Phytoremediation Potential of Native Plant Species in Mine Soils Polluted by Metal(loid)s and Rare Earth Elements
Abstract
1. Introduction
2. Results and Discussion
2.1. Physic-Chemical Characteristics of Soils
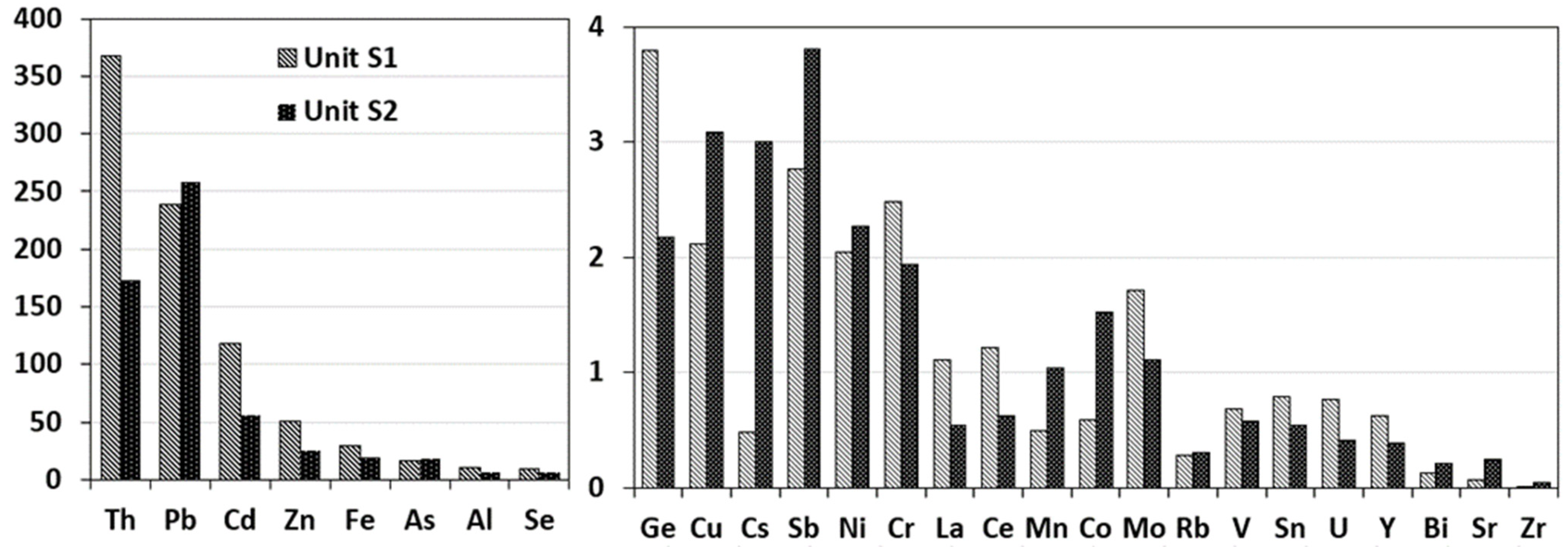
2.2. Metal(loid)s and REEs Concentrations in Soils
2.2.1. Distribution of Total Concentration in Soils
2.2.2. Contamination Factor
2.2.3. Relation and Behavior among Metal(loid)s and REEs
2.2.4. Available and Water-Soluble Metal(loid)s and Rees
2.3. Accumulation of Heavy Metals and Metalloids in Plant Tissues
3. Materials and Methods
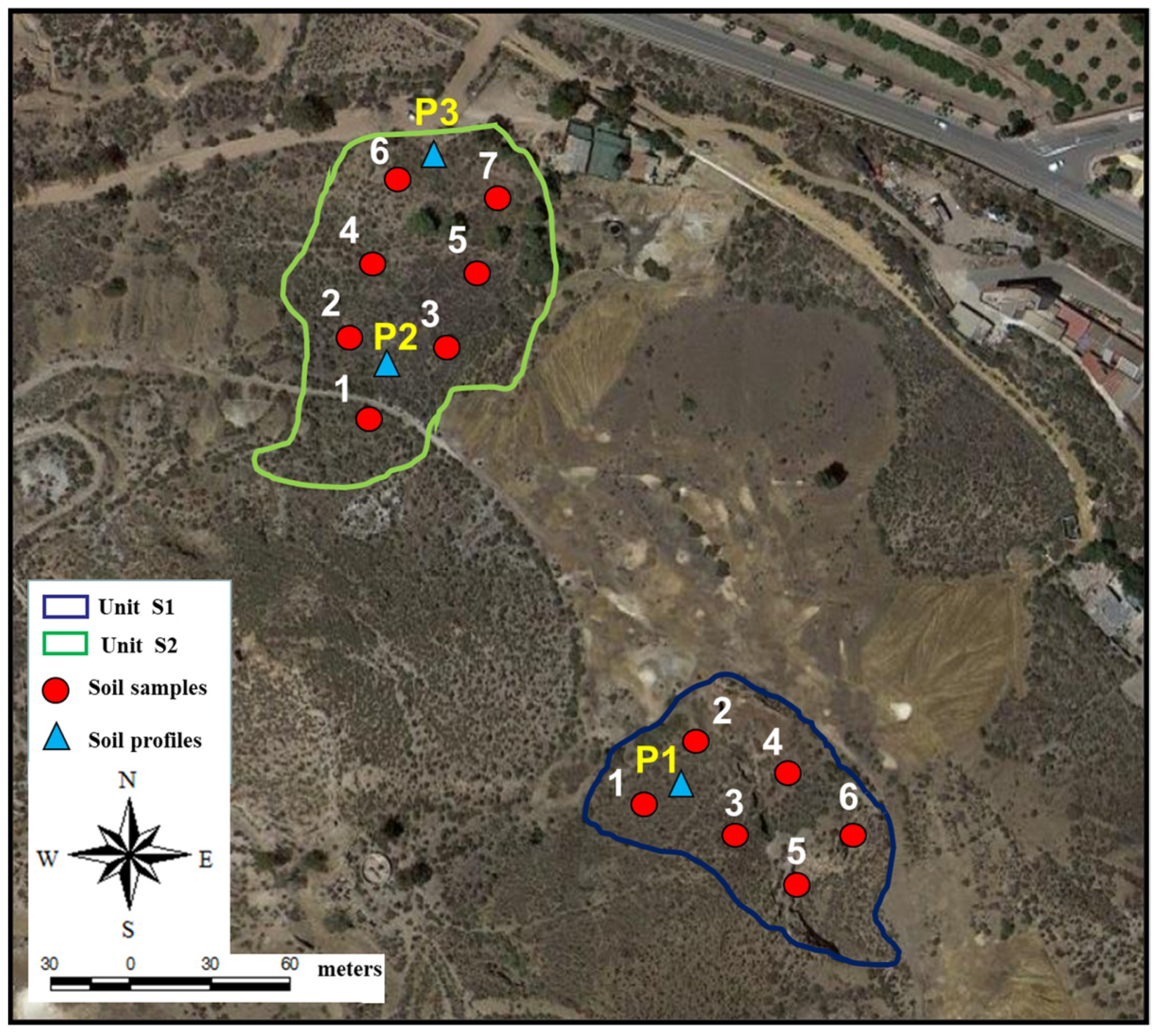
3.1. Site Characterization
3.2. Soil Sampling and Analysis
3.3. Plant Sampling and Analysis
3.4. Data Treatment and Statistical Analyses
3.4.1. Contamination, Bioconcentration, Translocation, and Accumulation Factors
3.4.2. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, O.; Sánchez de la Campa, A.M.; Sánchez-Rodas, D.; de la Rosa, J.D. Hazardous trace elements in thoracic fraction of airborne particulate matter: Assessment of temporal variations, sources, and health risks in a megacity. Sci. Total. Environ. 2020, 710, 136344. [Google Scholar] [CrossRef]
- Pagano, G.; Aliberti, F.; Guida, M.; Oral, R.; Siciliano, A.; Trifuoggi, M.; Tommasi, F. Rare earth elements in human and animal health: State of art and research priorities. Environ. Res. 2015, 142, 215–220. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, T.; Zhou, L.; Lou, W.; Zeng, W.; Liu, T.; Yin, H.; Liu, H.; Liu, X.; Mathivanan, K.; et al. Soil microbial community assembly model in response to heavy metal pollution. Environ. Res. 2022, 213, 113576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Long, J.; Wei, Z.; Alakangas, L. Vertical distribution and historical loss estimation of heavy metals in an abandoned tailings pond at HTM copper mine, northeastern China. Environ. Earth Sci. 2016, 75, 1–13. [Google Scholar] [CrossRef]
- Schaider, L.A.; Senn, D.B.; Estes, E.R.; Brabander, D.J.; Shine, J.P. Sources and fates of heavy metals in a mining-impacted stream: Temporal variability and the role of iron oxides. Sci. Total. Environ. 2014, 490, 456–466. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Z.; Hu, Y.; Cheng, H. Leaching of heavy metals from abandoned mine tailings brought by precipitation and the associated environmental impact. Sci. Total. Environ. 2019, 695, 133893. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Cheng, G.; Wang, Y.; Zhuang, D. Effects of natural factors on the spatial distribution of heavy metals in soils surrounding mining regions. Sci. Total. Environ. 2017, 578, 577–585. [Google Scholar] [CrossRef]
- Yun, S.-W.; Kang, D.-H.; Ji, W.-H.; Jung, M.-H.; Yu, C. Distinct Dispersion of As, Cd, Pb, and Zn in Farmland Soils near Abandoned Mine Tailings: Field Observation Results in South Korea. J. Chem. 2020, 2020, 9671871. [Google Scholar] [CrossRef]
- Acosta, J.A.; Jansen, B.; Kalbitz, K.; Faz, A.; Martínez-Martínez, S. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef]
- Dimirkou, A.; Ioannou, Z.; Golia, E.E.; Danalatos, N.; Mitsios, I.K. Sorption of Cadmium and Arsenic by Goethite and Clinoptilolite. Commun. Soil Sci. Plant Anal. 2003, 40, 1–6. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, M.L.; Lu, R.N.; Liao, Y.P.V. Tourism Development and Exploration of Abandoned Mine—Taking National Mine Park of Jianghe Coal Mine in Chongqing as an Example. Adv. Mater. Res. 2012, 599, 909–914. [Google Scholar] [CrossRef]
- Pascaud, G.; Leveque, T.; Soubrand, M.; Boussen, S.; Joussein, E.; Dumat, C. Environmental and health risk assessment of Pb, Zn, As and Sb in soccer field soils and sediments from mine tailings: Solid speciation and bioaccessibility. Environ. Sci. Pollut. Res. 2013, 21, 4254–4264. [Google Scholar] [CrossRef] [PubMed]
- Colín-Torres, C.G.; Murillo-Jiménez, J.M.; Del Razo, L.M.; Sánchez-Peña, L.C.; Becerra-Rueda, O.F.; Marmolejo-Rodríguez, A.J. Urinary arsenic levels influenced by abandoned mine tailings in the Southernmost Baja California Peninsula, Mexico. Environ. Geochem. Health 2014, 36, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Peñalver-Alcalá, A.; Álvarez-Rogel, J.; Peixoto, S.; Silva, I.; Silva, A.R.R.; González-Alcaraz, M.N. The relationships between functional and physicochemical soil parameters in metal(loid) mine tailings from Mediterranean semiarid areas support the value of spontaneous vegetation colonization for phytomanagement. Ecol. Eng. 2021, 168, 106293. [Google Scholar] [CrossRef]
- Anoopkumar, A.N.; Rebello, S.; Devassy, E.; Kavya Raj, K.; Puthur, S.; Aneesh, E.M.; Sindhu, R.; Binod, P.; Pandey, A. Phytoextraction of Heavy Metals. In Methods for Bioremediation of Water and Wastewater Pollution; Inamuddin, A.M.I., Lichtfouse, E., Asiri, A.M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 267–276. [Google Scholar] [CrossRef]
- Shackira, A.M.; Puthur, J.T. Phytostabilization of Heavy Metals: Understanding of Principles and Practices. In Plant-Metal Interactions; Srivastava, S., Srivastava, A.K., Suprasanna, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 263–282. [Google Scholar]
- Martínez-López, S.; Martínez-Sánchez, M.J.; Pérez-Sirvent, C.; Bech, J.; del Carmen Gómez Martínez, M.; García-Fernandez, A.J. Screening of wild plants for use in the phytoremediation of mining-influenced soils containing arsenic in semiarid environments. J. Soils Sediments 2014, 14, 794–809. [Google Scholar] [CrossRef]
- Kołodziej, B.; Antonkiewicz, J.; Bielińska, E.J.; Witkowicz, R.; Dubis, B. Recovery of microelements from municipal sewage sludge by reed canary grass and giant miscanthus. Int. J. Phytoremediation 2023, 25, 441–454. [Google Scholar] [CrossRef]
- Tarla, D.N.; Erickson, L.E.; Hettiarachchi, G.M.; Amadi, S.I.; Galkaduwa, M.; Davis, L.C.; Nurzhanova, A.; Pidlisnyuk, V. Remediation of soils on municipal rendering plant territories using Mscanthus x giganteus. Environ. Sci. Pollut. Res. 2022, 11, 1217. [Google Scholar]
- Martínez-Carlos, J.; Martínez-Martínez, S.; Faz, A.; Zornoza, R.; Gabarrón, M.; Soriano-Disla, M.; Gómez-López, M.D.; Acosta, J.A. Are the soils and vegetation of a forest close to tailings ponds affected by metals and arsenic? Environ. Geochem. Health 2021, 44, 15–28. [Google Scholar] [CrossRef]
- Suo, Y.; Tang, N.; Li, H.; Corti, G.; Jiang, L.; Huang, Z.; Zhang, Z.; Huang, J.; Wu, Z.; Feng, C.; et al. Long-term effects of phytoextraction by a poplar clone on the concentration, fractionation, and transportation of heavy metals in mine tailings. Environ. Sci. Pollut. Res. 2021, 28, 47528–47539. [Google Scholar] [CrossRef]
- Zhu, H.; Cheng, R.; Bañuelos, G.; Centofanti, T. Feasibility of growing halophyte “agretti” (Salsola soda) as an alternative boron-tolerant food crop in unproductive boron-laden regions. Plant Soil 2019, 445, 323–334. [Google Scholar] [CrossRef]
- Toderich, K.N.; Shuyskaya, E.V.; Khujanazarov, T.M.; Ismail, S.; Kawabata, Y. The Structural and Functional Characteristics of Asiatic Desert Halophytes for Phytostabilization of Polluted Sites. In Plant Adaptation and Phytoremediation; Ashraf, M., Ozturk, M., Ahmad, M.S.A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 245–274. [Google Scholar] [CrossRef]
- Heckenroth, A.; Rabier, J.; Dutoit, T.; Torre, F.; Prudent, P.; Laffont-Schwob, I. Selection of native plants with phytoremediation potential for highly contaminated Mediterranean soil restoration: Tools for a non-destructive and integrative approach. J. Environ. Manag. 2016, 183, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Parraga-Aguado, I.; Querejeta, J.-I.; González-Alcaraz, M.-N.; Jiménez-Cárceles, F.J.; Conesa, H.M. Usefulness of pioneer vegetation for the phytomanagement of metal(loid)s enriched tailings: Grasses vs. shrubs vs. trees. J. Environ. Manag. 2014, 133, 51–58. [Google Scholar] [CrossRef]
- Azizi, M.; Faz, A.; Zornoza, R.; Martínez-Martínez, S.; Shahrokh, V.; Acosta, J.A. Environmental pollution and depth distribution of metal(loid)s and rare earth elements in mine tailing. J. Environ. Chem. Eng. 2022, 10, 107526. [Google Scholar] [CrossRef]
- Gabarrón, M.; Faz, A.; Martínez-Martínez, S.; Acosta, J.A. Change in metals and arsenic distribution in soil and their bioavailability beside old tailing ponds. J. Environ. Manag. 2018, 212, 292–300. [Google Scholar] [CrossRef]
- Cobertera, E. Edafología Aplicada; Ediciones Cátedra S.A.: Madrid, Spain, 1993; p. 326. [Google Scholar]
- Porta, J.; López-Acevedo, M.; Roquero, C. Edafología Para la Agricultura y el Medio Ambiente, 2nd ed.; Ediciones Mundi-Prensa: Madrid, Spain, 1999. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; p. 234. [Google Scholar]
- Tuo, D.F.; Xu, M.X.; Ma, X.X.; Zheng, S.Q. Impact of wind-water alternate erosion on the characteristics of sediment particles. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2014, 25, 381–386. [Google Scholar]
- Midhat, L.; Ouazzani, N.; Hejjaj, A.; Ouhammou, A.; Mandi, L. Accumulation of heavy metals in metallophytes from three mining sites (Southern Centre Morocco) and evaluation of their phytoremediation potential. Ecotoxicol. Environ. Saf. 2019, 169, 150–160. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Shen, R.F. Aluminum–Nitrogen Interactions in the Soil–Plant System. Front. Plant Sci. 2018, 9, 807. [Google Scholar] [CrossRef]
- Balintova, M.; Petrilakova, A. Study of pH Influence on Selective Precipitation of Heavy Metals from Acid Mine Drainage. Chem. Eng. Trans. 2011, 25, 345–350. [Google Scholar]
- Alloway, B. Sources of Heavy Metals and Metalloids in Soils; Springer: Berlin/Heidelberg, Germany, 2013; Volume 22, pp. 11–50. [Google Scholar]
- Gilkes, R.J.; McKenzie, R.M. Geochemistry and Mineralogy of Manganese in Soils. In Manganese in Soils and Plants: Proceedings of the International Symposium on ‘Manganese in Soils and Plants’ held at the Waite Agricultural Research Institute, The University of Adelaide, Glen Osmond, Australia, 22–26 August 1988; as an Australian Bicentennial Event; Graham, R.D., Hannam, R.J., Uren, N.C., Eds.; Springer: Dordrecht, The Netherlands, 1988; pp. 23–35101007978. [Google Scholar]
- Queiroz, H.M.; Ying, S.C.; Abernathy, M.; Barcellos, D.; Gabriel, F.A.; Otero, X.L.; Nóbrega, G.N.; Bernardino, A.F.; Ferreira, T.O. Manganese: The overlooked contaminant in the world largest mine tailings dam collapse. Environ. Int. 2021, 146, 106284. [Google Scholar] [CrossRef]
- Martínez-Pagán, P.; Faz, A.; Acosta, J.; Carmona, D.M.; Martínez-Martínez, S. A multidisciplinary study for mining landscape reclamation: A study case on two tailing ponds in the Region of Murcia (SE Spain). Phys. Chem. Earth 2011, 36. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Ahmady-Birgani, H.; Engelbrecht, J.P.; Bazgir, M. How different source regions across the Middle East change aerosol and dust particle characteristics. Desert 2019, 24, 61–73. [Google Scholar] [CrossRef]
- Edahbi, M.; Plante, B.; Benzaazoua, M.; Ward, M.; Pelletier, M. Mobility of rare earth elements in mine drainage: Influence of iron oxides, carbonates, and phosphates. Chemosphere 2018, 199, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.; Bank, T.; Howard, B.; Granite, E. Rare Earth Elements in Alberta Oil Sand Process Streams. Energy Fuels 2017, 31, 4714–4720. [Google Scholar] [CrossRef]
- Balboni, E.; Simonetti, A.; Spano, T.; Cook, N.D.; Burns, P.C. Rare-earth element fractionation in uranium ore and its U(VI) alteration minerals. Appl. Geochem. 2017, 87, 84–92. [Google Scholar] [CrossRef]
- Ayora, C.; Macías, F.; Torres, E.; Lozano, A.; Carrero, S.; Nieto, J.-M.; Pérez-López, R.; Fernández-Martínez, A.; Castillo-Michel, H. Recovery of rare earth elements and yttrium from passive-remediation systems of acid mine drainage. Environ. Sci. Technol. 2016, 50, 8255–8262. [Google Scholar] [CrossRef]
- Pereira, W.V.d.S.; Ramos, S.J.; Melo, L.o.C.A.; Braz, A.M.d.S.; Dias, Y.N.; Almeida, G.V.d.; Fernandes, A.R. Levels and environmental risks of rare earth elements in a gold mining area in the Amazon. Environ. Res. 2022, 211, 113090. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Shen, L.; Feng, C.; Yang, R.; Qu, J.; Ju, H.; Zhang, Y. Distribution of rare earth elements (REEs) and their roles in plant growth: A review. Environ. Pollut. 2022, 298, 118540. [Google Scholar] [CrossRef]
- Mleczek, P.; Borowiak, K.; Budka, A.; Niedzielski, P. Relationship between concentration of rare earth elements in soil and their distribution in plants growing near a frequented road. Environ. Sci. Pollut. Res. 2018, 25, 23695–23711. [Google Scholar] [CrossRef]
- Cabral, A.R.; Lehmann, B.; Kwitko, R.; Costa, C.H.C. The Serra Pelada Au-Pd-Pt deposit, Carajas Mineral Province, Northern Brazil: Reconnaissance mineralogy and chemistry of very high grade palladian gold mineralization. Econ. Geol. 2002, 97, 1127–1138. [Google Scholar] [CrossRef]
- Dołȩgowska, S.; Migaszewski, Z.M. Anomalous Concentrations of Rare Earth Elements in the Moss-Soil System from South-Central Poland. Environ. Pollut. 2013, 178, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Fedotov, P.S.; Rogova, O.B.; Dzhenloda, R.K.; Karandashev, V.K. Metal-organic complexes as a major sink for rare earth elements in soils. Environ. Chem. 2019, 16, 323–332. [Google Scholar] [CrossRef]
- Ding, S.; Liang, T.; Zhang, C.; Yan, J.; Zhang, Z.; Sun, Q. Role of Ligands in Accumulation and Fractionation of Rare Earth Elements in Plants: Examples of Phosphate and Citrate. Biol. Trace Elem. Res. 2005, 107, 73–86. [Google Scholar] [CrossRef]
- Sanchez, M.J.; Sirvent, C.P.; Desarro, C.D.; Sosten, Y. Niveles de fondo y niveles genéricos de referencia de metales pesados en suelos de la Región de Murcia. Ord. Del. Territ. 2007, 1, 304. [Google Scholar]
- Instituto Geológico y Minero de España. Determinación de Niveles de Fondo y Niveles Genéricos de Referencia para Metales en Suelos de la Comunidad Autónoma de Aragon; Ministerio de Educación y Ciencia: Madrid, Spain, 2007. [Google Scholar]
- Sahoo, P.K.; Powell, M.A.; Martins, G.C.; Dall’Agnol, R.; Salomão, G.N.; Mittal, S.; Pontes, P.R.M.; Guimarães, J.T.F.; de Siqueira, J.O. Occurrence, distribution, and environmental risk assessment of heavy metals in the vicinity of Fe-ore mines: A global overview. Toxin Rev. 2021, 41, 675–698. [Google Scholar] [CrossRef]
- Ballesta, R.J.; Bueno, P.C.; Rubi, J.A.M.; Gim’enez, R.G. Pedo-geochemical baseline content levels and soil quality reference values of trace elements in soils from the Mediterranean (Castilla La Mancha, Spain). Cent. Eur. J. Geosci. 2010, 2, 441–454. [Google Scholar] [CrossRef]
- Galhardi, J.A.; Leles, B.P.; de Mello, J.W.V.; Wilkinson, K.J. Bioavailability of trace metals and rare earth elements (REE) from the tropical soils of a coal mining area. Sci. Total Environ. 2020, 717, 134484. [Google Scholar] [CrossRef]
- Li, G.; Lu, N.; Wei, Y.; Zhu, D. Relationship between Heavy Metal Content in Polluted Soil and Soil Organic Matter and pH in Mining Areas. IOP Conf. Ser. Mater. Sci. Eng. 2018, 394, 052081. [Google Scholar] [CrossRef]
- Zhou, S.; Hursthouse, A.; Chen, T. Pollution Characteristics of Sb, As, Hg, Pb, Cd, and Zn in Soils from Different Zones of Xikuangshan Antimony Mine. J. Anal. Methods Chem 2019, 2019, 2754385. [Google Scholar] [CrossRef]
- Burger, A.; Lichtscheidl-Schultz, I. Strontium in the environment: Review about reactions of plants towards stable and radioactive strontium isotopes. Sci. Total Environ. 2018, 653, 1458–1512. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace Elements in Terrestrial Environments; Springer: New York, NY, USA, 2001. [Google Scholar]
- Moreno-Jimenez, E.; Peñalosa, J.M.; Manzano, R.; Carpena-Ruiz, R.O.; Gamarra, R.; Esteban, E. Heavy metals distribution in soils surrounding an abandoned mine in NW Madrid (Spain) and their transference to wild flora. J. Hazard. Mater. 2009, 162, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Loell, M.; Albrecht, C.; Felix-Henningsen, P. Rare earth elements and relation between their potential bioavailability and soil properties, Nidda catchment (Central Germany). Plant Soil 2011, 349, 303–317. [Google Scholar] [CrossRef]
- Oyarzun, R.; Lillo, J.; López-García, J.A.; Esbrí, J.M.; Cubas, P.; Llanos, W.; Higueras, P. The Mazarrón Pb–(Ag)–Zn mining district (SE Spain) as a source of heavy metal contamination in a semiarid realm: Geochemical data from mine wastes, soils, and stream sediments. J. Geochem. Explor. 2011, 109, 113–124. [Google Scholar] [CrossRef]
- Martínez-Martínez, S.; Acosta, J.A.; Faz Cano, A.; Carmona, D.M.; Zornoza, R.; Cerda, C. Assessment of the lead and zinc contents in natural soils and tailing ponds from the Cartagena-La Unión mining district, SE Spain. J. Geochem. Explor. 2013, 124, 166–175. [Google Scholar] [CrossRef]
- Alvarez-Rogel, J.e.; Penalver-Alcala’, A.; Jim’enez-Carceles’, F.J.; Tercero, M.C.; Gonzalez-Alcaraz, M.N. Evidence supporting the value of spontaneous vegetation for phytomanagement of soil ecosystem functions in abandoned metal(loid) mine tailings. Catena 2021, 201, 105191. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton,, FL, USA, 2010. [Google Scholar] [CrossRef]
- Ha, N.T.H.; Ha, N.T.; Nga, T.T.H.; Minh, N.N.; Anh, B.T.K.; Hang, N.T.A.; Duc, N.A.; Nhuan, M.T.; Kim, K.-W. Uptake of arsenic and heavy metals by native plants growing near Nui Phao multi-metal mine, northern Vietnam. Appl. Geochem. 2019, 108, 104368. [Google Scholar] [CrossRef]
- Mahdavian, K.; Ghaderian, S.M.; Torkzadeh-Mahani, M. Accumulation and phytoremediation of Pb, Zn, and Ag by plants growing on Koshk lead–zinc mining area, Iran. J. Soils Sediments 2017, 17, 1310–1320. [Google Scholar] [CrossRef]
- Hasnaoui, S.E.; Fahr, M.; Keller, C.; Levard, C.; Angeletti, B.; Chaurand, P.; Triqui, Z.E.A.; Guedira, A.; Rhazi, L.; Colin, F.; et al. Screening of Native Plants Growing on a Pb/Zn Mining Area in Eastern Morocco: Perspectives for Phytoremediation. Plants 2020, 9, 1458. [Google Scholar] [CrossRef]
- Sinha, V.; Pakshirajan, K.; Chaturvedi, R. Chromium tolerance, bioaccumulation and localization in plants: An overview. J. Environ. Manag. 2018, 206, 715–730. [Google Scholar] [CrossRef]
- Wiche, O.; Heilmeier, H. Germanium (Ge) and rare earth element (REE) accumulation in selected energy crops cultivated on two different soils. Miner. Eng. 2016, 92, 208–215. [Google Scholar] [CrossRef]
- Wen-Shen, L. Phytoextraction of rare earth elements from ion-adsorption mine tailings by Phytolacca americana: Effects of organic material and biochar amendment. J. Clean. Prod. 2020, 275, 122959. [Google Scholar] [CrossRef]
- Saatz, J.; Vetterlein, D.; Mattusch, J.; Otto, M.; Daus, B. The influence of gadolinium and yttrium on biomass production and nutrient balance of maize plants. Environ. Pollut. 2015, 204, 32–38. [Google Scholar] [CrossRef]
- Dinh, T.; Dobo, Z.; Kovacs, H. Phytomining of rare earth elements—A review. Chemosphere 2022, 297, 134259. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.W. Integration of cerium chemical forms and subcellular distribution to understand cerium tolerance mechanism in the rice seedlings. Environ. Sci. Pollut. Control. Ser. 2017, 24, 16336–16343. [Google Scholar] [CrossRef]
- Ding, S.; Liang, T.; Zhang, C.; Yan, J.; Zhang, Z. Accumulation and fractionation of rare earth elements (REEs) in wheat: Controlled by phosphate precipitation, cell wall absorption and solution complexation. J. Exp. Bot. 2005, 56, 2765–2775. [Google Scholar] [CrossRef] [PubMed]
- Staff, S.S.D. Soil Survey Manual; United States Department of Agriculture: Washington DC, USA, 1993. [Google Scholar]
- Beretta, A.N.; Silbermann, A.V.; Paladino, L.; Torres, D.; Bassahun, D.; Musselli, R.; García-Lamohte, A. Soil texture analyses using a hydrometer: Modification of the Bouyoucos method. Cienc. Investig. Agrar. 2014, 41, 263–271. [Google Scholar] [CrossRef]
- Chapman, H.D. Cation-Exchange Capacity. In Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 1965; pp. 891–901. [Google Scholar] [CrossRef]
- Risser, J.A.; Baker, D.E. Testing Soils for Toxic Metals. In Soil Testing and Plant Analysis; American Society of Agronomy: Madison, WI, USA, 1990; pp. 275–298. [Google Scholar] [CrossRef]
- Crock, J.G.; Severson, R.C. Four Reference Soil and Rock Samples for Measuring Element Availability in the Western Energy Regions; U.S. Department of the Interior: Washington DC, USA, 1980. [Google Scholar]
- Terán-Mita, T.A.; Faz, A.; Salvador, F.; Arocena, J.M.; Acosta, J.A. High altitude artisanal small-scale gold mines are hot spots for Mercury in soils and plants. Environ. Pollut. 2013, 173, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Barriga, F.; Faz, Á.; Acosta, J.A.; Soriano-Disla, M.; Martínez-Martínez, S.; Zornoza, R. Use of Piptatherum miliaceum for the phytomanagement of biochar amended Technosols derived from pyritic tailings to enhance soil aggregation and reduce metal(loid) mobility. Geoderma 2017, 307, 159–171. [Google Scholar] [CrossRef]
- Acosta, J.A.; Abbaspour, A.; Martínez, G.R.; Martínez-Martínez, S.; Zornoza, R.; Gabarrón, M.; Faz, A. Phytoremediation of mine tailings with Atriplex halimus and organic/inorganic amendments: A five-year field case study. Chemosphere 2018, 234, 71–78. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Martínez-Martínez, S.M. Niveles de Fondo y de Referencia de Metales Pesados en Suelos Desarrollados de Material Parental Volcánico, Metamórfico y Sedimentario en la Región de Murcia Espana; En la Universidad Politécnica de Cartagena: Cartagena, Spain, 2009. [Google Scholar]

| Horizon | Depth | pH | EC * | OC * | TN * | CEC * | Carbonates | Sand | Silt | Clay | Soil Texture | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cm | dS m−1 | % | % | cmol+ kg−1 | % | |||||||
| Profile 1 | A1 | 0–10 | 7.67 | 0.347 | 3.1 | 0.24 | 9.36 | 2.38 | 61 | 18 | 21 | Sandy Clay Loam |
| A2 | 10–20 | 8.13 | 0.180 | 1.8 | 0.15 | 8.73 | 8.05 | 62 | 12 | 26 | Sandy Clay Loam | |
| C/R | 20–50 | 8.26 | 0.246 | 0.9 | 0.09 | 8.3 | 10.64 | 66 | 10 | 24 | Sandy Clay Loam | |
| Profile 2 | A | 0–10 | 8.04 | 0.591 | 2.4 | 0.2 | 8.9 | 0.84 | 62 | 10 | 28 | Sandy Clay Loam |
| AC | 10–20 | 8.18 | 0.480 | 1.7 | 0.15 | 9.87 | 15.02 | 66 | 12 | 22 | Sandy Clay Loam | |
| C/R | 20–40 | 8.18 | 0.503 | 0.8 | 0.1 | 10.01 | 19.71 | 78 | 8 | 14 | Sandy Loam | |
| Profile 3 | A | 0–20 | 7.92 | 0.298 | 3.1 | 0.19 | 9.48 | 6.36 | 68 | 10 | 22 | Sandy Clay Loam |
| C1 | 20–35 | 8.26 | 0.235 | 0.9 | 0.1 | 9.63 | 32.64 | 59 | 9 | 32 | Sandy Clay Loam | |
| C2 | 35–50 | 7.97 | 0.773 | 0.8 | 0.06 | 9.28 | 43.05 | 49 | 9 | 42 | Sandy Clay | |
| Unit S1 | Surface | 0–15 | 7.86 b | 0.168 a | - | - | - | - | 64 | 12 | 24 | Sandy Clay Loam |
| Sub-surface | 15–30 | 7.97 b | 0.138 a | - | - | - | - | 62 | 13 | 25 | Sandy Clay Loam | |
| Unit S2 | Surface | 0–15 | 8.13 a | 0.190 a | - | - | - | - | 62 | 15 | 23 | Sandy Clay Loam |
| Sub-surface | 15–30 | 8.27 a | 0.193 a | - | - | - | - | 57 | 14 | 29 | Sandy Clay Loam | |
| Unit S1 | Unit S2 | F Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Surface Soil | Subsurface Soil | Mean | Surface soil | Subsurface Soil | Mean | Unit | Depth | Unit × Depth | |
| V | 81.1 ± 10.6 ab | 86.8 ± 5.2 a | 83.9 A | 76.1 ± 9.7 ab | 68.5 ± 13.5 b | 72.3 B | 5.87 * | 0.04 ns | 1.93 ns |
| Cr | 102 ± 47.2 a | 119 ± 52 a | 110 A | 92.2 ± 18.2 a | 80.9 ± 15.6 a | 86.5 A | 3.00 ns | 0.04 ns | 1.01 ns |
| Mn | 369 ± 121 b | 287 ± 192 b | 328 B | 795 ± 254 a | 593 ± 308 ab | 694.5 A | 8.48 * | 1.26 ns | 0.23 ns |
| Co | 4.70 ± 0.42 c | 4.50 ± 1.37 c | 4.60 B | 13.4 ± 3.6 a | 10.0 ± 1.4 b | 11.7 A | 32.34 ** | 2.09 ns | 1.66 ns |
| Ni | 28.5 ± 3.76 b | 40.6 ± 19.8 ab | 34.5 A | 45.3 ± 14.0 a | 30.9 ± 3.6 b | 38.1 A | 0.46 ns | 0.04 ns | 6.29 * |
| Cu | 46.5 ± 14.2 b | 33.1 ± 13.7 b | 39.8 B | 82.2 ± 28.7 a | 59.9 ± 21.3 ab | 71.1 A | 8.37 * | 2.75 ns | 0.17 ns |
| As | 130 ± 12 a | 131 ± 54 a | 131 A | 153 ± 35 a | 144 ± 41 a | 149 A | 0.99 ns | 0.05 ns | 0.07 ns |
| Se | 1.87 ± 1.12 ab | 2.44 ± 1.04 a | 2.16 A | 1.42 ± 0.39 b | 1.22 ± 0.23 b | 1.32 B | 7.95 * | 0.42 ns | 1.72 ns |
| Sr | 130 ± 64 b | 140 ± 79 b | 135 B | 484 ± 106 a | 434 ± 93 a | 459 A | 50.13 ** | 0.19 ns | 0.43 ns |
| Y | 22.7 ± 2.9 ab | 24.92 ± 9.99 a | 23.8 A | 16.8 ± 3.8 bc | 13.1 ± 1.5 c | 14.9 B | 17.99 ** | 0.13 ns | 1.91 ns |
| Zr | 4.72 ± 0.88 b | 5.20 ± 2.14 b | 4.96 B | 16.1 ± 2.1 a | 15.4 ± 3.2 a | 15.8 A | 70.04 ** | 0.03 ns | 0.19 ns |
| Mo | 3.39 ± 0.94 a | 3.48 ± 0.92 a | 3.44 A | 2.82 ± 1.11 a | 1.63 ± 0.38 b | 2.22 B | 9.80 * | 1.97 ns | 2.78 ns |
| Cd | 15.4 ± 6.2 a | 12.8 ± 6.7 ab | 14.1 A | 7.21 ± 2.08 bc | 6.13 ± 2.48 c | 6.67 B | 15.31 * | 0.95 ns | 0.17 ns |
| In | 1.62 ± 0.81 a | 1.18 ± 0.86 ab | 1.40 A | 0.93 ± 0.34 ab | 0.81 ± 0.39 b | 0.87 A | 4.32 * | 1.17 ns | 0.42 ns |
| Sn | 5.93 ± 1.48 ab | 7.80 ± 2.63 a | 6.87 A | 6.55 ± 4.96 a | 2.84 ± 0.86 b | 4.70 A | 2.07 ns | 0.37 ns | 3.42 ns |
| Sb | 2.99 ± 1.05 a | 3.12 ± 0.42 a | 3.06 A | 5.00 ± 1.81 a | 3.39 ± 0.91 a | 4.20 A | 1.92 ns | 0.80 ns | 1.11 ns |
| La | 41.9 ± 5.1 b | 66.4 ± 6.6 a | 54.1 A | 28.9 ± 4.1 c | 23.6 ± 4.0 d | 26.3 B | 153.59 ** | 18.22 ** | 43.93 ** |
| Ce | 92.6 ± 9.3 b | 144 ± 14 a | 118 A | 65.9 ± 9.1 c | 56.3 ± 9.7 c | 61.2 B | 136.11 ** | 18.54 ** | 39.06 ** |
| Pr | 12.4 ± 1.5 b | 18.2 ± 1.64 a | 15.3 A | 8.43 ± 1.21 c | 7.34 ± 1.27 c | 7.89 B | 137.35 ** | 13.46 * | 29.03 ** |
| Bi | 0.37 ± 0.17 ab | 0.20 ± 0.01 b | 0.29 A | 0.56 ± 0.27 a | 0.38 ± 0.21 ab | 0.47 A | 3.19 ns | 2.44 ns | 0.01 ns |
| Th | 25.5 ± 3.5 b | 47.9 ± 4.8 a | 36.7 A | 18.4 ± 3.8 c | 16.2 ± 2.7 c | 17.3 B | 109.27 ** | 29.49** | 43.80 ** |
| Ge | 4.57 ± 0.09 b | 5.31 ± 0.19 a | 4.94 A | 3.10 ± 0.57 c | 2.56 ± 0.52 d | 2.84 B | 112.59 ** | 0.28 ns | 10.41* |
| Rb | 61.2 ± 13.61 a | 70.8 ± 4.3 a | 66.0 A | 71.5 ± 21.8 a | 76.0 ± 29.5 a | 73.8 A | 0.49 ns | 0.40 ns | 0.05 ns |
| Cs | 6.63 ± 1.51 b | 7.14 ± 0.54 b | 6.89 B | 38.7 ± 20.1 a | 46.5 ± 19.6 a | 42.6 A | 18.05 ** | 0.25 ns | 0.19 ns |
| U | 8.32 ± 1.62 a | 7.52 ± 1.78 a | 7.92 A | 4.45 ± 0.65 b | 4.08 ± 0.74 b | 4.26 B | 53.67 ** | 1.35 ns | 0.19 ns |
| Fe | 63,354 ± 6764 a | 53,283 ± 4005 a | 58,318 A | 40,828 ± 6003 b | 35,988 ± 8450 b | 38,408 B | 34.74 ** | 4.85 * | 0.60 ns |
| Pb | 2985 ± 1655 a | 1704 ± 1612 a | 2345 A | 2613 ± 920 a | 2443 ± 1134 a | 2528 A | 0.10 ns | 1.54 ns | 0.91 ns |
| Zn | 2533 ± 440 a | 2338 ± 289 a | 2436 A | 1283 ± 300 b | 1091 ± 366 b | 1187 B | 51.54 ** | 1.23 ns | 0.00 ns |
| Al | 44,179 ± 1791 a | 52,831 ± 3089 a | 48,505 A | 29,763 ± 5490 b | 26,491 ± 6692 b | 28,127 B | 58.60 ** | 1.02 ns | 5.02 * |
| Unit S1 | Unit S2 | F Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Surface Soil | Subsurface Soil | Mean | Surface Soil | Subsurface Soil | Mean | Unit | Depth | Unit × Depth | |
| V | 17.9 ± 6.5 b | 12.3 ± 10.4 b | 15.1 B | 81.5 ± 25.3 a | 72.5 ± 16.9 a | 77.0 A | 43.74 ** | 0.61 ns | 0.03 ns |
| Cr | 62.6 ± 69.0 ab | 10.5 ± 2.3 b | 36.6 A | 54.2 ± 56.5 ab | 136 ± 115 a | 95.3 A | 2.14 ns | 0.14 ns | 2.80 ns |
| Mn | 39.2 ± 7.1 a | 39.6 ± 12.1 a | 39.4 A | 44.4 ± 34.9 a | 26.0 ± 15.7 a | 35.2 A | 0.13 ns | 0.59 ns | 0.65 ns |
| Co | 221 ± 122 a | 228 ± 129 a | 225 A | 417 ± 318 a | 324 ± 197 a | 371 A | 1.58 ns | 0.14 ns | 0.19 ns |
| Ni | 277 ± 60 a | 364 ± 270 a | 321 A | 434 ± 117 a | 373 ± 163 a | 403 A | 1.15 ns | 0.03 ns | 0.92 ns |
| Cu * | 4.14 ± 1.98 ab | 2.17 ± 1.59 b | 3.16 A | 5.53 ± 2.31 a | 4.15 ± 1.51 ab | 4.84 A | 3.23 ns | 3.20 ns | 0.10 ns |
| As | 20.4 ± 2.1 b | 10.1 ± 4.8 b | 15.3 B | 80.4 ± 24.1 a | 60.5 ± 24.6 a | 70.5 A | 28.40 ** | 2.12 ns | 0.21 ns |
| Se | 30.8 ± 13.8 a | 20.8 ± 12.2 ab | 25.8 A | 14.0 ± 10.3 b | 12.4 ± 11.4 b | 13.2 B | 5.06 ns | 1.08 ns | 0.58 ns |
| Sr * | 7.83 ± 1.52 b | 9.26 ± 1.75 b | 8.54 B | 11.2 ± 2.3 ab | 15.2 ± 5.0 a | 13.2 A | 7.45 * | 2.49 ns | 0.55 ns |
| Y | 1.28 ± 0.35 a | 0.90 ± 0.53 a | 1.09 A | 0.33 ± 0.14 b | 0.24 ± 0.20 b | 0.28 B | 36.19 * | 3.05 ns | 1.17 ns |
| Zr | 6.93 ± 2.95 a | 6.67 ± 2.48 a | 6.80 A | 10.2 ± 4.8 a | 7.77 ± 6.32 a | 8.99 A | 0.78 ns | 0.30 ns | 0.20 ns |
| Mo | 13.8 ± 1.7 a | 10.5 ± 5.7 a | 12.1 A | 17.3 ± 7.3 a | 17.3 ± 4.9 a | 17.3 A | 3.24 ns | 0.33 ns | 0.32 ns |
| Cd * | 3.09 ± 1.61 a | 2.17 ± 1.63 a | 2.63 A | 0.83 ± 0.42 a | 0.61 ± 0.45 a | 0.72 B | 19.15 ** | 1.70 ns | 0.65 ns |
| In | 3.80 ± 1.69 a | 1.84 ± 1.62 a | 2.82 A | 2.17 ± 2.65 a | 1.61 ± 1.43 a | 1.89 A | 0.89 ns | 1.61 ns | 0.49 ns |
| Sn | nd | nd | nd | nd | nd | nd | - | - | - |
| Sb | 64.5 ± 22.3 b | 44.6 ± 39.8 b | 54.6 B | 143 ± 59 ab | 180 ± 95 a | 162 A | 9.72 * | 0.06 ns | 0.69 ns |
| La | 269 ± 120 a | 105 ± 57 b | 187 A | 223 ± 95 ab | 142 ± 79 ab | 182 A | 0.01 ns | 7.85 ns | 0.91 ns |
| Ce * | 0.62 ± 0.05 a | 0.32 ± 0.19 a | 0.47 A | 0.82 ± 0.46 a | 0.60 ± 0.45 a | 0.71 A | 1.54 ns | 1.74 ns | 0.03 ns |
| Pr | 88.5 ± 38.0 a | 36.8 ± 21.1 a | 62.6 A | 70.4 ± 30.2 a | 52.1 ± 39.1 a | 61.3 A | 0.01 ns | 4.46 ns | 1.02 ns |
| Bi | 8.40 ± 3.5 a | 5.17 ± 5.76 a | 6.79 A | 11.1 ± 10.7 a | 6.38 ± 5.35 a | 8.75 A | 0.27 ns | 1.11 ns | 0.04 ns |
| Th | 13.6 ± 1 a | 10.6 ± 4.02 a | 12.1 A | 16.2 ± 7.9 a | 12.0 ± 6.96 a | 14.1 A | 0.36 ns | 1.23 ns | 0.03 ns |
| Ge | 25.9 ± 7.7 a | 15.0 ± 8.42 a | 20.5 A | 51.0 ± 37.8 a | 21.6 ± 17.5 a | 36.3 A | 1.57 ns | 2.55 ns | 0.54 ns |
| Rb | 19.7 ± 6.3 b | 21.8 ± 1.84 b | 20.8 B | 58.6 ± 24.3 a | 34.3 ± 18.00 a | 46.4 A | 7.92 * | 1.48 ns | 2.11 ns |
| Cs | ndb | ndb | nd B | 0.63 ± 0.90 ab | 1.29 ± 0.55 a | 0.96 A | 9.19 * | 1.09 ns | 1.09 ns |
| U | 9.82 ± 7.07 a | 10.1 ± 14.5 a | 9.97 A | 6.38 ± 2.54 a | 7.56 ± 4.31 a | 6.97 A | 0.90 ns | 0.06 ns | 0.02 ns |
| Fe * | 9.78 ± 2.55 a | 6.18 ± 1.93 ab | 7.98 A | 8.20 ± 4.05 a | 4.93 ± 1.10 b | 6.56 A | 1.07 ns | 6.31 ns | 0.01 ns |
| Pb * | 581 ± 351 a | 329 ± 33 a | 455 A | 346 ± 208 a | 337 ± 228 a | 342 A | 0.83 ns | 1.10 ns | 0.96 ns |
| Zn * | 56.9 ± 13.0 a | 36.3 ± 26.7 a | 46.6 A | 57.5 ± 30.6 a | 50.1 ± 33.7 a | 53.8 A | 0.24 ns | 0.92 ns | 0.20 ns |
| Al * | 7.62 ± 7.41 a | 2.59 ± 3.30 ab | 5.11 A | 4.35 ± 2.33 ab | 1.67 ± 0.93 b | 3.01 A | 1.74 ns | 5.87 ns | 0.54 ns |
| Unit S1 | Unit S2 | F Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Surface Soil | Subsurface Soil | Mean | Surface Soil | Subsurface Soil | Mean | Unit | Depth | Unit × Depth | |
| V | 5.39 ± 1.01 b | 4.29 ± 0.76 b | 4.84 B | 25.1 ± 7.6 a | 19.6 ± 7.1 a | 22.4 A | 31.51 ** | 1.10 ns | 0.49 ns |
| Cr | 18.8 ± 5.7 a | 11.0 ± 1.5 b | 14.9 A | 13.9 ± 2.0 ab | 11.4 ± 2.6 b | 12.7 A | 1.51 ns | 11.9 * | 2.08 ns |
| Mn | 2842 ± 3378 a | 157 ± 145 b | 1500 A | 354 ± 316 b | 110 ± 66 b | 232 B | 4.83 * | 16.17 ** | 3.74 ns |
| Co | 14.5 ± 16.4 a | 1.47 ± 0.33 b | 8.03 A | 4.15 ± 2.59 b | 1.83 ± 0.63 b | 2.99 A | 1.79 ns | 17.57 ** | 3.56 ns |
| Ni | 48.5 ± 48.2 a | 10.6 ± 0.5 bc | 29.5 A | 18.6 ± 4.3 b | 10.6 ± 2.6 c | 14.6 A | 3.17 ns | 20.65 ** | 2.70 ns |
| Cu | 217 ± 191 a | 12.0 ± 8.3 a | 114 A | 70.4 ± 39.4 a | 34.5 ± 31.6 a | 52.5 A | 0.34 ns | 1.85 ns | 0.03 ns |
| As | 20.0 ± 2.9 c | 14.9 ± 3.6 c | 17.5 B | 108 ± 20 a | 72.3 ± 15.6 b | 90.4 A | 86.43 ** | 6.95 * | 3.91 ns |
| Se | 3.54 ± 3.06 ab | 2.32 ± 2.01 ab | 2.93 A | 3.83 ± 0.73 a | 1.61 ± 2.07 b | 2.72 A | 0.05 ns | 3.55 ns | 0.30 ns |
| Sr | 596 ± 138 bc | 494 ± 192 c | 545 B | 1147 ± 339 a | 986 ± 430 ab | 1066 A | 10.24 * | 0.65 ns | 0.03 ns |
| Y | 13.7 ± 13.2 a | 3.84 ± 2.5 bc | 8.18 A | 8.84 ± 4.78 ab | 2.56 ± 1.3 c | 5.70 A | 1.14 ns | 14.40 * | 0.02 ns |
| Zr | 2.54 ± 1.34 a | 4.16 ± 4.18 a | 3.35 A | 4.27 ± 1.65 a | 2.30 ± 1.11 a | 3.28 A | 0.01 ns | 0.03 ns | 3.47 ns |
| Mo | 10.3 ± 1.9 b | 12.11 ± 4.24 b | 11.2 B | 16.1 ± 1.8 b | 22.8 ± 8.5 a | 19.5 A | 9.08 * | 2.40 ns | 0.83 ns |
| Cd | 10.2 ± 7.2 a | 3.84 ± 1.05 a | 7.05 A | 9.75 ± 8.12 a | 2.08 ± 1.89 a | 5.91 A | 1.18 ns | 1.74 ns | 0.99 ns |
| In | 0.05 ± 0.43 a | nd | 0.25 A | 0.13 ± 0.24 b | 0.02 ± 0.07 b | 0.08 A | 2.51 ns | 8.24 * | 3.40 ns |
| Sn | nd | nd | ndA | 0.07 ± 0.18 | nd | 0.03 A | 0.40 ns | 0.40 ns | 0.40 ns |
| Sb | 30.8 ± 6.2 b | 25.6 ± 9.2 b | 28.2 B | 108 ± 41 a | 141 ± 75 a | 125 A | 58.13 * | 0 ns | 1.37 ns |
| La | 2.65 ± 0.52 b | 2.59 ± 1.63 b | 2.62 B | 10.7 ± 5.4 a | 4.0 ± 3.04 b | 7.38 A | 6.41 * | 3.30 ns | 3.18 ns |
| Ce | 7.96 ± 3.59 b | 5.58 ± 3.8 b | 6.77 B | 23.4 ± 11.6 a | 8.97 ± 6.16 b | 16.2 A | 5.46 * | 4.36 ns | 2.25 ns |
| Pr | 1.91 ± 1.79 ab | 0.62 ± 0.64 b | 1.26 A | 3.44 ± 1.69 a | 1.15 ± 0.88 b | 2.30 A | 2.45 ns | 7.34 * | 0.58 ns |
| Bi | nd | nd | nd | nd | nd | nd | - | - | - |
| Th | 1.54 ± 0.98 a | 2.08 ± 1.54 a | 1.81 A | 2.92 ± 1.17 a | 1.67 ± 1.72 a | 2.29 A | 0.48 ns | 0.27 ns | 1.64 ns |
| Ge | 0.58 ± 0.54 ab | ndb | 0.29 A | 1.96 ± 1.81 a | 0.49 ± 0.81 b | 1.23 A | 2.44 ns | 2.92 ns | 0.53 ns |
| Rb | 10.2 ± 2.9 b | 10.7 ± 2.4 b | 10.4 B | 29.0 ± 12.7 a | 16.7 ± 7.9 b | 22.8 A | 7.45 * | 1.68 ns | 1.99 ns |
| Cs | 0.52 ± 0.68 b | 0.10 ± 0.17 b | 0.31 B | 2.77 ± 1.32 a | 2.49 ± 1.43 a | 2.63 A | 15.19 * | 0.35 ns | 0.01 ns |
| U | 6.4 ± 3.77 a | 2.72 ± 2.63 a | 4.56 A | 4.46 ± 0.86 a | 3.72 ± 2.79 a | 4.09 A | 0.16 ns | 3.50 ns | 1.54 ns |
| Fe | 2418 ± 1207 ab | 1665 ± 2171 ab | 2042 A | 2870 ± 1742 a | 1140 ± 703 b | 2005 A | 0 ns | 3.09 ns | 0.48 ns |
| Pb | 850 ± 603 a | 324 ± 94 a | 587 A | 2209 ± 2051 a | 629 ± 528 a | 1419 A | 0.71 ns | 3.83 ns | 0.20 ns |
| Zn | 2231 ± 2361 a | 434 ± 298 bc | 1333 A | 951 ± 731 ab | 344 ± 198 c | 648 A | 1.02 ns | 9.55 * | 0.58 ns |
| Al | 2000 ± 2645 a | 666 ± 1154 a | 1333 A | 714 ± 951 a | 495 ± 763 a | 604 A | 0.1 ns | 0.43 ns | 0.39 ns |
| V | Cr | Mn * | Co | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root | Shoot | Mean | Root | Shoot | Mean | Root | Shoot | Mean | Root | Shoot | Mean | ||
| Unit S1 | S. o. | 2263 ± 2530 ab | 849 ± 256 bcd | 1556 AB | 976 ± 317 c | 1610 ± 550 abc | 1293 A | 124 ± 36 ab | 220 ± 42 a | 172 A | 659 ± 109 a | 439 ± 155 abc | 549 A |
| S. t. | 964 ± 230 bc | 729 ± 324 bcde | 846 BC | 807 ± 69 c | 3654 ± 1511 ab | 2231 A | 22.3 ± 2.6 def | 52.5 ± 2.1 bcd | 37.4 C | 201 ± 49 c–f | 157 ± 55 d–h | 179 BC | |
| P. m. | 2489 ± 687 a | 1036 ± 164 abc | 1762 A | 5030 ± 6660 abc | 6617 ± 4636 a | 5824 A | 68.4 ± 55.9 bcd | 24.3 ± 6.9 de | 46.4 C | 424 ± 426 abc | 213 ± 37 b–f | 318 B | |
| Unit S2 | S. o. | 530 ± 212 cde | 399 ± 158 de | 464 C | 1212 ± 923 c | 1301 ± 587 bc | 1256 A | 70.1 ± 36.9 bc | 130 ± 15 b | 100 B | 176 ± 128 d–h | 129 ± 77 e–h | 153 C |
| S. t. | 199 ± 1264 ab | 293 ± 99 ef | 1142 BC | 1783 ± 831 abc | 3633 ± 1272 ab | 2708 A | 30.9 ± 19.1 cde | 23.0 ± 5.7 def | 26.9 C | 283 ± 130 b–e | 74.7 ± 15 hi | 179 C | |
| P. m. | 1855 ± 1272 ab | 138 ± 47 f | 997 C | 9184 ± 11,567 a | 1449 ± 302 bc | 5317 A | 19.8 ± 20.9 ef | 8.9 ± 1.3 f | 14.4 E | 493 ± 205 ab | 48.3 ± 17 i | 270 BC | |
| A. h. | 607 ± 285 cde | 622 ± 99 cde | 614 C | 1437 ± 366 bc | 1037 ± 190 bc | 1237 A | 17.1 ± 6.3 ef | 20.8 ± 5.7 ef | 18.9 DE | 101 ± 45 ghi | 104 ± 5 fgh | 102 C | |
| Ni | Cu * | As * | Se | ||||||||||
| Root | Shoot | Mean | Root | Shoot | Mean | Root | Shoot | Mean | Root | Shoot | Mean | ||
| Unit S1 | S. o. | 2278 ± 708 ab | 2512 ± 782 ab | 2395 A | 10.6 ± 6.08 abc | 15.5 ± 5.1 a | 13.08 A | 2.11 ± 1.5 bcd | 0.60 ± 0.2 ef | 1.35 BC | 101 ± 64 ab | 89.4 ± 12 ab | 95.3 A |
| S. t. | 704 ± 82 bcd | 918 ± 319 a–d | 811 A | 6.8 ± 4.5 bcd | 3.54 ± 0.53 def | 5.22 BC | 2.44 ± 0.6 bc | 0.60 ± 0.1 def | 1.52 B | 41.6 ± 17 b–e | 40.5 ± 15 b–e | 41.1 B | |
| P. m. | 2124 ± 1676 abc | 2578 ± 1610 a | 2351 A | 12.13 ± 6.6 ab | 2.95 ± 0.66 efg | 7.54 B | 2.37 ± 1 a | 1.39 ± 0.2 be | 1.25 A | 99.3 ± 39 a | 58.7 ± 4 a–e | 79.1 A | |
| Unit S2 | S. o. | 922 ± 533 a–d | 945 ± 493 abcd | 933 A | 3.77 ± 0.99 def | 5.60 ± 1.27 cde | 4.68 BC | 0.95 ± 0.40 cde | 0.34 ± 0.1 fg | 0.65 C | 34.3 ± 1 cde | 45.7 ± 19 a–e | 40.0 B |
| S. t. | 1039 ± 407 a–d | 6571 ± 1564 ab | 3805 A | 4.01 ± 1.73 def | 2.56 ± 0.37 fg | 3.28 CD | 4.44 ± 3.6 b | 0.42 ± 0.1 fg | 2.43 B | 89.8 ± 72 abc | 39.9 ± 10 b–e | 64.9 B | |
| P. m. | 2921 ± 1620 a | 393 ± 53 d | 1657 A | 4.44 ± 2.08 def | 1.86 ± 0.57 g | 3.15 D | 3.20 ± 2.7 bc | 0.19 ± 0.03 g | 1.70 BC | 76.8 ± 53 a–e | 26.3 ± 11 e | 51.6 B | |
| A. h. | 674 ± 356 cd | 781 ± 115 abcd | 728 A | 10.48 ± 5.18 abc | 9.05 ± 2.38 abc | 9.77 A | 0.33 ± 0.77 c–e | 0.55 ± 0.07 ef | 0.94 BC | 27.6 ± 9 de | 67 ± 41 abc | 47.3 B | |
| Sr * | Y | Zr | Mo | ||||||||||
| Root | Shoot | Mean | Root | Shoot | Mean | Root | Shoot | Mean | Root | Shoot | Mean | ||
| Unit S1 | S. o. | 72.4 ± 41.8 bc | 157 ± 8 a | 115 A | 1160 ± 1057 ab | 519 ± 27 bcd | 839 AB | 200 ± 182 ab | 100 ± 51 a–d | 150 AB | 290 ± 218 cd | 521 ± 523 bcd | 405 CD |
| S. t. | 5.9 ± 0.51 f | 6.43 ± 1.22 f | 6.19 D | 946 ± 624 abc | 236 ± 73 de | 591 BC | 30.7 ± 0.7 ef | 56.7 ± 9 de | 43.7 D | 53.1 ± 20 e | 76.6 ± 2 e | 64.8 E | |
| P. m. | 44.2 ± 42.22 cd | 13.8 ± 1.6 e | 29.0 C | 2186 ± 1189 a | 517 ± 92 bcd | 1352 A | 100 ± 73 bcd | 68.4 ± 4 cde | 84.4 BC | 179 ± 18 d | 232 ± 43 cd | 206 D | |
| Unit S2 | S. o. | 120 ± 3 ab | 171 ± 11 a | 145 A | 248 ± 95 de | 146 ± 97 e | 206 DE | 132 ± 22 abc | 95.8 ± 45 a–d | 114 AB | 191 ± 71 d | 1289 ± 586 a | 740 BC |
| S. t. | 46.4 ± 26.3 c | 15.1 ± 1.4 e | 30.8 C | 926 ± 629 abc | 122 ± 27 ef | 524 CD | 188 ± 82 ab | 54.3 ± 32 de | 121 BC | 442 ± 44 bc | 653 ± 193 ab | 548 AB | |
| P. m. | 48.0 ± 21.5 c | 17.6 ± 4.4 de | 32.8 C | 527 ± 561 cde | 47.6 ± 24 f | 286 E | 169 ± 86 abc | 21.7 ± 5 f | 95.6 CD | 792 ± 183 ab | 1138 ± 503 a | 965 A | |
| A. h. | 61.7 ± 16.5 bc | 45.0 ± 3.7 c | 53.4 B | 191 ± 91 de | 203 ± 25 de | 197 DE | 191 ± 79 ab | 198 ± 29 a | 194 A | 661 ± 124 ab | 665 ± 221 ab | 663 AB | |
| Cd | In | Sn | Sb | ||||||||||
| Root | Shoot | Mean | Root | Shoot | Mean | Root | Shoot | Mean | Root | Shoot | Mean | ||
| Unit S1 | S. o. | 1290 ± 501 bc | 3487 ± 2788 b | 2389 A | 25.4 ± 16 bc | 12.2 ± 9 cde | 18.8 B | 104 ± 37 bcd | 111 ± 28 bc | 108 BC | 309 ± 291 cde | 157 ± 44 ef | 233 C |
| S. t. | 164 ± 55 de | 71 ± 4 ef | 117 C | 27.5 ± 7 b | 7.6 ± 3 ef | 17.6 BC | 157 ± 17 ab | 96.4 ± 16 bcd | 126 AB | 64.2 ± 42 gh | 58.6 ± 21 gh | 61.4 D | |
| P. m. | 17,694 ± 1803 a | 240 ± 117 de | 8967 A | 573 ± 272 a | 28.7 ± 6 b | 301.2 A | 280 ± 144 a | 127 ± 17 bc | 204 A | 2528 ± 207 a | 256 ± 28 cde | 1392 A | |
| Unit S2 | S. o. | 1662 ± 1336 bc | 2931 ± 3735 bc | 2297 A | 8.2 ± 3 ef | 12.5 ± 4 b–e | 10.3 BC | 108 ± 34 bcd | 88.9 ± 40 cd | 98.8 CD | 626 ± 292 bc | 132 ± 85 efg | 379 BC |
| S. t. | 813 ± 972 cd | 213 ± 165 de | 513 B | 20.4 ± 16 be | 3.6 ± 0.6 f | 12.1 CD | 119 ± 68 bcd | 58.6 ± 11 de | 88.9 CD | 548 ± 270 bcd | 72.9 ± 32 fgh | 310 C | |
| P. m. | 260 ± 313 de | 26.5 ± 2 f | 143 C | 21.2 ± 12 bcd | 1 ± 0.1 g | 11.1 D | 112 ± 58 bcd | 38.5 ± 8 e | 75.3 D | 1170 ± 725 ab | 41.7 ± 18 h | 606 C | |
| A. h. | 1784 ± 397 bc | 1032 ± 397 bc | 1408 A | 13.3 ± 12 cde | 7.2 ± 1 ef | 10.2 BC | 106 ± 50 bcd | 135 ± 6 abc | 120 AC | 981 ± 593 ab | 203 ± 33 de | 592 AB | |
| La | Ce | Pr | Bi | ||||||||||
| Root | Shoot | Mean | Root | Shoot | Mean | Root | Shoot | Mean | Root | Shoot | Mean | ||
| Unit S1 | S. o. | 701 ± 570 bcd | 497 ± 48 bcd | 599 B | 979 ± 886 cde | 519 ± 120 cde | 749 BC | 201 ± 154 bc | 110.2 ± 12 dc | 156 BC | 29.5 ± 22 ab | 9.2 ± 6 cde | 19.3 BC |
| S. t. | 479 ± 129 bcd | 375 ± 124 cd | 427 BC | 1073 ± 475 bcd | 542 ± 186 cde | 807 BC | 209 ± 105 bc | 92.9 ± 28 cd | 151 BC | 80.2 ± 90 a | 12.2 ± 1 b–e | 46.2 AB | |
| P. m. | 2958 ± 2307 a | 734 ± 69 bc | 1846 A | 4145 ± 3072 a | 1307 ± 190 abc | 2726 A | 744 ± 501 a | 198 ± 27 bc | 471 A | 55.7 ± 14 a | 21.4 ± 4 abc | 38.5 A | |
| Unit S2 | S. o. | 451 ± 122 cd | 255 ± 128 de | 353 BC | 596 ± 263 cde | 329 ± 162 ef | 463 C | 125 ± 47 dc | 58.8 ± 30 de | 92.1 BC | 6.8 ± 4 de | 9.7 ± 5 cde | 8.3 C |
| S. t. | 1852 ± 1850 ab | 272 ± 84 de | 1062 B | 4409 ± 4982 ab | 461 ± 124 cde | 2435 AB | 706 ± 764 ab | 69.6 ± 21 cde | 388 AB | 11.1 ± 4 b–e | 6.4 ± 1 de | 8.7 C | |
| P. m. | 678 ± 545 bcd | 112 ± 46 e | 395 C | 1426 ± 1089 bcd | 161 ± 71 f | 793 C | 337 ± 279 bc | 26.9 ± 11 e | 182 C | 7.2 ± 6 e | 0 f | 3.6 D | |
| A. h. | 270 ± 148 de | 369 ± 70 cd | 320 BC | 403 ± 170 def | 435 ± 56 def | 419 C | 80.2 ± 42 cde | 83 ± 17 cd | 81.6 C | 6.2 ± 3 de | 14.9 ± 3 bcd | 10.6 C | |
| Th | Ge | Rb * | Cs | ||||||||||
| Root | Shoot | Mean | Root | Shoot | Mean | Root | Shoot | Mean | Root | Shoot | Mean | ||
| Unit S1 | S. o. | 131 ± 131 de | 41.4 ± 18 ef | 86.3 C | 803 ± 539 a | 133 ± 9 bc | 468 A | 7.58 ± 2.55 bc | 38.7 ± 13.4 a | 23.1 A | 362 ± 126 def | 3331 ± 1917 ab | 1847 B |
| S. t. | 88.2 ± 28 def | 80.7 ± 22 def | 84.5 C | 74.9 ± 19 cd | 36.1 ± 10 de | 55.6 C | 2.31 ± 0.96 e | 2.04 ± 1.1 ef | 2.17 BC | 512 ± 271 cde | 398 ± 230 def | 455 CD | |
| P. m. | 657 ± 559 a | 186 ± 37 bcd | 421.9 A | 276 ± 64 ab | 261.7 ± 72 b | 268.9 A | 2.98 ± 0.69 cde | 1.38 ± 0.4 ef | 2.1 BC | 763 ± 406 cd | 334 ± 88 def | 549 BC | |
| Unit S2 | S. o. | 301 ± 134 abc | 50.1 ± 40 ef | 176 BC | 61.1 ± 16 cde | 25.6 ± 11 e | 43.4 C | 1.18 ± 0.64 b | 64.1 ± 32.2 a | 38.0 A | 1782 ± 1677 bc | 10,748 ± 7663 a | 6265 A |
| S. t. | 677 ± 609 ab | 84 ± 27 def | 380 AB | 231 ± 109 b | 71.6 ± 29 cd | 151 B | 3.51 ± 0.31 de | 0.81 ± 0.3 f | 2.1 C | 644 ± 543 cde | 273 ± 223 ef | 458 CD | |
| P. m. | 274 ± 186 a–d | 32.5 ± 11 f | 153 C | 186 ± 181 bc | 64.7 ± 76 de | 125 BC | 3.34 ± 2.84 e | 1.95 ± 0.3 ef | 2.6 BC | 354 ± 185 def | 117 ± 8 f | 235 D | |
| A. h. | 141 ± 57 cd | 109 ± 29 cde | 125 BC | 63.6 ± 32 cde | 31.2 ± 4 de | 47.4 C | 1.79 ± 0.43 ef | 6.42 ± 2.6 bcd | 4.1 B | 255 ± 94 def | 320 ± 282 def | 287 CD | |
| U | Pb * | Zn * | Al * | ||||||||||
| Root | Shoot | Mean | Root | Shoot | Mean | Root | Shoot | Mean | Root | Shoot | Mean | ||
| Unit S1 | S. o. | 256.6 ± 201 abc | 61.7 ± 15 cde | 159.2 A | 46.7 ± 28 b–f | 31.4 ± 6 c–f | 39.1 B | 236.9 ± 121 a | 547 ± 339 a | 402.2 A | 49.3 ± 39 f | 33.7 ± 19 f | 41.5 E |
| S. t. | 949.8 ± 799 a | 69.8 ± 9 cde | 509.8 A | 92.6 ± 7 bc | 4.3 ± 1 g | 48.5 B | 24.6 ± 4 de | 26.0 ± 6.2 de | 25.3 CD | 750 ± 175 a | 407 ± 22 abc | 578 A | |
| P. m. | 861.1 ± 443 a | 137.5 ± 34 bcd | 499.3 A | 1943.6 ± 694 a | 31.1 ± 17 def | 987.4 A | 687.3 ± 484 a | 92.4 ± 29.3 b | 389 A | 60.9 ± 29 ef | 171 ± 50 cd | 116 D | |
| Unit S2 | S. o. | 488.6 ± 249 ab | 52.5 ± 23 de | 270.6 A | 51.4 ± 20 b–e | 16.8 ± 6 f | 34.1 B | 60.9 ± 27 bcd | 84.9 ± 43 bc | 72.9 B | 242 ± 76 bcd | 108 ± 28 de | 175 CD |
| S. t. | 2437.1 ± 3997 ab | 43.8 ± 7 de | 1240.5 A | 162.7 ± 164 b | 14.2 ± 1 f | 88.5 B | 55.8 ± 32 bcd | 36.2 ± 13.9 cd | 46.0 BC | 311 ± 225 bcd | 240 ± 187 cd | 275 BC | |
| P. m. | 451.8 ± 382 ab | 27.1 ± 7 e | 239.5 A | 55.3 ± 61 c–f | 1.8 ± 0.2 g | 28.5 C | 49.2 ± 64 de | 12.5 ± 3.8 e | 30.8 D | 660 ± 506 ab | 236 ± 121 bcd | 448 AB | |
| A. h. | 345 ± 204 ab | 64.5 ± 22 cde | 204.8 A | 112.6 ± 144 bcd | 17.2 ± 4 ef | 64.9 B | 60.5 ± 22.4 bcd | 53.3 ± 10.9 bcd | 56.9 B | 163 ± 43 cd | 237 ± 63 bcd | 200 BC | |
| Fe * | |||||||||||||
| Root | Shoot | Mean | |||||||||||
| Unit S1 | S. o. | 159 ± 124 bcd | 238 ± 43 abc | 199 B | |||||||||
| S. t. | 131 ± 30 bcd | 146 ± 45 bcd | 135 BC | ||||||||||
| P. m. | 495 ± 309 a | 261 ± 40 abc | 378 A | ||||||||||
| Unit S2 | S. o. | 206 ± 83 bcd | 138 ± 67 cd | 172 BC | |||||||||
| S. t. | 283 ± 109 ab | 96.3 ± 33 d | 190 BC | ||||||||||
| P. m. | 295 ± 137 ab | 37.9 ± 8 e | 166 C | ||||||||||
| A. h. | 170 ± 86 bcd | 251 ± 48 abc | 210 B | ||||||||||
| BCFroot | BCFshoot | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit 1 | Unit 2 | Unit 1 | Unit 2 | |||||||||||
| S.o. | S. t. | P. m. | S.o. | S. t. | P. m. | A. h. | S.o. | S. t. | P. m. | S.o. | S. t. | P. m. | A. h. | |
| V | 95 | 41 | 90 | 3.4 | 13 | 12 | 3.9 | 31 | 31 | 42 | 2.6 | 1.9 | 0.9 | 4 |
| Cr | 30 | 20 | 226 | 6.4 | 9.4 | 48 | 7.5 | 51 | 79 | 218 | 6.8 | 19 | 7.6 | 5.4 |
| Mn | 1.9 | 0.3 | 0.9 | 1 | 0.4 | 0.3 | 0.2 | 3.4 | 0.8 | 0.4 | 1.9 | 0.3 | 0.1 | 0.3 |
| Co | 1.5 | 0.4 | 0.9 | 0.2 | 0.4 | 0.7 | 0.1 | 1 | 0.4 | 0.5 | 0.2 | 0.1 | 0.1 | 0.1 |
| Ni | 3.8 | 1.2 | 4.1 | 1.1 | 1.3 | 3.6 | 0.8 | 4.6 | 1.5 | 5.1 | 1.2 | 8.1 | 0.5 | 1 |
| Cu | 1.8 | 2 | 2.6 | 0.4 | 0.4 | 0.5 | 1.1 | 4 | 0.7 | 0.6 | 0.6 | 0.3 | 0.2 | 0.9 |
| As | 69 | 81 | 790 | 6.8 | 32 | 23 | 9.5 | 19 | 21 | 47 | 2.5 | 3 | 1.4 | 3.9 |
| Se | 2.1 | 0.9 | 2.4 | 1.3 | 3.4 | 2.9 | 1 | 1.9 | 0.8 | 1.3 | 1.7 | 1.5 | 1 | 2.5 |
| Sr | 4.3 | 0.4 | 2.9 | 4.6 | 1.8 | 1.8 | 2.3 | 9.4 | 0.4 | 0.8 | 6.5 | 0.6 | 0.7 | 1.7 |
| Y | 0.5 | 0.5 | 1.2 | 0.4 | 1.6 | 0.9 | 0.3 | 0.3 | 0.1 | 0.3 | 0.3 | 0.2 | 0.1 | 0.4 |
| Zr | 15 | 2.6 | 11 | 7.4 | 10 | 9.4 | 11 | 8.3 | 4.6 | 5.8 | 5.3 | 3 | 1.2 | 11 |
| Mo | 12 | 2.4 | 7.5 | 5.5 | 13 | 23 | 19 | 22 | 3.2 | 10 | 37 | 19 | 33 | 19 |
| Cd | 0.3 | 0 | 3 | 1.2 | 0.6 | 0.2 | 1.2 | 0.7 | 0 | 0.1 | 2 | 0.1 | 0 | 0.7 |
| In | 5.8 | 8.2 | 125 | 2.2 | 5.4 | 5.6 | 3.5 | 2.4 | 2 | 8.3 | 3.3 | 1 | 0.3 | 1.9 |
| Sb | 3.2 | 0.6 | 25 | 1.9 | 1.7 | 3.6 | 3 | 1.6 | 0.7 | 3 | 0.4 | 0.2 | 0.1 | 0.6 |
| La | 1.7 | 1.5 | 9.9 | 1.2 | 5.1 | 1.9 | 0.7 | 1.5 | 1 | 2.2 | 0.7 | 0.7 | 0.3 | 1 |
| Ce | 1 | 1.2 | 4.3 | 0.4 | 3.1 | 1 | 0.3 | 0.5 | 0.6 | 1.4 | 0.2 | 0.3 | 0.1 | 0.3 |
| Pr | 1.5 | 1.9 | 7.6 | 1 | 5.8 | 2.8 | 0.7 | 1 | 0.8 | 1.8 | 0.5 | 0.6 | 0.2 | 0.7 |
| Pb | 0.1 | 0.2 | 3.7 | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 | 0 | 0.1 | 0 | 0 | 0 | 0 |
| Bi | 1.1 | 3 | 2.4 | 0.2 | 0.4 | 0.3 | 0.2 | 0.4 | 0.5 | 0.9 | 0.3 | 0.2 | 0 | 0.5 |
| Th | 3 | 2.4 | 16 | 4.2 | 9.3 | 3.8 | 1.9 | 1 | 2.1 | 5.5 | 0.7 | 1.2 | 0.4 | 1.5 |
| Ge | 20 | 1.8 | 6.7 | 0.7 | 2.5 | 2 | 0.7 | 3.3 | 0.9 | 6.3 | 0.3 | 0.8 | 0.7 | 0.3 |
| Cs | 41 | 38 | 76 | 128 | 46 | 25 | 18 | 372 | 34 | 29 | 770 | 20 | 8.4 | 23 |
| U | 24 | 157 | 85 | 28 | 139 | 26 | 20 | 6.3 | 7.8 | 18 | 3 | 2.5 | 1.5 | 3.7 |
| Zn | 2.9 | 0.3 | 7.6 | 0.6 | 0.5 | 0.5 | 0.6 | 6.5 | 0.3 | 1.1 | 0.8 | 0.3 | 0.1 | 0.5 |
| Al | 4.7 | 112 | 7.1 | 40 | 52 | 110 | 27 | 3.5 | 55 | 20 | 18 | 40 | 39 | 39 |
| Fe | 9.6 | 8.4 | 33 | 16 | 22 | 22 | 13 | 15 | 9 | 17 | 11 | 7.3 | 2.9 | 19 |
| Background Level (mg kg−1) | Soil Quality Reference Values (mg kg−1) | ||||
|---|---|---|---|---|---|
| Sanchez et al. [53] | Martínez-Martínez [87] | IGME [54] | Ballesta et al. [56] | ||
| Zn | 55.2 | 48 | - | Mo | 2.0 |
| Fe | - | - | 2.02 | Rb | 234 |
| Al | - | - | 4540 | Cs | 14.2 |
| Pb | 9.8 | - | - | V | 123 |
| Mn | 664 | - | 359 | Sr | 1868 |
| Cd | 0.12 | 0.13 | - | Zr | 413 |
| Co | 7.7 | 24.4 | - | Sn | 8.7 |
| Ni | 16.8 | - | - | Y | 38.3 |
| Cr | 44.6 | 115 | - | La | 48.4 |
| Cu | 18.7 | 43.6 | - | Ce | 97.9 |
| As | 8.1 | - | 14 | Bi | 2.2 |
| Se | 0.22 | - | 0.7 | Ge | 1.3 |
| Sb | 1.1 | - | 1 | U | 10.3 |
| Th | 0.1 | - | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azizi, M.; Faz, A.; Zornoza, R.; Martinez-Martinez, S.; Acosta, J.A. Phytoremediation Potential of Native Plant Species in Mine Soils Polluted by Metal(loid)s and Rare Earth Elements. Plants 2023, 12, 1219. https://doi.org/10.3390/plants12061219
Azizi M, Faz A, Zornoza R, Martinez-Martinez S, Acosta JA. Phytoremediation Potential of Native Plant Species in Mine Soils Polluted by Metal(loid)s and Rare Earth Elements. Plants. 2023; 12(6):1219. https://doi.org/10.3390/plants12061219
Chicago/Turabian StyleAzizi, Mitra, Angel Faz, Raul Zornoza, Silvia Martinez-Martinez, and Jose A. Acosta. 2023. "Phytoremediation Potential of Native Plant Species in Mine Soils Polluted by Metal(loid)s and Rare Earth Elements" Plants 12, no. 6: 1219. https://doi.org/10.3390/plants12061219
APA StyleAzizi, M., Faz, A., Zornoza, R., Martinez-Martinez, S., & Acosta, J. A. (2023). Phytoremediation Potential of Native Plant Species in Mine Soils Polluted by Metal(loid)s and Rare Earth Elements. Plants, 12(6), 1219. https://doi.org/10.3390/plants12061219









