The High-Elevation Peatlands of the Northern Andes, Colombia
Abstract
1. Introduction
2. Results
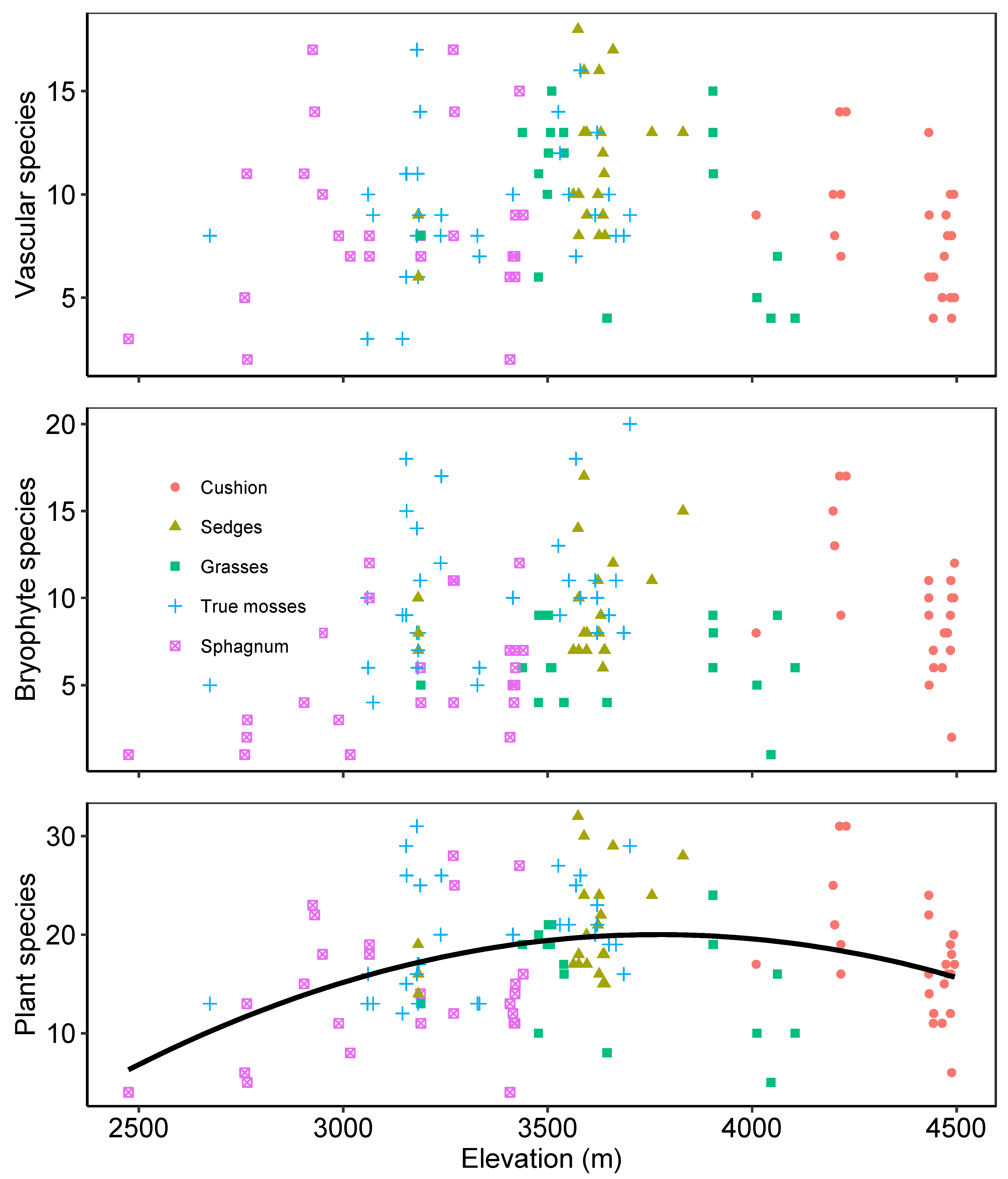
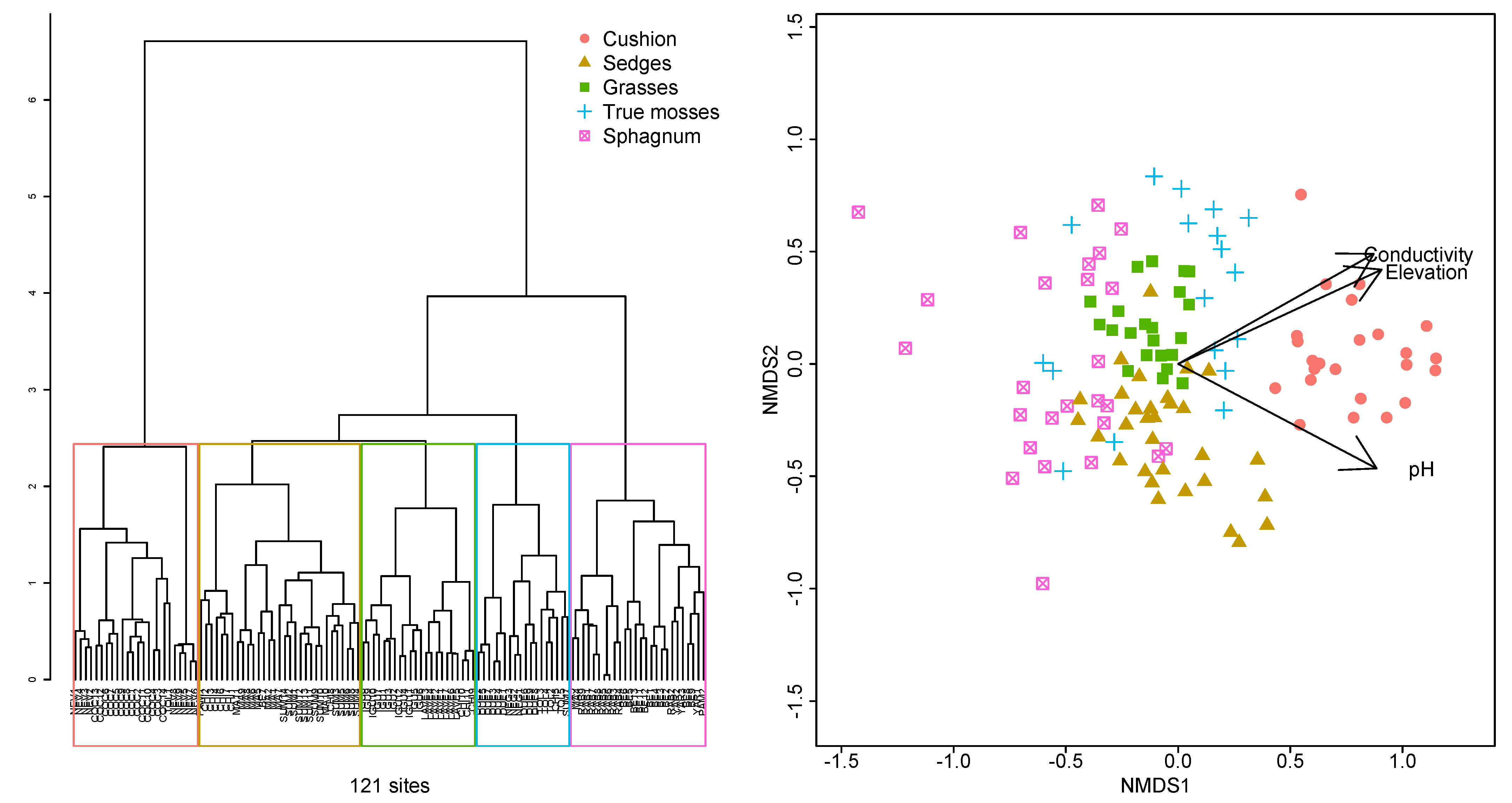
2.1. Plant Diversity and Vegetation Types
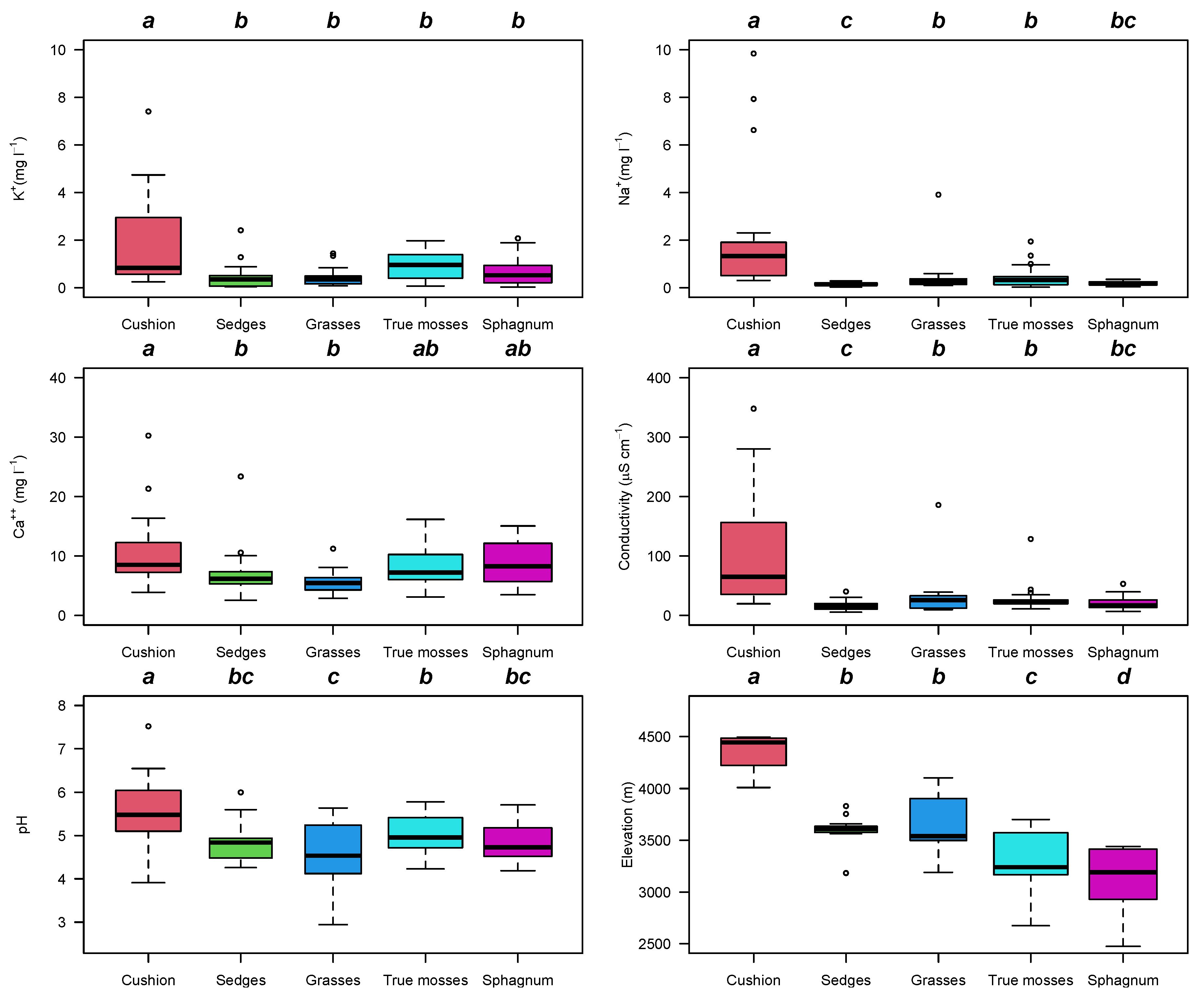
2.2. Water Chemistry
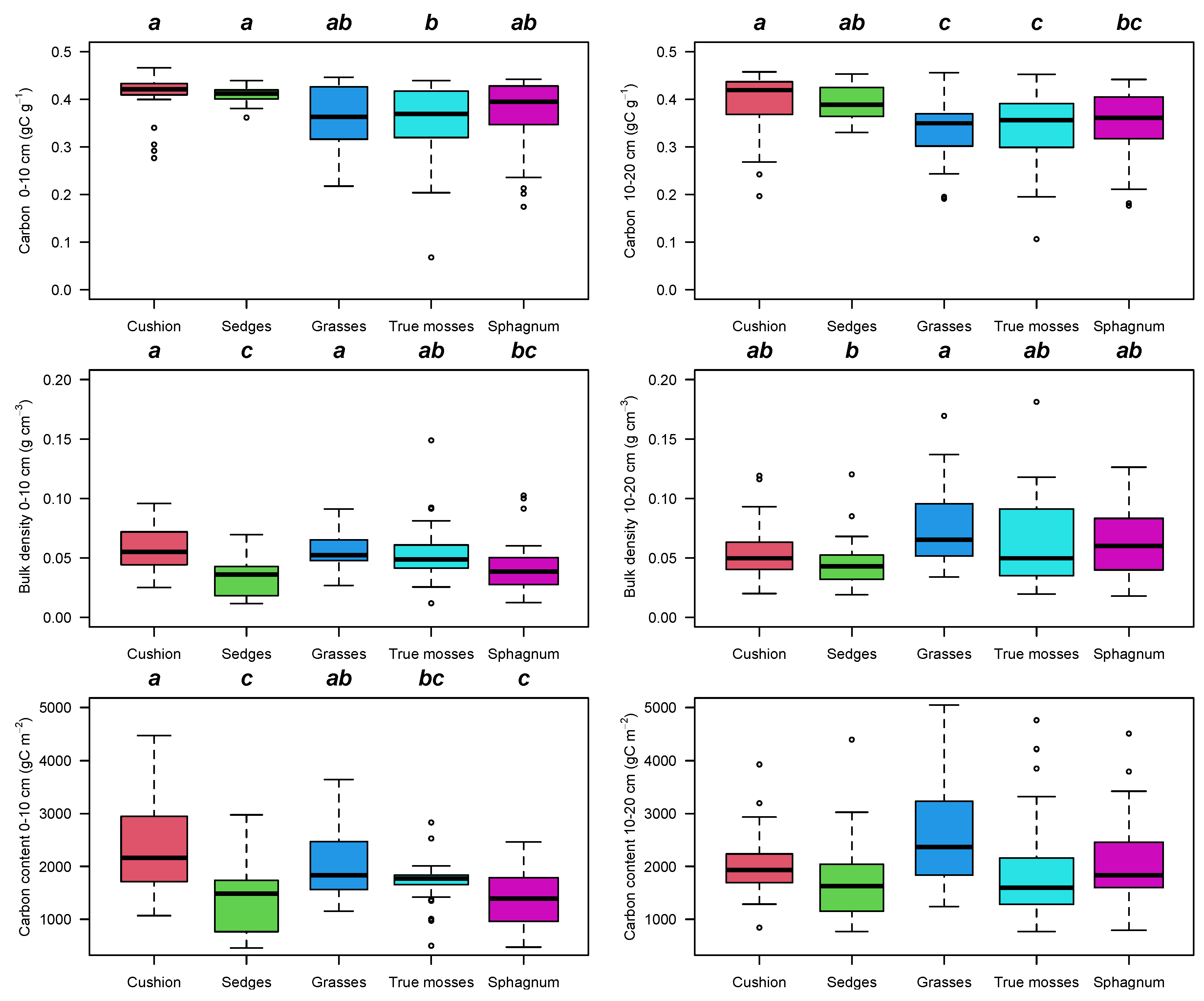
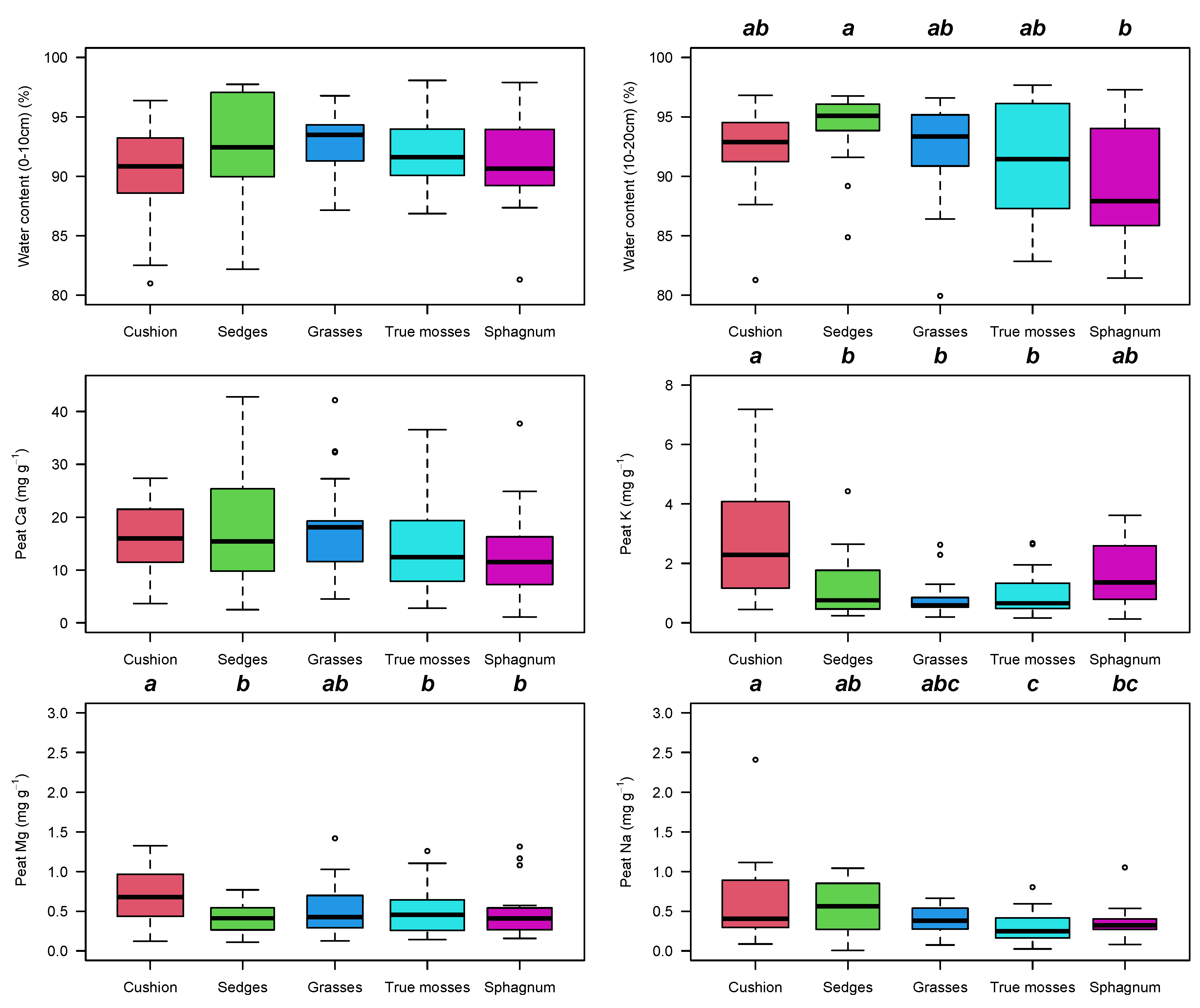
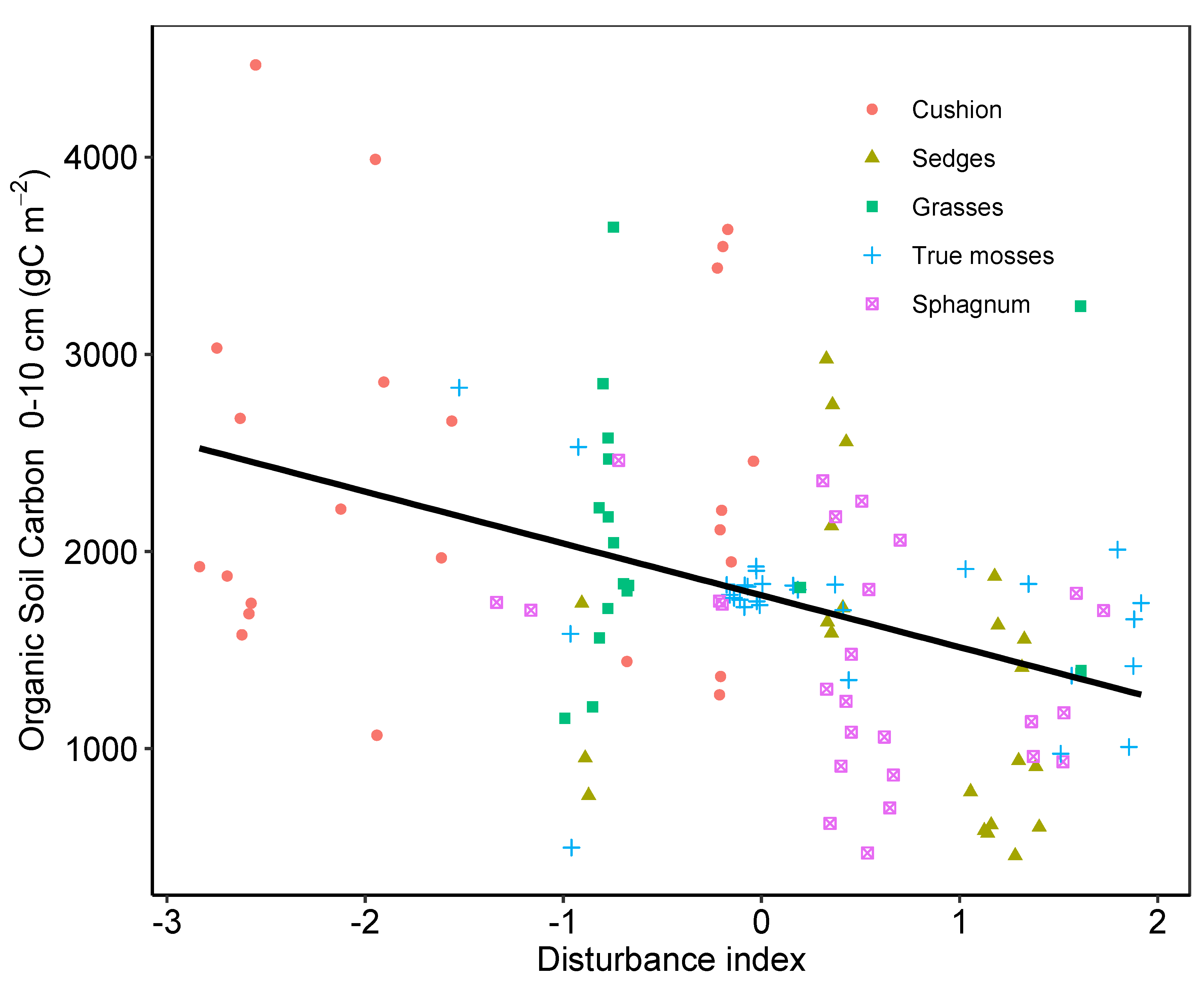
2.3. Peat Soil
3. Discussion
4. Materials and Methods
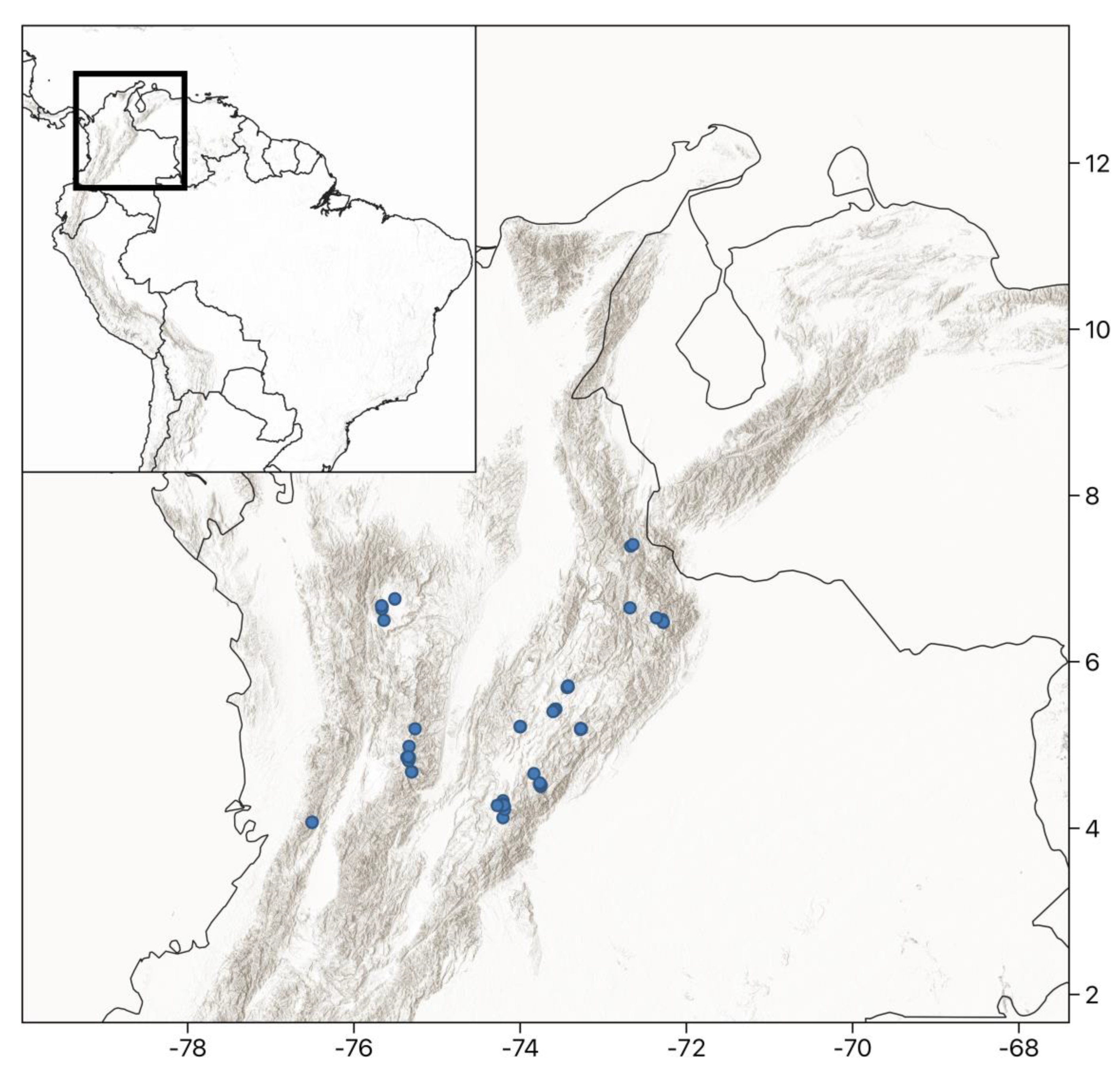
4.1. Study Area and Site Selection
4.2. Field Data Collection and Laboratory Analysis
4.3. Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, D.J.; Wolf, E.C.; Colson, C.; Vering, W.; Granda, A.; Meyer, M. Alpine peatlands of the Andes, Cajamarca, Peru. Arct. Antarct. Alp. Res. 2010, 42, 19–33. [Google Scholar] [CrossRef]
- Vitt, D.H.; Chee, W.-L. The relationships of vegetation to surface water chemistry and peat chemistry in fens of Alberta, Canada. Vegetatio 1990, 89, 87–106. [Google Scholar] [CrossRef]
- Vitt, D.H.; House, M. Bryophytes as key indicators of ecosystem function and structure of northern peatlands. Bryophyt. Divers. Evol. 2021, 43, 253–264. [Google Scholar] [CrossRef]
- Bosman, A.F.; Vandermolen, P.C.; Young, R.; Cleef, A.M. Ecology of a paramo cushion mire. J. Veg. Sci. 1993, 4, 633–640. [Google Scholar] [CrossRef]
- Cooper, D.J.; Sueltenfuss, J.; Oyague, E.; Yager, K.; Slayback, D.; Caballero, E.M.C.; Argollo, J.; Mark, B.G. Drivers of peatland water table dynamics in the central Andes, Bolivia and Peru. Hydrol. Process. 2019, 33, 1913–1925. [Google Scholar] [CrossRef]
- Cleef, A. The Vegetation of the Páramos of the Colombian Cordillera Oriental; Cramer: Vaduz, Liechtenstein, 1981; Volume 61, p. 320. [Google Scholar]
- Moscol Olivera, M.C.; Cleef, A.M. A phytosociological study of the paramo along two altitudinal transects in El Carchi province, northern Ecuador. Phytocoenologia 2009, 39, 79–107. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global biodiversity conservation: The critical role of hotspots. In Biodiversity Hotspots; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. [Google Scholar]
- Pennington, R.T.; Lavin, M.; Särkinen, T.; Lewis, G.P.; Klitgaard, B.B.; Hughes, C.E. Contrasting plant diversification histories within the Andean biodiversity hotspot. Proc. Natl. Acad. Sci. USA 2010, 107, 13783–13787. [Google Scholar] [CrossRef]
- Izquierdo, A.E.; Carilla, J.; Nieto, C.; Osinaga Acosta, O.; Martin, E.; Grau, H.R.; Reynaga, M.C. Multi-taxon patterns from high Andean peatlands: Assessing climatic and landscape variables. Community Ecol. 2020, 21, 317–332. [Google Scholar] [CrossRef]
- Polk, M.H.; Young, K.; Cano, A.; León, B. Vegetation of Andean wetlands (bofedales) in Huascarán National Park, Peru. Mires Peat 2019, 24, 1–26. [Google Scholar]
- Ruthsatz, B.; Schittek, K.; Backes, B. The vegetation of cushion peatlands in the Argentine Andes and changes in their floristic composition across a latitudinal gradient from 39 S to 22 S. Phytocoenologia 2020, 50, 249–278. [Google Scholar] [CrossRef]
- Sklenár, P.; Ramsay, P.M. Diversity of zonal páramo plant communities in Ecuador. Divers. Distrib. 2001, 7, 113–124. [Google Scholar] [CrossRef]
- Diaz, H.F.; Bradley, R.S.; Ning, L. Climatic changes in mountain regions of the American Cordillera and the tropics: Historical changes and future outlook. Arct. Antarct. Alp. Res. 2014, 46, 735–743. [Google Scholar] [CrossRef]
- Young, B.E.; Young, K.R.; Josse, C. Vulnerability of Tropical Andean ecosystems to climate change. In Climate Change and Biodiversity in the Tropical Andes; Herzog, S.K., Martínez, R., Jørgensen, P.M., Tiessen, H., Eds.; Inter-American Institute for Global Change Research: Montevideo, Uruguay, 2011; pp. 170–181. [Google Scholar]
- Benavides, J.C.; Vitt, D.H. Response curves and the environmental limits for peat-forming species in the northern Andes. Plant Ecol. 2014, 215, 937–952. [Google Scholar] [CrossRef]
- Valencia, J.; Lassaletta, L.; Velázquez, E.; Nicolau, J.M.; Gómez-Sal, A. Factors controlling compositional changes in a Northern Andean páramo (La Rusia, Colombia). Biotropica 2013, 45, 18–26. [Google Scholar] [CrossRef]
- Xu, J.; Morris, P.J.; Liu, J.; Holden, J. Hotspots of peatland-derived potable water use identified by global analysis. Nat. Sustain. 2018, 1, 246–253. [Google Scholar] [CrossRef]
- Holden, J. Peatland hydrology and carbon release: Why small-scale process matters. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2005, 363, 2891–2913. [Google Scholar] [CrossRef]
- Hribljan, J.; Cooper, D.; Sueltenfuss, J.; Wolf, E.; Heckman, K.; Lilleskov, E.; Chimner, R. Carbon storage and long-term rate of accumulation in high-altitude Andean peatlands of Bolivia. Mires Peat 2015, 15, 1–14. [Google Scholar]
- Benavides, J. The effect of drainage on organic matter accumulation and plant communities of high-altitude peatlands in the Colombian tropical Andes. Mires Peat 2014, 15, 1–15. [Google Scholar]
- Hribljan, J.A.; Suárez, E.; Heckman, K.A.; Lilleskov, E.A.; Chimner, R.A. Peatland carbon stocks and accumulation rates in the Ecuadorian páramo. Wetl. Ecol. Manag. 2016, 24, 113–127. [Google Scholar] [CrossRef]
- Müller, J.; Joos, F. Peatland area and carbon over the past 21,000 years—A global process based model investigation. Biogeosciences 2020, 17, 5285–5308. [Google Scholar] [CrossRef]
- Loisel, J.; Gallego-Sala, A.V.; Amesbury, M.; Magnan, G.; Anshari, G.; Beilman, D.; Benavides, J.; Blewett, J.; Camill, P.; Charman, D. Expert assessment of future vulnerability of the global peatland carbon sink. Nat. Clim. Chang. 2021, 11, 70–77. [Google Scholar] [CrossRef]
- Clymo, R. The limits to peat bog growth. Philos. Trans. R. Soc. London B Biol. Sci. 1984, 303, 605–654. [Google Scholar]
- Yu, Z.; Beilman, D.W.; Frolking, S.; MacDonald, G.M.; Roulet, N.T.; Camill, P.; Charman, D.J. Peatlands and their role in the global Carbon cycle. Eos Trans. AGU 2011, 92, 97–98. [Google Scholar] [CrossRef]
- Clymo, R.S.; Turunen, J.; Tolonen, K. Carbon accumulation in peatland. Oikos 1998, 81, 368–388. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Bond-Lamberty, B.; Euskirchen, E.; Talbot, J.; Frolking, S.; McGuire, A.D.; Tuittila, E.S. The resilience and functional role of moss in boreal and arctic ecosystems. New Phytol. 2012, 196, 49–67. [Google Scholar] [CrossRef]
- Loisel, J.; Yu, Z.; Beilman, D.W.; Camill, P.; Alm, J.; Amesbury, M.J.; Anderson, D.; Andersson, S.; Bochicchio, C.; Barber, K. A database and synthesis of northern peatland soil properties and Holocene carbon and nitrogen accumulation. Holocene 2014, 24, 1028–1042. [Google Scholar] [CrossRef]
- Loisel, J.; Bunsen, M. Abrupt fen-bog transition across southern Patagonia: Timing, causes, and impacts on carbon sequestration. Front. Ecol. Evol. 2020, 8, 273. [Google Scholar] [CrossRef]
- Valderrama, M.M.; Buitrago, D.; Bedoya, M.M.; Benavides, J.C. Changes in macrofossil distribution and recent dinamics of Distichia muscoides peatlands in the Sierra Nevada del Cocuy, Colombia. Caldasia 2017, 39, 79–90. [Google Scholar] [CrossRef]
- Cuello, N.L.; Cleef, A.M. The paramo vegetation of Ramal de Guaramacal, Trujillo State, Venezuela. 2. Azonal vegetation. Phytocoenologia 2009, 39, 389–409. [Google Scholar] [CrossRef]
- Ruthsatz, B. Vegetation and ecology of the high Andean peatlands of Bolivia. Phytocoenologia 2012, 42, 133–179. [Google Scholar] [CrossRef]
- Kleinebecker, T.; Hölzel, N.; Vogel, A. Patterns and gradients of diversity in South Patagonian ombrotrophic peat bogs. Austral Ecol. 2010, 35, 1–12. [Google Scholar] [CrossRef]
- Benavides, J.C.; Campos, L.V.; Uribe, J. A new terrestrial species of Colura (Marchantiophyta: Lejeuneaceae) at tropical high elevations (Boyacá, Colombia). Cryptogam. Bryol. 2020, 41, 141–145. [Google Scholar] [CrossRef]
- Crum, H. Miscellaneous notes on the genus Sphagnum. 6. Contrib. Univ. Mich. Herb. 1995, 20, 129–140. [Google Scholar] [CrossRef]
- Sjörs, H. On the relation between vegetation and electrolytes in North Swedish mire waters. Oikos 1950, 2, 241–258. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Crow, S.E.; Evans, R.J.; Vitt, D.H.; Wieder, R.K. Trade-offs in resource allocation among moss species control decomposition in boreal peatlands. J. Ecol. 2008, 96, 1297–1305. [Google Scholar] [CrossRef]
- Vitt, D.H. An overview of factors that influence the development of Canadian peatlands. Mem. Entomol. Soc. Can. 1994, 126, 7–20. [Google Scholar] [CrossRef]
- Benavides, J.; Crandall-Stotler, B.; Stotler, R. Morphological annotations of rare liverworts from the Northern Andean Paramos. In Proceedings of the Botany, Providence, RI, USA, 31 July–4 August 2010. [Google Scholar]
- Crum, H.; Crosby, M.R. A New Sphagnum from High Altitude Costa Rica. Ann. Mo. Bot. Gard. 1974, 61, 904–906. [Google Scholar] [CrossRef]
- Fritz, C.; van Dijk, G.; Smolders, A.J.P.; Pancotto, V.A.; Elzenga, T.J.T.M.; Roelofs, J.G.M.; Grootjans, A.P. Nutrient additions in pristine Patagonian Sphagnum bog vegetation: Can phosphorus addition alleviate (the effects of) increased nitrogen loads. Plant Biol. 2012, 14, 491–499. [Google Scholar] [CrossRef]
- Vitt, D.H.; Halsey, L.A.; Bauer, I.E.; Campbell, C. Spatial and temporal trends in carbon storage of peatlands of continental western Canada through the Holocene. Can. J. Earth Sci. 2000, 37, 683–693. [Google Scholar] [CrossRef]
- Urbina, J.C.; Benavides, J.C. Simulated Small Scale Disturbances Increase Decomposition Rates and Facilitates Invasive Species Encroachment in a High Elevation Tropical Andean Peatland. Biotropica 2015, 47, 143–151. [Google Scholar] [CrossRef]
- Vitt, D.H.; Wieder, R.K.; Scott, K.D.; Faller, S. Decomposition and peat accumulation in rich fens of boreal Alberta, Canada. Ecosystems 2009, 12, 360–373. [Google Scholar] [CrossRef]
- Sklenář, P. Searching for altitudinal zonation: Species distribution and vegetation composition in the superpáramo of Volcán Iliniza, Ecuador. Plant Ecol. 2006, 184, 337–350. [Google Scholar] [CrossRef]
- Ricaurte, L.F.; Patiño, J.E.; Zambrano, D.F.R.; Arias-G, J.C.; Acevedo, O.; Aponte, C.; Medina, R.; González, M.; Rojas, S.; Flórez, C. A Classification System for Colombian Wetlands: An Essential Step Forward in Open Environmental Policy-Making. Wetlands 2019, 39, 971–990. [Google Scholar] [CrossRef]
- Junk, W.J.; Piedade, M.T.F.; Lourival, R.; Wittmann, F.; Kandus, P.; Lacerda, L.D.; Bozelli, R.L.; Esteves, F.; Nunes da Cunha, C.; Maltchik, L. Brazilian wetlands: Their definition, delineation, and classification for research, sustainable management, and protection. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 24, 5–22. [Google Scholar] [CrossRef]
- van der Hammen, T.; Cleef, A.M. Development of the high phamos flora and vegetation. In High Altitude Tropical Biogeography; Vuilleumier, F., Monasterio, M., Eds.; Oxford University Press: New York, NY, USA, 1986; pp. 153–201. [Google Scholar]
- Squeo, F.A.; Warner, B.G.; Aravena, R.; Espinoza, D. Bofedales: High altitude peatlands of the central Andes. Rev. Chil. Hist. Nat. 2006, 79, 245–255. [Google Scholar] [CrossRef]
- Gumbricht, T.; Roman-Cuesta, R.M.; Verchot, L.; Herold, M.; Wittmann, F.; Householder, E.; Herold, N.; Murdiyarso, D. An expert system model for mapping tropical wetlands and peatlands reveals South America as the largest contributor. Glob. Chang. Biol. 2017, 23, 3581–3599. [Google Scholar] [CrossRef]
- Yu, Z.; Loisel, J.; Brosseau, D.P.; Beilman, D.W.; Hunt, S.J. Global peatland dynamics since the Last Glacial Maximum. Geophys. Res. Lett. 2010, 37, L13402. [Google Scholar] [CrossRef]
- Keddy, P.A. Competitive hierarchies and centrifugal organization in plant communities. Perspect. Plant Compet. 1990, 265, 90. [Google Scholar]
- Burt, R. Soil Survey Laboratory Methods Manual. Version 4.0; United States Department of Agriculture: Washington, DC, USA, 2004.
- Turetsky, M.; Wieder, R.; Williams, C.; Vitt, D. Organic matter accumulation, peat chemistry, and permafrost melting in peatlands of boreal Alberta. Ecoscience 2000, 7, 379–392. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R package version 1.3-5. 2021. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 15 September 2022).
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R, 2nd ed.; Springer: New York, NY, USA, 2018; p. 440. [Google Scholar]
- Anderson, M.J.; Walsh, D.C. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- Faith, D.P.; Minchin, P.R.; Belbin, L. Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 1987, 69, 57–68. [Google Scholar] [CrossRef]
- Kantvilas, G.; Minchin, P.R. An analysis of epiphytic lichen communities in Tasmanian cool temperate rainforest. Vegetatio 1989, 84, 99–112. [Google Scholar] [CrossRef]
- Crawley, M. The R Book; Wiley: Chichester, UK, 2007; p. 950. [Google Scholar]

| Species | Cushion n = 24 | Sedges n = 22 | Sphagnum n = 26 | True mosses n = 31 | Grasses n = 18 |
|---|---|---|---|---|---|
| Ageratina tinifolia | 0 | 0 | 2 * | 1.5 | 0 |
| Arcytophyllum muticum | 0 | 4.3 | 6 | 4.4 * | 0 |
| Astrophyllum nigrescens | 3.9 * | 0 | 4 | 2 | 1 |
| Blechnum loxense | 0 | 2 | 2.8 * | 1.3 | 0 |
| Breutelia chrysea | 0 | 0 | 0 | 0 * | 0 |
| Calamagrostis effussa | 2.1 | 5.4 | 6.1 | 3.7 | 6.4 * |
| Campylopus cuspidatus | 3.4 | 4.4 * | 4 | 4 | 2.9 |
| Carex pygmaea | 4.3 | 5.4 | 2.8 | 1.7 | 11.5 * |
| Disterigma alaternoides | 0 | 1 | 0 | 3 * | 1 |
| Distichia muscoides | 18.8 * | 0 | 0 | 0 | 6 |
| Espeletia grandiflora | 0 | 4.1 * | 3 | 1 | 2.5 |
| Espeletia occidentalis | 0 | 0 | 0 | 0 | 3.7 * |
| Hypnum cupressiforme | 1 | 2 | 1.7 | 3.3 * | 0 |
| Juncus effusus | 0 | 0 | 1.9 * | 1 | 0 |
| Lophozia sp | 3 * | 0 | 0 | 0 | 1 |
| Nertera gradensis | 1 | 2.3 | 3.5 * | 2.8 | 3 |
| Oreobolus cleefii | 0 | 0 | 0 | 0 | 6.7 * |
| Paepalanthus colombianus | 0 | 2.3 | 2.7 * | 2.3 | 2.8 |
| Pernettya prostrata | 3 | 5.7 * | 3.6 | 2 | 6.4 |
| Puya goudutia | 0 | 2.5 * | 1.6 | 1.7 | 0 |
| Rhacoccarpus purpurascens | 4.5 | 4.5 | 0 | 4 | 8.1 * |
| Rhynchospora oreoboloidea | 1.9 | 3.3 | 0 | 4.4 | 6.6 * |
| Riccardia paramorum | 5.7 | 5.9 * | 3.6 | 3.4 | 3.9 |
| Sphagnum magellanicum | 0 | 9.4 * | 8.4 | 4.6 | 5.5 |
| Sphagnum sancto-josephense | 8 | 8.4 | 12.7 | 10.6 * | 9.8 |
| Warnstorfia exannulata | 4.6 * | 0 | 0 | 1 | 3 |
| Werneria pygmaea | 6.4 * | 0 | 0 | 1 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benavides, J.C.; Vitt, D.H.; Cooper, D.J. The High-Elevation Peatlands of the Northern Andes, Colombia. Plants 2023, 12, 955. https://doi.org/10.3390/plants12040955
Benavides JC, Vitt DH, Cooper DJ. The High-Elevation Peatlands of the Northern Andes, Colombia. Plants. 2023; 12(4):955. https://doi.org/10.3390/plants12040955
Chicago/Turabian StyleBenavides, Juan C., Dale H. Vitt, and David J. Cooper. 2023. "The High-Elevation Peatlands of the Northern Andes, Colombia" Plants 12, no. 4: 955. https://doi.org/10.3390/plants12040955
APA StyleBenavides, J. C., Vitt, D. H., & Cooper, D. J. (2023). The High-Elevation Peatlands of the Northern Andes, Colombia. Plants, 12(4), 955. https://doi.org/10.3390/plants12040955






