Phytosterols Are Involved in Sclareol-Induced Chlorophyll Reductions in Arabidopsis
Abstract
1. Introduction
2. Results and Discussion
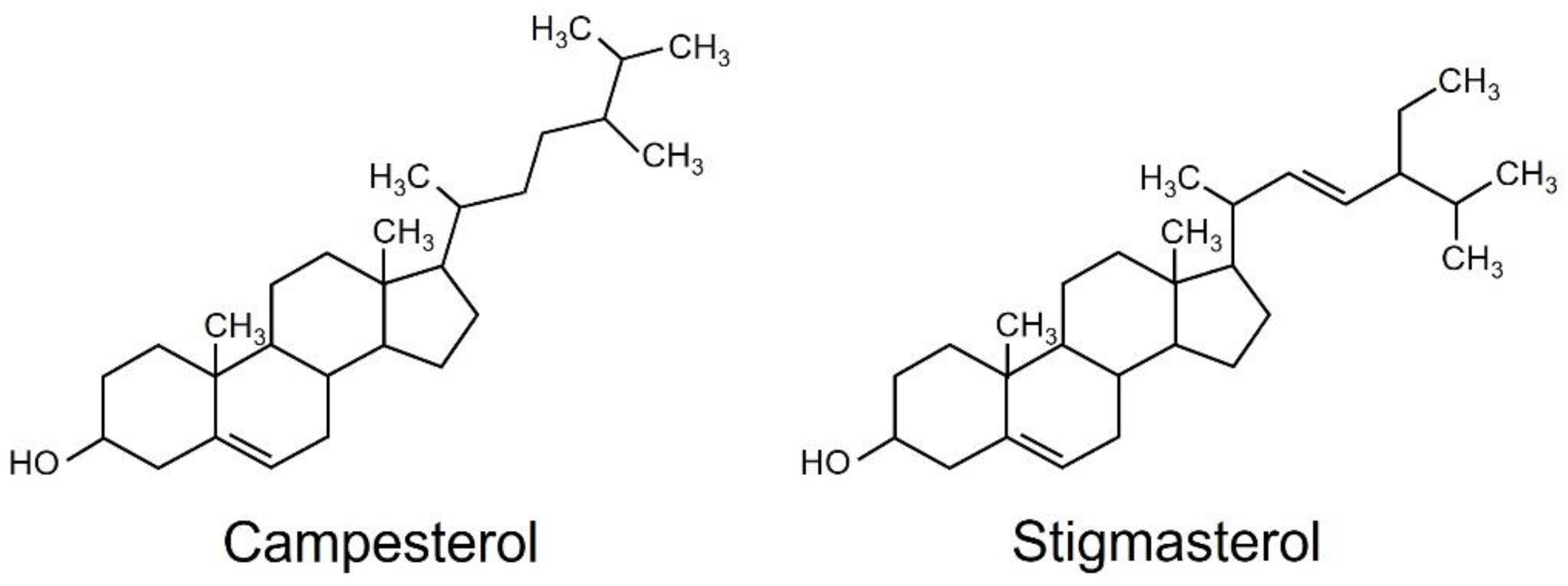
2.1. Isolation and Identification of Chlorophyll-Content-Reducing Substances
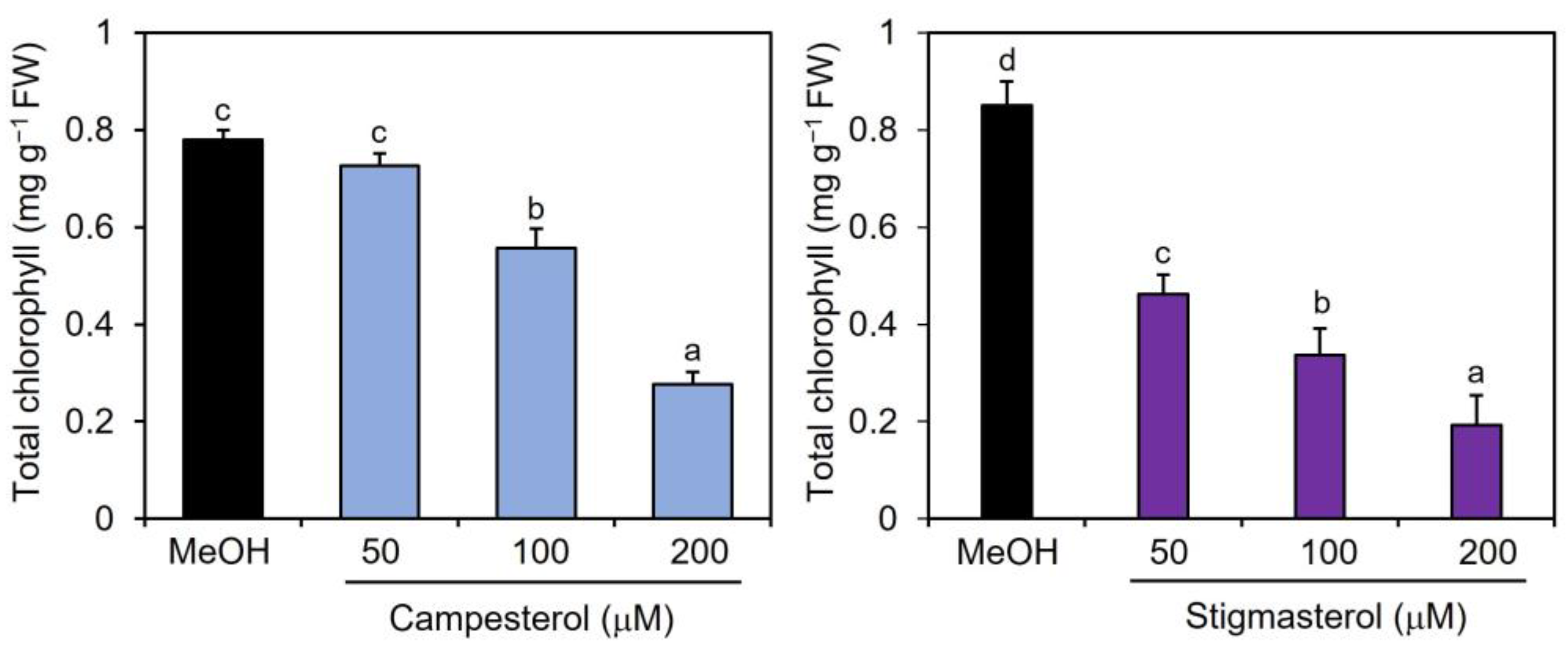
2.2. Effects of Campesterol and Stigmasterol on Chlorophyll Reductions
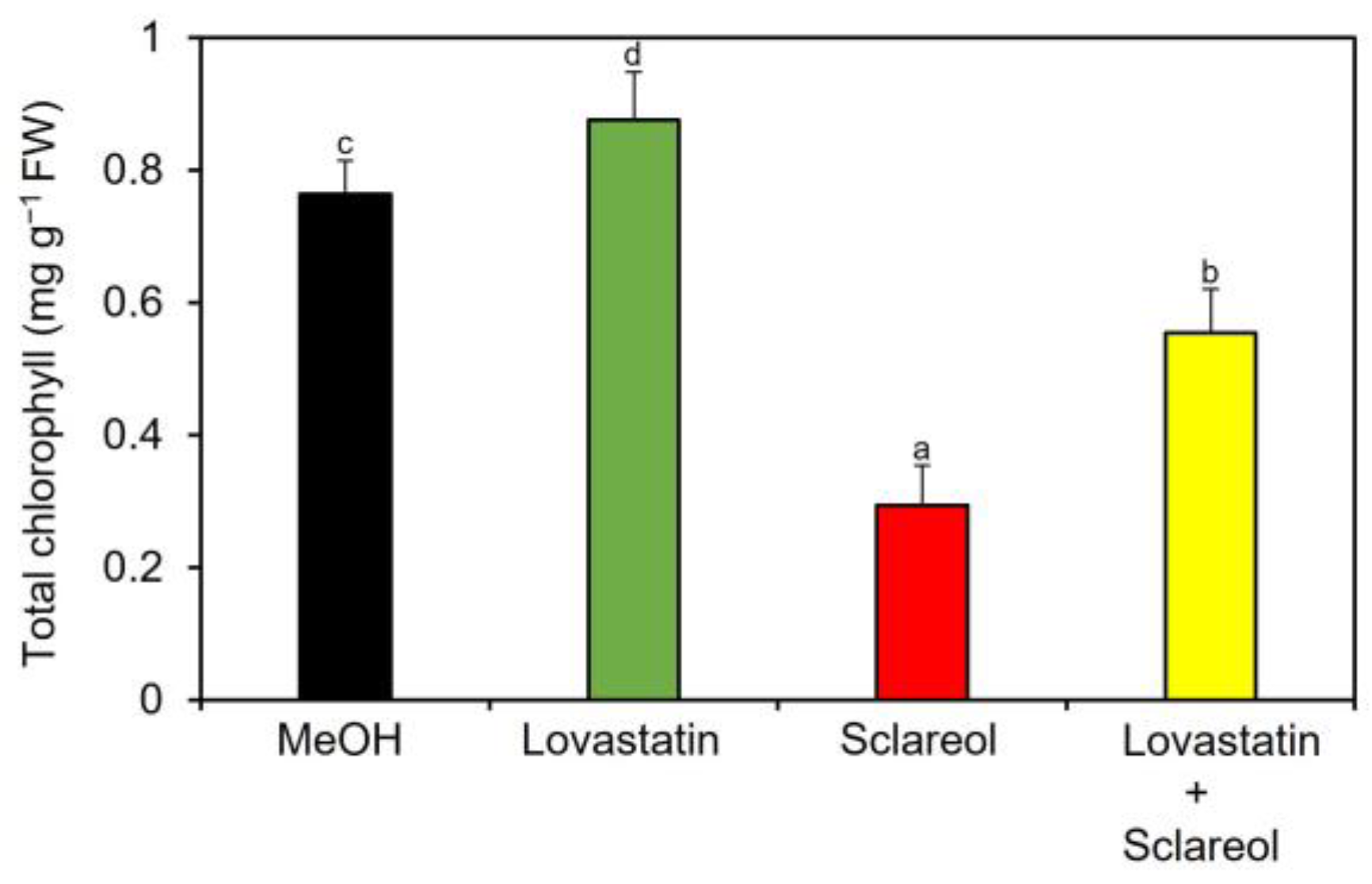
2.3. Effects of Sclareol on Accumulation of Campesterol and Stigmasterol, and Expression of Phytosterol Biosynthetic Genes
3. Materials and Methods
3.1. Plant Materials
3.2. Extraction, Fractionation, and Purification of Active Substances
3.2.1. Small-Scale Extraction
3.2.2. Large-Scale Extraction
3.3. Chemical Treatments
3.4. Chlorophyll Measurement
3.5. Structural Analyses of Active Compounds
3.5.1. NMR
3.5.2. TLC
3.5.3. Gas Chromatography–Mass Spectrometry
3.5.4. Silylation of Samples
3.5.5. Compound 1
3.5.6. Compound 2
3.6. Quantification of Phytosterols
3.7. Quantitative Real-Time PCR
3.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akhter, M.D.; Nakahara, K.S.; Masuta, C. Resistance induction based on the understanding of molecular interactions between plant viruses and host plants. Viol. J. 2021, 18, 176. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Yang, Y. Mechanisms of silicon-induced fungal disease resistance in plants. Plant Physiol. Biochem. 2021, 165, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Mubeen, B.; Hasnain, A.; Rizwan, M.; Adrees, M.; Naqvi, S.A.H.; Iqbal, S.; Kamran, M.; El-Sabrout, A.M.; Elansary, H.O.; et al. Role of promising secondary metabolites to confer resistance against environmental stresses in crop plants: Current scenario and future perspectives. Front. Plant Sci. 2022, 13, 881032. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Guo, M.; Song, J.; Ma, Y.; Xu, Z. Signals in systemic acquired resistance of plants against microbial pathogens. Mol. Plant Biol. Rep. 2021, 48, 3747–3759. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wagner, G.J. Biosynthesis of labdenediol and sclareol in cell-free extracts from trichomes of Nicotiana glutinosa. Planta 1995, 197, 627–632. [Google Scholar] [CrossRef]
- Caniard, A.; Zerbe, P.; Legrand, S.; Cohade, A.; Valot, N.; Magnard, J.L.; Bohlmann, J.; Legendre, L. Discovery and functional characterization of two diterpene synthases for sclareol biosynthesis in Salvia sclarea (L.) and their relevance for perfume manufacture. BMC Plant Biol. 2012, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.A.; Carter, G.A.; Burden, R.S.; Wain, R.L. Control of rust disease by diterpenes from Nicotiana glutinosa. Nature 1975, 255, 328–329. [Google Scholar] [CrossRef]
- Kennedy, B.S.; Nielsen, M.T.; Severson, R.F.; Sisson, V.A.; Stephenson, M.K.; Jackson, D.M. Leaf surface chemicals from Nicotiana affecting germination of Peronospora tabacina (adam) sporangia. J. Chem. Ecol. 1992, 18, 1467–1479. [Google Scholar] [CrossRef]
- Jackson, D.M.; Danehower, D.A. Integrated case study: Nicotiana leaf surface components and their effects on insect pests and disease. In Plant Cuticles: An Integrated Functional Approach; Kerstiens, G., Ed.; BIOS Scientific Publishers, Ltd.: Oxford, UK, 1996; pp. 231–254. Available online: https://www.ars.usda.gov/research/publications/publication/?seqNo115=70621 (accessed on 1 August 2022).
- Popova, V.; Ivanova, T.; Stoyanova, A.; Nikolova, V.; Hristeva, T.; Gochev, V.; Yonchev, Y.; Nikolov, N.; Zheljazkov, V.D. Terpenoids in the essential oil and concentrated aromatic products obtained from Nicotiana glutinosa L. leaves. Molecules 2019, 25, 30. [Google Scholar] [CrossRef] [PubMed]
- Kroumova, A.B.; Artiouchine, I.; Wagner, G.J. Use of several natural products from selected Nicotiana species to prevent black shank disease in tobacco. Contrib. Tob. Nicotine Res. 2016, 27, 113–125. [Google Scholar] [CrossRef]
- Campbell, E.; Schenk, P.M.; Kazan, K.; Penninck, I.A.; Anderson, J.P.; Maclean, D.J.; Cammue, B.P.A.; Ebert, P.R.; Manners, J.M. Pathogen-responsive expression of a putative ATP-binding cassette transporter gene conferring resistance of the diterpenoid sclareol is regulated by multiple defense signaling pathways in Arabidopsis. Plant Physiol. 2003, 133, 1272–1284. [Google Scholar] [CrossRef]
- Grec, S.; Vanham, D.; De Ribaucourt, J.C.; Purnelle, B.; Boutry, M. Identification of regulatory sequence elements within the transcription promoter region of NpABC1, a gene encoding a plan ABC transporter induced by diterpenes. Plant J. 2003, 35, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Jasiński, M.; Stukkens, Y.; Degand, H.; Purnelle, B.; Marchand-Brynaert, J.; Boutry, M. A plant plasma membrane ATP binding cassette-type transporter is involved in antifungal terpenoid secretion. Plant Cell 2001, 13, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Gomi, K.; Kaku, H.; Abe, H.; Seto, H.; Nakatsu, S.; Neya, M.; Kobayashi, M.; Nakaho, K.; Ichinose, Y.; et al. Identification of natural diterpenes that inhibit bacterial wilt disease in tobacco, tomato and Arabidopsis. Plant Cell Physiol. 2012, 53, 1432–1444. [Google Scholar] [CrossRef]
- Fujimoto, T.; Mizukubo, T.; Abe, H.; Seo, S. Sclareol induces plant resistance to root-knot nematode partially through ethylene-dependent enhancement of lignin accumulation. Mol. Plant-Microbe Interact. 2015, 28, 398–407. [Google Scholar] [CrossRef]
- Caissard, J.C.; Oliver, T.; Delbecque, C.; Palle, S.; Garry, P.P.; Audran, A.; Valot, N.; Moja, S.; Nicole, F.; Magnard, J.L.; et al. Extracellular localization of the diterpene sclareol in Clary Sage (Salvia sclarea L., Lamiaceae). PLoS ONE 2012, 7, e48253. [Google Scholar] [CrossRef]
- Fujimoto, T.; Mizukubo, T.; Abe, H.; Seo, S. Phytol, a constituent of chlorophyll, induces root-knot nematode resistance in Arabidopsis via the ethylene signaling pathway. Mol. Plant-Microbe Interact. 2021, 34, 279–285. [Google Scholar] [CrossRef]
- Du, Y.; Fu, X.; Chu, Y.; Wu, P.; Liu, Y.; Ma, L.; Tian, H. Biosynthesis and the roles of plant sterols in development and stress responses. Int. J. Mol. Sci. 2022, 23, 2332. [Google Scholar] [CrossRef]
- Tsukagoshi, Y.; Suzuki, H.; Seki, H.; Muranaka, T.; Ohyama, K.; Fujimoto, Y. Ajuga Δ24-sterol reductase catalyzes the direct reductive conversion of 24-methylenecholesterol to campesterol. J. Biol. Chem. 2016, 291, 8189–8198. [Google Scholar] [CrossRef]
- Lange, I.; Poirier, B.C.; Herron, B.K.; Lange, B.M. Comprehensive assessment of transcriptional regulation facilitates metabolic engineering of isoprenoid accumulation in Arabidopsis. Plant Physiol. 2015, 169, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Darnet, S.; Rahier, A. Plant sterol biosynthesis: Identification of two distinct families of sterol 4α-methyl oxidases. Biochem. J. 2004, 378, 889–898. [Google Scholar] [CrossRef]
- Rahier, A. Dissecting the sterol C-4 demethylation process in higher plants. From structures and genes to catalytic mechanism. Steroids 2011, 76, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Laule, O.; Fürfholz, A.; Chang, H.S.; Zhu, T.; Wang, X.; Heifetz, P.B.; Gruissem, W.; Lange, B.M. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 6866–6871. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.L.; Shimada, T.; Okazaki, Y.; Higashi, Y.; Saito, K.; Kuwata, K.; Oyama, K.; Kato, M.; Ueda, H.; Nakano, A.; et al. HIGH STEROL ESTER 1 is a key factor in plant sterol homeostasis. Nature Plants 2019, 5, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Yamaguchi, K.; Shigenobu, S.; Takahashi, H.; Murase, M.; Fukuyoshi, S.; Hara-Nishimura, I. Excess sterols disrupt plant cellular activity by inducing stress-responsive gene expression. J. Plant Res. 2020, 133, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophy. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Lytovchenko, A.; Beleggia, R.; Schauer, N.; Isaacson, T.; Leuendorf, J.E.; Hellmann, H.; Rose, J.K.C.; Fernie, A.R. Application of GC–MS for the detection of lipophilic compounds in diverse plant tissues. Plant Meth. 2009, 5, 4. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hmidene, A.B.; Ono, H.; Seo, S. Phytosterols Are Involved in Sclareol-Induced Chlorophyll Reductions in Arabidopsis. Plants 2023, 12, 1282. https://doi.org/10.3390/plants12061282
Hmidene AB, Ono H, Seo S. Phytosterols Are Involved in Sclareol-Induced Chlorophyll Reductions in Arabidopsis. Plants. 2023; 12(6):1282. https://doi.org/10.3390/plants12061282
Chicago/Turabian StyleHmidene, Asma Ben, Hiroshi Ono, and Shigemi Seo. 2023. "Phytosterols Are Involved in Sclareol-Induced Chlorophyll Reductions in Arabidopsis" Plants 12, no. 6: 1282. https://doi.org/10.3390/plants12061282
APA StyleHmidene, A. B., Ono, H., & Seo, S. (2023). Phytosterols Are Involved in Sclareol-Induced Chlorophyll Reductions in Arabidopsis. Plants, 12(6), 1282. https://doi.org/10.3390/plants12061282







