Improvement in Essential Oil Quantity and Quality of Thyme (Thymus vulgaris L.) by Integrative Application of Chitosan Nanoparticles and Arbuscular Mycorrhizal Fungi under Water Stress Conditions
Abstract
1. Introduction
2. Results
2.1. Analysis of Variance
2.2. AMF Colonization
2.3. Nutrient Concentration
2.4. Agronomic Traits
2.4.1. Plant Height
2.4.2. Canopy Diameter
2.5. Fresh Yield
2.6. Dry Yield
2.7. Essential Oil Content
2.8. Essential Oil Yield
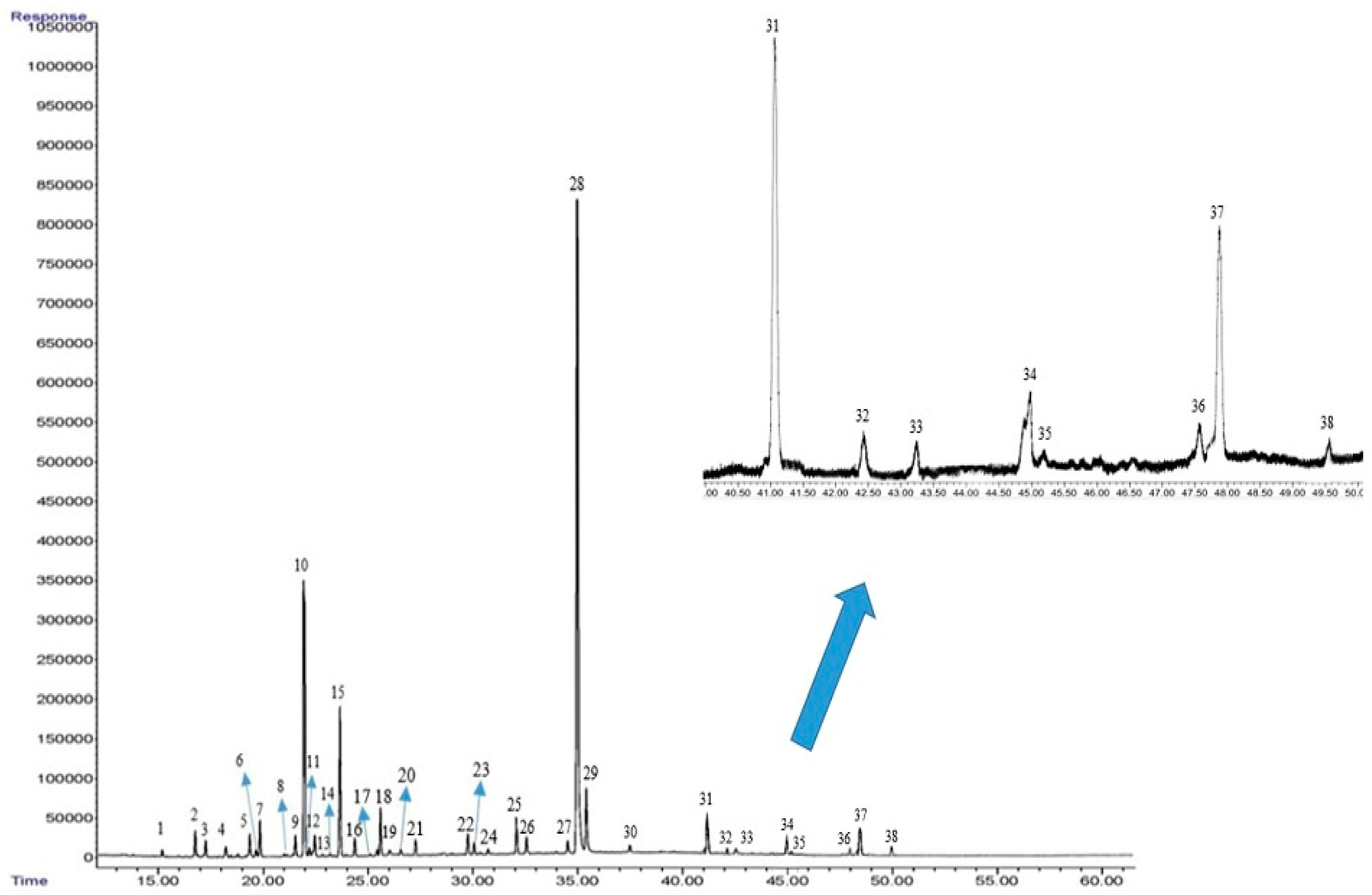
2.9. Essential Oil Constituents
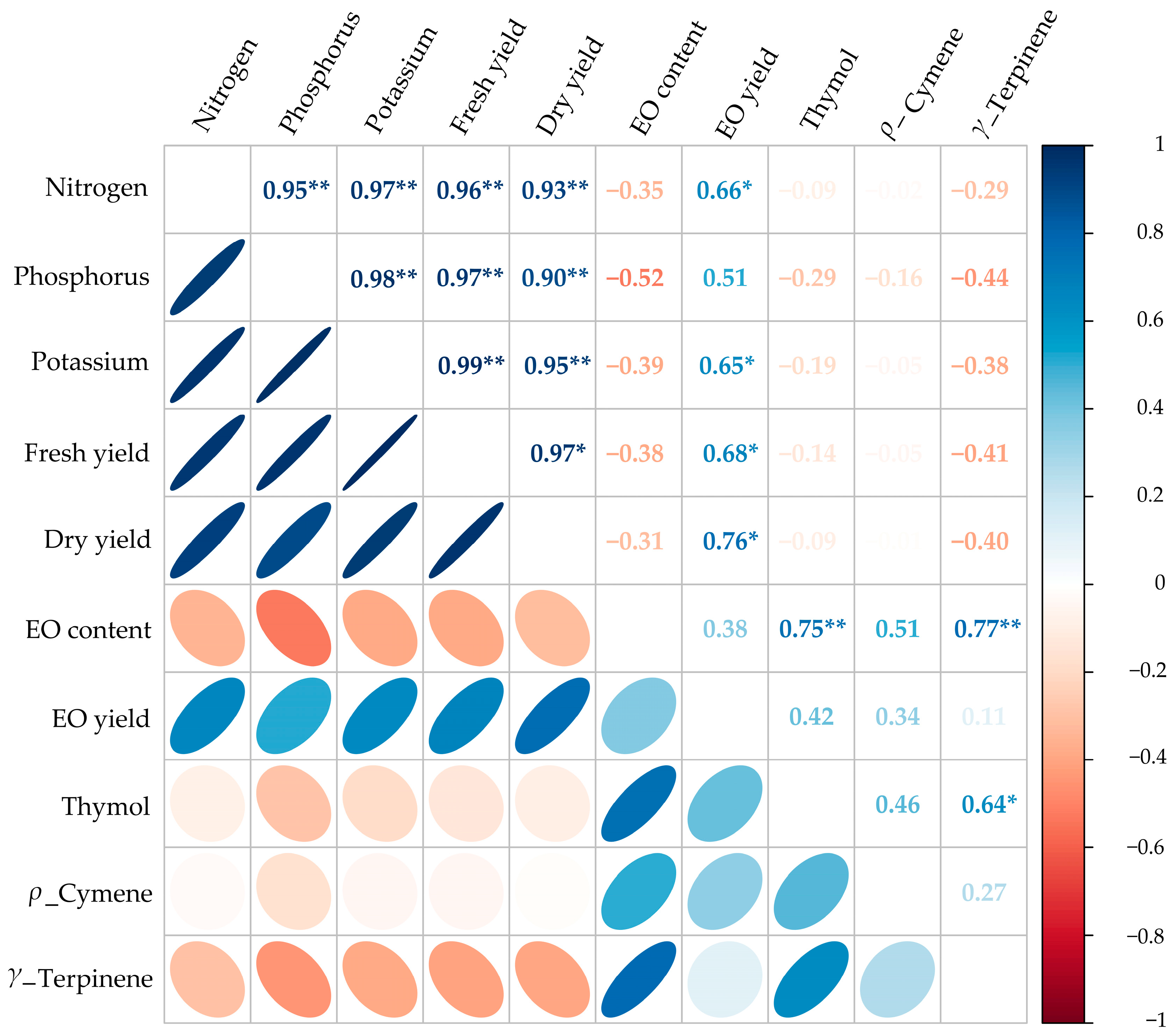
2.10. Correlation
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Experiment Design and Crop Management
4.3. Measurements
4.3.1. Fresh and Dry Yield
4.3.2. Essential Oil Content, Yield and Constituents
4.3.3. Nutrient Content
4.3.4. Root AMF Colonization
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amani Machiani, M.; Javanmard, A.; Habibi Machiani, R.; Sadeghpour, A. Arbuscular mycorrhizal fungi and changes in primary and secondary metabolites. Plants 2022, 11, 2183. [Google Scholar] [CrossRef]
- Alavi-Samani, S.M.; Kachouei, M.A.; Pirbalouti, A.G. Growth, yield, chemical composition, and antioxidant activity of essential oils from two thyme species under foliar application of jasmonic acid and water deficit conditions. Hortic. Environ. Biotechnol. 2015, 56, 411–420. [Google Scholar] [CrossRef]
- da Cunha Honorato, A.; Maciel, J.F.A.; de Assis, R.M.A.; Nohara, G.A.; de Carvalho, A.A.; Pinto, J.E.B.P.; Bertolucci, S.K.V. Combining green manure and cattle manure to improve biomass, essential oil, and thymol production in Thymus vulgaris L. Ind. Crops Prod. 2022, 187, 115469. [Google Scholar] [CrossRef]
- Askary, M.; Behdani, M.A.; Parsa, S.; Mahmoodi, S.; Jamialahmadi, M. Water stress and manure application affect the quantity and quality of essential oil of Thymus daenensis and Thymus vulgaris. Ind. Crops Prod. 2018, 111, 336–344. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Morshedloo, M.R.; Aghaee, A.; Maggi, F. Funneliformis mosseae inoculation under water deficit stress improves the yield and phytochemical characteristics of thyme in intercropping with soybean. Sci. Rep. 2021, 11, 15279. [Google Scholar] [CrossRef]
- Pavela, R.; Žabka, M.; Vrchotová, N.; Tříska, J. Effect of foliar nutrition on the essential oil yield of Thyme (Thymus vulgaris L.). Ind. Crops Prod. 2018, 112, 762–765. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Athar, M.T.; Al-Faraidy, A.A.; Almuhaiza, M.S. Chemical composition of essential oil of Thymus vulgaris collected from Saudi Arabian market. Asian Pac. J. Trop. Biomed. 2017, 7, 147–150. [Google Scholar] [CrossRef]
- Ostadi, A.; Javanmard, A.; Amani Machiani, M.; Kakaei, K. Optimizing antioxidant activity and phytochemical properties of Peppermint (Mentha piperita L.) by integrative application of biofertilizer and stress-modulating nanoparticles under drought stress conditions. Plants 2023, 12, 151. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Morshedloo, M.R.; Janmohammadi, M.; Maggi, F. Funneliformis mosseae application improves the oil quantity and quality and eco-physiological characteristics of soybean (Glycine max L.) under water stress conditions. J. Soil Sci. Plant Nutr. 2021, 21, 3076–3090. [Google Scholar] [CrossRef]
- Sharma, B.; Tiwari, S.; Kumawat, K.C.; Cardinale, M. Nano-biofertilizers as bio-emerging strategies for sustainable agriculture development: Potentiality and their limitations. Sci. Total Environ. 2022, 860, 160476. [Google Scholar] [CrossRef]
- He, X.; Deng, H.; Hwang, H.-M. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef]
- Ingle, A.P. Nanotechnology in Plant Growth Promotion and Protection; Wiley Online Library: Hoboken, NJ, USA, 2021. [Google Scholar]
- Ostadi, A.; Javanmard, A.; Amani Machiani, M.; Sadeghpour, A.; Maggi, F.; Nouraein, M.; Morshedloo, M.R.; Hano, C.; Lorenzo, J.M. Co-application of TiO2 nanoparticles and arbuscular mycorrhizal fungi improves essential oil quantity and quality of sage (Salvia officinalis L.) in drought stress conditions. Plants 2022, 11, 1659. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.-P.; Joshi, A.; Tian, D.-D.; Rajput, V.D.; Singh, M.; Arora, J.; Minkina, T.; Li, Y.-R. Recent trends in nano-fertilizers for sustainable agriculture under climate change for global food security. J. Nanomater. 2022, 12, 173. [Google Scholar] [CrossRef]
- Ingle, P.U.; Shende, S.S.; Shingote, P.R.; Mishra, S.S.; Sarda, V.; Wasule, D.L.; Rajput, V.D.; Minkina, T.; Rai, M.; Sushkova, S. Chitosan nanoparticles (ChNPs): A versatile growth promoter in modern agricultural production. Heliyon 2022, 8, e11893. [Google Scholar] [CrossRef]
- Attaran Dowom, S.; Karimian, Z.; Mostafaei Dehnavi, M.; Samiei, L. Chitosan nanoparticles improve physiological and biochemical responses of Salvia abrotanoides (Kar.) under drought stress. BMC Plant Biol. 2022, 22, 364. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Manikandan, A. Foliar application of chitosan nanoparticle improves yield, mineral content and boost innate immunity in finger millet plants. Carbohydr. Polym. 2021, 258, 117691. [Google Scholar] [CrossRef]
- Cavagnaro, T.R.; Bender, S.F.; Asghari, H.R.; van der Heijden, M.G.A. The role of arbuscular mycorrhizas in reducing soil nutrient loss. Trends Plant Sci. 2015, 20, 283–290. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Varma, A.; Prasad, R.; Tuteja, N. Mycorrhiza-Nutrient Uptake, Biocontrol, Ecorestoration; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Hafez, M.; Popov, A.I.; Rashad, M. Integrated use of bio-organic fertilizers for enhancing soil fertility–plant nutrition, germination status and initial growth of corn (Zea mays L.). Environ. Technol. Innov. 2021, 21, 101329. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Logvinenko, L.; Novitsky, M.; Zamana, S.; Sokolov, S.; Molchanova, A.; Shevchuk, O.; Sekara, A.; Tallarita, A.; Caruso, G. Yield, essential oil and quality performances of Artemisia dracunculus, Hyssopus officinalis and Lavandula angustifolia as affected by arbuscular mycorrhizal fungi under organic management. Plants 2020, 9, 375. [Google Scholar] [CrossRef]
- Thokchom, S.D.; Gupta, S.; Kapoor, R. Arbuscular mycorrhiza augments essential oil composition and antioxidant properties of Ocimum tenuiflorum L.—A popular green tea additive. Ind Crops Prod. 2020, 153, 112418. [Google Scholar] [CrossRef]
- Strzemski, M.; Dzida, K.; Dresler, S.; Sowa, I.; Kurzepa, J.; Szymczak, G.; Wójciak, M. Nitrogen fertilisation decreases the yield of bioactive compounds in Carlina acaulis L. grown in the field. Ind. Crops Prod. 2021, 170, 113698. [Google Scholar] [CrossRef]
- Abdel-Salam, E.; Alatar, A.; El-Sheikh, M.A. Inoculation with arbuscular mycorrhizal fungi alleviates harmful effects of drought stress on damask rose. Saudi J. Biol. Sci. 2018, 25, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Sanaullah, M.; Rumpel, C.; Charrier, X.; Chabbi, A. How does drought stress influence the decomposition of plant litter with contrasting quality in a grassland ecosystem? Plant Soil 2012, 352, 277–288. [Google Scholar] [CrossRef]
- Khaliq, A.; Perveen, S.; Alamer, K.H.; Zia Ul Haq, M.; Rafique, Z.; Alsudays, I.M.; Althobaiti, A.T.; Saleh, M.A.; Hussain, S.; Attia, H. Arbuscular mycorrhizal fungi symbiosis to enhance plant–soil interaction. Sustainability 2022, 14, 7840. [Google Scholar] [CrossRef]
- Hafez, Y.; Attia, K.; Alamery, S.; Ghazy, A.; Al-Doss, A.; Ibrahim, E.; Rashwan, E.; El-Maghraby, L.; Awad, A.; Abdelaal, K. Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agron. 2020, 10, 630. [Google Scholar] [CrossRef]
- Dzung, N.A.; Khanh, V.T.P.; Dzung, T.T. Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydr. Polym. 2011, 84, 751–755. [Google Scholar] [CrossRef]
- Javanmard, A.; Ashrafi, M.; Morshedloo, M.R.; Machiani, M.A.; Rasouli, F.; Maggi, F. Optimizing phytochemical and physiological characteristics of Balangu (Lallemantia iberica) by foliar application of chitosan nanoparticles and Myco-Root inoculation under water supply restrictions. Horticulturae 2022, 8, 695. [Google Scholar] [CrossRef]
- Shehzad, M.A.; Nawaz, F.; Ahmad, F.; Ahmad, N.; Masood, S. Protective effect of potassium and chitosan supply on growth, physiological processes and antioxidative machinery in sunflower (Helianthus annuus L.) under drought stress. Ecotoxicol. Environ. Saf. 2020, 187, 109841. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, M.; Kuiry, R.; Pal, P.K. Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100255. [Google Scholar] [CrossRef]
- Mazrou, R.; Ali, E.F.; Hassan, S.; Hassan, F.A. A pivotal role of chitosan nanoparticles in enhancing the essential oil productivity and antioxidant capacity in Matricaria chamomilla L. Horticulturae 2021, 7, 574. [Google Scholar] [CrossRef]
- Jie, W.-G.; Yao, Y.-X.; Guo, N.; Zhang, Y.-Z.; Qiao, W. Effects of Rhizophagus intraradices on plant growth and the composition of microbial communities in the roots of continuous cropping soybean at maturity. Sustainability 2021, 13, 6623. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Saber, M.; Akbari, A.; Mahdavinia, G.R. Encapsulation of Satureja hortensis L.(Lamiaceae) in chitosan/TPP nanoparticles with enhanced acaricide activity against Tetranychus urticae Koch (Acari: Tetranychidae). Ecotoxicol. Environ. Saf. 2018, 161, 111–119. [Google Scholar] [CrossRef]
- Ostadi, A.; Javanmard, A.; Amani Machiani, M.; Morshedloo, M.R.; Nouraein, M.; Rasouli, F.; Maggi, F. Effect of different fertilizer sources and harvesting time on the growth characteristics, nutrient uptakes, essential oil productivity and composition of Mentha x piperita L. Ind. Crops Prod. 2020, 148, 112290. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Khan, M.; Abdullah, M.M.S.; Mousa, A.A.; Hamad, Z.A. Chemical composition of vegetative parts and flowers essential oils of wild Anvillea garcinii grown in Saudi Arabia. Rec. Nat. Prod. 2016, 10, 251. [Google Scholar]
- Khan, M.; Al-Saleem, M.S.; Alkhathlan, H.Z. A detailed study on chemical characterization of essential oil components of two Plectranthus species grown in Saudi Arabia. J. Saudi Chem. Soc. 2016, 20, 711–721. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, M.; Mousa, A.A.; Mahmood, A.; Alkhathlan, H.Z. Chemical diversity in leaf and stem essential oils of Origanum vulgare L. and their effects on microbicidal activities. J AMB Express 2019, 9, 176. [Google Scholar] [CrossRef]
- Kovàts, E. Characterization of organic compounds by gas chromatography. Part 1. Retention indices of aliphatic halides, alcohols, aldehydes and ketones. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North Africa Region; International Center for Agricultural Research in the Dry Areas (ICARDA) Publication: Beirut, Lebanon, 2013. [Google Scholar]
- Kalra, Y. Handbook of Reference Methods for Plant Analysis; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Koske, R.; Gemma, J. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989, 92, 486. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
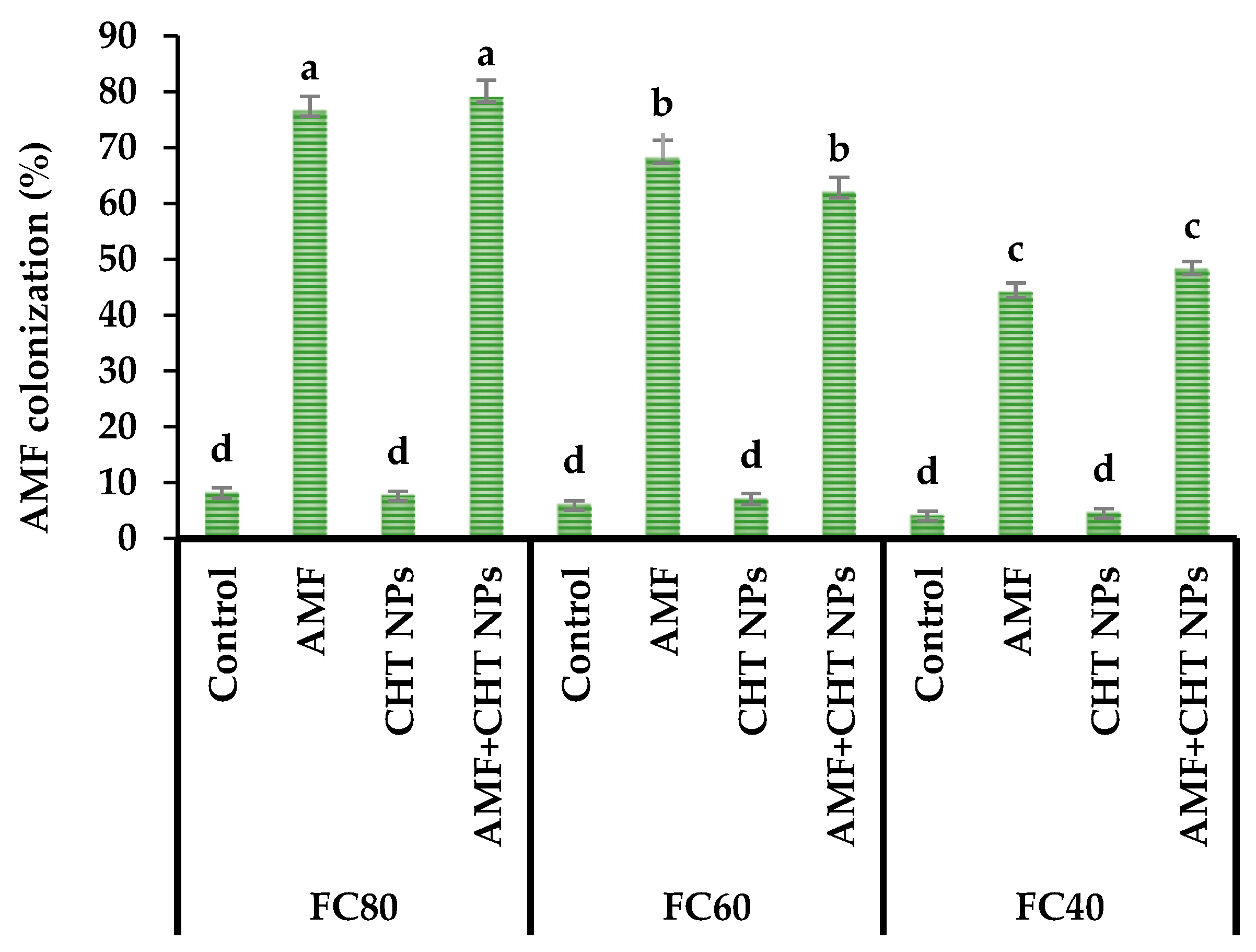
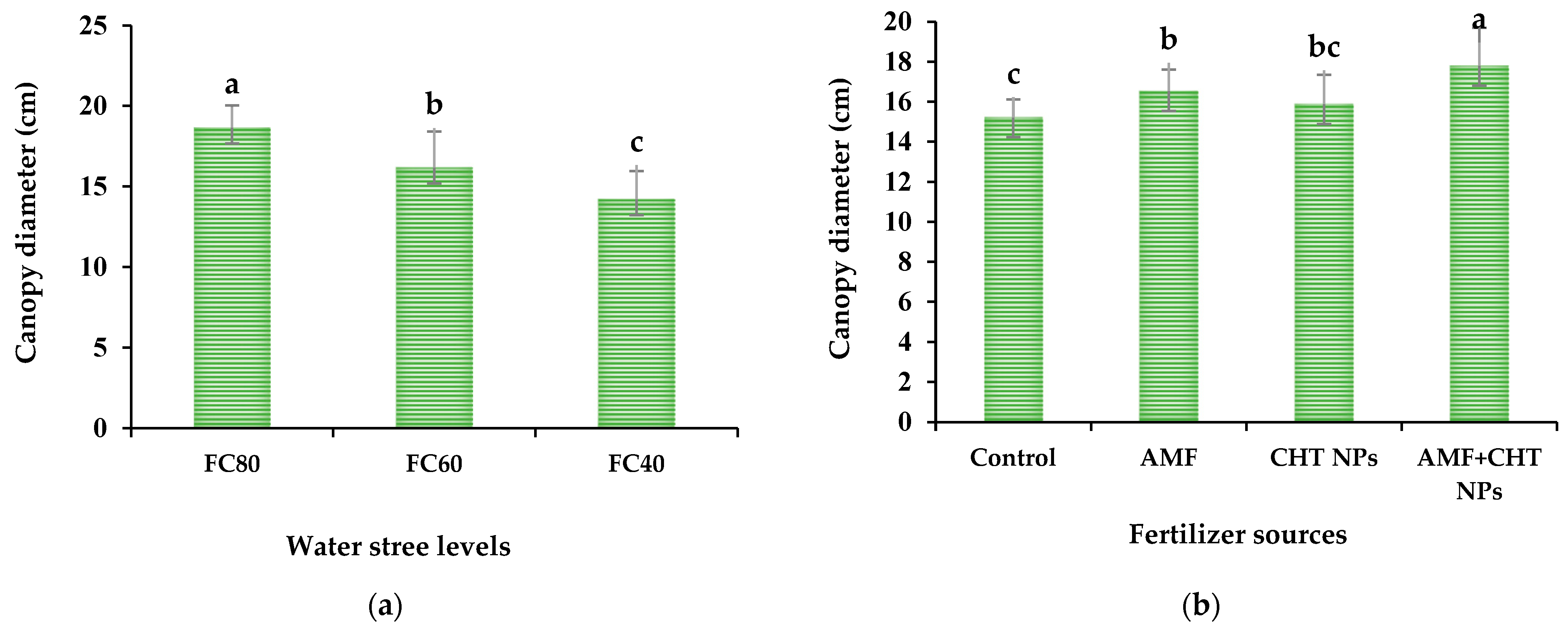
| Source of Variation | Root Colonization | N Content | P Content | K Content | Plant Height | Canopy Diameter | Fresh Yield | Dry Yield | EO Content | EO Yield |
|---|---|---|---|---|---|---|---|---|---|---|
| I | <0.0001 ** | <0.0001 ** | <0.0001 ** | <0.0001 ** | <0.0001 ** | <0.0001 ** | <0.002 ** | <0.005 ** | <0.0001 ** | <0.009 ** |
| F | 0.018 * | <0.0001 ** | <0.0001 ** | <0.0001 ** | <0.0001 ** | <0.0001 ** | <0.003 ** | <0.002 ** | <0.0001 ** | <0.002 ** |
| I × F | <0.0001 ** | 0.006 ** | <0.0001 ** | 0.002 ** | <0.0001 ** | 0.845 ns | 0.004 ** | 0.002** | <0.0001 ** | 0.001 ** |
| Treatments | N (g kg−1) | P (g kg−1) | K (g kg−1) | |
|---|---|---|---|---|
| FC80 | Control | 19.39 cd | 2.09 b | 16.36 bc |
| AMF | 20.11 bc | 2.19 b | 17.03 b | |
| CHT | 20.98 b | 2.18 b | 16.99 b | |
| AMF+CHT | 22.31 a | 2.49 a | 19.06 a | |
| FC60 | Control | 17.25 fg | 1.61 def | 13.86 ef |
| AMF | 18.23 def | 1.80 c | 15.16 cde | |
| CHT | 18.65 de | 1.68 cde | 14.35 de | |
| AMF+CHT | 19.84 bc | 1.83 c | 15.68 bcd | |
| FC40 | Control | 14.99 h | 1.36 g | 11.49 g |
| AMF | 16.62 g | 1.56 ef | 12.82 fg | |
| CHT | 17.07 fg | 1.49 fg | 12.36 g | |
| AMF+CHT | 18.2 ef | 1.74 cd | 14.70 de | |
| LSD0.05 | 1.16 | 0.166 | 1.36 | |
| Treatments | Plant Height (cm) | Fresh Yield (g m−2) | Dry Yield (g m−2) | Essential Oil Content (%) | Essential Oil Yield (g m−2) | |
|---|---|---|---|---|---|---|
| FC80 | Control | 20.95 bcd | 1535.69 cd | 480.12 cd | 1.34 g | 6.43 ef |
| AMF | 22.27 ab | 1703.50 ab | 536.05 ab | 1.39 g | 7.45 de | |
| CHT | 21.58 bc | 1632.50 ab | 545.72 ab | 1.39 g | 7.58 de | |
| AMF+CHT | 23.65 a | 1806.53 a | 570.4 a | 1.58 f | 9.01 abc | |
| FC60 | Control | 18.33 fg | 1328.23 fg | 434.02 d | 1.75 de | 7.58 de |
| AMF | 19.63 def | 1457.56 ef | 477.13 cd | 1.99 ab | 9.49 ab | |
| CHT | 18.82 ef | 1376.96 ef | 450.27 cd | 1.85 bcd | 8.33 bcd | |
| AMF+CHT | 20.15 cde | 1510.44 cde | 494.76 bc | 2.03 a | 10.04 a | |
| FC40 | Control | 15.96 h | 1049.43 h | 273.55 f | 1.66 ef | 4.54 h |
| AMF | 17.29 gh | 1181.61 gh | 317.60 f | 1.83 cd | 5.81 fg | |
| CHT | 16.83 h | 1135.52 h | 302.24 f | 1.67 ef | 5.05 gh | |
| AMF+CHT | 19.17 ef | 1369.85 ef | 380.35 e | 1.95 abc | 7.42 de | |
| LSD0.05 | 1.48 | 154.04 | 52.64 | 0.14 | 1.28 | |
| No | Components | LRI Exp. a | LRI Lit. b | FC80 Control | FC80 AMF | FC80 CHT | FC80 AMF+CHT | FC60 Control | FC60 AMF | FC60 CHT | FC60 AMF+CHT | FC40 Control | FC40 AMF | FC40 CHT | FC40 AMF+CHT | ID c |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tricyclene | 920 | 921 | 0.09 | 0.02 | 0.11 | 0.06 | 0 | 0.03 | 0.07 | 0.05 | 0 | 0 | 0.03 | 0.07 | RI-MS |
| 2 | α-Thujene | 929 | 930 | 1.66 | 1.34 | 1.43 | 1.52 | 1.33 | 1.49 | 1.94 | 1.78 | 1.7 | 1.65 | 1.76 | 2.03 | RI-MS |
| 3 | α-Pinene | 931 | 932 | 1.21 | 0.56 | 0.49 | 0.54 | 0.68 | 0.55 | 1.06 | 0.84 | 0.86 | 1.21 | 0.59 | 0.92 | Std |
| 4 | Camphene | 944 | 946 | 0.88 | 0.65 | 0.41 | 0.84 | 0.78 | 0.59 | 0.92 | 1.04 | 0.42 | 0.65 | 0.37 | 0.44 | Std |
| 5 | Sabinene | 968 | 969 | 1.1 | 0.73 | 0.68 | 0.85 | 0.71 | 0.89 | 1.04 | 0.98 | 0.78 | 0.82 | 0.83 | 0.68 | Std |
| 6 | 1-Octen-3-ol | 972 | 974 | 0.79 | 0.63 | 0.66 | 0.5 | 0.46 | 0.6 | 0.7 | 0.61 | 0.61 | 0.48 | 0.38 | 0.42 | RI-MS |
| 7 | β-Myrcene | 985 | 988 | 2.09 | 1.83 | 1.96 | 2.02 | 1.88 | 2.12 | 1.98 | 2.21 | 1.79 | 2.08 | 1.98 | 2.16 | Std |
| 8 | 3-Octanol | 997 | 998 | 0.05 | 0.06 | 0 | 0.09 | 0 | 0.04 | 0.03 | 0 | 0.05 | 0.04 | 0.03 | 0 | RI-MS |
| 9 | α-Terpinene | 1011 | 1014 | 1.22 | 1.18 | 1.02 | 1.21 | 1.08 | 1.15 | 1.27 | 1.3 | 1.28 | 1.25 | 1.16 | 1.33 | RI-MS |
| 10 | p-Cymene | 1019 | 1020 | 16.35 | 16.72 | 18.3 | 18.05 | 17.68 | 18.84 | 18.73 | 18.5 | 18.19 | 19.32 | 18.34 | 19.38 | Std |
| 11 | Limonene | 1022 | 1024 | 0.56 | 0.39 | 0.18 | 0.41 | 0.23 | 0.29 | 0.46 | 0.52 | 0.19 | 0.27 | 0.23 | 0.37 | RI-MS |
| 12 | 1,8-Cineole | 1024 | 1026 | 3.02 | 3.15 | 2.89 | 2.63 | 3.18 | 3.26 | 2.89 | 2.63 | 3.69 | 3.18 | 1.85 | 2.63 | Std |
| 13 | Z-β-Ocimene | 1030 | 1032 | 0.08 | 0.09 | 0.02 | 0 | 0.02 | 0 | 0.03 | 0 | 0 | 0.03 | 0.04 | 0.02 | Std |
| 14 | E-β-Ocimene | 1042 | 1044 | 1.12 | 0.78 | 0.39 | 0.49 | 0.77 | 0.61 | 0.42 | 0.39 | 0.86 | 0.42 | 0.44 | 0.69 | Std |
| 15 | γ-Terpinene | 1052 | 1054 | 13.11 | 12.83 | 13.12 | 12.61 | 12.87 | 13.7 | 12.83 | 13.98 | 13.07 | 12.46 | 13.29 | 13.89 | Std |
| 16 | cis-Sabinene hydrate | 1064 | 1065 | 0.63 | 0.21 | 0.35 | 0.36 | 0.44 | 0.51 | 0.41 | 0.27 | 0.54 | 0.47 | 0.51 | 0.29 | RI-MS |
| 17 | α-Terpinolene | 1066 | 1068 | 0.04 | 0.06 | 0.08 | 0.08 | 0.09 | 0.11 | 0.06 | 0.05 | 0.07 | 0.06 | 0.06 | 0.08 | RI-MS |
| 18 | Linalool | 1094 | 1095 | 2.06 | 2.09 | 1.83 | 2.16 | 2.18 | 1.54 | 1.97 | 2.07 | 1.84 | 2.09 | 2.15 | 1.37 | Std |
| 19 | α-Campholenal | 1124 | 1126 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | RI-MS |
| 20 | trans-Sabinol | 1135 | 1137 | 0.02 | 0.01 | 0 | 0 | 0.02 | 0.03 | 0.01 | 0 | 0 | 0.01 | 0.03 | 0 | RI-MS |
| 21 | Camphor | 1137 | 1141 | 1.54 | 1.06 | 1.28 | 1.46 | 1.03 | 1.51 | 0.75 | 1.09 | 1.04 | 0.6 | 1.31 | 1.75 | RI-MS |
| 22 | Borneol | 1161 | 1165 | 0.8 | 0.7 | 0.45 | 0.96 | 0.72 | 0.82 | 0.57 | 0.72 | 0.54 | 0.81 | 0.7 | 0.53 | Std |
| 23 | Terpinen-4-ol | 1171 | 1174 | 0.62 | 0.54 | 0.47 | 0.18 | 0.52 | 0.4 | 0.32 | 0.46 | 0.7 | 0.38 | 0.28 | 0.45 | RI-MS |
| 24 | α-terpineol | 1193 | 1195 | 0.07 | 0.05 | 0.02 | 0.03 | 0 | 0.05 | 0.05 | 0 | 0.08 | 0.02 | 0.02 | 0.04 | RI-MS |
| 25 | Neral | 1234 | 1235 | 0.06 | 0.08 | 0.04 | 0.04 | 0.03 | 0.07 | 0.02 | 0.03 | 0.03 | 0.05 | 0.06 | 0.04 | Std |
| 26 | Carvacrol, methyl ether | 1238 | 1241 | 1.39 | 1.17 | 1.06 | 1.25 | 1.1 | 0.73 | 1.46 | 1.37 | 0.89 | 1.33 | 1.13 | 1.03 | RI-MS |
| 27 | Geranial | 1260 | 1264 | 0.33 | 0.31 | 0.31 | 0.28 | 0.27 | 0.29 | 0.32 | 0.33 | 0.34 | 0.31 | 0.28 | 0.29 | RI-MS |
| 28 | Thymol | 1287 | 1289 | 35.64 | 38.74 | 38.15 | 37.87 | 37.99 | 39.55 | 38.91 | 41.31 | 38.25 | 39.3 | 38.86 | 39.02 | Std |
| 29 | Carvacrol | 1297 | 1298 | 2.94 | 2.99 | 3.19 | 3.32 | 3.23 | 3.35 | 3.7 | 2.78 | 3.35 | 3.07 | 3.03 | 3.93 | Std |
| 30 | Thymol acetate | 1347 | 1349 | 0.71 | 0.53 | 0.61 | 0.88 | 0.68 | 0.4 | 0.31 | 0.34 | 0.35 | 0.56 | 0.58 | 0.59 | RI-MS |
| 31 | (E)-Caryophyllene | 1415 | 1417 | 3.23 | 3.55 | 3.48 | 2.29 | 3.22 | 2.4 | 2.29 | 2.05 | 2.9 | 2.27 | 2.85 | 3.38 | Std |
| 32 | α-Humulene | 1451 | 1452 | 0.06 | 0 | 0 | 0.04 | 0 | 0 | 0 | 0.05 | 0 | 0.04 | 0 | 0 | RI-MS |
| 33 | Linalool isovalerate | 1463 | 1466 | 0.07 | 0.11 | 0.06 | 0.03 | 0.04 | 0.03 | 0 | 0.02 | 0 | 0.03 | 0.03 | 0.05 | RI-MS |
| 34 | Germacrene D | 1481 | 1484 | 0.48 | 0.25 | 0.53 | 0.41 | 0.31 | 0.34 | 0.38 | 0.4 | 0.23 | 0.58 | 0.51 | 0.39 | RI-MS |
| 35 | Bicyclogermacrene | 1498 | 1500 | 0.03 | 0 | 0 | 0.02 | 0 | 0.02 | 0 | 0 | 0.04 | 0.03 | 0 | 0 | RI-MS |
| 36 | δ-Cadinene | 1521 | 1522 | 0.23 | 0.36 | 0.39 | 0.23 | 0.15 | 0.18 | 0.09 | 0.15 | 0.39 | 0.28 | 0.42 | 0.39 | RI-MS |
| 37 | Caryophyllene oxide | 1581 | 1582 | 0.69 | 0.58 | 0.72 | 1.22 | 1.16 | 1.02 | 0.67 | 0.55 | 0.32 | 0.91 | 0.83 | 0.64 | Std |
| 38 | β-Bisabolene | 1766 | 1768 | 0 | 0 | 0 | 0.03 | 0 | 0.05 | 0 | 0 | 0.04 | 0 | 0 | 0.03 | RI-MS |
| Total identified (%) | 94.78 | 94.16 | 95.49 | 94.78 | 94.66 | 97.37 | 96.47 | 98.68 | 95.24 | 96.88 | 94.77 | 99.06 |
| Soil Texture | Sand (%) | Silt (%) | Clay (%) | OM (g kg−1) | EC (ds.m−1) | pH | FC (%) | PWP (%) | N (g kg−1) | P (mg kg−1) | K (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sandy clay loam | 56.1 | 17 | 26.9 | 8.2 | 1.16 | 7.71 | 26.8 | 13.7 | 0.85 | 9.8 | 549.34 |
| Year | April | May | June | July | August | September |
|---|---|---|---|---|---|---|
| Monthly average temperature (°C) | ||||||
| 2020 | 11.8 | 19.1 | 24.2 | 28.0 | 25.1 | 23.8 |
| 2021 | 16.3 | 21.3 | 27.2 | 28.3 | 28.1 | 23.02 |
| 2-year mean | 14.1 | 20.2 | 25.7 | 28.1 | 26.6 | 23.4 |
| 10-year mean | 12.9 | 18.5 | 24.4 | 28.1 | 27.5 | 22.7 |
| Total monthly precipitation (mm) | ||||||
| 2020 | 63.3 | 12.0 | 2.6 | 0.1 | 1.2 | 0.0 |
| 2021 | 12.01 | 13.3 | 0.01 | 3.10 | 0.02 | 0.1 |
| 2-year mean | 37.6 | 12.7 | 1.3 | 1.6 | 0.6 | 0.05 |
| 10-year mean | 41.8 | 19.9 | 1.5 | 0.7 | 0.3 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amani Machiani, M.; Javanmard, A.; Ostadi, A.; Alizadeh, K. Improvement in Essential Oil Quantity and Quality of Thyme (Thymus vulgaris L.) by Integrative Application of Chitosan Nanoparticles and Arbuscular Mycorrhizal Fungi under Water Stress Conditions. Plants 2023, 12, 1422. https://doi.org/10.3390/plants12071422
Amani Machiani M, Javanmard A, Ostadi A, Alizadeh K. Improvement in Essential Oil Quantity and Quality of Thyme (Thymus vulgaris L.) by Integrative Application of Chitosan Nanoparticles and Arbuscular Mycorrhizal Fungi under Water Stress Conditions. Plants. 2023; 12(7):1422. https://doi.org/10.3390/plants12071422
Chicago/Turabian StyleAmani Machiani, Mostafa, Abdollah Javanmard, Ali Ostadi, and Khoshnood Alizadeh. 2023. "Improvement in Essential Oil Quantity and Quality of Thyme (Thymus vulgaris L.) by Integrative Application of Chitosan Nanoparticles and Arbuscular Mycorrhizal Fungi under Water Stress Conditions" Plants 12, no. 7: 1422. https://doi.org/10.3390/plants12071422
APA StyleAmani Machiani, M., Javanmard, A., Ostadi, A., & Alizadeh, K. (2023). Improvement in Essential Oil Quantity and Quality of Thyme (Thymus vulgaris L.) by Integrative Application of Chitosan Nanoparticles and Arbuscular Mycorrhizal Fungi under Water Stress Conditions. Plants, 12(7), 1422. https://doi.org/10.3390/plants12071422







