Physiological and Transcriptomic Evaluation of Drought Effect on Own-Rooted and Grafted Grapevine Rootstock (1103P and 101-14MGt)
Abstract
1. Introduction
2. Results
2.1. Phenotypic Response of Own-Rooted Plants to Water Deficit
2.2. Phenotypic Response of Grafted Plants to Water Deficit
2.3. Interaction of Genotype and Presence of Scion on Phenotypic Traits
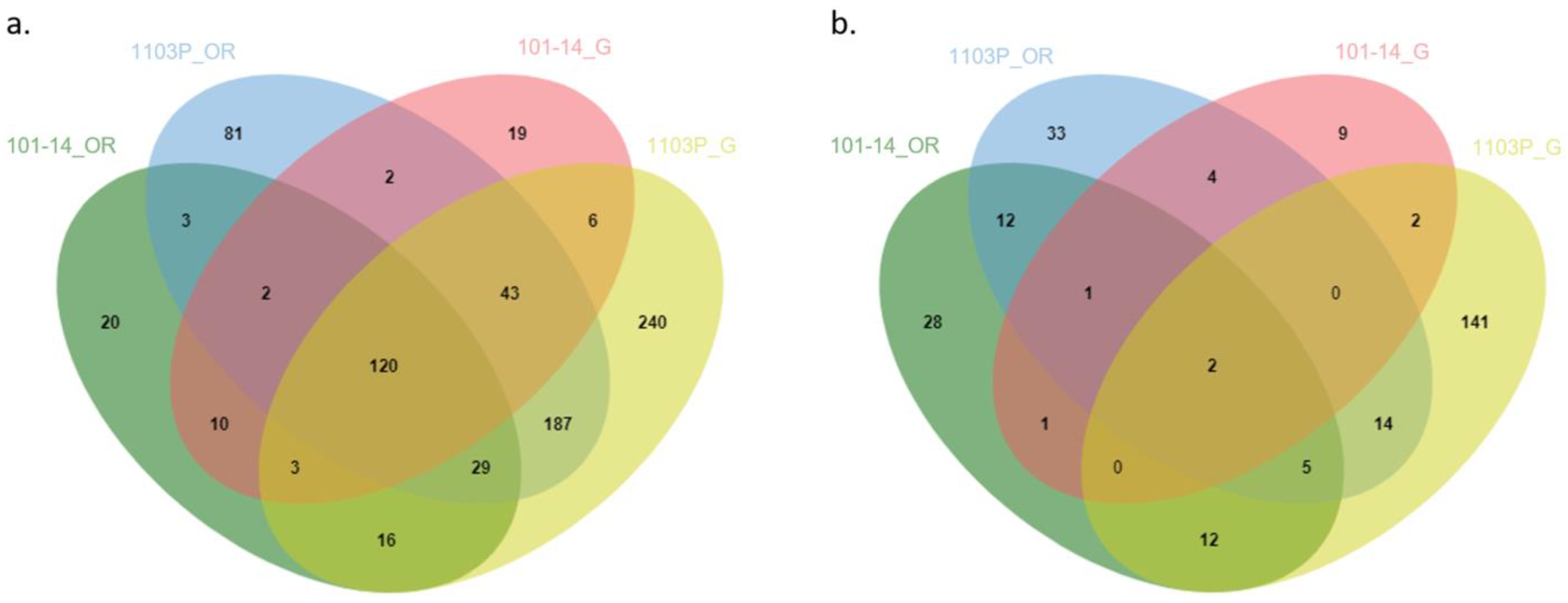
2.4. Transcriptomic Response of Own-Rooted and Grafted Plants to Water Deficit
2.5. Gene Expression Pattern of Genes Involved in ABA Metabolism in Both Own-Rooted and Grafted Plants
3. Discussion
3.1. Two Rootstock Genotype Models for Water Deficit Response
3.2. Grafting Combinations with V. vinifera Reflects the Rootstock Responses
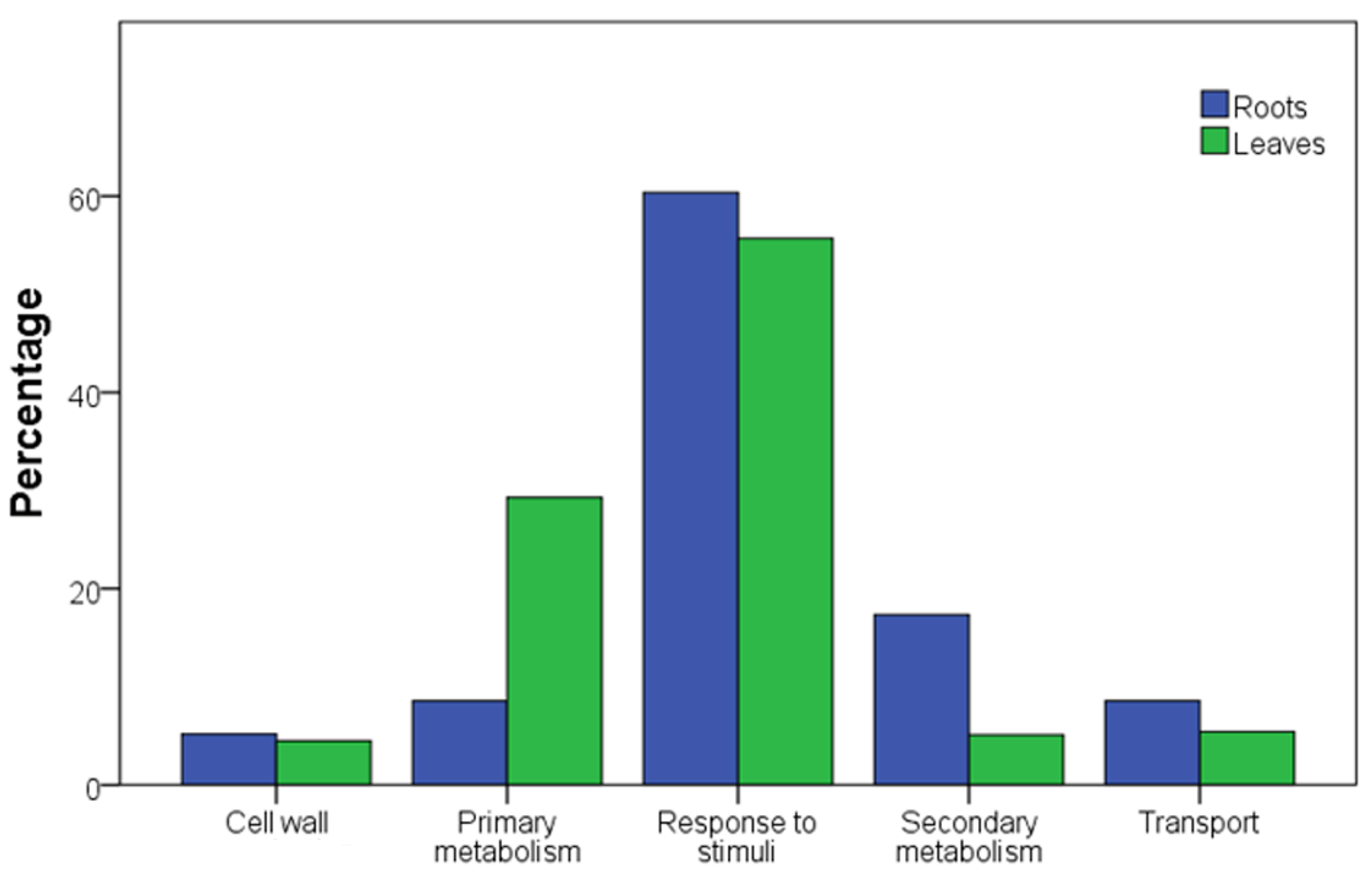
3.3. Roots Are the More Responsive Organ in Perceiving the Water Deficit
3.4. A Core Set of Genes Is Regulated in Roots of Both Rootstock Genotypes under Severe Water Deficit
3.5. 1103P Shows a Greater Transcriptomic Responsiveness at the Root Level
3.6. Rootstock Influences the Scion Leaf Transcriptome under Water Deficit
3.7. ABA Content Is Affected by Grafting under Water Deficit
4. Material and Methods
4.1. Plant Material and Growth Conditions
4.2. Irrigation Management
4.3. Plant Phenotyping for Drought Response
4.4. Abscisic Acid (ABA) Detection in Roots and Leaves
4.5. Total RNA Extraction
4.6. Library Construction and Sequencing
4.7. Sequence Annotation
4.8. Identification of Differentially Expressed Genes (DEGs)
4.9. Real-Time RT-PCR of Genes Involved in the ABA Metabolism
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, E.A. Plant responses to water deficit. Trends Plant Sci. 1997, 2, 48–54. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef] [PubMed]
- Charrier, G.; Delzon, S.; Domec, J.; Zhang, L.; Delmas, C.E.L.; Merlin, I.; Corso, D.; King, A.; Ojeda, H.; Ollat, N.; et al. Drought will not leave your glass empty: Low risk of hydraulic failure revealed by long-term drought observations in world’s top wine regions. Sci. Adv. 2018, 4, eaao6969. [Google Scholar] [CrossRef] [PubMed]
- Shultz, H.R.; Matthews, M.A. Vegetative Growth Distribution during Water Deficits in Vitis vinifera L. Aust. J. Plant Physiol. 1988, 15, 641–656. [Google Scholar] [CrossRef]
- Lovisolo, C.; Schubert, A. Effects of water stress on vessel size and xylem hydraulic conductivity in Vitis vinifera L. J. Exp. Bot. 1998, 49, 693–700. [Google Scholar]
- Serra, I.; Strever, A.; Myburgh, P.A.; Deloire, A. Review: The interaction between rootstocks and cultivars (Vitis vinifera L.) to enhance drought tolerance in grapevine. Aust. J. Grape Wine Res. 2014, 20, 1–14. [Google Scholar] [CrossRef]
- Tyree, M.T.; Sperry, J.S. Vulnerability of xylem to cavitation and embolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 19–38. [Google Scholar] [CrossRef]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Zhang, L.; Marguerit, E.; Rossdeutsch, L.; Ollat, N.; Gambetta, G.A. The influence of grapevine rootstocks on scion growth and drought resistance. Theory Exp. Plant Physiol. 2016, 28, 143–157. [Google Scholar] [CrossRef]
- Munemasa, S.; Hauser, F.; Park, J.; Waadt, R.; Brandt, B.; Schroeder, J.I. Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr. Opin. Plant Biol. 2015, 28, 154–162. [Google Scholar] [CrossRef]
- Allen, G.J.; Chu, S.P.; Harrington, C.L.; Schumacher, K.; Hoffmann, T.; Tang, Y.Y.; Grill, E.; Schroeder, J.I. A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 2001, 411, 1053–1057. [Google Scholar] [CrossRef]
- Yordanov, I.; VelikoVa, V.; Tsonev, T. Plant responses to drought, acclimation, and stress tolerance. Photosynthetica. 2000, 38, 171–186. [Google Scholar] [CrossRef]
- Escalona, J.M.; Flexas, J.; Medrano, H. Stomatal and non-stomatal limitations of photosynthesis under water stress in field-grown grapevines. Funct. Plant Biol. 2000, 27, 87. [Google Scholar] [CrossRef]
- Salleo, S.; Trifilò, P.; Esposito, S.; Andrea, N.; Lo Gullo, M. Starch-to-sugar conversion in wood parenchyma of field-growing Laurus nobilis plants: A component of the signal pathway for embolism repair? Funct. Plant Biol. 2009, 36, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Keller, M. The Science of Grapevines. Anatomy and Physiology; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780124199873. [Google Scholar]
- Carvalho, L.C.; Vidigal, P.; Amâncio, S. Oxidative stress homeostasis in grapevine (Vitis vinifera L.). Front. Environ. Sci. 2015, 3, 20. [Google Scholar] [CrossRef]
- Vorwerk, S.; Forneck, A. Reproductive mode of grape phylloxera (Daktulosphaira vitifoliae, Homoptera: Phylloxeridae) in Europe: Molecular evidence for predominantly asexual populations and a lack of gene flow between them. Genome 2006, 49, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, L.; Tarczal, E.; Kàllay, M. The effect of rootstocks on the productivity and fruit composition of Vitis vinifera L. ‘Cabernet Sauvignon’ and ‘Kékfrankos’. Acta Hortic. 2012, 931, 403–412. [Google Scholar] [CrossRef]
- Mullins, M.G.; Bouquet, A.; Williams, L.E. Biology of the Grapevine. In The Biology of Horticultural Crops; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Rossdeutsch, L.; Edwards, E.; Cookson, S.J.; Barrieu, F.; Gambetta, G.A.; Delrot, S.; Ollat, N. ABA-mediated responses to water deficit separate grapevine genotypes by their genetic background. BMC Plant Biol. 2016, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Marguerit, E.; Brendel, O.; Lebon, E.; Van Leeuwen, C.; Ollat, N. Rootstock control of scion transpiration and its acclimation to water deficit are controlled by different genes. New Phytol. 2012, 194, 416–429. [Google Scholar] [CrossRef]
- Corso, M.; Vannozzi, A.; Maza, E.; Vitulo, N.; Meggio, F.; Pitacco, A.; Telatin, A.; D’Angelo, M.; Feltrin, E.; Negri, A.S.; et al. Comprehensive transcript profiling of two grapevine rootstock genotypes contrasting in drought susceptibility links the phenylpropanoid pathway to enhanced tolerance. J. Exp. Bot. 2015, 66, 5739–5752. [Google Scholar] [CrossRef]
- Bianchi, D.; Caramanico, L.; Grossi, D.; Brancadoro, L.; De Lorenzis, G. How do novel m-rootstock (Vitis spp.) genotypes cope with drought? Plants 2020, 9, 1385. [Google Scholar] [CrossRef]
- Soar, C.J.; Dry, P.R.; Loveys, B.R. Scion photosynthesis and leaf gas exchange in Vitis vinifera L. cv. Shiraz: Mediation of rootstock effects via xylem sap ABA. Aust. J. Grape Wine Res. 2006, 12, 82–96. [Google Scholar] [CrossRef]
- Lovisolo, C.; Lavoie-lamoureux, A.; Tramontini, S.; Ferrandino, A. Grapevine adaptations to water stress: New perspectives about soil/plant interactions. Theor. Exp. Plant Physiol. 2016, 28, 53–66. [Google Scholar] [CrossRef]
- Alsina, M.M.; Smart, D.R.; Bauerle, T.; De Herralde, F.; Biel, C.; Stockert, C.; Negron, C.; Save, R. Seasonal changes of whole root system conductance by a drought-tolerant grape root system. J. Exp. Bot. 2011, 62, 99–109. [Google Scholar] [CrossRef]
- Koundouras, S.; Tsialtas, I.T.; Zioziou, E.; Nikolaou, N. Rootstock effects on the adaptive strategies of grapevine (Vitis vinifera L. cv. Cabernet-Sauvignon) under contrasting water status: Leaf physiological and structural responses. Agric. Ecosyst. Environ. 2008, 128, 86–96. [Google Scholar] [CrossRef]
- Reingwirtz, I.; Uretsky, J.; Cuneo, I.F.; Knipfer, T.; Reyes, C.; Walker, M.A.; McElrone, A.J. Inherent and stress-induced responses of fine root morphology and anatomy in commercial grapevine rootstocks with contrasting drought resistance. Plants 2021, 10, 1121. [Google Scholar] [CrossRef]
- Meggio, F.; Prinsi, B.; Negri, A.S.; Simone Di Lorenzo, G.; Lucchini, G.; Pitacco, A.; Failla, O.; Scienza, A.; Cocucci, M.; Espen, L. Biochemical and physiological responses of two grapevine rootstock genotypes to drought and salt treatments. Aust. J. Grape Wine Res. 2014, 20, 310–323. [Google Scholar] [CrossRef]
- Chitarra, W.; Perrone, I.; Avanzato, C.G.; Minio, A.; Boccacci, P.; Santini, D.; Gilardi, G.; Siciliano, I.; Gullino, M.L.; Delledonne, M.; et al. Grapevine Grafting: Scion Transcript Profiling and Defense-Related Metabolites Induced by Rootstocks. Front. Plant Sci. 2017, 8, 654. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Min, Z.; Zhang, Y.; Zhang, K.; Liu, M.; Fang, Y. Transcriptome profiling provide new insights into the molecular mechanism of grapevine response to heat, drought, and combined stress. Sci. Hortic. (Amst.) 2021, 286, 110076. [Google Scholar] [CrossRef]
- Barrios-Masias, F.H.; Knipfer, T.; Walker, M.A.; McElrone, A.J. Differences in hydraulic traits of grapevine rootstocks are not conferred to a common Vitis vinifera scion. Funct. Plant Biol. 2019, 46, 228–235. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; Van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Ollat, N.; Cookson, S.J.; Destrac-Irvine, A.; Lauvergeat, V.; Ouaked-Lecourieux, F.; Marguerit, E.; Barrieu, F.; Dai, Z.; Duchêne, E.; Gambetta, G.A.; et al. Grapevine adaptation to abiotic stress: An overview. Acta Hortic. 2019, 1248, 497–512. [Google Scholar] [CrossRef]
- Delzon, S. New insight into leaf drought tolerance. Funct. Ecol. 2015, 29, 1247–1249. [Google Scholar] [CrossRef]
- Frioni, T.; Biagioni, A.; Squeri, C.; Tombesi, S.; Gatti, M.; Poni, S. Grafting cv. grechetto gentile vines to new m4 rootstock improves leaf gas exchange and water status as compared to commercial 1103p rootstock. Agronomy 2020, 10, 708. [Google Scholar] [CrossRef]
- Herrera, J.C.; Calderan, A.; Gambetta, G.A.; Peterlunger, E.; Forneck, A.; Sivilotti, P.; Cochard, H.; Hochberg, U. Stomatal responses in grapevine become increasingly more tolerant to low water potentials throughout the growing season. Plant J. 2022, 109, 804–815. [Google Scholar] [CrossRef]
- Hochberg, U.; Degu, A.; Fait, A.; Rachmilevitch, S. Near isohydric grapevine cultivar displays higher photosynthetic efficiency and photorespiration rates under drought stress as compared with near anisohydric grapevine cultivar. Physiol. Plant. 2013, 147, 443–452. [Google Scholar] [CrossRef]
- Rattanakon, S.; Ghan, R.; Gambetta, G.A.; Deluc, L.G.; Schlauch, K.A.; Cramer, G.R. Abscisic acid transcriptomic signaling varies with grapevine organ. BMC Plant Biol. 2016, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Gautier, A.T.; Cochetel, N.; Merlin, I.; Hevin, C.; Lauvergeat, V.; Vivin, P.; Mollier, A.; Ollat, N.; Cookson, S.J. Scion genotypes exert long distance control over rootstock transcriptome responses to low phosphate in grafted grapevine. BMC Plant Biol. 2020, 20, 367. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Guzman, M.; Pizzio, G.A.; Antoni, R.; Vera-Sirera, F.; Merilo, E.; Bassel, G.W.; Fernández, M.A.; Holdsworth, M.J.; Perez-Amador, M.A.; Kollist, H. Arabidopsis PYR/PYL/RCAR Receptors Play a Major Role in Quantitative Regulation of Stomatal Aperture and Transcriptional Response to Abscisic Acid. Plant Cell 2012, 24, 2483–2496. [Google Scholar] [CrossRef]
- Dunwell, J.M.; Gibbings, J.G.; Mahmood, T.; Saqlan Naqvi, S.M. Germin and Germin-like Proteins: Evolution, Structure, and Function. CRC. Crit. Rev. Plant Sci. 2008, 27, 342–375. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Loosening of plant cell walls by expansins. Nature 2000, 407, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, J.; Pak, S.; Zeng, M.; Sun, J.; Yu, S.; He, Y.; Li, C. PuC3H35 confers drought tolerance by enhancing lignin and proanthocyanidin biosynthesis in the roots of Populus ussuriensis. New Phytol. 2022, 233, 390–408. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, J.; Wang, C.; Pang, Y.; Li, L.; Tang, X.; Li, B.; Sun, Q. Genome-wide analysis of the strigolactone biosynthetic and signaling genes in grapevine and their response to salt and drought stresses. PeerJ 2022, 10, e13551. [Google Scholar] [CrossRef]
- Gautam, T.; Dutta, M.; Jaiswal, V.; Zinta, G.; Gahlaut, V.; Kumar, S. Emerging Roles of SWEET Sugar Transporters in Plant Development and Abiotic Stress Responses. Cells 2022, 11, 1303. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and Raffinose Constitute a Novel Function to Protect Plants from Oxidative Damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Frei, B.; Eisenach, C.; Martinoia, E.; Hussein, S.; Chen, X.-Z.; Arrivault, S.; Neuhaus, H.E. Purification and functional characterization of the vacuolar malate transporter tDT from Arabidopsis. J. Biol. Chem. 2018, 293, 4180–4190. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Li, S.; Zhang, L.; Qi, C.; Weeda, S.; Zhao, B.; Ren, S.; Guo, Y.D. Plasma membrane intrinsic proteins SlPIP2;1, SlPIP2;7 and SlPIP2;5 conferring enhanced drought stress tolerance in Tomato. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Nolan, T.M.; Jiang, H.; Yin, Y. AP2/ERF Transcription Factor Regulatory Networks in Hormone and Abiotic Stress Responses in Arabidopsis. Front. Plant Sci. 2019, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Shouyi, C. Plant protein kinase genes induced by drought, high salt and cold stresses. Chin. Sci. Bull. 2000, 45, 1153–1157. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Q.; Li, P.; Zhang, S.; Liu, C.; Jin, J.; Cao, P.; Yang, Y. Carotenoid Cleavage Dioxygenases: Identification, Expression, and Evolutionary Analysis of This Gene Family in Tobacco. Int. J. Mol. Sci. 2019, 20, 5796. [Google Scholar] [CrossRef]
- Wei, K.; Chen, H. Global identification, structural analysis and expression characterization of cytochrome P450 monooxygenase superfamily in rice. BMC Genom. 2018, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Laxmi, A. Transcriptional regulation of drought response: A tortuous network of transcriptional factors. Front. Plant Sci. 2015, 6, 895. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Meng, F.; Qi, H.; Mo, F.; Wang, P.; Chen, X.; Wang, A. Escaping drought: The pectin methylesterase inhibitor gene Slpmei27 can significantly change drought resistance in tomato. Plant Physiol. Biochem. 2022, 192, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Braam, J. Diversity of Genes, Enzymes and Potential Wall-Modifying Functions. Trends Plant Sci. 1999, 4, 361–366. [Google Scholar] [CrossRef]
- Rocheta, M.; Becker, J.D.; Coito, J.L.; Carvalho, L.; Amâncio, S. Heat and water stress induce unique transcriptional signatures of heat-shock proteins and transcription factors in grapevine. Funct. Integr. Genom. 2014, 14, 135–148. [Google Scholar] [CrossRef]
- Kumar, D.; Al Hassan, M.; Naranjo, M.A.; Agrawal, V.; Boscaiu, M.; Vicente, O. Effects of salinity and drought on growth, ionic relations, compatible solutes and activation of antioxidant systems in oleander (Nerium oleander L.). PLoS ONE 2017, 12, e0185017. [Google Scholar] [CrossRef]
- Watkins, J.; Chapman, J.M.; Muday, G.K. Abscisic acid-induced reactive oxygen species are modulated by flavonols to control stomata aperture. Plant Physiol. 2017, 175, 1807–1825. [Google Scholar] [CrossRef]
- Marè, C.; Aprile, A.; Roncaglia, E.; Tocci, E. Rootstock and soil induce transcriptome modulation of phenylpropanoid pathway in grape leaves. J. Plant Interact. 2012, 8, 334–349. [Google Scholar] [CrossRef]
- Johnson, K.L.; Jones, B.J.; Bacic, A.; Schultz, C.J. The Fasciclin-Like Arabinogalactan Proteins of Arabidopsis. A Multigene Family of Putative Cell Adhesion Molecules. Plant Physiol. 2003, 133, 1911–1925. [Google Scholar] [CrossRef]
- Yao, X.; Xiong, W.; Ye, T.; Wu, Y. Overexpression of the aspartic protease ASPG1 gene confers drought avoidance in Arabidopsis. J. Exp. Bot. 2012, 63, 2579–2593. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, W.; Zhou, B.; Wang, Z.; Qian, Y.; Wang, B.; Yan, L. Genome-wide analysis of the SCPL gene family in grape (Vitis vinifera L.). J. Integr. Agric. 2021, 20, 2666–2679. [Google Scholar] [CrossRef]
- Marusig, D.; Tombesi, S. Abscisic Acid Mediates Drought and Salt Stress Responses in Vitis vinifera—A Review. Int. J. Mol. Sci. 2020, 21, 8648. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.M.K.; Robinson, D.; Blyth, K.; Cooper, J.D. Soil Water Content. In Soil and Environmental Analysis: Physical Methods; Smith, K.A., Mullins, C.E., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2001; pp. 13–20. [Google Scholar]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap Pressure in Vascular Plants: Negative hydrostatic pressure can be measured in plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Mairata, A.; Tortosa, I.; Douthe, C.; Escalona, J.M.; Pou, A.; Medrano, H. Comparing Selection Criteria to Select Grapevine Clones by Water Use Efficiency. Agronomy 2022, 12, 1963. [Google Scholar] [CrossRef]
- Fischer, R.A.; Turner, N.C. Plant Productivity in the Arid and Semiarid Zones. Annu. Rev. Plant Physiol. 1978, 29, 277–317. [Google Scholar] [CrossRef]
- Enoki, S.; Hattori, T.; Ishiai, S.; Tanaka, S.; Mikami, M.; Arita, K.; Nagasaka, S.; Suzuki, S. Vanillylacetone up-regulates anthocyanin accumulation and expression of anthocyanin biosynthetic genes by inducing endogenous abscisic acid in grapevine tissues. J. Plant Physiol. 2017, 219, 22–27. [Google Scholar] [CrossRef]
- Toffolatti, S.L.; De Lorenzis, G.; Costa, A.; Maddalena, G.; Passera, A.; Bonza, M.C.; Pindo, M.; Stefani, E.; Cestaro, A.; Casati, P.; et al. Unique resistance traits against downy mildew from the center of origin of grapevine (Vitis vinifera). Sci. Rep. 2018, 8, 12523. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- R Core Team. R: A language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. Available online: http://www.R-project.org/ (accessed on 20 February 2023).
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Alexa, A.; Rahnenfuhrer, J. TopGO: Enrichment Analysis for Gene Ontology. R Package Version 2.24.0. 2016. Available online: http://bioinformatics.cau.edu.cn/easygo/ (accessed on 20 February 2023).
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. Jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Warnes, G.R.; Bolker, B.; Lumley, T. Gplots: Various R programming Tools for Plotting Data. 2005. Available online: https://cran.microsoft.com/snapshot/2016-03-30/web/packages/gplots/gplots.pdf (accessed on 20 February 2023).
- Merilo, E.; Jalakas, P.; Kollist, H.; Brosché, M. The Role of ABA Recycling and Transporter Proteins in Rapid Stomatal Responses to Reduced Air Humidity, Elevated CO2, and Exogenous ABA. Mol. Plant 2015, 8, 657–659. [Google Scholar] [CrossRef]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A.M. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef] [PubMed]
- Fujita, A.; Soma, N.; Goto-yamamoto, N.; Mizuno, A.; Kiso, K.; Hashizume, K. Effect of Shading on Proanthocyanidin Biosynthesis in the Grape Berry. J. Jpn. Soc. Hortic. Sci. 2007, 76, 112–119. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tandonnet, J.P.; Cookson, S.J.; Vivin, P.; Ollat, N. Scion genotype controls biomass allocation and root development in grafted grapevine. Aust. J. Grape Wine Res. 2010, 16, 290–300. [Google Scholar] [CrossRef]
- Migicovsky, Z.; Cousins, P.; Jordan, L.M.; Myles, S.; Striegler, R.K.; Verdegaal, P.; Chitwood, D.H. Grapevine rootstocks affect growth-related scion phenotypes. Plant Direct 2021, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis—Bookreview. J. Stat. Softw. 2010, 35, 1–3. [Google Scholar]
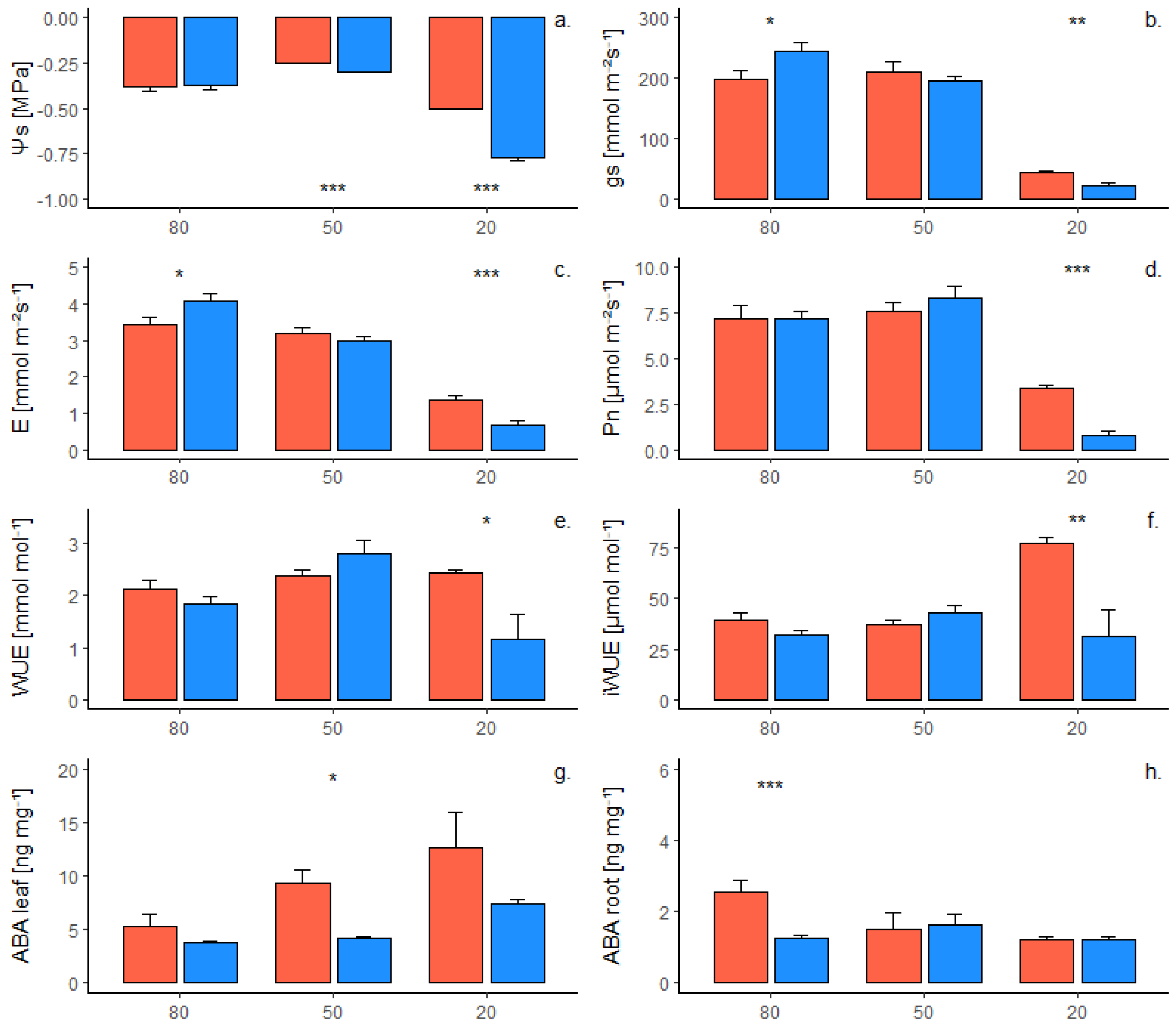
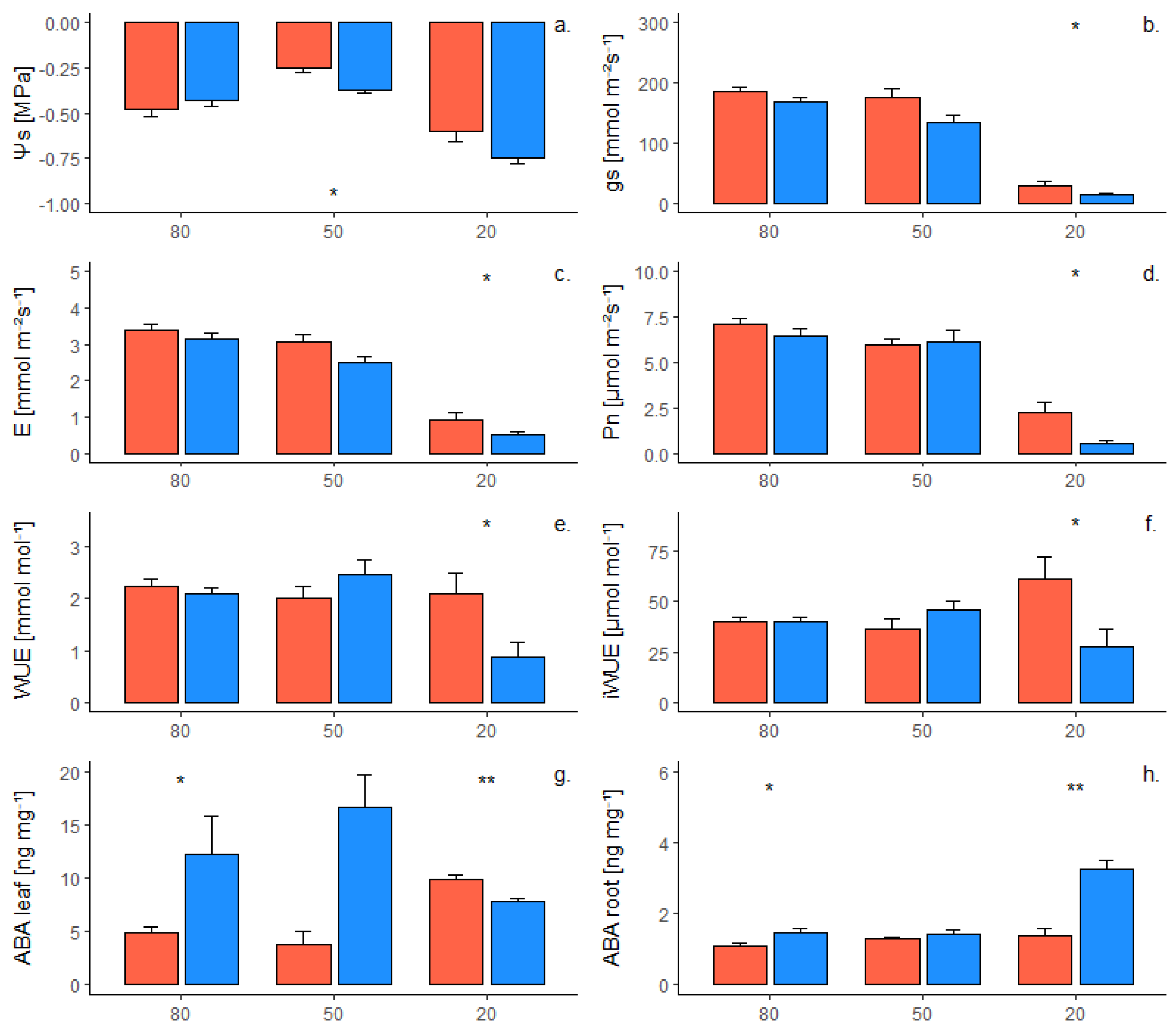
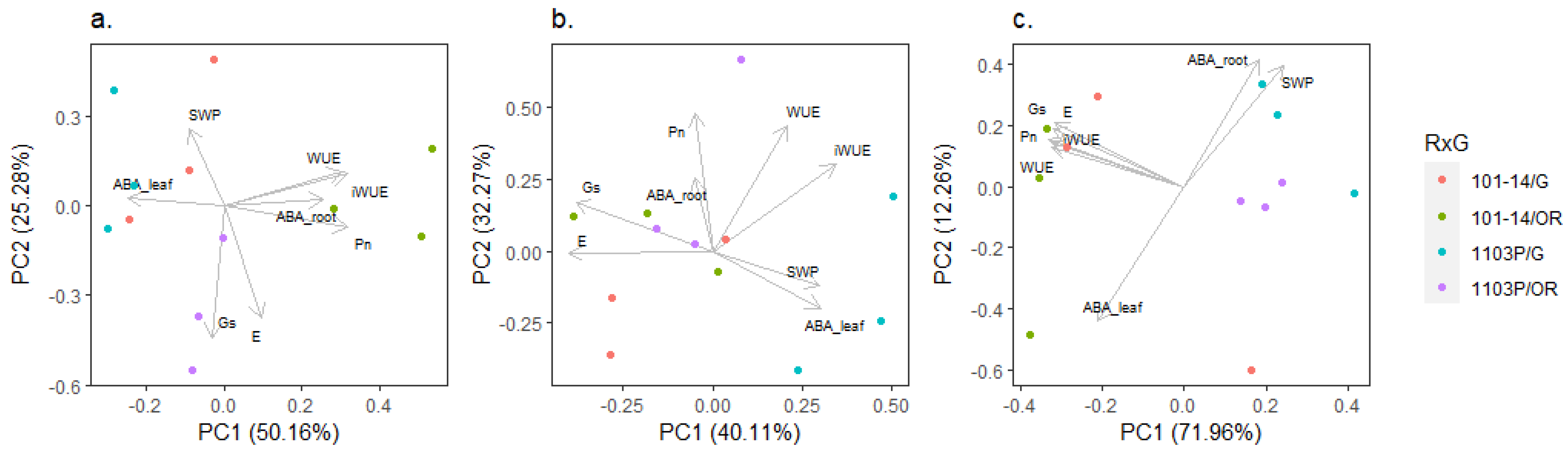


| Trait | Genotype | a | b | R2 | p Value | Sig. |
|---|---|---|---|---|---|---|
| gs 1 | 101-14MGt | 323.80 | 375.50 | 0.235 | 0.042 | * |
| 1103P | 375.30 | 404.40 | 0.575 | 0.000 | *** | |
| Pn 2 | 101-14MGt | 3.90 | 1.88 | 0.031 | 0.484 | |
| 1103P | 5.78 | 5.65 | 0.491 | 0.001 | ** | |
| E 3 | 101-14MGt | 8.79 | 4.62 | 0.024 | 0.538 | |
| 1103P | 11.93 | 13.44 | 0.656 | 0.047 | *** | |
| WUE 4 | 101-14MGt | 2.18 | −0.13 | 0.001 | 0.925 | |
| 1103P | 2.79 | 2.24 | 0.293 | 0.020 | * | |
| ABA 5 leaf | 101-14MGt | 5.76 | −3.06 | 0.004 | 0.805 | |
| 1103P | 1.92 | −5.68 | 0.413 | 0.004 | ** | |
| ABA root | 101-14MGt | 2.25 | 0.30 | 0.001 | 0.915 | |
| 1103P | 1.48 | 0.42 | 0.050 | 0.374 |
| Trait | Genotype | a | b | R2 | p Value | Sig. |
|---|---|---|---|---|---|---|
| gs 1 | 101-14MGt | 198.39 | 80.38 | 0.034 | 0.465 | |
| 1103P | 243.50 | 211.60 | 0.223 | 0.048 | * | |
| Pn 2 | 101-14MGt | 2.89 | −0.14 | 0.000 | 0.939 | |
| 1103P | 4.30 | 3.44 | 0.221 | 0.049 | * | |
| E 3 | 101-14MGt | 7.67 | 3.55 | 0.077 | 0.263 | |
| 1103P | 9.83 | 9.46 | 0.346 | 0.010 | * | |
| WUE 4 | 101-14MGt | 2.61 | 1.09 | 0.088 | 0.233 | |
| 1103P | 3.09 | 2.46 | 0.311 | 0.016 | * | |
| ABA 5 leaf | 101-14MGt | 1.20 | −9.22 | 0.308 | 0.017 | * |
| 1103P | 18.98 | 14.17 | 0.072 | 0.282 | ||
| ABA root | 101-14MGt | 1.29 | 0.25 | 0.029 | 0.501 | |
| 1103P | 0.39 | −2.88 | 0.323 | 0.014 | * |
| Trait | SWC 8 | Grafting (G) | RIstock (R) | Interaction (R ∗ G) | Error |
|---|---|---|---|---|---|
| Ψs 1 | 80 | 69.2 * | 14.6 | 4.6 | 11.6 |
| 50 | 13.1 * | 71.4 *** | 13.1 * | 2.4 | |
| 20 | 2.7 | 87.6 *** | 7.6 | 2.1 | |
| gs 2 | 80 | 62.6 *** | 4.0 | 29.2 ** | 4.1 |
| 50 | 66.3 ** | 23.2 | 5.0 | 5.5 | |
| 20 | 22.2 * | 72.3 *** | 1.5 | 4.0 | |
| Pn 3 | 80 | 28.5 | 21.5 | 17.8 | 32.2 |
| 50 | 86.4 ** | 4.5 | 1.7 | 7.4 | |
| 20 | 7.5 | 85.9 *** | 3.8 | 2.9 | |
| E 4 | 80 | 51.9 ** | 6.7 | 35.5 * | 5.9 |
| 50 | 30.7 | 50.1* | 9.5 | 9.8 | |
| 20 | 20.6 * | 70.2 *** | 5.1 | 4.2 | |
| WUE 5 | 80 | 34.1 | 41.4 | 4.3 | 20.2 |
| 50 | 33.3 | 52.2 | 0.1 | 14.4 | |
| 20 | 5.1 | 87.9** | 0.1 | 7.0 | |
| iWUE 6 | 80 | 38.8 | 25.1 | 22.9 | 13.2 |
| 50 | 1.4 | 73.0 | 4.6 | 21.0 | |
| 20 | 4.8 | 87.2 *** | 2.0 | 6.1 | |
| ABA 7 leaf | 80 | 35.9 * | 10.3 | 48.1 ** | 5.6 |
| 50 | 17.5 * | 6.4 | 73.9 ** | 2.2 | |
| 20 | 3.8 | 69.6 | 11.1 | 15.5 | |
| ABA root | 80 | 27.2 ** | 15.3* | 55.3 *** | 2.2 |
| 50 | 23.9 | 9.3 | 0.1 | 66.7 | |
| 20 | 40.9 *** | 30.1 *** | 28.1 *** | 0.8 |
| Genotype | Differentially Expressed Genes | ||||||
|---|---|---|---|---|---|---|---|
| T1 (80% SWC) | T2 (50% SWC) | T3 (20% SWC) | |||||
| Roots | Leaves | Roots | Leaves | Roots | Leaves | ||
| Own-rooted | |||||||
| 101-14MGt | Up | - | - | 46 (0.11) | 282 (0.84) | 3157 (8.21) | 976 (2.50) |
| Down | - | - | 44 (0.10) | 155 (0.38) | 1900 (5.63) | 831 (2.23) | |
| Total | - | - | 90 (0.21) | 437 (1.22) | 5057 (13.84) | 1807 (4.73) | |
| 1103P | Up | - | - | 114 (0.24) | 1 (0.00) | 3164 (8.32) | 1463 (3.91) |
| Down | - | - | 98 (0.22) | - | 2166 (6.25) | 1341 (3.56) | |
| Total | - | - | 212 (0.46) | 1 (0.00) | 5330 (14.57) | 2804 (7.37) | |
| Grafted | |||||||
| 101-14MGt | Up | - | - | 42 (0.08) | 56 (0.13) | 2509 (6.32) | 1195 (3.02) |
| Down | - | - | 72 (0.16) | 84 (0.21) | 1478 (4.27) | 492 (1.35) | |
| Total | - | - | 114 (0.25) | 140 (0.34) | 3987 (10.59) | 1687 (4.37) | |
| 1103P | Up | - | - | 350 (0.82) | - | 4162 (11.12) | 1440 (3.93) |
| Down | - | - | 638 (1.50) | 11 (0.02) | 2978 (8.44) | 846 (2.47) | |
| Total | - | - | 988 (2.32) | 11 (0.02) | 7140 (19.56) | 2286 (6.40) | |
| Rootstock | Own-Rooted | Grafted | ||
|---|---|---|---|---|
| Up | Down | Up | Down | |
| Roots | ||||
| 101-14MGt |
|
|
|
|
| 1103P |
|
|
|
|
| Leaves | ||||
| 101-14MGt |
|
|
|
|
| 1103P |
|
|
|
|
| Rootstock | Grafting | Treatment | T1 1 | T2 1 | T3 1 |
|---|---|---|---|---|---|
| 101-14MGt | own-rooted | well-watered | 80% | 80% | 80% |
| water stressed | 80% | 50% | 20% | ||
| grafted with CS | well-watered | 80% | 80% | 80% | |
| water stressed | 80% | 50% | 20% | ||
| 1103P | own-rooted | well-watered | 80% | 80% | 80% |
| water stressed | 80% | 50% | 20% | ||
| grafted with CS | well-watered | 80% | 80% | 80% | |
| water stressed | 80% | 50% | 20% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, D.; Ricciardi, V.; Pozzoli, C.; Grossi, D.; Caramanico, L.; Pindo, M.; Stefani, E.; Cestaro, A.; Brancadoro, L.; De Lorenzis, G. Physiological and Transcriptomic Evaluation of Drought Effect on Own-Rooted and Grafted Grapevine Rootstock (1103P and 101-14MGt). Plants 2023, 12, 1080. https://doi.org/10.3390/plants12051080
Bianchi D, Ricciardi V, Pozzoli C, Grossi D, Caramanico L, Pindo M, Stefani E, Cestaro A, Brancadoro L, De Lorenzis G. Physiological and Transcriptomic Evaluation of Drought Effect on Own-Rooted and Grafted Grapevine Rootstock (1103P and 101-14MGt). Plants. 2023; 12(5):1080. https://doi.org/10.3390/plants12051080
Chicago/Turabian StyleBianchi, Davide, Valentina Ricciardi, Carola Pozzoli, Daniele Grossi, Leila Caramanico, Massimo Pindo, Erika Stefani, Alessandro Cestaro, Lucio Brancadoro, and Gabriella De Lorenzis. 2023. "Physiological and Transcriptomic Evaluation of Drought Effect on Own-Rooted and Grafted Grapevine Rootstock (1103P and 101-14MGt)" Plants 12, no. 5: 1080. https://doi.org/10.3390/plants12051080
APA StyleBianchi, D., Ricciardi, V., Pozzoli, C., Grossi, D., Caramanico, L., Pindo, M., Stefani, E., Cestaro, A., Brancadoro, L., & De Lorenzis, G. (2023). Physiological and Transcriptomic Evaluation of Drought Effect on Own-Rooted and Grafted Grapevine Rootstock (1103P and 101-14MGt). Plants, 12(5), 1080. https://doi.org/10.3390/plants12051080







