Nanocapsules of ZnO Nanorods and Geraniol as a Novel Mean for the Effective Control of Botrytis cinerea in Tomato and Cucumber Plants
Abstract
1. Introduction
2. Results
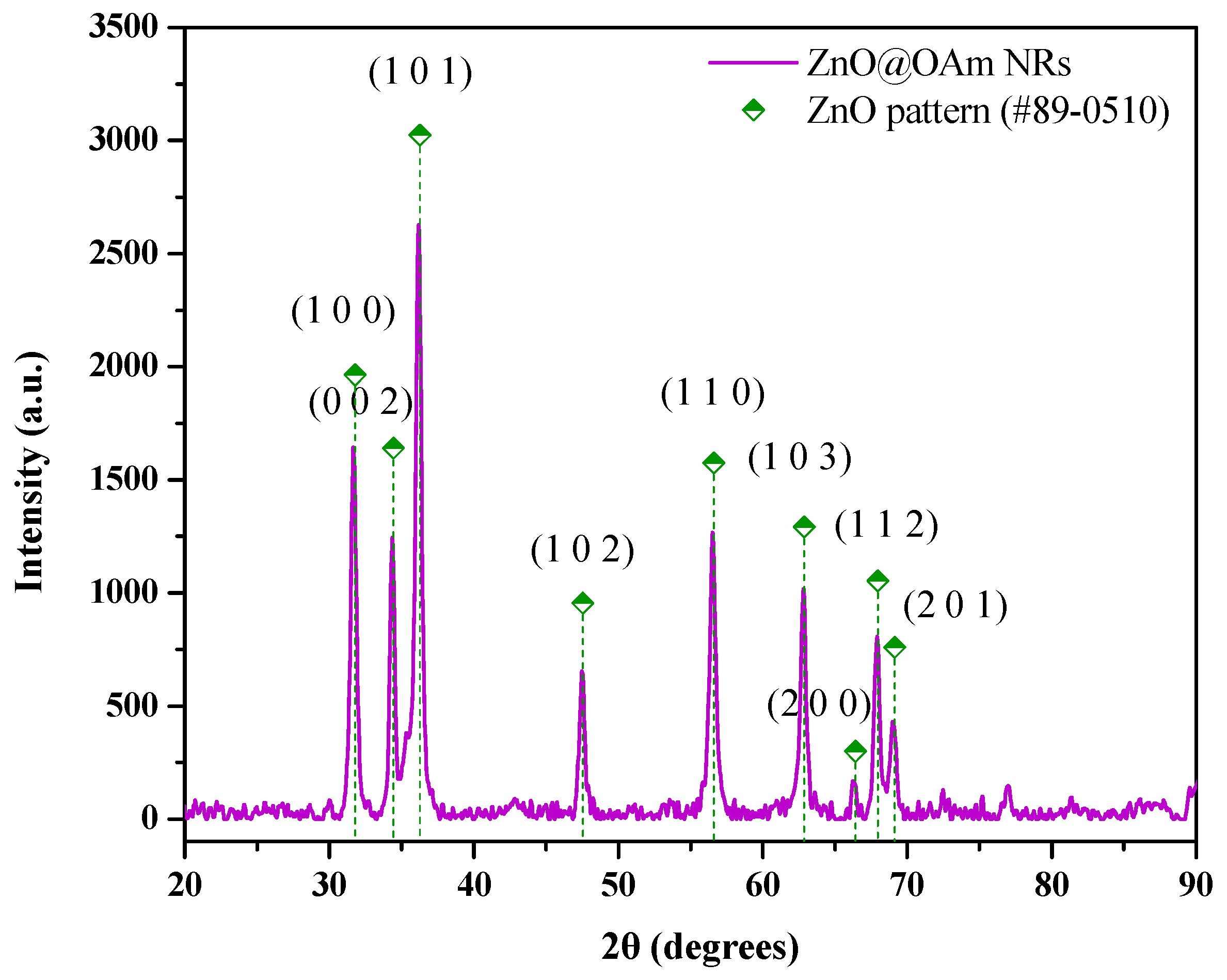
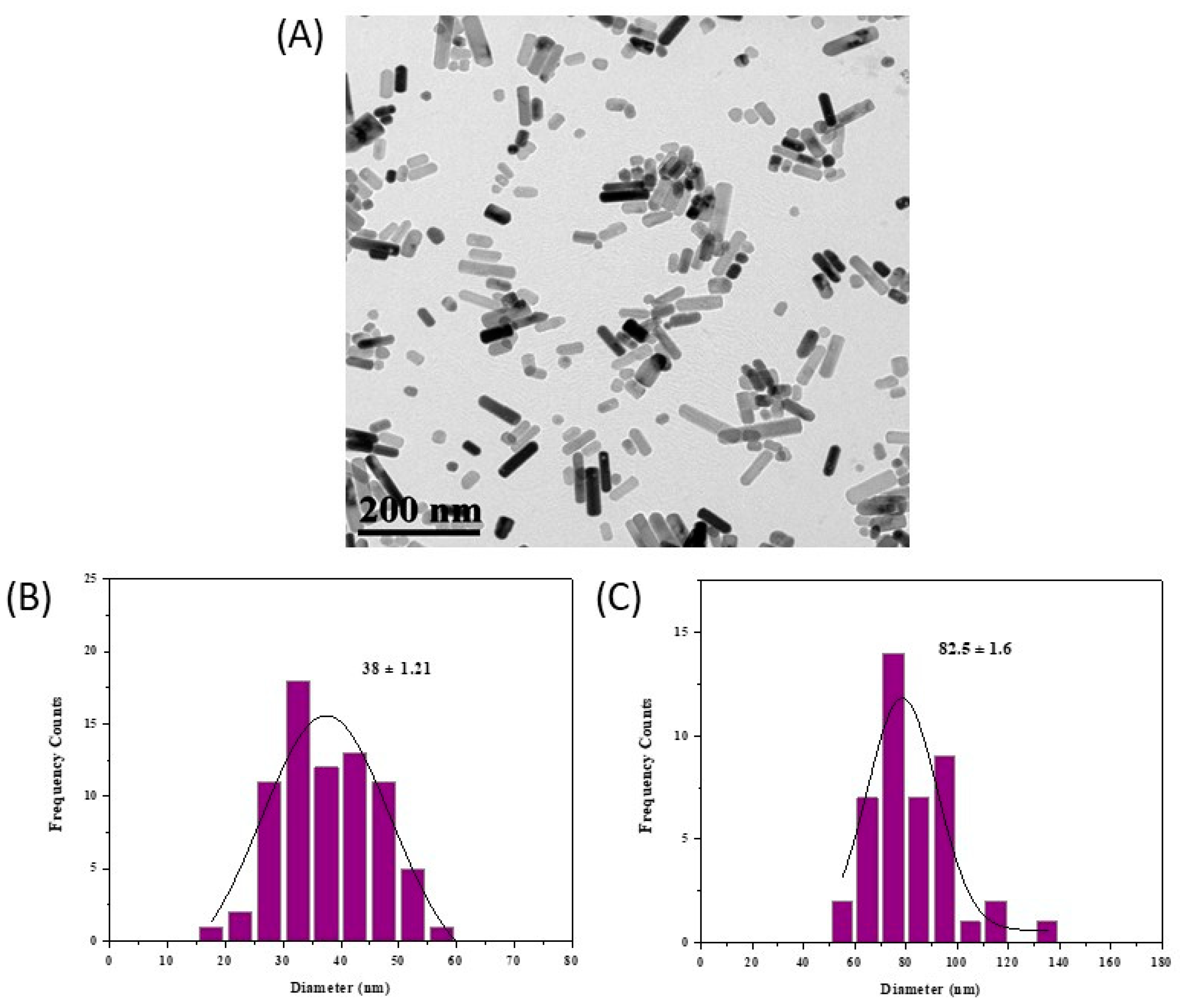
2.1. Physicochemical Characterization of ZnO@OAm NRs
2.2. Physicochemical Characterization of ZnO-Based Nanocapsules
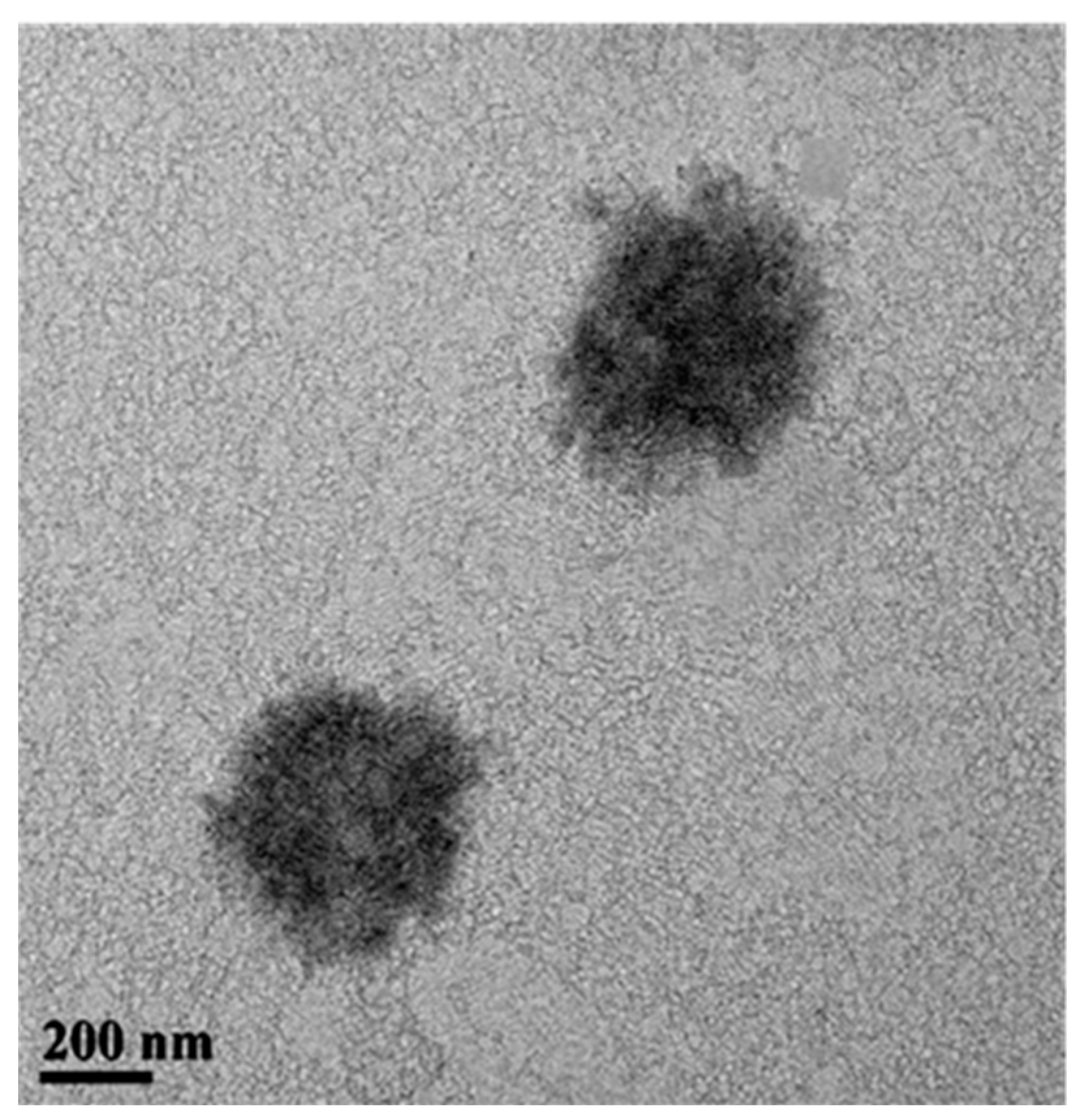
2.2.1. Morphology of ZnO NCs
2.2.2. Encapsulation Efficiency, Loading Capacity of NCs, and Release Profile of Geraniol from NCs
2.2.3. Hydrodynamic Size, ζ-Potential, and Polydispersity
2.3. Bioassays Results
2.3.1. In Vitro Antifungal Activity
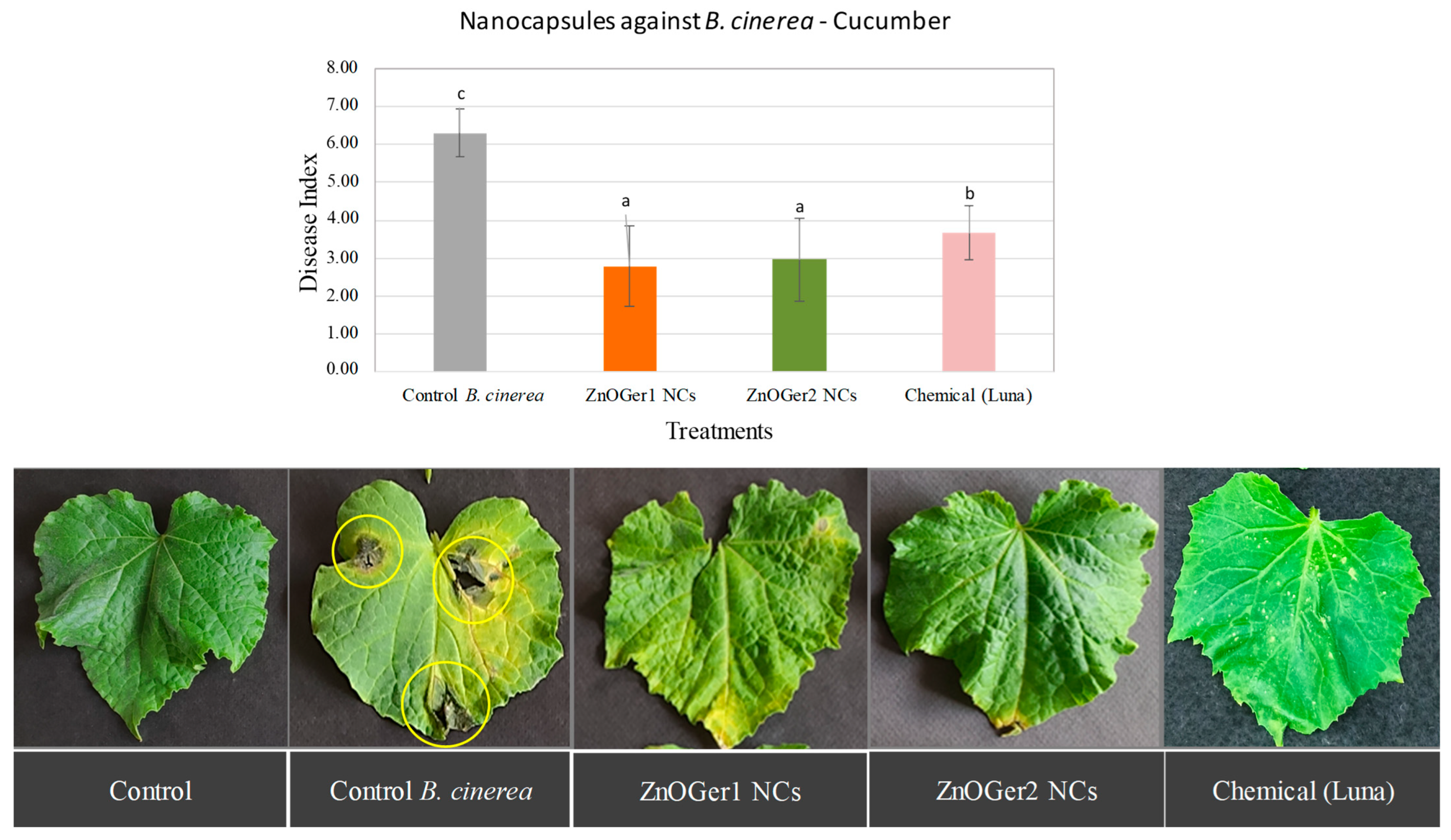
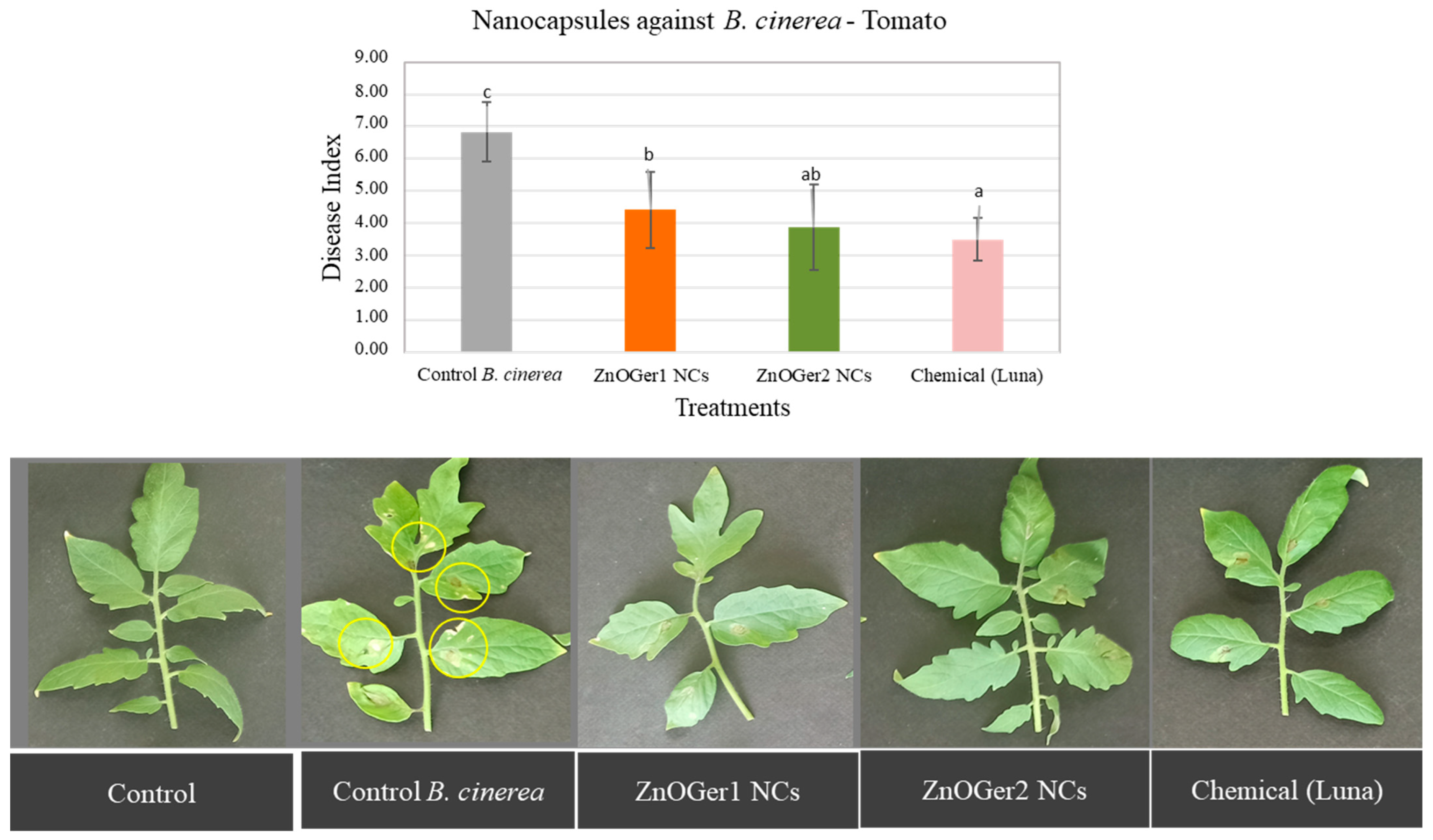
2.3.2. In Planta Antifungal Activity
3. Discussion
4. Materials and Methods
4.1. Materials
Chemicals and Reagents
4.2. Synthesis of ZnO@OAm Nanorods
4.3. Preparation ZnO-Based Nanocapsules
4.4. Physicochemical Characterization of Nanorods and ZnO-Based Nanocapsules
4.4.1. Encapsulation Efficiency and Loading Capacity
4.4.2. Geraniol Release Studies from ZnO-Based NCs
4.5. In Vitro Antifungal Activity against B. cinerea
4.6. In Planta Experiments on Cucumbers and Tomatoes
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; van Kan, J.A. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology: Top 10 fungal pathogens. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. FAOSTAT. 2020. Available online: https://www.fao.org/faostat/en/#search/Tomatoes (accessed on 7 December 2022).
- Panno, S.; Davino, S.; Caruso, A.G.; Bertacca, S.; Crnogorac, A.; Mandić, A.; Noris, E.; Matić, S.A. review of the most common and economically important diseases that undermine the cultivation of tomato crop in the mediterranean basin. Agronomy 2021, 11, 2188. [Google Scholar] [CrossRef]
- Aissat, K.; Nicot, P.C.; Guechi, A.; Bardin, M.; Chibane, M. Grey mold development in greenhouse tomatoes under drip and furrow irrigation. Agron. Sustain. Dev. 2008, 28, 403–409. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological control of plant pathogens: Aglobal perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Caboni, P. Botanical nematicides: A review. J. Agric. Food Chem. 2012, 60, 9929–9940. [Google Scholar] [CrossRef]
- dos Santos Silva, G.; de Jesus Marques, J.N.; Linhares, E.P.M.; Carolina Martinez Bonora, C.M.; Costa, É.T.; Saraiva, M.F. Review of anticancer activity of monoterpenoids: Geraniol, nerol, geranial and neral. Chem.-Biol. Interact. 2022, 362, 109994. [Google Scholar] [CrossRef]
- Kamou, N.N.; Kalogiouri, N.P.; Tryfon, P.; Papadopoulou, A.; Karamanoli, K.; Dendrinou-Samara, C.; Menkissoglu-Spiroudi, U. Impact of geraniol and geraniol nanoemulsions on Botrytis cinerea and effect of geraniol on cucumber plants’ metabolic profile analyzed by LC-QTOF-MS. Plants 2022, 11, 2513. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Das, S.; Dubey, N.K. Nanoencapsulation of essential oils and their bioactive constituents: A novel strategy to control mycotoxin contamination in food system. Food Chem. Toxicol. 2021, 149, 112019. [Google Scholar] [CrossRef]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.P. An overview of micro- and nanoemulsions as vehicles for essential oils: Formulation, preparation and stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef]
- Montes de Oca-Ávalos, J.M.; Candal, R.J.; Herrera, M.L. Nanoemulsions: Stability and physical properties. COFS 2017, 16, 1–6. [Google Scholar] [CrossRef]
- Kaur, G.; Ganjewala, D.; Bist, V.; Verma, P.C. Antifungal and larvicidal activities of two acyclic monoterpenes; citral and geraniol against phytopathogenic fungi and insects. Arch. Phytopathol. Plant Prot. 2019, 52, 458–469. [Google Scholar] [CrossRef]
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current research on zinc oxide nanoparticles: Synthesis, characterization, and biomedical applications. Nanomaterials 2022, 12, 3066. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.A.; Alsaad, A.M.; Al-Bataineh, Q.M.; Al-Akhras, M.-A.H.; Albataineh, Z.; Alizzy, K.A.; Daoud, N.S. Synthesis and characterization of ZnO NPs-doped PMMA-BDK-MR polymer-coated thin films with UV curing for optical data storage applications. Polym. Bull. 2021, 78, 1189–1211. [Google Scholar] [CrossRef]
- U.S Food and Drug Administration (FDA). GRAS Notice. Available online: https://www.accessdata.fda.gov (accessed on 20 December 2022).
- Pariona, N.A.I.M.; Paraguay-Delgado, F.; Basurto-Cereceda, S.; Morales-Mendoza, J.E.; Hermida-Montero, L.A. Shape-dependent antifungal activity of ZnO particles against phytopathogenic fungi. Appl. Nanosci. 2020, 10, 435–443. [Google Scholar] [CrossRef]
- Tryfon, P.; Kamou, N.N.; Mourdikoudis, S.; Karamanoli, K.; Menkissoglu-Spiroudi, U.; Dendrinou-Samara, C. CuZn and ZnO Nanoflowers as nano-fungicides against Botrytis cinerea and Sclerotinia sclerotiorum: Phytoprotection, translocation, and impact after foliar application. Materials 2021, 14, 7600. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, S.; Bandyopadhyay, A.; Debnath, T.; Chakraborty, T.; Majumdar, S.; Chakraborty, S.; Das, S. Effect of hydrothermal synthesis on physical property modulation and biological activity of ZnO nanorods. Mater. Res. Express 2019, 6, 1250f7. [Google Scholar] [CrossRef]
- Enayati, S.; Davari, M.; Habibi-Yangjeh, A.; Ebadollahi, A.; Feizpoor, S. Enhancement of the antifungal properties of Zataria multiflora essential oil through nanoencapsulation with ZnO nanomaterial. Res. Sq. 2021, 42, 503–513. [Google Scholar] [CrossRef]
- Akhtari, A.; Davari, M.; Habibi-Yangjeh, A.; Ebadollahi, A.; Feizpour, S. Antifungal activities of pure and ZnO-encapsulated essential oil of Zataria multiflora on Alternaria solani as the pathogenic agent of tomato early blight disease. Front. Plant Sci. 2022, 13, 932475. [Google Scholar] [CrossRef]
- Ahammed, K.R.; Ashaduzzaman, M.; Paul, S.C.; Nath, M.R.; Bhowmik, S.; Saha, O.; Rahaman, M.M.; Bhowmik, S.; Aka, T.D. Microwave assisted synthesis of zinc oxide (ZnO) nanoparticles in a noble approach: Utilization for antibacterial and photocatalytic activity. SN Appl. Sci. 2020, 2, 955. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Y.; Cortie, M.B.; Liu, X.; Xie, Q.; Wang, X.; Peng, D.L. Shape-selective formation of monodisperse copper nanospheres and nanocubes via disproportionation reaction route and their optical properties. J. Phys. Chem. C 2014, 118, 9801–9808. [Google Scholar] [CrossRef]
- Zhu, X.; Vo, C.; Taylor, M.; Smith, B.R. Non-spherical micro-and nanoparticles in nanomedicine. Mater. Horiz. 2019, 6, 1094–1121. [Google Scholar] [CrossRef]
- Abbas, Q.; Yousaf, B.; Ullah, H.; Ali, M.U.; Ok, Y.S.; Rinklebe, J. Environmental transformation and nano-toxicity of engineered nano-particles (ENPs) in aquatic and terrestrial organisms. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2523–2581. [Google Scholar] [CrossRef]
- Kutawa, A.B.; Ahmad, K.; Ali, A.; Hussein, M.Z.; Wahab, M.A.A.; Adamu, A.; Ismaila, A.A.; Gunasena, M.T.; Rahman, M.Z.; Hossain, M.I. Trends in nanotechnology and its potentialities to control plant pathogenic fungi: A review. Biology 2021, 10, 881. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Liz-Marzán, L.M. Oleylamine in Nanoparticle Synthesis. Chem. Mater. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Dumestre, F.; Chaudret, B.; Amiens, C.; Fromen, M.C.; Casanove, M.J.; Renaud, P.; Zurcher, P. Shape control of thermodynamically stable cobalt nanorods through organometallic chemistry. Angew. Chem. Int. Ed. Engl. 2002, 41, 4286–4289. [Google Scholar] [CrossRef]
- Mintcheva, N.; Aljulaih, A.A.; Wunderlich, W.; Kulinich, S.A.; Iwamori, S. Laser-Ablated ZnO nanoparticles and their photocatalytic activity toward organic pollutants. Materials 2018, 11, 1127. [Google Scholar] [CrossRef]
- Schlur, L.; Calado, J.R.; Spitzer, D. Synthesis of zinc oxide nanorods or nanotubes on oneside of a microcantilever. R. Soc. Open Sci. 2018, 5, 180510. [Google Scholar] [CrossRef]
- Mishra, S.M.; Satpati, B. Morphology of ZnO nanorods and Au-ZnO heterostructures on different seed layers and their influence on the optical behavior. J. Lumin. 2022, 246, 118813. [Google Scholar] [CrossRef]
- Srikant, V.; Clarke, D.R. On the optical band gap of zinc oxide. J. Appl. Phys. 1998, 83, 5447. [Google Scholar] [CrossRef]
- Goyal, M.; Singh, M. Size and shape dependence of optical properties of nanostructures. Appl. Phys. A 2020, 126, 176. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z. Facile synthesis of quasi spherical ZnO nanoparticles with excellent photo catalytic activity. J. Clust. Sci. 2015, 26, 1187–1201. [Google Scholar] [CrossRef]
- Chong, Y.; Ge, C. Rational design of metal-based antimicrobial nanomaterials in environmental applications. Environ. Sci.-Nano 2021, 8, 3478–3492. [Google Scholar] [CrossRef]
- Yegin, Y.; Perez-Lewis, K.L.; Zhang, M.; Akbulut, M.; Taylor, T.M. Development and characterization of geraniol-loaded polymeric nanoparticles with antimicrobial activity against foodborne bacterial pathogens. J. Food Eng. 2016, 170, 64–71. [Google Scholar] [CrossRef]
- Fery, A.; Weinkamer, R. Mechanical properties of micro- and nanocapsules: Single-capsule measurements. Polymer 2007, 48, 7221–7235. [Google Scholar] [CrossRef]
- Liao, W.; Badri, W.; Dumas, E.; Ghnimi, S.; Elaissari, A.; Saurel, R.; Gharsallaoui, A. Nanoencapsulation of essential oils as natural food antimicrobial agents: An overview. Appl. Sci. 2021, 11, 5778. [Google Scholar] [CrossRef]
- Wang, D.; Saleh, N.B.; Byro, A.; Zepp, R.; Sahle-Demessie, E.; Luxton, T.P.; Ho, K.T.; Burgess, R.M.; Flury, M.; White, J.C.; et al. Nano-enabled pesticides for sustainable agriculture and global food security. Nat. Nanotechnol. 2022, 17, 347–360. [Google Scholar] [CrossRef]
- Huo, Q. Protein complexes/aggregates as potential cancer biomarkers revealed by a nanoparticle aggregation immunoassay. Colloids Surf. B Biointerfaces 2010, 78, 259–265. [Google Scholar] [CrossRef]
- Bernardi, D.S.; Pereira, T.A.; Maciel, N.R.; Bortoloto, J.; Viera, G.S.; Oliveira, G.C.; Rocha-Filho, P.A. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: In vitro and in vivo assessments. J. Nanobiotechnol. 2011, 9, 44. [Google Scholar] [CrossRef]
- Li, B.; Du, W.; Jin, J.; Du, Q. Preservation of (−)-epigallocatechin-3-gallate antioxidant properties loaded in heat treated β-lactoglobulin nanoparticles. J. Agric. Food Chem. 2012, 60, 3477–3484. [Google Scholar] [CrossRef] [PubMed]
- Loosli, F.; Stoll, S. Effect of surfactants, pH and water hardness on the surface properties and agglomeration behavior of engineered TiO2 nanoparticles. Environ. Sci. Nano 2017, 1, 203–211. [Google Scholar] [CrossRef]
- Pincus, M.R. Physiological structure and function of proteins. In Cell Physiology Source Book, 4th ed.; A Molecular Approach; Academic Press: Cambridge, MA, USA, 2001; pp. 19–42. [Google Scholar] [CrossRef]
- Tang, X.; Shao, Y.-L.; Tang, Y.-J.; Zhou, W.-W. Antifungal activity of essential oil compounds (geraniol and citral) and inhibitory mechanisms on grain pathogens (Aspergillus flavus and Aspergillus ochraceus). Molecules 2018, 23, 2108. [Google Scholar] [CrossRef] [PubMed]
- Biondo, F.; Baldassarre, F.; Vergaro, V.; Ciccarella, G. Controlled biocide release from smart delivery systems: Materials engineering to tune release rate, biointeractions, and responsiveness. In Nanotechnology-Based Sustainable Alternatives for the Management of Plant Diseases; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2022; chapter 3; pp. 31–147. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.; Lei, J.; Ma, Y.; Du, F. The wetting behavior of aqueous surfactant solutions on wheat (Triticum aestivum). Soft Matter. 2017, 13, 503–513. [Google Scholar] [CrossRef]
- Dolores Ibanez, M.; Amparo Blazquez, M. Phytotoxic effects of commercial essential oils on selected vegetable crops: Cucumber and tomato. Sustain. Chem. Pharm. 2020, 15, 100209. [Google Scholar] [CrossRef]
- Cabot, C.; Martos, S.; Llugany, M.; Gallego, B.; Poschenrieder, C. A role for zinc in plant defense against pathogens and herbivores. Front. Plant Sci. 2019, 10, 1171. [Google Scholar] [CrossRef]
- Moghtader, M. Antifungal effects of the essential oil from Thymus vulgaris L. and comparison with synthetic thymol on Aspergillus niger. J. Yeast Fungal Res. 2012, 3, 83–88. [Google Scholar] [CrossRef]
- Spielman-Sun, E.; Avellan, A.; Bland, G.D.; Clement, E.T.; Tappero, R.V.; Acerbo, A.S.; Lowry, G.V. Protein coating composition targets nanoparticles to leaf stomata and trichomes. Nanoscale 2020, 12, 3630–3636. [Google Scholar] [CrossRef]
- Tryfon, P.; Kamou, N.N.; Ntalli, N.; Mourdikoudis, S.; Karamanoli, K.; Karfaridis, D.; Menkissoglu-Spiroudi, U.; Dendrinou-Samara, C. Coated Cu-doped ZnO and Cu nanoparticles as control agents against plant pathogenic fungi and nematodes. NanoImpact 2022, 28, 100430. [Google Scholar] [CrossRef]
- Liu, X.; Mak, M.; Babla, M.; Wang, F.; Chen, G.; Veljanoski, F.; Wang, G.; Shabala, S.; Zhou, M.; Chen, Z.H. Linking stomatal traits and expression of slow anion channel genes HvSLAH1 and HvSLAC1 with grain yield for increasing salinity tolerance in barley. Front. Plant Sci. 2014, 5, 634. [Google Scholar] [CrossRef]
- Kardiman, R.; Ræbild, A. Relationship between stomatal density, size and speed of opening in Sumatran rainforest species. Tree Physiol. 2018, 38, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Pathare, V.S.; Koteyeva, N.; Cousins, A.B. Increased adaxial stomatal density is associated with greater mesophyll surface area exposed to intercellular air spaces and mesophyll conductance in diverse C4 grasses. New Phytol. 2020, 225, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Bussieres, P. Estimating the number and size of phloem sieve plate pores using longitudinal views and geometric reconstruction. Sci. Rep. 2014, 4, 4929. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.F.M.; Torresi, R.M.; Emmerling, F.; Camargo, P.H.C. Challenges and opportunities in the bottom-up mechanochemical synthesis of noble metal nanoparticles. J. Mater. Chem. A 2020, 8, 16114–16141. [Google Scholar] [CrossRef]
- Ghezzia, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Dash, V. Release kinetic studies of aspirin microcapsules from ethyl cellulose, cellulose acetate phthalate and their mixtures by emulsion solvent evaporation method. Sci. Pharm. 2010, 78, 93–101. [Google Scholar] [CrossRef]
- Lokhandwala, H.; Deshpande, A.; Deshpande, S. Kinetic modeling and dissolution profiles comparison: An overview. Int. J. Pharm. Bio. Sci. 2013, 4, 728–773. [Google Scholar]
- Ramteke, K.H.; Dighe, P.A.; Kharat, A.R.; Patil, S.V. Mathematical models of drug dissolution: A review. Sch. Acad. J. Pharm. 2014, 3, 388396. [Google Scholar]

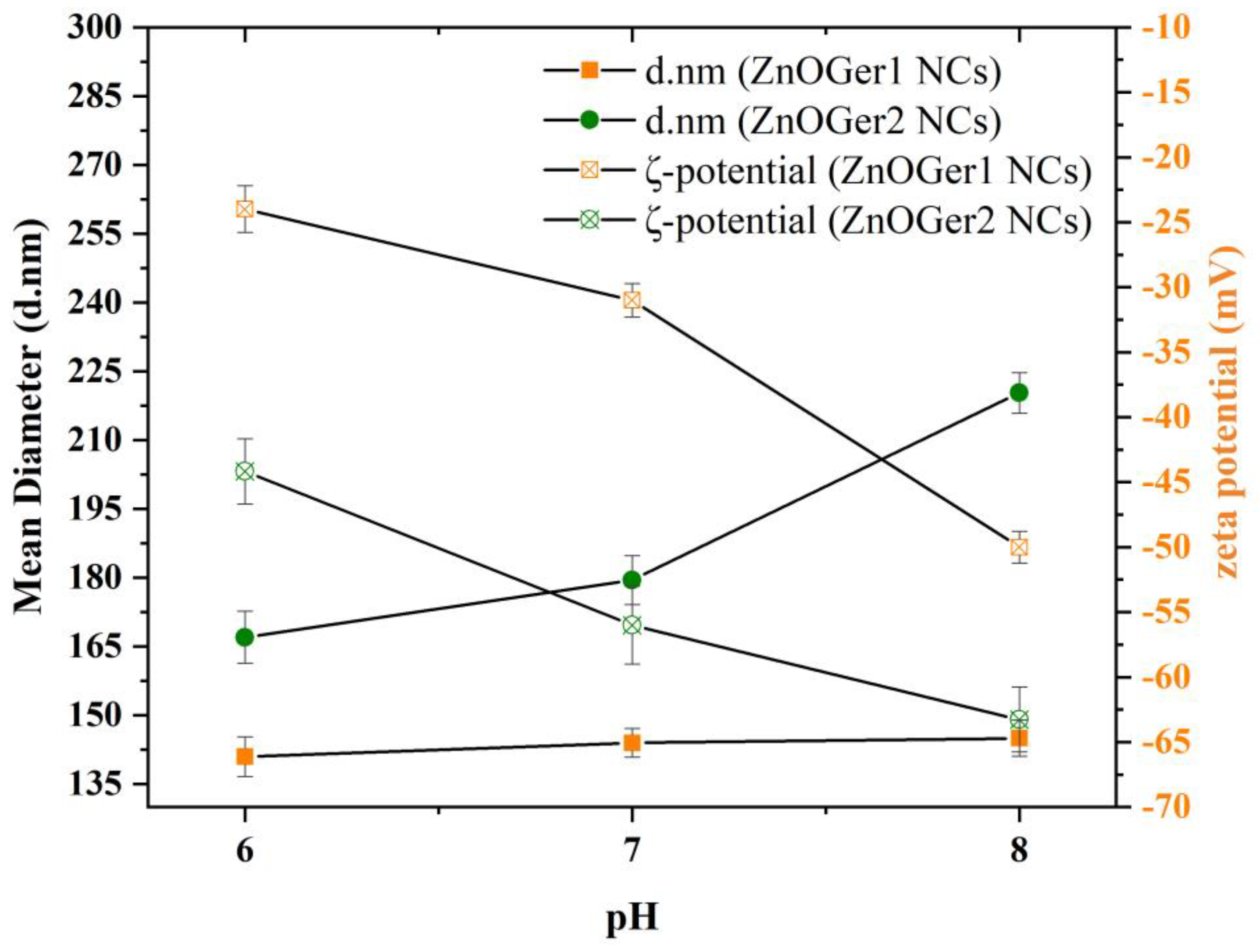
| Nanoemulsions | pH Value | Z-Average (d.nm) ± SD | PDI ± SD | z Potential (mV) ± SD |
|---|---|---|---|---|
| ZnOGer1 NCs | 8 | 145 ± 2.91 | 0.37 ± 0.04 | −50 ± 1.23 |
| 7 | 144 ± 1.51 | 0.31 ± 0.08 | −31 ± 1.29 | |
| 6 | 141 ± 4.33 | 0.25 ± 0.03 | −24 ± 0.27 | |
| ZnOGer2 NCs | 8 | 220 ± 2.43 | 0.42 ± 0.01 | −63 ± 1.55 |
| 7 | 180 ± 5.31 | 0.39 ± 0.08 | −56 ± 3.00 | |
| 6 | 167 ± 5.69 | 0.34 ± 0.02 | −44 ± 1.55 | |
| ZnO NCs | 8 | 256 ± 2.27 | 0.48 ± 0.03 | −68 ± 2.42 |
| 7 | 234 ± 2.35 | 0.45 ± 0.06 | −53 ± 3.16 | |
| 6 | 222 ± 3.51 | 0.43 ± 0.04 | −51 ± 2.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tryfon, P.; Kamou, N.N.; Pavlou, A.; Mourdikoudis, S.; Menkissoglu-Spiroudi, U.; Dendrinou-Samara, C. Nanocapsules of ZnO Nanorods and Geraniol as a Novel Mean for the Effective Control of Botrytis cinerea in Tomato and Cucumber Plants. Plants 2023, 12, 1074. https://doi.org/10.3390/plants12051074
Tryfon P, Kamou NN, Pavlou A, Mourdikoudis S, Menkissoglu-Spiroudi U, Dendrinou-Samara C. Nanocapsules of ZnO Nanorods and Geraniol as a Novel Mean for the Effective Control of Botrytis cinerea in Tomato and Cucumber Plants. Plants. 2023; 12(5):1074. https://doi.org/10.3390/plants12051074
Chicago/Turabian StyleTryfon, Panagiota, Nathalie N. Kamou, Akrivi Pavlou, Stefanos Mourdikoudis, Urania Menkissoglu-Spiroudi, and Catherine Dendrinou-Samara. 2023. "Nanocapsules of ZnO Nanorods and Geraniol as a Novel Mean for the Effective Control of Botrytis cinerea in Tomato and Cucumber Plants" Plants 12, no. 5: 1074. https://doi.org/10.3390/plants12051074
APA StyleTryfon, P., Kamou, N. N., Pavlou, A., Mourdikoudis, S., Menkissoglu-Spiroudi, U., & Dendrinou-Samara, C. (2023). Nanocapsules of ZnO Nanorods and Geraniol as a Novel Mean for the Effective Control of Botrytis cinerea in Tomato and Cucumber Plants. Plants, 12(5), 1074. https://doi.org/10.3390/plants12051074








