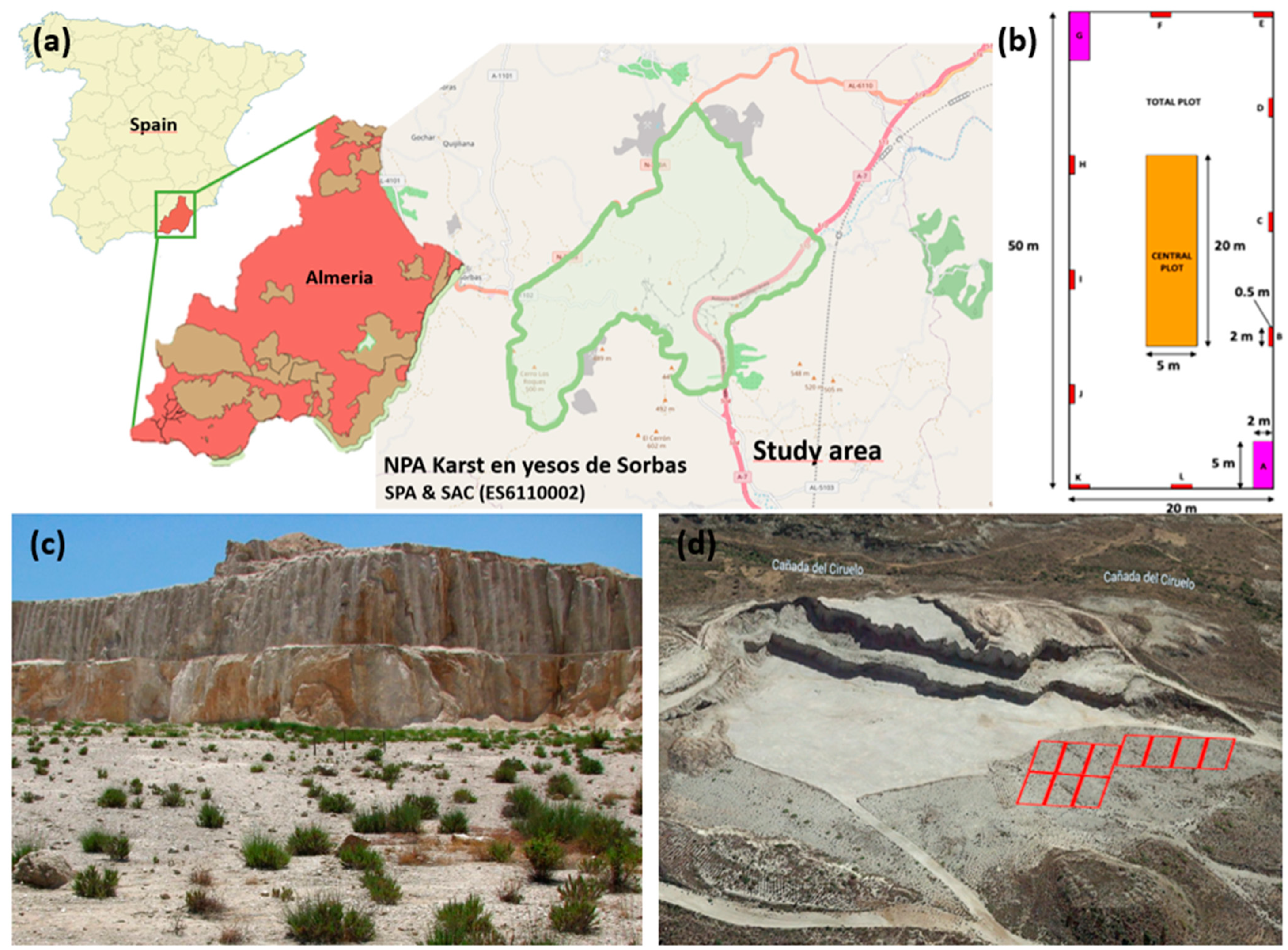
4.1. Study Area and Data Collection
4.1.1. Location of Permanent Plots for Diachronic Study
4.1.2. Study of the Successional Pattern in Iberian Gypsum Quarries
4.2. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, L.R. Patterns and processes in primary succession. In Ecosystems of Disturbed Ground. Ecosystems of the World 16; Walker, L.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 585–610. [Google Scholar]
- Prach, K.; Tolvanen, A. How can we restore biodiversity and ecosystem services in mining and industrial sites? Environ. Sci. Pollut. Res. 2016, 23, 13587–13590. [Google Scholar] [CrossRef]
- Thoreau, H.D. The Succession of Forest Trees: An Address Read to the Middlesex Agricultural Society in Concord, September 1860; 8th Annual Report of the Massachusetts Board of Agriculture; Massachusetts Board of Agriculture: Boston, MA, USA, 1956; p. 72.
- Warming, E. Plantesamfund: Grundtrk af den Økologiska Plantegeografi; Philipsen: Copenhagen, Denmark, 1895. [Google Scholar]
- Cowles, H.C. The ecological relations of the vegetation on the sand dunes of Lake Michigan. Bot. Gaz. 1899, 27, 167–202. [Google Scholar] [CrossRef]
- Clements, F.E. Plant Succession: An Analysis of the Development of Vegetation; Publication 242; Carnegie Institute of Washington: Washington, DC, USA, 1916. [Google Scholar]
- Hobbs, R.J.; Walker, L.R.; Walker, J. Integrating Restoration and Succession. In Linking Restoration and Ecological Succession Springer Series on Environmental Management; Walker, L.R., Walker, J., Hobbs, R.J., Eds.; Springer: New York, NY, USA, 2007; pp. 168–179. [Google Scholar]
- Walker, R.L.; del Moral, R. Primary Succession and Ecosystem Rehabilitation; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Prach, K.; Walker, L.R. Four opportunities for studies of ecological succession. Trends Ecol. Evol. 2011, 26, 119–123. [Google Scholar] [CrossRef]
- Meiners, S.J.; Pickett, S.T.; Cadenasso, M.L. An Integrative Approach to Successional Dynamics; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Johnson, E.A.; Miyanishi, K. (Eds.) Plant Disturbance Ecology: The Process and the Response, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Prach, K.; Walker, L.R. Comparative Plant Succession among Terrestrial Biomes of the World; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- Prach, K. Spontaneous succession in Central–European man–made habitats, what information can be used in restoration practice? Appl. Veg. Sci. 2003, 6, 125–129. [Google Scholar] [CrossRef]
- Prach, K.; Pyšek, P.; Bastl, M. Spontaneous vegetation succession in human-disturbed habitats: A pattern across seres. Appl. Veg. Sci. 2001, 4, 83–88. [Google Scholar] [CrossRef]
- Walker, L.R.; Wardle, D.A.; Bardgett, R.D.; Clarkson, B.D. The use of chronosequences in studies of ecological succession and soil development. J. Ecol. 2010, 98, 725–736. [Google Scholar] [CrossRef]
- Walker, L.R.; del Moral, R. Lessons from primary succession for restoration of severely damaged habitats. Appl. Veg. Sci. 2009, 12, 55–67. [Google Scholar] [CrossRef]
- Aronson, J.; Floret, C.; LeFloch’h, E.; Ovalle, C.; Pontanier, R. Restoration and rehabilitation of degraded ecosystems in arid and semiarid regions. I. A view from the South. Restor. Ecol. 1993, 1, 8–17. [Google Scholar] [CrossRef]
- Bradshaw, A.D.; Chadwick, M.J. The Restoration of Land: The Ecology and Reclamation of Derelict and Degraded Land; University of California Press: Berkeley, CA, USA, 1980. [Google Scholar]
- Walker, L.R.; Walker, J.; del Moral, R. Forging a new alliance between succession and restoration. In Linking Restoration and Ecological Succession; Walker, L.R., Walker, J., Hobbs, R.J., Eds.; Springer: New York, NY, USA, 2007; pp. 1–18. [Google Scholar]
- Mota, J.F.; Sola, A.J.; Dana, E.D.; Jiménez-Sánchez, M.L. Plant succession in abandoned gypsum quarries in SE Spain. Phytocoenologia 2003, 33, 13–28. [Google Scholar] [CrossRef]
- Dana, E.; Mota, J.F. Vegetation and soil recovery on gypsum outcrops in semi–arid Spain. J. Arid Environ. 2006, 65, 444–459. [Google Scholar] [CrossRef]
- Glenn-Lewin, D.C.; Peet, R.K.; Veblen, T.T. (Eds.) Plant Succession: Theory and Prediction; Chapman and Hall: London, UK, 1992; Volume 11. [Google Scholar]
- Prach, K.; Walker, L.R. Differences between primary and secondary plant succession among biomes of the world. J. Ecol. 2019, 107, 510–516. [Google Scholar] [CrossRef]
- Mota, J.F.; Merlo, M.E.; Peñas de Giles, J.; Oyonarte-Gutiérrez, C.; Pérez-García, F.J.; Aguilera-Lirola, A.M.; Rodríguez-Tamayo, M.L.; Bonilla Parrón, G.; Cabello Piñar, J.; Cueto Romero, M.; et al. Vegetación, sucesión y restauración ecológica en canteras de yeso. In Minería, Industria y Medio Ambiente en la Cuenca Mediterránea: Homenaje al Prof. Antonio Marañés; Sánchez Garrido, J.A., Collado Fernández, D.M., Navarro Flores, A., Marañés, A., Eds.; Servicio de Publicaciones de la Universidad de Almería: Almería, Spain, 1999; pp. 83–96. [Google Scholar]
- Matthews, J.A.; Hill, J.L.; Winkler, S.; Owen, G.; Vater, A.E. Autosuccession in alpine vegetation: Testing the concept on an altitudinal bioclimatic gradient, Jotunheimen, southern Norway. Catena 2018, 170, 169–182. [Google Scholar] [CrossRef]
- Muller, C.H. Plant succession in the Larrea–Flourensia climax. Ecology 1940, 21, 206–212. [Google Scholar] [CrossRef]
- Matthews, J.A. The vegetation of the Storbreen gletschervorfeld, Jotunheimen, Norway, II. Approaches involving ordination and general conclusions. J. Biogeogr. 1979, 6, 133–167. [Google Scholar] [CrossRef]
- Matthews, J.A. The Ecology of Recently-Deglaciated Terrain: A Geoecological Approach to Glacier Forelands and Primary Succession; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Matthews, J.A. Disturbance regimes and ecosystem response on recently–deglaciated substrates. In Ecosystems of Disturbed Ground: Ecosystems of the World; Walker, L.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 16, pp. 17–37. [Google Scholar]
- Robbins, J.A.; Matthews, J.A. Regional variation in successional trajectories and rates of vegetation change on glacier forelands in south-central Norway. Arct. Antarct. Alp. Res. 2010, 42, 351–361. [Google Scholar] [CrossRef]
- Muller, C.H. Plant succession in Arctic heath and tundra in northern Scandinavia. Bull. Torrey Bot. Club 1952, 79, 296–309. [Google Scholar] [CrossRef]
- Svoboda, J.; Henry, G.H.R. Succession in marginal Arctic environments. Arct. Alp. Res. 1987, 19, 373–384. [Google Scholar] [CrossRef]
- Shreve, F. The desert vegetation of North America. Bot. Rev. 1942, 8, 195–246. [Google Scholar] [CrossRef]
- Whittaker, R.H.; Levin, S. The role of mosaic phenomena in natural communities. Theor. Popul. Biol. 1977, 12, 117–139. [Google Scholar] [CrossRef]
- Moore, M.J.; Mota, J.F.; Douglas, N.A.; Olvera, H.F.; Ochoterena, H. 2014. The ecology, assembly, and evolution of gypsophile floras. In Plant Ecology and Evolution in Harsh Environments; Rajakaruna, N., Boyd, R.S., Harris, T.B., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 97–128. [Google Scholar]
- Zahawi, R.A.; Reid, J.L.; Holl, K.D. Hidden costs of passive restoration. Restor. Ecol. 2014, 22, 284–287. [Google Scholar] [CrossRef]
- Erskine, P.D. Land clearing and forest rehabilitation in the wet tropics of north Queensland, Australia. Ecol. Manag. Restor. 2002, 3, 135–137. [Google Scholar]
- Rey Benayas, J.M.; Bullock, J.M.; Newton, A.C. Creating Woodland islets to reconcile ecological restoration, conservation, and agricultural land use. Front. Ecol. Environ. 2008, 6, 329–336. [Google Scholar] [CrossRef]
- Prach, K.; Šebelíková, L.; Řehounková, K.; del Moral, R. Possibilities and limitations of passive restoration of heavily disturbed sites. Landsc. Res. 2020, 45, 247–253. [Google Scholar] [CrossRef]
- Rey Benayas, J.M. Restoring forests after land abandonment. In Forest Restoration in Landscapes: Beyond Planting Trees; Mansourian, S., Vallauri, D., Dudley, N., Eds.; Springer: New York, NY, USA, 2000; pp. 356–360. [Google Scholar]
- Melo, F.P.L.; Pinto, S.R.R.; Brancalion, P.H.S.; Castro, P.S.; Rodrigues, R.R.; Aronson, J.; Taborelli, M. Priority setting for scaling–up tropical forest restoration projects, early lessons from the Atlantic forest restoration pact. Environ. Sci. Policy 2013, 33, 395–404. [Google Scholar] [CrossRef]
- Wright, S.J.; Muller-Landau, H.C. The future of tropical forest species. Biotropica 2006, 38, 287–301. [Google Scholar] [CrossRef]
- Holl, K.D.; Aide, T.M. When and where to actively restore ecosystems? For. Ecol. Manag. 2011, 261, 1558–1563. [Google Scholar] [CrossRef]
- McDonald, T.; Gann, G.D.; Jonson, J.; Dixon, K.W. International Standards for the Practice of Ecological Restoration—Including Principles and Key Concepts; Society for Ecological Restoration: Washington, DC, USA, 2016. [Google Scholar]
- Mota, J.F.; Sánchez-Gómez, P.; Guirado, J.S. (Eds.) Diversidad Vegetal de las Yeseras Ibéricas: El Reto de los Archipiélagos Edáficos para la Biología de la Conservación; ADIF–Mediterráneo Asesores Consultores: Almería, Spain, 2011. [Google Scholar]
- Cortina, J.; Sebastià, M.T.; Soriano i Tomàs, I.; Casals i Tortras, P.; Álvarez de la Campa, J.M.; Vallejo, V.R. Datos sobre la variabilidad de algunos parámetros ecológicos en cuatro comunidades nitrófilas barcelonesas. Acta Bot. Barcinonensia 1988, 37, 79–94. [Google Scholar]
- Mota, J.F.; Sola, A.J.; Jiménez-Sánchez, M.L.; Pérez-García, F.J.; Merlo, M.E. Gypsicolous flora, conservation and restoration of quarries in the southeast of the Iberian Peninsula. Biodivers. Conserv. 2004, 13, 1797–1808. [Google Scholar] [CrossRef]
- Mota, J.F.; Garrido-Becerra, J.A.; Merlo, M.E.; Medina-Cazorla, J.M.; Sánchez-Gómez, P. The edaphism, gypsum, dolomite and serpentine flora and vegetation. In The Vegetation of the Iberian Peninsula; Loidi, J., Ed.; Springer: Cham, Switzerland, 2017; pp. 277–354. [Google Scholar]
- Ballesteros, M.; Cañadas, E.M.; Foronda, A.; Fernández-Ordoño, E.; Peñas de Giles, J.; Lorite, J. Vegetation recovery of gypsum quarries: Short–term sowing response to different soil treatments. Appl. Veg. Sci. 2012, 15, 187–197. [Google Scholar] [CrossRef]
- Ballesteros, M.; Cañadas, E.M.; Foronda, A.; Peñas de Giles, J.; Valle, F.; Lorite, J. Central role of bedding materials for gypsum–quarry restoration: An experimental planting of gypsophile species. Ecol. Eng. 2014, 70, 470–476. [Google Scholar] [CrossRef]
- Ballesteros, M.; Ayerbe, J.; Casares, M.; Cañadas, E.M.; Lorite, J. Successful lichen translocation on disturbed gypsum areas. A test with adhesives to promote the recovery of biological soil crusts. Sci. Rep. 2017, 7, 45606. [Google Scholar] [CrossRef] [PubMed]
- Cañadas, E.M.; Ballesteros, M.; Foronda, A.; Navarro, F.B.; Jiménez, M.N.; Lorite, J. Enhancing seedling production of native species to restore gypsum habitats. J. Environ. Manag. 2015, 163, 109–114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Foronda, A.; Pueyo, Y.; Castillejo, J.M.; de la Luz Giner, M.; Alados, C.L. Substrate–specialist plants for restoring vegetation in post–mining gypsum substrates. Catena 2020, 186, 104308. [Google Scholar] [CrossRef]
- Pérez-García, F.J.; Salmerón-Sánchez, E.; Martínez-Hernández, F.; Mendoza-Fernández, A.; Merlo, E.; Mota, J.F. Towards an Eco–Compatible Origin of Construction Materials. Case Study, Gypsum. In International Symposium, New Metropolitan Perspectives; Bevilacqua, C., Calabrò, F., Della Spina, L., Eds.; Springer: Cham, Switzerland, 2020; pp. 1259–1267. [Google Scholar]
- Lorite, J.; Agea, D.; García-Robles, H.; Cañadas, E.M.; Rams, S.; Sánchez-Castillo, P. Plant recovery techniques do not ensure biological soil-crust recovery after gypsum quarrying: A call for active restoration. Restor. Ecol. 2020, 28, S86–S95. [Google Scholar] [CrossRef]
- Lorite, J.; Ballesteros, M.; García-Robles, H.; Cañadas, E.M. Economic evaluation of ecological restoration options in gypsum habitats after mining. J. Nat. Conserv. 2021, 59, 125935. [Google Scholar] [CrossRef]
- Navarro-Cano, J.A.; Goberna, M.; Valiente-Banuet, A.; Montesinos-Navarro, A.; García, C.; Verdú, M. Plant phylodiversity enhances soil microbial productivity in facilitation–driven communities. Oecologia 2014, 174, 909–920. [Google Scholar] [CrossRef]
- Mueller-Dombois, D. Pacific Island Forests: Successionally Impoverished and Now Threatened to Be Overgrown by Aliens? Pac. Sci. 2008, 62, 303–308. [Google Scholar] [CrossRef]
- Pitz, C.; Mahy, G.; Vermeulen, C.; Marlet, C.; Séleck, M. Developing biodiversity indicators on a stakeholders’ opinions basis: The gypsum industry Key Performance Indicators framework. Environ. Sci. Pollut. Res. 2016, 23, 13661–13671. [Google Scholar] [CrossRef]
- Prach, K.; Durigan, G.; Fennessy, S.; Overbeck, G.E.; Torezan, J.M.; Murphy, S.D. A primer on choosing goals and indicators to evaluate ecological restoration success. Restor. Ecol. 2019, 27, 917–923. [Google Scholar] [CrossRef]
- Dale, V.H.; Beyeler, S.C. Challenges in the development and use of ecological indicators. Ecol. Indic. 2001, 1, 3–10. [Google Scholar] [CrossRef]
- Scheiner, S.M. Six types of species-area curves. Glob. Ecol. Biogeogr. 2003, 12, 441–447. [Google Scholar] [CrossRef]
- Westman, W.E. Measuring the inertia and resilience of ecosystems. BioScience 1978, 28, 705–710. [Google Scholar] [CrossRef]
- Siebert, F.; Van Staden, N.; Komape, D.M.; Swemmer, A.M.; Siebert, S.J. Effects of land-use change on herbaceous vegetation in a semi-arid Mopaneveld savanna. Bothalia 2021, 51, a8. [Google Scholar] [CrossRef]
- Dovčiak, M.; Frelich, L.E.; Reich, P.B. Pathways in old-field succession to white pine: Seed rain, shade, and climate effects. Ecol. Monogr. 2005, 75, 363–378. [Google Scholar] [CrossRef]
- Prach, K.; Hobbs, R.J. Spontaneous succession versus technical reclamation in the restoration of disturbed sites. Restor. Ecol. 2008, 16, 363–366. [Google Scholar] [CrossRef]
- Baasch, A.; Kirmer, A.; Tischew, S. Nine years of vegetation development in a postmining site: Effects of spontaneous and assisted site recovery. J. Appl. Ecol. 2012, 49, 251–260. [Google Scholar] [CrossRef]
- SER (Society for Ecological Restoration). The SER International Primer on Ecological Restoration, Version 2; Society for Ecological Restoration: Tucson, AZ, USA, 2004. [Google Scholar]
- Jones, H.P.; Schmitz, O.J. Rapid recovery of damaged ecosystems. PLoS ONE 2009, 4, e5653. [Google Scholar] [CrossRef]
- Salgueiro, P.A.; Prach, K.; Branquinho, C.; Mira, A. Enhancing biodiversity and ecosystem services in quarry restoration–challenges, strategies, and practice. Restor. Ecol. 2020, 28, 655–660. [Google Scholar] [CrossRef]
- Merlo, M.E.; Garrido-Becerra, J.A.; Mota, J.F.; Salmerón-Sánchez, E.; Martínez-Hernández, F.; Mendoza-Fernández, A.; Pérez-García, F.J. Threshold ionic contents for defining the nutritional strategies of gypsophile flora. Ecol. Indic. 2019, 97, 247–259. [Google Scholar] [CrossRef]
- Mota, J.; Merlo, E.; Martínez-Hernández, F.; Mendoza-Fernández, A.J.; Pérez-García, F.J.; Salmerón-Sánchez, E. Plants on rich–magnesium dolomite barrens: A global phenomenon. Biology 2021, 10, 38. [Google Scholar] [CrossRef]
- Castillejo, J.M.; Castelló, R. Influence of the application rate of an organic amendment (municipal solid waste [MSW] compost) on gypsum quarry rehabilitation in semiarid environments. Arid Land Res. Manag. 2010, 24, 344–364. [Google Scholar] [CrossRef]
- Anthelme, F.; Cauvy-Fraunié, S.; Francou, B.; Cáceres, B.; Dangles, O. Living at the edge: Increasing stress for plants 2–13 years after the retreat of a tropical glacier. Front. Ecol. Evol. 2021, 9, 584872. [Google Scholar] [CrossRef]
- Osbornová, J.; Kovárová, M.; Leps, J.; Prach, K. (Eds.) Succession in Abandoned Fields: Studies in Central Bohemia, Czechoslovakia; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1990. [Google Scholar]
- Lepš, J. Scale-and time-dependent effects of fertilization, mowing and dominant removal on a grassland community during a 15-year experiment. J. Appl. Ecol. 2014, 51, 978–987. [Google Scholar] [CrossRef]
- Lepš, J.; Štursa, J. Species-area curve, life history strategies, and succession: A field test of relationships. Vegetatio 1989, 83, 249–257. [Google Scholar] [CrossRef]
- Matthews, T.J.; Guilhaumon, F.; Triantis, K.A.; Borregaard, M.K.; Whittaker, R.J. On the form of species–area relationships in habitat islands and true islands. Glob. Ecol. Biogeogr. 2016, 25, 847–858. [Google Scholar] [CrossRef]
- Fattorini, S. On the general dynamic model of oceanic island biogeography. J. Biogeogr. 2009, 36, 1100–1110. [Google Scholar] [CrossRef]
- Cramer, V.A.; Hobbs, R.J. (Eds.) Old Fields: Dynamics and Restoration of Abandoned Farmland; Island Press: Washington, DC, USA, 2007; Volume 101. [Google Scholar]
- Yodzis, P. Environmental randomness and the tenacity of equilibria. J. Theor. Biol. 1978, 72, 185–189. [Google Scholar] [CrossRef]
- Verschuuren, J. Climate change: Rethinking restoration in the European Union’s Birds and Habitats Directives. Ecol. Restor. 2010, 28, 431–439. [Google Scholar] [CrossRef]
- Trouwborst, A. Conserving European biodiversity in a changing climate: The Bern convention, the European Union Birds and Habitats Directives and the adaptation of nature to climate change. Rev. Eur. Comp. Int. Environ. Law 2011, 20, 62–77. [Google Scholar] [CrossRef]
- Merlo, M.E.; Gil de Carrasco, C.; Sola, A.J.; Jiménez-Sánchez, M.L.; Rodríguez-Tamayo, M.L.; Mota, J.F. Can gypsophytes distinguish different types of gypsum habitats? Acta Bot. Gall. 2009, 156, 63–78. [Google Scholar] [CrossRef]
- Stohlgren, T.J. Measuring Plant Diversity: Lessons from the Field; Oxford University Press: New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- Castroviejo, S. (Ed.) Flora Iberica; Real Jardín Botánico, CSIC: Madrid, Spain, 1986. [Google Scholar]
- Arrhenius, O. Species and area. J. Ecol. 1921, 9, 95–99. [Google Scholar] [CrossRef]
- Schenk, J.J. Evolution of limited seed dispersal ability on gypsum islands. Am. J. Bot. 2013, 100, 1811–1822. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, F.; Medina-Cazorla, J.M.; Mendoza-Fernández, A.J.; Pérez-García, F.J.; Sánchez-Gómez, P.; Garrido-Becerra, J.A.; Gil, C.; Mota, J.F. Preliminary essay on the chorology of the Iberian gypsicolous flora: Rarity and richness of the gypsum outcrops. Acta Bot. Gall. 2009, 156, 9–18. [Google Scholar] [CrossRef]
- Martínez-Hernández, F.; Pérez-García, F.J.; Garrido-Becerra, J.A.; Mendoza-Fernández, A.J.; Medina-Cazorla, J.M.; Martínez-Nieto, M.I.; Merlo-Calvente, M.E.; Mota, J.F. The distribution of Iberian gypsophilous flora as a criterion for conservation policy. Biodivers. Conserv. 2011, 20, 1353–1364. [Google Scholar] [CrossRef]
- Martínez-Hernández, F.; Mendoza-Fernández, A.J.; Pérez-García, F.J.; Martínez-Nieto, M.I.; Garrido-Becerra, J.A.; Salmerón-Sánchez, E.; Merlo, M.E.; Gil, C.; Mota, J.F. Areas of endemism as a conservation criterion for Iberian gypsophilous flora: A multi–scale test using the NDM/VNDM program. Plant Biosyst. 2015, 149, 483–493. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. PC-ORD: Multivariate Analysis of Ecological Data, Version 7.07; MjM Software Design: Gleneden Beach, OR, USA, 2018. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; Plymouth Marine Laboratory: Plymouth, UK, 2015. [Google Scholar]
- Musarella, C.M.; Mendoza-Fernández, A.J.; Mota, J.F.; Alessandrini, A.; Bacchetta, G.; Brullo, S.; Caldarella, O.; Ciaschetti, G.; Conti, F.; Di Martino, L.; et al. Checklist of gypsophilous vascular flora in Italy. PhytoKeys 2018, 103, 61–82. [Google Scholar] [CrossRef]
| Source | df | SS | MS | Pseudo-F | p (perm) | Unique Perms |
|---|---|---|---|---|---|---|
| YE | 12 | 31,670 | 2639.2 | 31.295 | 0.0001 | 9787 |
| Res | 117 | 98,668 | 843.32 | |||
| Total | 129 | 130,000 |
| Source | df | SS | MS | Pseudo-F | p (perm) | Unique Perms |
|---|---|---|---|---|---|---|
| OR | 9 | 65,641 | 7293.4 | 13.528 | 0.0001 | 9839 |
| Res | 120 | 64,698 | 539.15 | |||
| Total | 129 | 130,000 |
| PAIR-WISE TESTS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | df | SS | MS | Pseudo-F | p (perm) | Unique Perms | Groups | t | p (perm) | Unique Perms |
| SI | 2 | 214.25 | 107.13 | 22.173 | 0.0001 | 9894 | S, R | 3.5393 | 0.0001 | 9916 |
| Res | 137 | 661.89 | 4.8313 | S, M | 5.8556 | 0.0001 | 9907 | |||
| Total | 139 | 876.14 | R, M | 2.2991 | 0.0093 | 126 | ||||
| Province | Locality | Quarries | Scrubs |
|---|---|---|---|
| Albacete | Las Minas | 1 | 5 |
| Alicante | Cabecicos de Villena | 3 | 6 |
| Alicante | Villena | 1 | 4 |
| Almería | Los Castaños | 4 | 4 |
| Almería | Sierra de Almagro | 3 | 3 |
| Almería | Sorbas | 2 | 2 |
| Almería | Topares | 1 | 2 |
| Almería | Venta de los Yesos | 2 | 2 |
| Almería | Zurgena | 1 | 1 |
| Guadalajara | Sacedón | 1 | 9 |
| Huesca | Azanuy | 1 | 9 |
| Huesca | Planos de Elena | 3 | 6 |
| Jaén | Cabra de Santo Cristo | 1 | 8 |
| Toledo | Yepes | 1 | 13 |
| Yepes | Cofrentes | 3 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, J.F.; Martínez-Hernández, F.; Salmerón-Sánchez, E.; Mendoza-Fernández, A.J.; Pérez-García, F.J.; Merlo, M.E. Spontaneous Primary Succession and Vascular Plant Recovery in the Iberian Gypsum Quarries: Insights for Ecological Restoration in an EU Priority Habitat. Plants 2023, 12, 1162. https://doi.org/10.3390/plants12051162
Mota JF, Martínez-Hernández F, Salmerón-Sánchez E, Mendoza-Fernández AJ, Pérez-García FJ, Merlo ME. Spontaneous Primary Succession and Vascular Plant Recovery in the Iberian Gypsum Quarries: Insights for Ecological Restoration in an EU Priority Habitat. Plants. 2023; 12(5):1162. https://doi.org/10.3390/plants12051162
Chicago/Turabian StyleMota, Juan Francisco, Fabián Martínez-Hernández, Esteban Salmerón-Sánchez, Antonio Jesús Mendoza-Fernández, Francisco Javier Pérez-García, and M. Encarna Merlo. 2023. "Spontaneous Primary Succession and Vascular Plant Recovery in the Iberian Gypsum Quarries: Insights for Ecological Restoration in an EU Priority Habitat" Plants 12, no. 5: 1162. https://doi.org/10.3390/plants12051162
APA StyleMota, J. F., Martínez-Hernández, F., Salmerón-Sánchez, E., Mendoza-Fernández, A. J., Pérez-García, F. J., & Merlo, M. E. (2023). Spontaneous Primary Succession and Vascular Plant Recovery in the Iberian Gypsum Quarries: Insights for Ecological Restoration in an EU Priority Habitat. Plants, 12(5), 1162. https://doi.org/10.3390/plants12051162


.png)







