Evaluations of Andrographolide-Rich Fractions of Andrographis paniculata with Enhanced Potential Antioxidant, Anticancer, Antihypertensive, and Anti-Inflammatory Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Fractionation and Diterpenoid Quantification of A. paniculata
2.2. Total Phenolic and Flavonoid Content
2.3. Antioxidant Activity
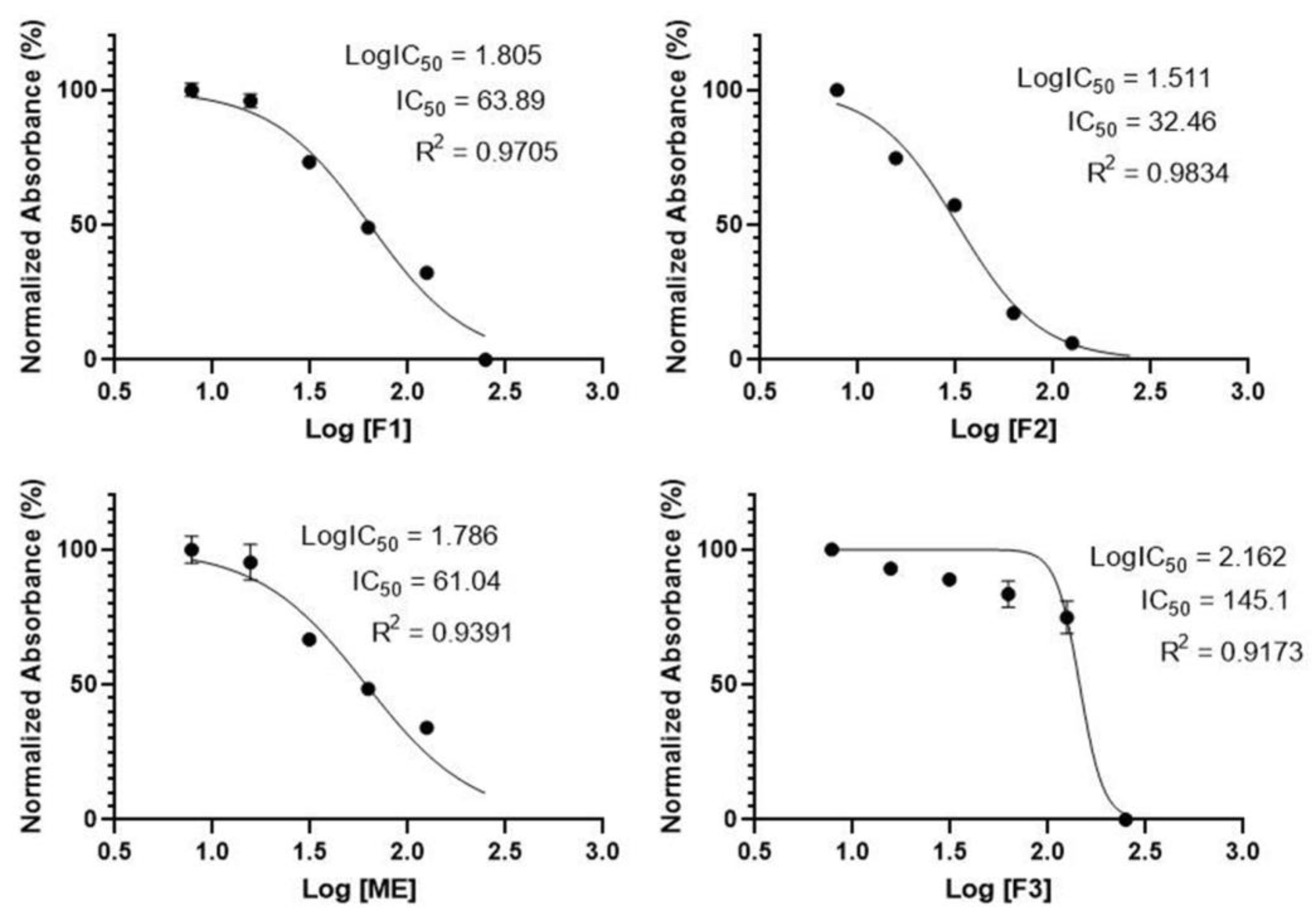
2.4. Cellular Nitric Oxide (NO) Activity
2.5. Cytotoxic Activity
2.6. Angiotensin-Converting Enzyme (ACE) Inhibition Activity
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Sample Preparation and Extraction Method
3.2.2. HPLC Analysis and Quantification of Andrographolide and Its Derivatives
3.2.3. Total Phenolic Content (TPC) by Folin-Ciocalteu Reagent Assay
3.2.4. Total Flavonoid Content (TFC) by Aluminum Chloride Colorimetric Assay
3.2.5. 2,2′-Azino-bis-3ethylbenzothiazoline-6-sulfonic Acid (ABTS) Assay
3.2.6. Ferric Reducing-Antioxidant Power (FRAP) Assay
3.2.7. Angiotensin-Converting Enzyme (ACE) Inhibition Assay
3.2.8. Cell Culture
3.2.9. Cellular NO Production and Quantification
3.2.10. Cytotoxic Screening
3.2.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saxena, R.C.; Singh, R.; Kumar, P.; Yadav, S.C.; Negi, M.P.S.; Saxena, V.S.; Joshua, A.J.; Vijayabalaji, V.; Goudar, K.S.; Venkateshwarlu, K.; et al. A Randomized Double Blind Placebo Controlled Clinical Evaluation of Extract of Andrographis paniculata (KalmCold™) in Patients with Uncomplicated Upper Respiratory Tract Infection. Phytomedicine 2010, 17, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Barua, P.M.B.; Sahu, P.R.; Mondal, E.; Bose, G.; Khan, A.T. A Mild and Environmentally Benign Synthetic Protocol for Catalytic Hydrolysis of Thioglycosides. Synlett 2002, 2002, 0081–0084. [Google Scholar] [CrossRef]
- Marcos, I.; Pedrero, A.; Sexmero, M.; Diez, D.; Basabe, P.; Hernández, F.; Urones, J. Synthesis and Absolute Configuration of Three Natural Ent-Halimanolides with Biological Activity. Tetrahedron Lett. 2003, 44, 369–372. [Google Scholar] [CrossRef]
- Siedle, B.; García-Piñeres, A.J.; Murillo, R.; Schulte-Mönting, J.; Castro, V.; Rüngeler, P.; Klaas, C.A.; Da Costa, F.B.; Kisiel, W.; Merfort, I. Quantitative Structure–Activity Relationship of Sesquiterpene Lactones as Inhibitors of the Transcription Factor NF-ΚB. J. Med. Chem. 2004, 47, 6042–6054. [Google Scholar] [CrossRef] [PubMed]
- Surowiak, A.K.; Balcerzak, L.; Lochyński, S.; Strub, D.J. Biological Activity of Selected Natural and Synthetic Terpenoid Lactones. Int. J. Mol. Sci. 2021, 22, 5036. [Google Scholar] [CrossRef]
- Salminen, A.; Lehtonen, M.; Suuronen, T.; Kaarniranta, K.; Huuskonen, J. Terpenoids: Natural Inhibitors of NF-ΚB Signaling with Anti-Inflammatory and Anticancer Potential. Cell. Mol. Life Sci. 2008, 65, 2979–2999. [Google Scholar] [CrossRef]
- Adiguna, S.P.; Panggabean, J.A.; Atikana, A.; Untari, F.; Izzati, F.; Bayu, A.; Rosyidah, A.; Rahmawati, S.I.; Putra, M.Y. Antiviral Activities of Andrographolide and Its Derivatives: Mechanism of Action and Delivery System. Pharmaceuticals 2021, 14, 1102. [Google Scholar] [CrossRef]
- Suriyo, T.; Chotirat, S.; Rangkadilok, N.; Pholphana, N.; Satayavivad, J. Interactive Effects of Andrographis paniculata Extracts and Cancer Chemotherapeutic 5-Fluorouracil on Cytochrome P450s Expression in Human Hepatocellular Carcinoma HepG2 Cells. J. Herb. Med. 2021, 26, 100421. [Google Scholar] [CrossRef]
- Banerjee, M.; Parai, D.; Chattopadhyay, S.; Mukherjee, S.K. Andrographolide: Antibacterial Activity against Common Bacteria of Human Health Concern and Possible Mechanism of Action. Folia Microbiol. 2017, 62, 237–244. [Google Scholar] [CrossRef]
- Murugan, K.; Selvanayaki, K.; Al-Sohaibani, S. Antibiofilm Activity of Andrographis paniculata against Cystic Fibrosis Clinical Isolate Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2011, 27, 1661–1668. [Google Scholar] [CrossRef]
- Krithika, R.; Verma, R.J.; Shrivastav, P.S. Antioxidative and Cytoprotective Effects of Andrographolide against CCl4-Induced Hepatotoxicity in HepG2 Cells. Hum. Exp. Toxicol. 2013, 32, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Churiyah; Pongtuluran, O.B.; Rofaani, E.; Tarwadi. Antiviral and Immunostimulant Activities of Andrographis paniculata. HAYATI J. Biosci. 2015, 22, 67–72. [Google Scholar] [CrossRef]
- Imani, S.F.; Khairani, A.A.; Arbianti, R.; Utami, T.S.; Hermansyah, H. The Effect of Fermentation Time and Sonication Temperatures on Extraction Process of Bitter Leaves (Andrographis paniculata) against Antidiabetic Activity through α-Glucosidase Enzyme Inhibition Test. AIP Conf. Proc. 2020, 2255, 030047. [Google Scholar] [CrossRef]
- Kaushik, S.; Dar, L.; Kaushik, S.; Yadav, J.P. Identification and Characterization of New Potent Inhibitors of Dengue Virus NS5 Proteinase from Andrographis paniculata Supercritical Extracts on in Animal Cell Culture and in Silico Approaches. J. Ethnopharmacol. 2021, 267, 113541. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Zhong, L.-Y.; Yu, N.-N.; Ouyang, L.; Fang, R.-D.; Wang, Y.; He, Q.-Y. Structure-Based Discovery of Neoandrographolide as a Novel Inhibitor of Rab5 to Suppress Cancer Growth. Comput. Struct. Biotechnol. J. 2020, 18, 3936–3946. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Zhao, F.; Liu, Z.; Li, J.; Qiu, F. Microbial Transformation of Neoandrographolide by Mucor Spinosus (AS 3.2450). J. Mol. Catal. B Enzym. 2011, 68, 13. [Google Scholar] [CrossRef]
- Jiang, M.; Sheng, F.; Zhang, Z.; Ma, X.; Gao, T.; Fu, C.; Li, P. Andrographis paniculata (Burm. f.) Nees and Its Major Constituent Andrographolide as Potential Antiviral Agents. J. Ethnopharmacol. 2021, 272, 113954. [Google Scholar] [CrossRef]
- Tajidin, N.E.; Shaari, K.; Maulidiani, M.; Salleh, N.S.; Ketaren, B.R.; Mohamad, M. Metabolite Profiling of Andrographis paniculata (Burm. f.) Nees. Young and Mature Leaves at Different Harvest Ages Using 1H NMR-Based Metabolomics Approach. Sci. Rep. 2019, 9, 16766. [Google Scholar] [CrossRef]
- Lim, X.Y.; Chan, J.S.W.; Tan, T.Y.C.; Teh, B.P.; Mohd Abd Razak, M.R.; Mohamad, S.; Syed Mohamed, A.F. Andrographis paniculata (Burm. F.) Wall. Ex Nees, Andrographolide, and Andrographolide Analogues as SARS-CoV-2 Antivirals? A Rapid Review. Nat. Prod. Commun. 2021, 16, 1934578X2110166. [Google Scholar] [CrossRef]
- Hossain, S.; Urbi, Z.; Karuniawati, H.; Mohiuddin, R.B.; Moh Qrimida, A.; Allzrag, A.M.M.; Ming, L.C.; Pagano, E.; Capasso, R. Andrographis paniculata (Burm. f.) Wall. Ex Nees: An Updated Review of Phytochemistry, Antimicrobial Pharmacology, and Clinical Safety and Efficacy. Life 2021, 11, 348. [Google Scholar] [CrossRef]
- Ali, J.S.; Khan, I.; Zia, M. Antimicrobial, Cytotoxic, Phytochemical and Biological Properties of Crude Extract and Solid Phase Fractions of Monotheca Buxifolia. Adv. Tradit. Med. 2020, 20, 115–122. [Google Scholar] [CrossRef]
- Chao, W.-W.; Kuo, Y.-H.; Lin, B.-F. Anti-Inflammatory Activity of New Compounds from Andrographis paniculata by NF-ΚB Transactivation Inhibition. J. Agric. Food Chem. 2010, 58, 2505–2512. [Google Scholar] [CrossRef]
- Rao, P.R.; Rathod, V.K. Rapid Extraction of Andrographolide from Andrographis paniculata Nees by Three Phase Partitioning and Determination of Its Antioxidant Activity. Biocatal. Agric. Biotechnol. 2015, 4, 586–593. [Google Scholar] [CrossRef]
- Chao, W.-W.; Lin, B.-F. Isolation and Identification of Bioactive Compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef]
- Bhaskar Reddy, M.V.; Kishore, P.H.; Rao, C.V.; Gunasekar, D.; Caux, C.; Bodo, B. New 2′-Oxygenated Flavonoids from Andrographis Affinis. J. Nat. Prod. 2003, 66, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Radhika, P.; Prasad, Y.R.; Lakshmi, K.R. Flavones from the Stem of Andrographis paniculata Nees. Nat. Prod. Commun. 2010, 5, 59–60. [Google Scholar] [CrossRef]
- Chia, V.V.; Pang, S.F.; Gimbun, J. Mass Spectrometry Analysis of Auxiliary Energy-Induced Terpenes Extraction from Andrographis paniculata. Ind. Crops Prod. 2020, 155, 112828. [Google Scholar] [CrossRef]
- Xiang, J.; Li, W.; Ndolo, V.U.; Beta, T. A Comparative Study of the Phenolic Compounds and in Vitro Antioxidant Capacity of Finger Millets from Different Growing Regions in Malawi. J. Cereal Sci. 2019, 87, 143–149. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Akowuah, G.A.; Zhari, I.; Norhayati, I.; Mariam, A. HPLC and HPTLC Densitometric Determination of Andrographolides and Antioxidant Potential of Andrographis paniculata. J. Food Compos. Anal. 2006, 19, 118–126. [Google Scholar] [CrossRef]
- Chiou, W.-F.; Chen, C.-F.; Lin, J.-J. Mechanisms of Suppression of Inducible Nitric Oxide Synthase (INOS) Expression in RAW 264.7 Cells by Andrographolide. Br. J. Pharmacol. 2000, 129, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-X.; Zhuang, Y.-L.; Shen, L.; Ma, E.-L.; Zhu, H.-J.; Zhao, F.; Qiu, F. Microbial Transformation of 14-Deoxy-11, 12-Didehydroandrographolide and 14-Deoxyandrographolide and Inhibitory Effects on Nitric Oxide Production of the Transformation Products. J. Mol. Catal. B Enzym. 2011, 72, 248–255. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.-T.; Ji, L.-L.; Ge, B.-X. Inhibitory Effects of Neoandrographolide on Nitric Oxide and Prostaglandin E2 Production in LPS-Stimulated Murine Macrophage. Mol. Cell. Biochem. 2007, 298, 49–57. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.-T.; Ji, L.-L. In Vivo and In Vitro Anti-Inflammatory Activities of Neoandrographolide. Am. J. Chin. Med. 2007, 35, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z.-T.; Ge, B.-X. Andrograpanin, Isolated from Andrographis paniculata, Exhibits Anti-Inflammatory Property in Lipopolysaccharide-Induced Macrophage Cells through down-Regulating the P38 MAPKs Signaling Pathways. Int. Immunopharmacol. 2008, 8, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.; Jha, A.; Youssef, D.; Rupasinghe, H. Curcumin and Its Carbocyclic Analogs: Structure-Activity in Relation to Antioxidant and Selected Biological Properties. Molecules 2013, 18, 5389–5404. [Google Scholar] [CrossRef]
- Dalawai, D.; Aware, C.; Jadhav, J.P.; Murthy, H.N. RP-HPLC Analysis of Diterpene Lactones in Leaves and Stem of Different Species of Andrographis. Nat. Prod. Res. 2021, 35, 2239–2242. [Google Scholar] [CrossRef]
- Sekhon-Loodu, S.; Rupasinghe, H.P.V. Evaluation of Antioxidant, Antidiabetic and Antiobesity Potential of Selected Traditional Medicinal Plants. Front. Nutr. 2019, 6, 53. [Google Scholar] [CrossRef]
- Chatatikun, M.; Chiabchalard, A. Phytochemical Screening and Free Radical Scavenging Activities of Orange Baby Carrot and Carrot (Daucus Carota Linn.) Root Crude Extracts. J. Chem. Pharm. Res. 2013, 5, 97–102. [Google Scholar]
- Xiang, J.; Apea-Bah, F.B.; Ndolo, V.U.; Katundu, M.C.; Beta, T. Profile of Phenolic Compounds and Antioxidant Activity of Finger Millet Varieties. Food Chem. 2019, 275, 361–368. [Google Scholar] [CrossRef]
- Li, H.-B.; Wong, C.-C.; Cheng, K.-W.; Chen, F. Antioxidant Properties in Vitro and Total Phenolic Contents in Methanol Extracts from Medicinal Plants. LWT-Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Hettihewa, S.; Hemar, Y.; Rupasinghe, H. Flavonoid-Rich Extract of Actinidia Macrosperma (A Wild Kiwifruit) Inhibits Angiotensin-Converting Enzyme In Vitro. Foods 2018, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, R.; Zarei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Ismail, A.; Saari, N. Angiotensin-I Converting Enzyme (ACE) Inhibitory and Anti-Oxidant Activities of Sea Cucumber (Actinopyga lecanora) Hydrolysates. Int. J. Mol. Sci. 2015, 16, 28870–28885. [Google Scholar] [CrossRef] [PubMed]




| Compound | Samples (mg g−1) | |||
|---|---|---|---|---|
| ME | F1 | F2 | F3 | |
| Andrographolide | 38.15 | 31.45 | 85.65 | 12.16 |
| 14-deoxy-11,12-didehydroandrographolide | 8.60 | 6.43 | 49.19 | - |
| Neoandrographolide | 19.87 | 16.86 | 110.77 | - |
| Andrograpanin | 4.98 | - | 17.39 | - |
| Extract | Antioxidant Capacity (mmol TE g−1 d.w.) | TPC (µg GAE g−1 d.w.) | TFC (µg QE g−1 d.w.) | |
|---|---|---|---|---|
| ABTS | FRAP | |||
| ME | 0.35 ± 0.01 | 0.47 ± 0.01 | 30.68 ± 2.34 | 33.50 ± 4.57 |
| F1 | 0.23 ± 0.01 | 0.25 ± 0.02 | 30.21 ± 1.13 | 14.68 ± 5.11 |
| F2 | 0.22 ± 0.00 | 0.08 ± 0.00 | 20.02 ± 2.30 | 19.57 ± 4.17 |
| F3 | 0.12 ± 0.00 | 0.06 ± 0.01 | 16.81 ± 0.21 | 147.77 ± 5.30 |
| Sample | NO Production (µM) | % Inhibition |
|---|---|---|
| Dexamethasone 10 ppm | 3.80 ± 0.21 | 84.82 |
| ME | 0.81 ± 0.18 | 96.78 |
| F1 | 1.33 ± 0.46 | 94.70 |
| F2 | 0.41 ± 0.11 | 98.36 |
| F3 | 2.18 ± 0.24 | 91.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adiguna, S.P.; Panggabean, J.A.; Swasono, R.T.; Rahmawati, S.I.; Izzati, F.; Bayu, A.; Putra, M.Y.; Formisano, C.; Giuseppina, C. Evaluations of Andrographolide-Rich Fractions of Andrographis paniculata with Enhanced Potential Antioxidant, Anticancer, Antihypertensive, and Anti-Inflammatory Activities. Plants 2023, 12, 1220. https://doi.org/10.3390/plants12061220
Adiguna SP, Panggabean JA, Swasono RT, Rahmawati SI, Izzati F, Bayu A, Putra MY, Formisano C, Giuseppina C. Evaluations of Andrographolide-Rich Fractions of Andrographis paniculata with Enhanced Potential Antioxidant, Anticancer, Antihypertensive, and Anti-Inflammatory Activities. Plants. 2023; 12(6):1220. https://doi.org/10.3390/plants12061220
Chicago/Turabian StyleAdiguna, Sya’ban Putra, Jonathan Ardhianto Panggabean, Respati Tri Swasono, Siti Irma Rahmawati, Fauzia Izzati, Asep Bayu, Masteria Yunovilsa Putra, Carmen Formisano, and Chianese Giuseppina. 2023. "Evaluations of Andrographolide-Rich Fractions of Andrographis paniculata with Enhanced Potential Antioxidant, Anticancer, Antihypertensive, and Anti-Inflammatory Activities" Plants 12, no. 6: 1220. https://doi.org/10.3390/plants12061220
APA StyleAdiguna, S. P., Panggabean, J. A., Swasono, R. T., Rahmawati, S. I., Izzati, F., Bayu, A., Putra, M. Y., Formisano, C., & Giuseppina, C. (2023). Evaluations of Andrographolide-Rich Fractions of Andrographis paniculata with Enhanced Potential Antioxidant, Anticancer, Antihypertensive, and Anti-Inflammatory Activities. Plants, 12(6), 1220. https://doi.org/10.3390/plants12061220








