Bacillus-Loaded Biochar as Soil Amendment for Improved Germination of Maize Seeds
Abstract
1. Introduction
2. Results and Discussion
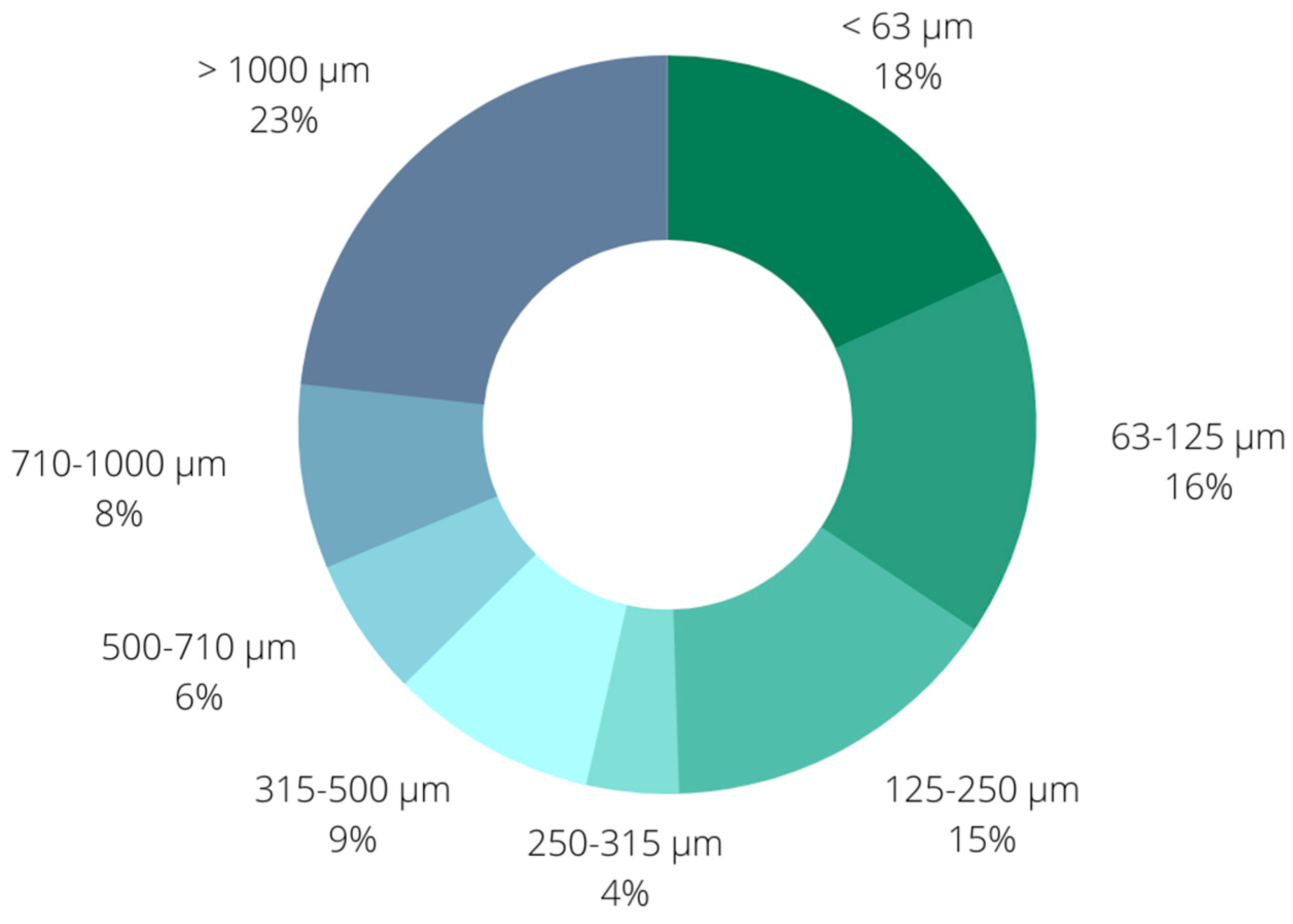
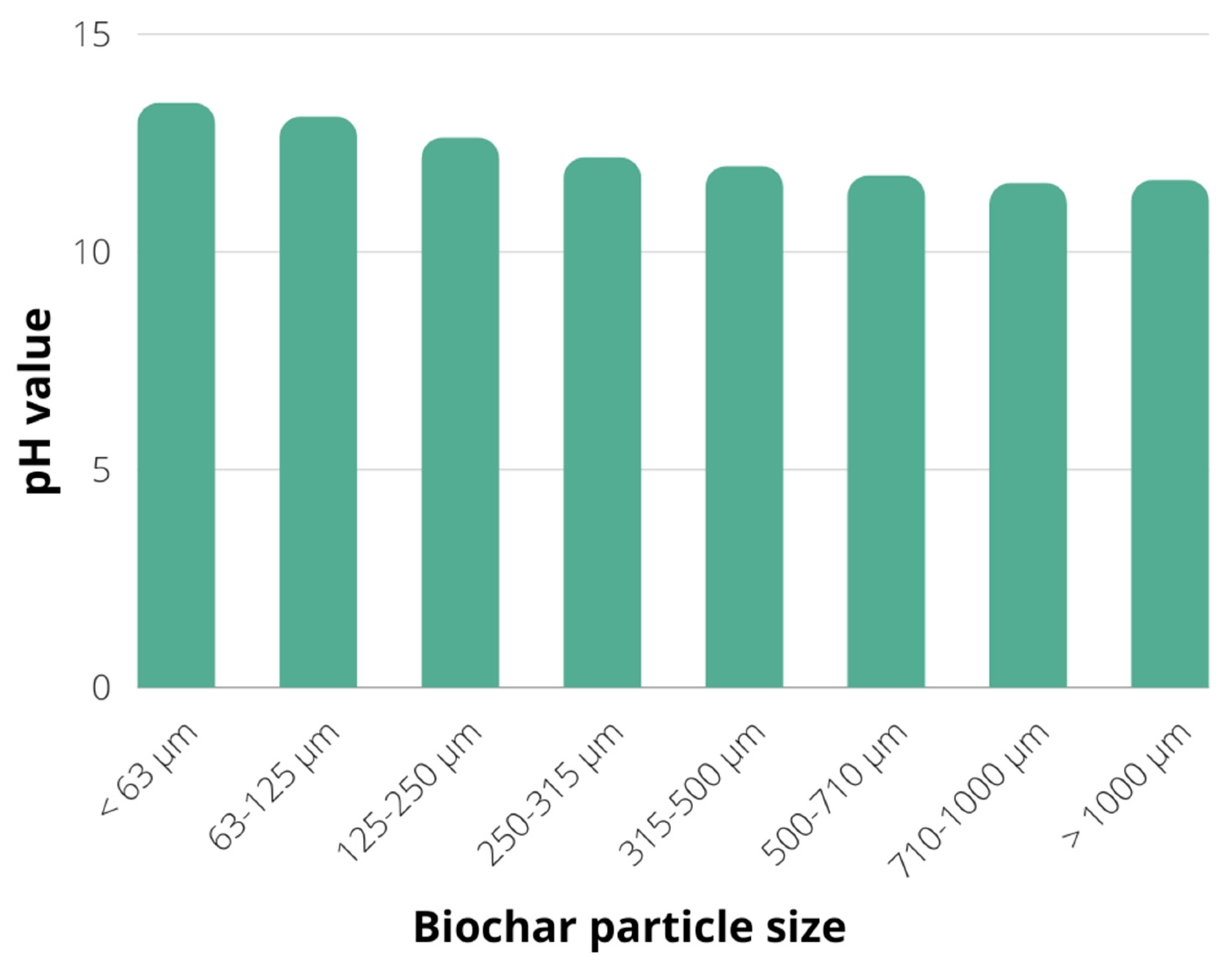
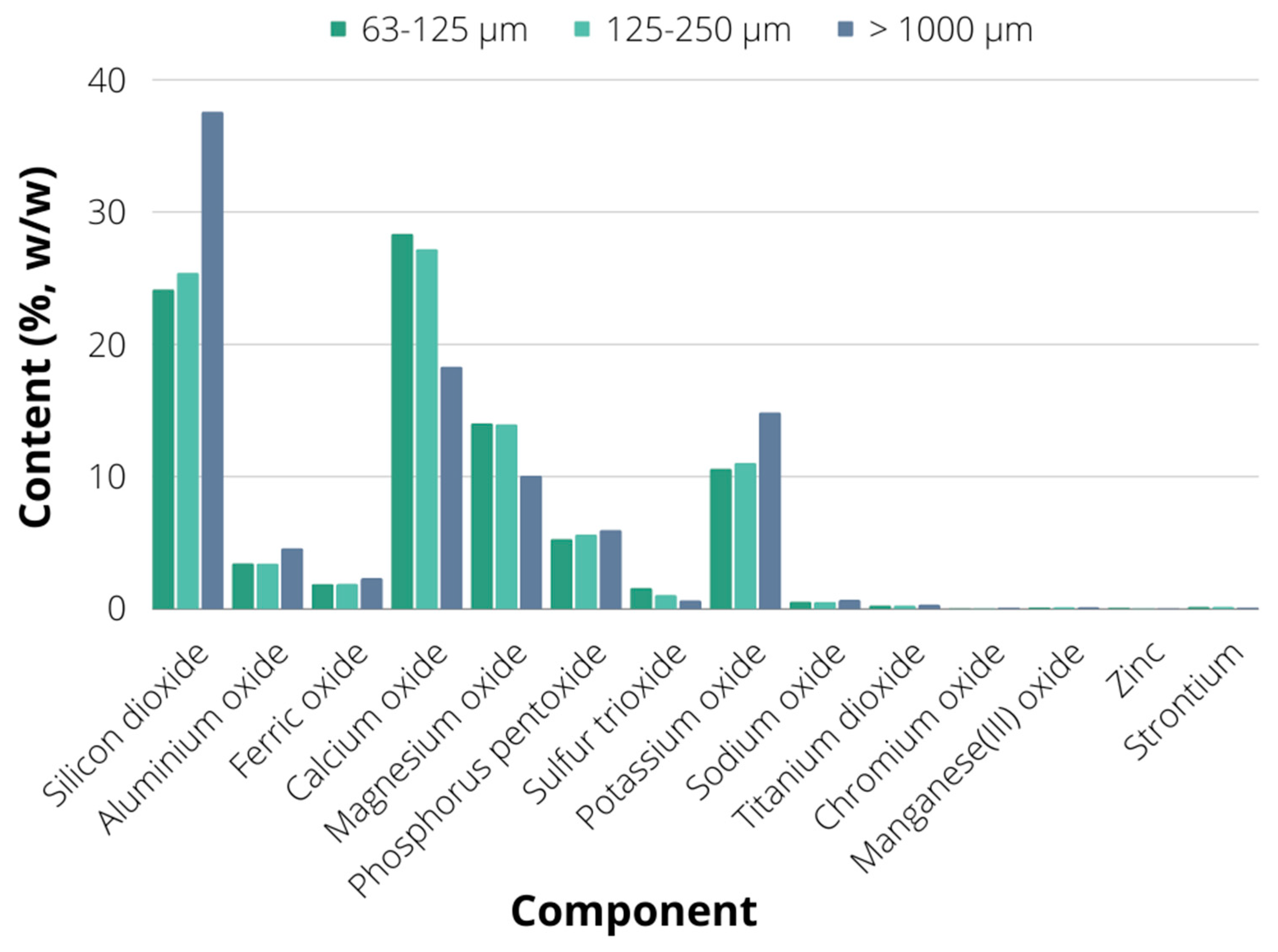
2.1. Biochar Characterization
2.2. Composition of the Bacillus sp. BioSol021 Cultivation Broth
2.3. PGP and Biocontrol Traits of the Bacillus BioSol021 Cultivation Broth
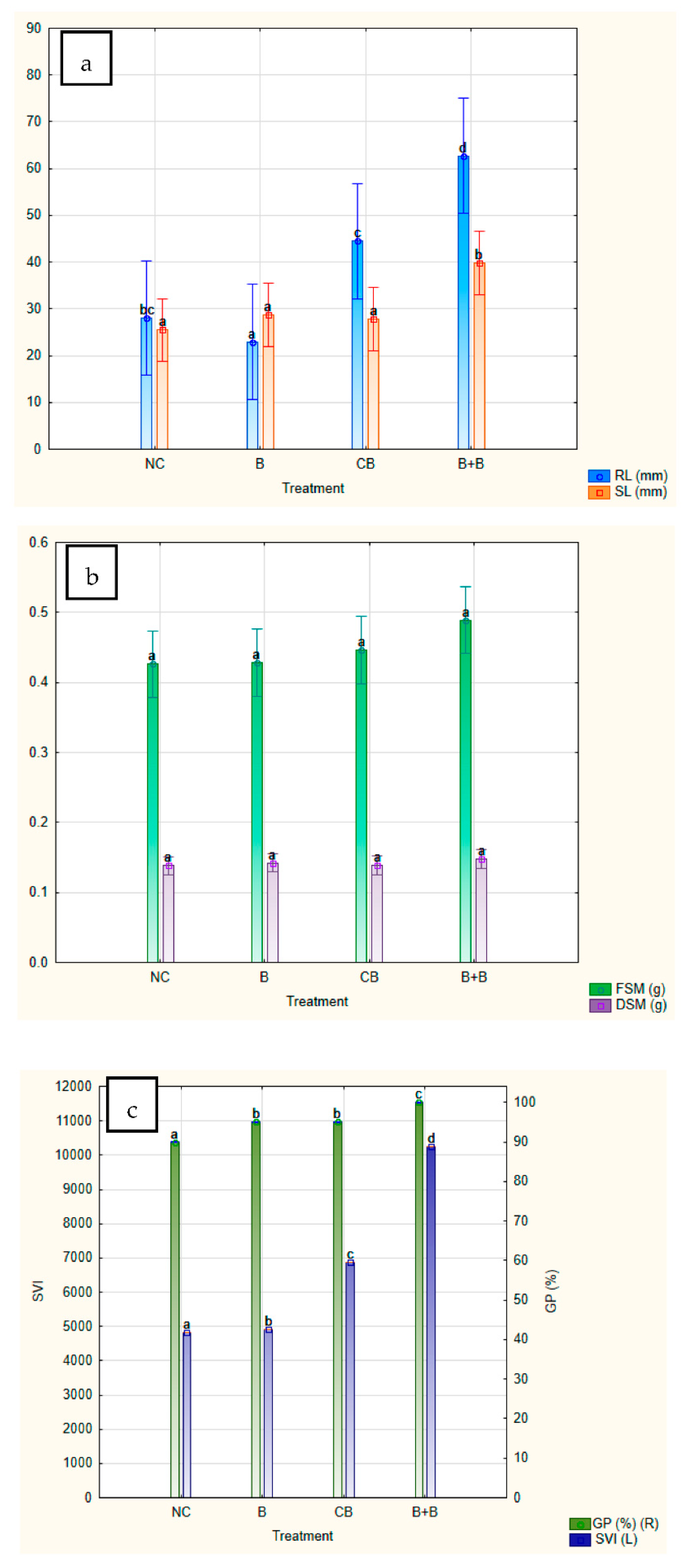
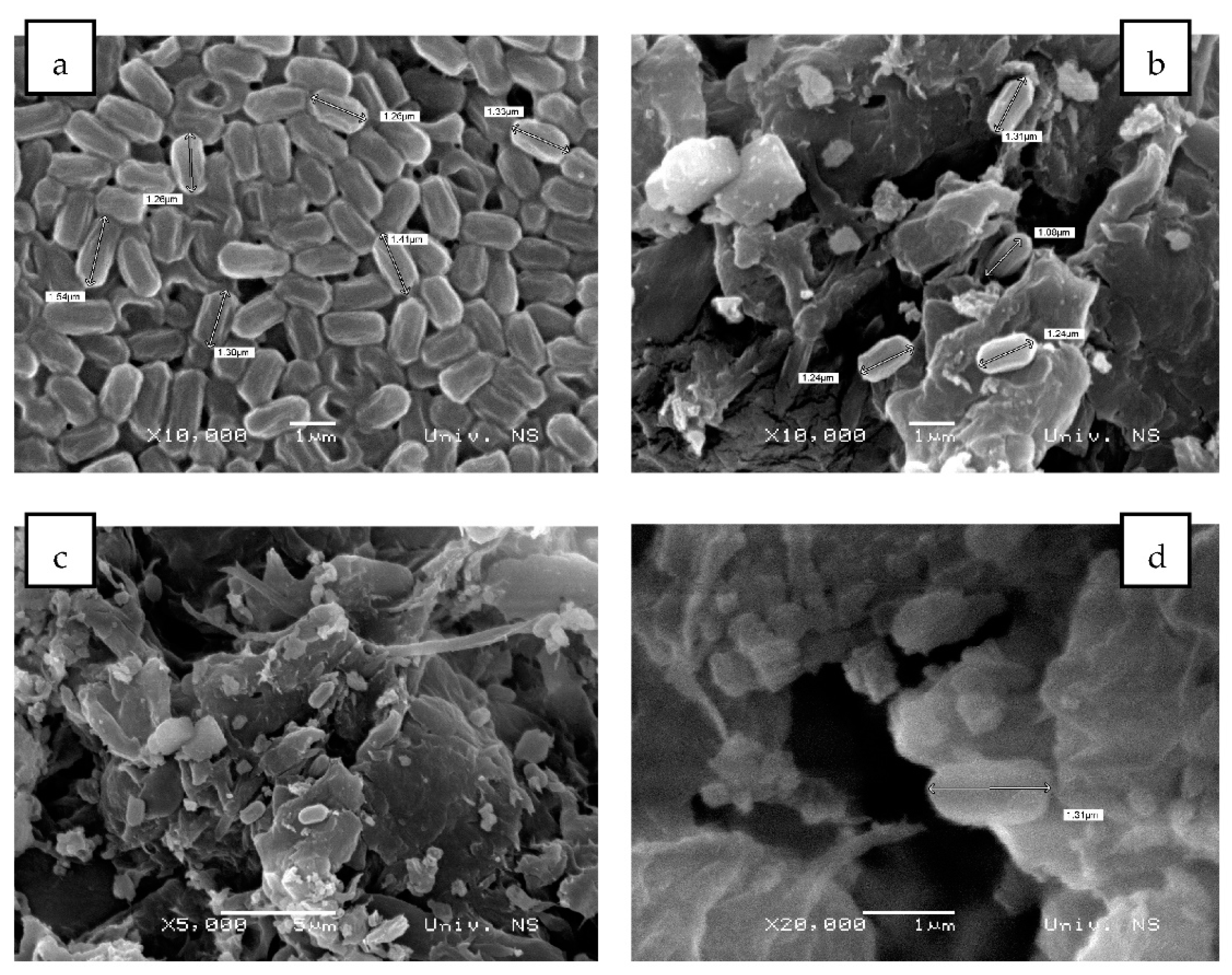
2.4. Bacillus BioSol021 Immobilisation to Biochar and Maize Seed Germination
3. Materials and Methods
3.1. Production and Characterisation of Biochar
3.2. Producing Microorganism, Cultivation Medium and Cultivation Conditions
3.3. Characterisation of Cultivation Broth—Biomass, Residual Nutrients, pH Value
3.4. Characterisation of Cultivation Broth—Plant Growth Promoting and Biocontrol Traits
3.4.1. CPC-BTB Method for Surfactin Quantification
3.4.2. PGP Traits Screening
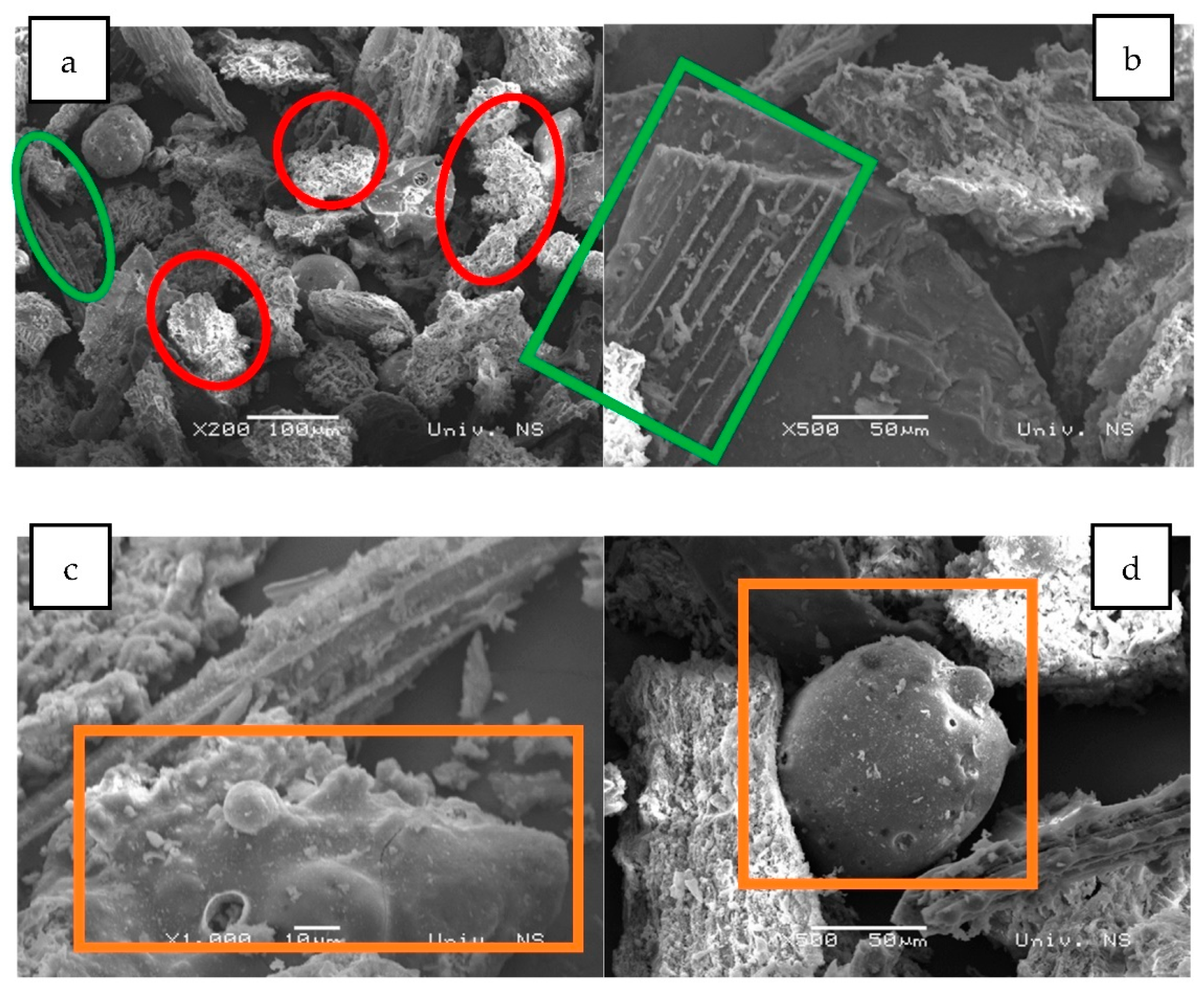
3.5. Immobilisation of Producing Microorganism on Biochar
SEM Imaging of the Free and Biochar-Bound Bacillus sp. BioSol021 Cells
3.6. Seed Germination Activity Assay
3.7. Experimental Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Di Benedetto, N.A.; Corbo, M.R.; Campaniello, D.; Cataldi, M.P.; Bevilacqua, A.; Sinigaglia, M.; Flagella, Z. The role of plant growth promoting bacteria in improving nitrogen use efficiency for sustainable crop production: A focus on wheat. AIMS Microbiol. 2017, 3, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial Plant Biostimulants: A Sustainable Way towards Improving Growth, Productivity, and Health of Crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Singh, S.B.; Shilpa, N.; Aiyaz, M.; Nataraj, K.; Udayashankar, A.C.; Amruthesh, K.N.; Murali, M.; Poczai, P.; Gafur, A.; et al. Insight into Recent Progress and Perspectives in Improvement of Antioxidant Machinery upon PGPR Augmentation in Plants under Drought Stress: A Review. Antioxidants 2022, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Aloo, B.N.; Makumba, B.A.; Mbega, E.R. The potential of Bacilli rhizobacteria for sustainable crop production and environmental sustainability. Microbiol. Res. 2019, 219, 26–39. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, J.D.; Suriani, N.L. Recent Understanding of Soil Acidobacteria and Their Ecological Significance: A Critical Review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef] [PubMed]
- Manasa, M.; Ravinder, P.; Gopalakrishnan, S.; Srinivas, V.; Sayyed, R.Z.; El Enshasy, H.A.; Yahayu, M.; Kee Zuan, A.T.; Kassem, H.S.; Hameeda, B. Co-Inoculation of Bacillus spp. for Growth Promotion and Iron Fortification in Sorghum. Sustainability 2021, 13, 12091. [Google Scholar] [CrossRef]
- Rabari, A.; Ruparelia, J.; Jha, C.K.; Sayyed, R.Z.; Mitra, D.; Priyadarshini, A.; Senapati, A.; Panneerselvam, P.; das Mohapatra, P.K. Articulating beneficial rhizobacteria mediated plant defenses through induced systemic resistance. Pedosphere 2022, in press. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Dubey, R.C.; Maheshwari, D.K. Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol. Res. 2012, 167, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Omar, A.F.; Rehan, M.; Al-Turki, A.; Sagar, A.; Ilyas, N.; Sayyed, R.Z.; Hasanuzzaman, M. Crop microbiome: Their role and advances in molecular and omic techniques for the sustenance of agriculture. Planta 2023, 257, 27. [Google Scholar] [CrossRef] [PubMed]
- Stamenković Stojanović, S.; Karabegović, I.; Beškoski, V.; Nikolić, N.; Lazić, M. Bacillus based microbial formulations: Optimization of the production process. Hem. Ind. 2019, 73, 169–182. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Martos, V.; Garcia del Moral, L.F.; Kowalska, J.; Tylkowski, B.; Malusá, E. Formulation of microbial inoculants by encapsulation in natural polysaccharides: Focus on beneficial properties of carrier additives and derivatives. Front. Plant Sci. 2020, 11, 270. [Google Scholar] [CrossRef]
- Schisler, D.A.; Slininger, P.J.; Behle, R.W.; Jackson, M.A. Formulation of Bacillus spp. for biological control of plant diseases. Phytopathology 2004, 94, 1267–1271. [Google Scholar] [CrossRef]
- Gašić, S.; Tanović, B. Biopesticide formulations, possibility of application and future trends. Pestic. Phytomed. 2013, 28, 97–102. [Google Scholar] [CrossRef]
- Grahovac, M.; Indjić, D.; Lazić, S.; Vuković, S. Biofungicides and their applicability in modern agricultural practice. Pestic. Phytomed. 2009, 24, 245–258. [Google Scholar] [CrossRef]
- Yánez-Mendizábal, V.; Viñas, I.; Usall, J.; Torres, R.; Solsona, C.; Abadias, M.; Teixidó, N. Formulation development of the biocontrol agent Bacillus subtilis strain CPA-8 by spray-drying. J. Appl. Microbiol. 2012, 112, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Wu, Z.; Wei, M.; Liu, X.; He, Y.; Ye, B.C. Bacillus subtilis SL-13 Biochar formulation promotes pepper plant growth and soil improvement. Can. J. Microbiol. 2019, 65, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef] [PubMed]
- Živanov, M.; Šeremešić, S.; Vasin, J.; Milić, S.; Ninkov, J.; Jakšić, S.; Vojnov, B. Uticaj primene biouglja u kontrolisanim uslovima na hemijska svojstva zemljišta. Soil Plant 2016, 65, 31–44. [Google Scholar]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Lebrun, M.; Miard, F.; Bucci, A.; Trupiano, D.; Nandillon, R.; Naclerio, G.; Scippa, G.S.; Morabito, D.; Bourgerie, S. Evaluation of direct and biochar carrier-based inoculation of Bacillus sp. on As- and Pb-contaminated technosol: Effect on Metal(Loid) Availability, Salix Viminalis Growth, and Soil Microbial Diversity/Activity. Environ. Sci. Pollut. Res. 2021, 28, 11195–11204. [Google Scholar] [CrossRef]
- Azeem, M.; Hassan, T.U.; Tahir, M.I.; Ali, A.; Jeyasundar, P.G.S.A.; Hussain, Q.; Bashir, S.; Mehmood, S.; Zhang, Z. Tea leaves biochar as a carrier of Bacillus cereus improves the soil function and crop productivity. Appl. Soil Ecol. 2021, 157, 103732. [Google Scholar] [CrossRef]
- Saxena, J.; Rana, G.; Pandey, M. Impact of addition of biochar along with Bacillus sp. on growth and yield of French beans. Sci. Hortic. 2013, 162, 351–356. [Google Scholar] [CrossRef]
- Ahmad, M.; Wang, X.; Hilger, T.H.; Luqman, M.; Nazli, F.; Hussain, A.; Zahir, Z.A.; Latif, M.; Saeed, Q.; Malik, H.A.; et al. Evaluating biochar-microbe synergies for improved growth, yield of maize, and post-harvest soil characteristics in a semi-arid climate. Agronomy 2020, 10, 1055. [Google Scholar] [CrossRef]
- Tu, C.; Wei, J.; Guan, F.; Liu, Y.; Sun, Y.; Luo, Y. Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ. Int. 2020, 137, 105576. [Google Scholar] [CrossRef]
- Maftuah, E.; Saleh, M.; Pratiwi, E. The potentials of biochar from agricultural waste as a carrier material of biofertilizer for swamplands. IOP Conf. Ser. Mater. Sci. Eng. 2020, 980, 012064. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Sec. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Sari, N.A.; Ishak, C.F.; Bakar, R.A. Characterization of oil palm empty fruit bunch and rice husk biochars and their potential to adsorb arsenic and cadmium. Am. J. Agric. Biol. Sci. 2014, 9, 450–456. [Google Scholar] [CrossRef]
- Abdullah, R.; Ishak, C.F.; Osman, N.; Halim, N.S.A.; Panhwar, Q.A. Determining the characteristics and potential of plant-based biochars to reduce copper uptake in maize. Bragantia 2021, 80, e2221. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.-K.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]
- Shaaban, M.; Van Zwieten, L.; Bashir, S.; Younas, A.; Núñez-Delgado, A.; Chhajro, M.A.; Kubar, K.A.; Ali, U.; Rana, M.S.; Mehmood, M.A.; et al. A concise review of biochar application to agricultural soils to improve soil conditions and fight pollution. J. Environ. Manag. 2018, 228, 429–440. [Google Scholar] [CrossRef]
- Chen, W.; Meng, J.; Han, X.; Lan, Y.; Zhang, W. Past, present, and future of biochar. Biochar 2019, 1, 75–87. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Murtaza, B.; Bibi, I.; Natasha; Naeem, M.A.; Niazi, N.K. A critical review of different factors governing the fate of pesticides in soil under biochar application. Sci. Total Environ. 2020, 711, 134645. [Google Scholar] [CrossRef]
- Morales, L.F.; Herrera, K.; Lopez, J.E.; Saldarriaga, J.F. Use of biochar from rice husk pyrolysis: Assessment of reactivity in lime pastes. Heliyon 2021, 7, e08423. [Google Scholar] [CrossRef]
- Waqas, M.; Aburiazaiza, A.S.; Miandad, R.; Rehan, M.; Barakat, M.A.; Nizami, A.S. Development of biochar as fuel and catalyst in energy recovery technologies. J. Clean. Prod. 2018, 188, 477–488. [Google Scholar] [CrossRef]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A review of silicon in soils and plants and its role in US agriculture: History and future perspectives. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef]
- Khawkomol, S.; Neamchan, R.; Thongsamer, T.; Vinitnantharat, S.; Panpradit, B.; Sohsalam, P.; Werner, D.; Mrozik, W. Potential of biochar derived from agricultural residues for sustainable management. Sustainability 2021, 13, 8147. [Google Scholar] [CrossRef]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef]
- Ishfaq, M.; Wang, Y.; Yan, M.; Wang, Z.; Wu, L.; Li, C.; Li, X. Physiological essence of magnesium in plants and its widespread deficiency in the farming system of China. Front. Plant Sci. 2022, 13, 802274. [Google Scholar] [CrossRef]
- Li, D.; Ma, W.; Wei, J.; Mao, Y.; Peng, Z.; Zhang, J.; Kong, X.; Han, Q.; Fan, W.; Yang, Y.; et al. Magnesium promotes root growth and increases aluminum tolerance via modulation of nitric oxide production in Arabidopsis. Plant Soil 2020, 457, 83–95. [Google Scholar] [CrossRef]
- Tian, X.Y.; He, D.-D.; Bai, S.; Zeng, W.-Z.; Wang, Z.; Wang, M.; Wu, L.-Q.; Chen, Z.-C. Physiological and molecular advances in magnesium nutrition of plants. Plant Soil 2021, 468, 1–17. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P. Characterization of biochars produced from residues from biogas production. J. Anal. Appl. Pyrolysis 2015, 115, 157–165. [Google Scholar] [CrossRef]
- Onia, B.A.; Oziegbeb, O.; Olawole, O.O. Significance of biochar application to the environment and economy. Ann. Agric. Sci. 2019, 64, 222–236. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Sodium in plants: Perception, signalling, and regulation of sodium fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef]
- Narayan, O.P.; Kumar, P.; Yadav, B.; Dua, M.; Johri, A.K. Sulfur nutrition and its role in plant growth and development. Plant Signal. Behav. 2022, 2022, 2030082. [Google Scholar] [CrossRef]
- Aarabi, F.; Naake, T.; Fernie, A.R.; Hoefgen, R. Coordinating sulfur pools under sulfate deprivation. Trends Plant Sci. 2020, 25, 1227–1239. [Google Scholar] [CrossRef]
- Yadav, B.; Jogawat, A.; Lal, S.K.; Lakra, N.; Mehta, S.; Shabek, N.; Narayan, O.P. Plant mineral transport systems and the potential for crop improvement. Planta 2021, 253, 45. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, M.; Liu, X.; Mao, Q.; Shi, C.; Kochian, L.V.; Liao, H. Aluminium is essential for root growth and development of tea plants (Camellia sinensis). J. Integr. Plant Biol. 2020, 62, 984–997. [Google Scholar] [CrossRef]
- Malta, P.G.; Arcanjo-Silva, S.; Ribeiro, C.; Campos, N.V.; Alves-Azevedo, A. Rudgea viburnoides (Rubiaceae) overcomes the low soil fertility of the Brazilian Cerrado and hyperaccumulates aluminum in cell walls and chloroplast. Plant Soil 2016, 408, 369–384. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, N.; Siddique, K.H.M.; Nayyar, H. Beneficial elements for agricultural crops and their functional relevance in defense against stresses. Arch. Agron. Soil Sci. 2016, 62, 905–920. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Kar, S.; Regon, P.; Panda, S.K. Physiology and biochemistry of Fe excess in acidic Asian soils on crop soil. J. Soil Sci. Agroclimatol. 2019, 16, 112–126. [Google Scholar] [CrossRef]
- Dey, S.; Regon, P.; Kar, S.; Panda, S.K. Chelators of iron and their role in plant’s iron management. Physiol. Mol. Biol. Plants 2020, 26, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.P.; Bucheli, T.; Kammann, C.; Glaser, B.; Abiven, S.; Leifeld, J.; Soja, G.; Hagemann, N. EBC (2012–2022) European Biochar Certificate—Guidelines for a Sustainable Production of Biochar, Version 10.1; European Biochar Foundation (EBC): Arbaz, Switzerland, 2022; pp. 22–23. [Google Scholar]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Moon, D.H.; Hwang, I.; Chang, Y.Y.; Koutsospyros, A.; Cheong, K.H.; Ji, W.H.; Park, J.H. Quality improvement of acidic soils by biochar derived from renewable materials. Environ. Sci. Pollut. Res. 2017, 24, 4194–4199. [Google Scholar] [CrossRef]
- Diatta, A.A.; Fike, J.H.; Battaglia, M.L.; Galbraith, J.M.; Baig, M.B. Effects of biochar on soil fertility and crop productivity in arid regions: A review. Arab. J. Geosci. 2020, 13, 595. [Google Scholar] [CrossRef]
- Yang, H.; Yu, H.; Shen, Z. A novel high-throughput and quantitative method based on visible color shifts for screening Bacillus subtilis THY-15 for surfactin production. J. Ind. Microbiol. Biotechnol. 2015, 42, 1139–1147. [Google Scholar] [CrossRef]
- Sun, D.; Liao, J.; Sun, L.; Wang, Y.; Liu, Y.; Deng, Q.; Zhang, N.; Xu, D.; Fang, Z.; Wang, W.; et al. Effect of media and fermentation conditions on surfactin and iturin homologues produced by Bacillus natto NT-6: LC–MS analysis. AMB Express 2019, 9, 120. [Google Scholar] [CrossRef]
- Gayathiri, E.; Prakash, P.; Karmegam, N.; Varjani, S.; Awasthi, M.K.; Ravindran, B. Biosurfactants: Potential and eco-friendly material for sustainable agriculture and environmental safety—A review. Agronomy 2022, 12, 662. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, N.; Yang, J.; Yang, Y.; Wang, R.; Liu, L.; Yuan, H. Lipopeptide produced from Bacillus sp. W112 improves the hydrolysis of lignocellulose by specifically reducing non-productive binding of cellulases with and without CBMs. Biotechnol. Biofuels 2017, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, F.; Zhang, X.; Zhou, X.; Zhong, Z.; Huaiyi, S.; Jin, L.; Haozhou, L.; Feng, F.; Lan, J.; et al. Cellulose-dependent expression and antibacterial characteristics of surfactin from Bacillus subtilis HH2 isolated from the giant panda. PLoS ONE 2018, 13, e0191991. [Google Scholar] [CrossRef]
- Iqbal, H.M.N.; Kyazze, G.; Keshavarz, T. Advances in the valorization of lignocellulosic materials by biotechnology: An overview. BioResources 2013, 8, 3157–3176. [Google Scholar] [CrossRef]
- Prado, A.A.O.S.; Santos, B.L.P.; Vieira, I.M.M.; Ramos, L.C.; de Souza, R.R.; Silva, D.P.; Ruzene, D.S. Evaluation of a new strategy in the elaboration of culture media to produce surfactin from hemicellulosic corncob liquor. Biotechnol. Rep. 2019, 24, e00364. [Google Scholar] [CrossRef]
- Ali, S.A.M.; Sayyed, R.Z.; Mir, M.I.; Khan, M.Y.; Hameeda, B.; Alkhanani, M.F.; Haque, S.; Mohammad Al Tawaha, A.R.; Poczai, P. Induction of systemic resistance in maize and antibiofilm activity of surfactin from Bacillus velezensis MS20. Front. Microbiol. 2022, 13, 879739. [Google Scholar] [CrossRef] [PubMed]
- Théatre, A.; Cano-Prieto, C.; Bartolini, M.; Laurin, Y.; Deleu, M.; Niehren, J.; Fida, T.; Gerbinet, S.; Alanjary, M.; Medema, M.H.; et al. The surfactin-like lipopeptides from Bacillus spp.: Natural biodiversity and synthetic biology for a broader application range. Front. Bioeng. Biotechnol. 2021, 9, 623701. [Google Scholar] [CrossRef]
- Li, M.S.M.; Piccoli, D.A.; McDowell, T.; MacDonald, J.; Renaud, J. Evaluating the biocontrol potential of Canadian strain Bacillus velezensis 1B-23 via its surfactin production at various pHs and temperatures. BMC Biotechnol. 2021, 21, 31. [Google Scholar] [CrossRef]
- Choub, V.; Maung, C.E.H.; Won, S.-J.; Moon, J.-H.; Kim, K.Y.; Han, Y.S.; Cho, J.-Y.; Ahn, Y.S. Antifungal activity of cyclic tetrapeptide from Bacillus velezensis CE 100 against plant pathogen Colletotrichum gloeosporioides. Pathogens 2021, 10, 209. [Google Scholar] [CrossRef]
- Krishnan, N.; Velramar, B.; Velu, R.K. Investigation of antifungal activity of surfactin against mycotoxigenic phytopathogenic fungus Fusarium moniliforme and its impact in seed germination and mycotoxicosis. Pestic. Biochem. Physiol. 2019, 155, 101–107. [Google Scholar] [CrossRef]
- Mohammadipour, M.; Mousivand, M.; Jouzani, G.S.; Abbasalizadeh, S. Molecular and biochemical characterization of Iranian surfactin-producing Bacillus subtilis isolates and evaluation of their biocontrol potential against Aspergillus flavus and Colletotrichum gloeosporioides. Can. J. Microbiol. 2009, 55, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Geissler, M.; Kühle, I.; Morabbi Heravi, K.; Altenbuchner, J.; Henkel, M.; Hausmann, R. Evaluation of surfactin synthesis in a genome reduced Bacillus subtilis strain. AMB Express 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.F.; Wei, J.Y.; Chen, H.W.; Liu, Y.Y.; Lu, H.Y.; Chou, J.Y. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant Signal. Behav. 2015, 10, e1048052. [Google Scholar] [CrossRef]
- Mohite, B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J. Soil Sci. Plant Nutr. 2013, 13, 638–649. [Google Scholar] [CrossRef]
- Majda, M.; Robert, S. The role of auxin in cell wall expansion. Int. J. Mol. Sci. 2018, 19, 951. [Google Scholar] [CrossRef]
- Bhattacharyya, C.; Banerjee, S.; Acharya, U.; Mitra, A.; Mallick, I.; Haldar, A.; Haldar, S.; Ghosh, A.; Ghosh, A. Evaluation of plant growth promotion properties and induction of antioxidative defense mechanism by tea rhizobacteria of Darjeeling, India. Sci. Rep. 2020, 10, 15536. [Google Scholar] [CrossRef]
- Cabra Cendales, T.; Rodríguez González, C.A.; Villota Cuásquer, C.P.; Tapasco Alzate, O.A.; Hernández Rodríguez, A. Bacillus effect on the germination and growth of tomato seedlings (Solanum lycopersicum L.). Acta Biol. Colomb. 2017, 22, 37–44. [Google Scholar] [CrossRef]
- Wagi, S.; Ahmed, A. Bacillus spp.: Potent microfactories of bacterial IAA. PeerJ 2019, 7, e7258. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Nargotra, P.; Chen, C.-W.; Kuo, C.-H.; Sun, P.-P.; Dong, C.-D. Agro-industrial food waste as a low-cost substrate for sustainable production of industrial enzymes: A critical review. Catalysts 2022, 12, 1373. [Google Scholar] [CrossRef]
- Shrestha, S.; Kognou, A.L.M.; Zhang, J.; Qin, W. Different facets of lignocellulosic biomass including pectin and its perspectives. Waste Biomass Valorization 2021, 12, 4805–4823. [Google Scholar] [CrossRef]
- Hoff, G.; Arguelles Arias, A.; Boubsi, F.; Pršić, J.; Meyer, T.; Ibrahim, H.M.M.; Steels, S.; Luzuriaga, P.; Legras, A.; Franzil, L.; et al. Surfactin stimulated by pectin molecular patterns and root exudates acts as a key driver of the Bacillus-plant mutualistic interaction. mBio 2021, 12, e01774-21. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, S.; Petrillo, C.; Donadio, G.; Piaz, F.D.; Cimmino, A.; Masi, M.; Evidente, A.; Isticato, R. Plant growth promotion function of Bacillus sp. strains isolated from salt-pan rhizosphere and their biocontrol potential against Macrophomina phaseolina. Int. J. Mol. Sci. 2021, 22, 3324. [Google Scholar] [CrossRef] [PubMed]
- Østby, H.; Hansen, L.D.; Horn, S.J.; Eijsink, V.G.H.; Várnai, A. Enzymatic processing of lignocellulosic biomass: Principles, recent advances and perspectives. J. Ind. Microbiol. Biotechnol. 2020, 47, 623–657. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kumar, B.; Verma, P.A. Detailed overview of xylanases: An emerging biomolecule for current and future prospective. Bioresour. Bioprocess. 2019, 6, 40. [Google Scholar] [CrossRef]
- Kabir, M.S.; Tasmim, T. Isolation of pectinase producing bacteria from the rhizosphere of Andrographis paniculata nees and 16S rRNA gene sequence comparison of some potential strains. Adv. Microbiol. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Oumer, O.J.; Abate, D. Screening and molecular identification of pectinase producing microbes from coffee pulp. Biomed. Res. Int. 2018, 2018, 2961767. [Google Scholar] [CrossRef] [PubMed]
- Pathak, E.; Sanjyal, A.; Regmi, C.R.; Paudel, S.; Shrestha, A. Screening of potential plant growth promoting properties of Bacillus species isolated from different regions of Nepal. Nepal J. Biotechnol. 2021, 9, 79–84. [Google Scholar] [CrossRef]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Bouket, A.C.; Boudechicha, A.; Luptakova, L.; Alenezi, F.N.; Belbahri, L. Screening of cellulolytic bacteria from various ecosystems and their cellulases production under multi-stress conditions. Catalysts 2022, 12, 769. [Google Scholar] [CrossRef]
- Batubara, U.M.; Mardalisa, M.; Suparjo, S.; Maritsa, H.U.; Pujianto, E.; Herlini, M. Isolation and characterization of cellulolytic bacteria diversity in peatland ecosystem and their cellulolytic activities. IOP Conf. Ser. Earth Environ. Sci. 2021, 934, 012028. [Google Scholar] [CrossRef]
- Raza, A.; Bashir, S.; Tabassum, R. Evaluation of cellulases and xylanases production from Bacillus spp. isolated from buffalo digestive system. Kafkas Univ. Vet. Fak. Derg. 2019, 25, 39–46. [Google Scholar] [CrossRef]
- Hashmi, S.; Iqbal, S.; Ahmed, I.; Janjua, H.A. Production, optimization, and partial purification of alkali-thermotolerant proteases from newly isolated Bacillus subtilis S1 and Bacillus amyloliquefaciens KSM12. Processes 2022, 10, 1050. [Google Scholar] [CrossRef]
- Kotlar, C.; Ponce, A.; Roura, S. Characterization of a novel protease from Bacillus cereus and evaluation of an eco-friendly hydrolysis of a brewery byproduct. J. Inst. Brew. 2015, 121, 558–565. [Google Scholar] [CrossRef]
- Suberu, Y.; Akande, I.; Samuel, T.; Lawal, A.; Olaniran, A. Optimization of protease production in indigenous Bacillus species isolated from soil samples in Lagos, Nigeria using response surface methodology. Biocatal. Agric. Biotechnol. 2019, 18, 101011. [Google Scholar] [CrossRef]
- Pertiwiningrum, A.; Anggraini, F.D.; Fitrianto, N.A.; Rochijan, R. Isolation and Identification of bacterial protease enzyme of leather waste. J. Indones. Trop. Anim. Agric. 2017, 42, 33–41. [Google Scholar] [CrossRef]
- Ariaeenejad, S.; Mousivand, M.; Dezfouli, P.M.; Hashemi, M.; Kavousi, K.; Salekdeh, G.H.A. Computational method for prediction of xylanase enzymes activity in strains of Bacillus subtilis based on pseudo amino acid composition features. PLoS ONE 2018, 13, e205796. [Google Scholar] [CrossRef] [PubMed]
- Tamariz-Angeles, C.; Lázaro-Palomino, J.; Olivera-Gonzales, P.; Castañeda-Barreto, A.; Villena, G.K. Isolation of thermotolerant Bacillus subtilis DCH4 from Chancos hot spring (Carhuaz, Peru) with potential to degrade lignocellulosic agriculture wastes. Rev. Peru Biol. 2020, 27, 67–78. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 171–190. [Google Scholar] [CrossRef]
- Joe, M.M.; Deivaraj, S.; Benson, A.; Henry, A.J.; Narendrakumar, G. Soil extract calcium phosphate media for screening of phosphate-solubilizing bacteria. Agric. Nat. Resour. 2018, 52, 305–308. [Google Scholar] [CrossRef]
- Janati, W.; Mikou, K.; El Ghadraoui, L.; Errachidi, F. Isolation and characterization of phosphate solubilizing bacteria naturally colonizing legumes rhizosphere in Morocco. Front. Microbiol. 2022, 13, 958300. [Google Scholar] [CrossRef]
- Mohamed, A.E.; Nessim, M.G.; Abou-el-seoud, I.I.; Darwish, K.M.; Shamseldin, A. Isolation and selection of highly effective phosphate solubilizing bacterial strains to promote wheat growth in Egyptian calcareous soils. Bull. Natl. Res. Cent. 2019, 43, 203. [Google Scholar] [CrossRef]
- Johnson, S.E.; Loeppert, R.H. Role of organic acids in phosphate mobilization from iron oxide. Soil Sci. Soc. Am. J. 2006, 70, 222–234. [Google Scholar] [CrossRef]
- Behera, B.C.; Yadav, H.; Singh, S.K.; Mishra, R.R.; Sethi, B.K.; Dutta, S.K.; Thatoi, H.N. Phosphate solubilization and acid phosphatase activity of Serratia sp. isolated from mangrove soil of Mahanadi river delta, Odisha, India. J. Genet. Eng. Biotechnol. 2017, 15, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Dmitrović, S.; Pajčin, I.; Vlajkov, V.; Grahovac, M.; Jokić, A.; Grahovac, J. Dairy and wine industry effluents as alternative media for the production of Bacillus-based biocontrol agents. Bioengineering 2022, 9, 663. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Sinha, S.N. Phosphate solubilization potential and phosphate activity of some bacterial strains isolated from thermal power plant effluent exposed water of river Ganga. CIBTech J. Microbiol. 2013, 2, 1–7. [Google Scholar]
- Mosela, M.; Andrade, G.; Massucato, L.R.; de Araújo Almeida, S.R.; Nogueira, A.F.; de Lima Filho, R.B.; Zeffa, D.M.; Mian, S.; Higashi, A.Y.; Shimizu, G.D.; et al. Bacillus velezensis strain Ag75 as a new multifunctional agent for biocontrol, phosphate solubilization and growth promotion in maize and soybean crops. Sci. Rep. 2022, 12, 15284. [Google Scholar] [CrossRef]
- Izhar Shafi, M.; Adnan, M.; Fahad, S.; Wahid, F.; Khan, A.; Yue, Z.; Danish, S.; Zafar-ul-Hye, M.; Brtnicky, M.; Datta, R. Application of single superphosphate with humic acid improves the growth, yield and phosphorus uptake of wheat (Triticum aestivum L.) in calcareous soil. Agronomy 2020, 10, 1224. [Google Scholar] [CrossRef]
- Marques, A.P.G.C.; Pires, C.; Moreira, H.; Rangel, A.O.S.S.; Castro, P.M.L. Assessment of the plant growth promotion abilities of six bacterial isolates using Zea mays as indicator plant. Soil Biol. Biochem. 2010, 42, 1229–1235. [Google Scholar] [CrossRef]
- Gohil, R.B.; Raval, V.H.; Panchal, R.R.; Rajput, K.N. Plant growth-promoting activity of Bacillus sp. PG-8 isolated from fermented panchagavya and its effect on the growth of arachis hypogea. Front. Agron. 2022, 4, 805454. [Google Scholar] [CrossRef]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant growth enhancement using rhizospheric halotolerant phosphate solubilizing bacterium Bacillus licheniformis QA1 and Enterobacter asburiae QF11 isolated from Chenopodium quinoa Willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 2013, 118, 10–15. [Google Scholar] [CrossRef]
- Patil, C.; Suryawanshi, R.; Koli, S.; Patil, S. Improved method for effective screening of ACC (1-aminocyclopropane-1-carboxylate) deaminase producing microorganisms. J. Microbiol. Methods 2016, 131, 102–104. [Google Scholar] [CrossRef]
- Zarei, T.; Moradi, A.; Kazemeini, S.A.; Akhgar, A.; Rahi, A.A. The role of ACC deaminase producing bacteria in improving sweet corn (Zea mays L. var saccharata) productivity under limited availability of irrigation water. Sci. Rep. 2020, 10, 20361. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Chauhan, P.S. ACC deaminase-producing rhizosphere competent Bacillus spp. mitigate salt stress and promote Zea mays growth by modulating ethylene metabolism. 3 Biotech 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Zakavi, M.; Askari, H.; Shahrooei, M. Maize growth response to different Bacillus strains isolated from a salt-marshland area under salinity stress. BMC Plant Biol. 2022, 22, 367. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.A.; Rasmussen, A.; Traini, R.; Voß, U.; Sturrock, C.; Mooney, S.J.; Wells, D.M.; Bennett, M.J. Branching out in roots: Uncovering form, function, and regulation. Plant Physiol. 2014, 166, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Hochholdinger, F.; Marcon, C.; Baldauf, J.A.; Yu, P.; Frey, F.P. Proteomics of maize root development. Front. Plant Sci. 2018, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, S.M.; de Oliveira, C.A.; Andrade, D.L.; de Carvalho, C.G.; Ribeiro, V.P.; Pastina, M.M.; Marriel, I.E.; de Paula Lana, U.G.; Gomes, E.A. Tropical Bacillus strains inoculation enhances maize root surface area, dry weight, nutrient uptake and grain yield. J. Plant Growth Regul. 2021, 40, 867–877. [Google Scholar] [CrossRef]
- Zerrouk, I.Z.; Rahmoune, B.; Auer, S.; Rößler, S.; Lin, T.; Baluska, F.; Dobrev, P.I.; Motyka, V.; Ludwig-Müller, J. Growth and aluminum tolerance of maize roots mediated by auxin- and cytokinin-producing Bacillus toyonensis requires polar auxin transport. Environ. Exp. Bot. 2020, 176, 104064. [Google Scholar] [CrossRef]
- Hou, Y.; Zeng, W.; Ao, C.; Luo, Y.; Wang, Z.; Hou, M.; Huang, J. Bacillus atrophaeus WZYH01 and Planococcus soli WZYH02 improve salt tolerance of maize (Zea mays L.) in saline soil. Front. Plant Sci. 2022, 13, 891372. [Google Scholar] [CrossRef]
- Vardharajula, S.; Zulfikar Ali, S.; Grover, M.; Reddy, G.; Bandi, V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, H.; Luo, S.; Yang, W.; Zhang, L.; Li, S.; Jin, Q.; Cao, Q.; Sun, S.; Xiao, M. Role of maize root exudates in promotion of colonization of Bacillus velezensis strain S3-1 in rhizosphere soil and root tissue. Curr. Microbiol. 2019, 76, 855–862. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, D.; Wang, D.; Miao, Y.; Shao, J.; Zhou, X.; Xu, Z.; Li, Q.; Feng, H.; Li, S.; et al. Whole transcriptomic analysis of the plant-beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 during enhanced biofilm formation regulated by maize root exudates. BMC Genom. 2015, 16, 685. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, E.; Asamoah, G.; Kofi, B.; Abunyewa, A.A. Effect of biochar type and rate of application on maize yield indices and water use efficiency on an Ultisol in Ghana. Energy Procedia 2016, 93, 14–18. [Google Scholar] [CrossRef]
- Pandit, N.R.; Mulder, J.; Hale, S.E.; Martinsen, V.; Schmidt, H.P.; Cornelissen, G. Biochar improves maize growth by alleviation of nutrient stress in a moderately acidic low-input Nepalese soil. Sci. Total Environ. 2018, 625, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Benkova, M.; Nenova, L.; Simeonova, T.; Petkova, G.; Mikova, A.; Atanassova, I. Influence of biochar addition to fluvisol on maize yield and soil microbiota. J. Cent. Eur. Agric. 2022, 23, 413–422. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Liu, C.; Sun, B.; Zheng, J.; Bian, R.; Drosos, M.; Zhang, X.; Li, L.; Pan, G. Biochar increases maize yield by promoting root growth in the rainfed region. Arch. Agron. Soil Sci. 2021, 67, 1411–1424. [Google Scholar] [CrossRef]
- Kätterer, T.; Roobroeck, D.; Kimutai, G.; Karltun, E.; Nyberg, G.; Sundberg, C.; de Nowina, K.R. Maize grain yield responses to realistic biochar application rates on smallholder farms in Kenya. Agron. Sustain. Dev. 2022, 42, 63. [Google Scholar] [CrossRef]
- Luo, Y.; Jiao, Y.-J.; Zhao, X.-R.; Li, G.-T.; Zhao, L.-X.; Meng, H.-B. Improvement to maize growth caused by biochars derived from six feedstocks prepared at three different temperatures. J. Integr. Agric. 2014, 13, 533–540. [Google Scholar] [CrossRef]
- Jabborova, D.; Wirth, S.; Kannepalli, A.; Narimanov, A.; Desouky, S.; Davranov, K.; Sayyed, R.Z.; El Enshasy, H.; Malek, R.A.; Syed, A.; et al. Co-Inoculation of Rhizobacteria and Biochar Application Improves Growth and Nutrientsin Soybean and Enriches Soil Nutrients and Enzymes. Agronomy 2020, 10, 1142. [Google Scholar] [CrossRef]
- Irfan, M.; Mudassir, M.; Khan, M.J.; Dawar, K.M.; Muhammad, D.; Mian, I.A.; Ali, W.; Fahad, S.; Saud, S.; Hayat, Z.; et al. Heavy metals immobilization and improvement in maize (Zea mays L.) growth amended with biochar and compost. Sci. Rep. 2021, 11, 18416. [Google Scholar] [CrossRef]
- Zhang, X.; Al-Dossary, A.; Hussain, M.; Setlow, P.; Li, J. Applications of Bacillus subtilis spores in biotechnology and advanced materials. Appl. Environ. Microbiol. 2020, 86, e01096-20. [Google Scholar] [CrossRef]
- Vlajkov, V.; Grahovac, M.; Budakov, D.; Loc, M.; Pajčin, I.; Milić, D.; Novaković, T.; Grahovac, J. Distribution, Genetic Diversity and Biocontrol of Aflatoxigenic Aspergillus Flavus in Serbian Maize Fields. Toxins 2021, 13, 687. [Google Scholar] [CrossRef]
- Dmitrović, S.; Pajčin, I.; Lukić, N.; Vlajkov, V.; Grahovac, M.; Grahovac, J.; Jokić, A. Taguchi grey relational analysis for multi-response optimization of Bacillus bacteria flocculation recovery from fermented broth by chitosan to enhance biocontrol efficiency. Polymers 2022, 14, 3282. [Google Scholar] [CrossRef] [PubMed]
- Vlajkov, V.; Andelić, S.; Pajčin, I.; Grahovac, M.; Budakov, D.; Jokić, A.; Grahovac, J. Medium for the production of Bacillus-based biocontrol agent effective against aflatoxigenic Aspergillus flavus: Dual approach for modelling and optimization. Microorganisms 2022, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Malbaša, R.; Vitas, J.; Vukmanović, S. Analytical Chemistry: A Practicum with a Workbook; Faculty of Technology Novi Sad: Novi Sad, Serbia, 2021. [Google Scholar]
- Cunniff, P. Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Valenzuela-Ávila, L.; Miliar, Y.; Moya-Ramirez, I.; Chyhyrynets, O.; Garcia-Román, M.; Altmajer-Vaz, D. Effect of emulsification and hydrolysis pretreatments of waste frying oil on surfactin production. J. Chem. Technol. Biotechnol. 2019, 95, 223–231. [Google Scholar] [CrossRef]
- Syed Ab Rahman, S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef]
- Adinarayana, K.; Ellaiah, P.; Prasad, D.S. Purification and partial characterization of thermostable serine alkaline protease from a newly isolated Bacillus subtilis PE-11. AAPS PharmSciTech 2003, 4, E56. [Google Scholar] [CrossRef]
- Amore, A.; Parameswaran, B.; Kumar, R.; Birolo, L.; Vinciguerra, R.; Marcolongo, L.; Ionata, E.; La Cara, F.; Pandey, A.; Faraco, V. Application of a new xylanase activity from Bacillus amyloliquefaciens XR44A in brewer’s spent grain saccharification. J. Chem. Technol. Biotechnol. 2015, 90, 573–581. [Google Scholar] [CrossRef]
- Mohandas, A.; Raveendran, S.; Parameswaran, B.; Abraham, A.; Athira, R.S.R.; Mathew, A.K.; Pandey, A. Production of pectinase from Bacillus sonorensis MPTD1. Food Technol. Biotechnol. 2018, 56, 110–116. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Fahsi, N.; Mahdi, I.; Mesfioui, A.; Biskri, L.; Allaoui, A. Plant growth-promoting rhizobacteria isolated from the jujube (Ziziphus lotus) plant enhance wheat growth, Zn uptake, and heavy metal tolerance. Agriculture 2021, 11, 316. [Google Scholar] [CrossRef]
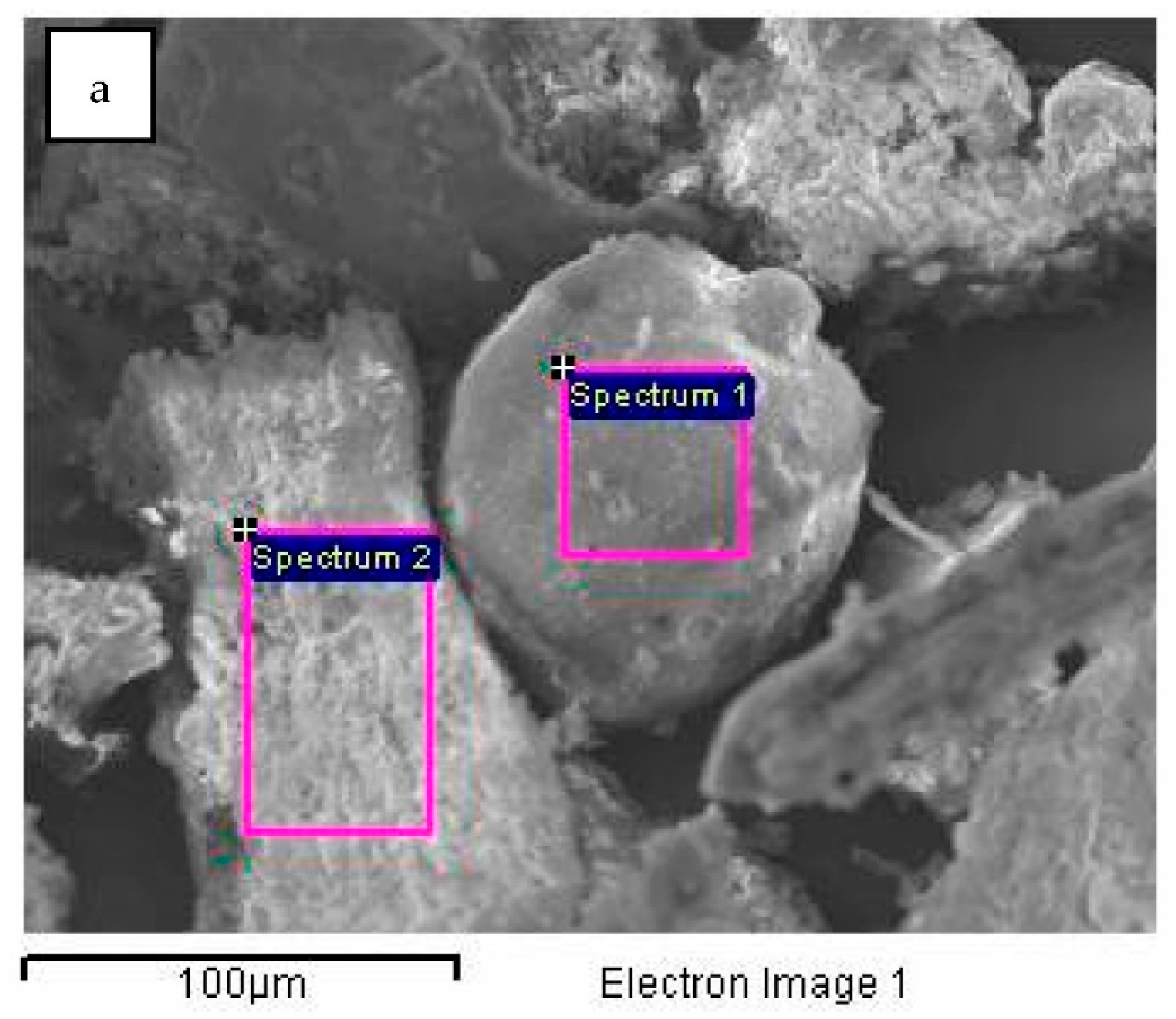

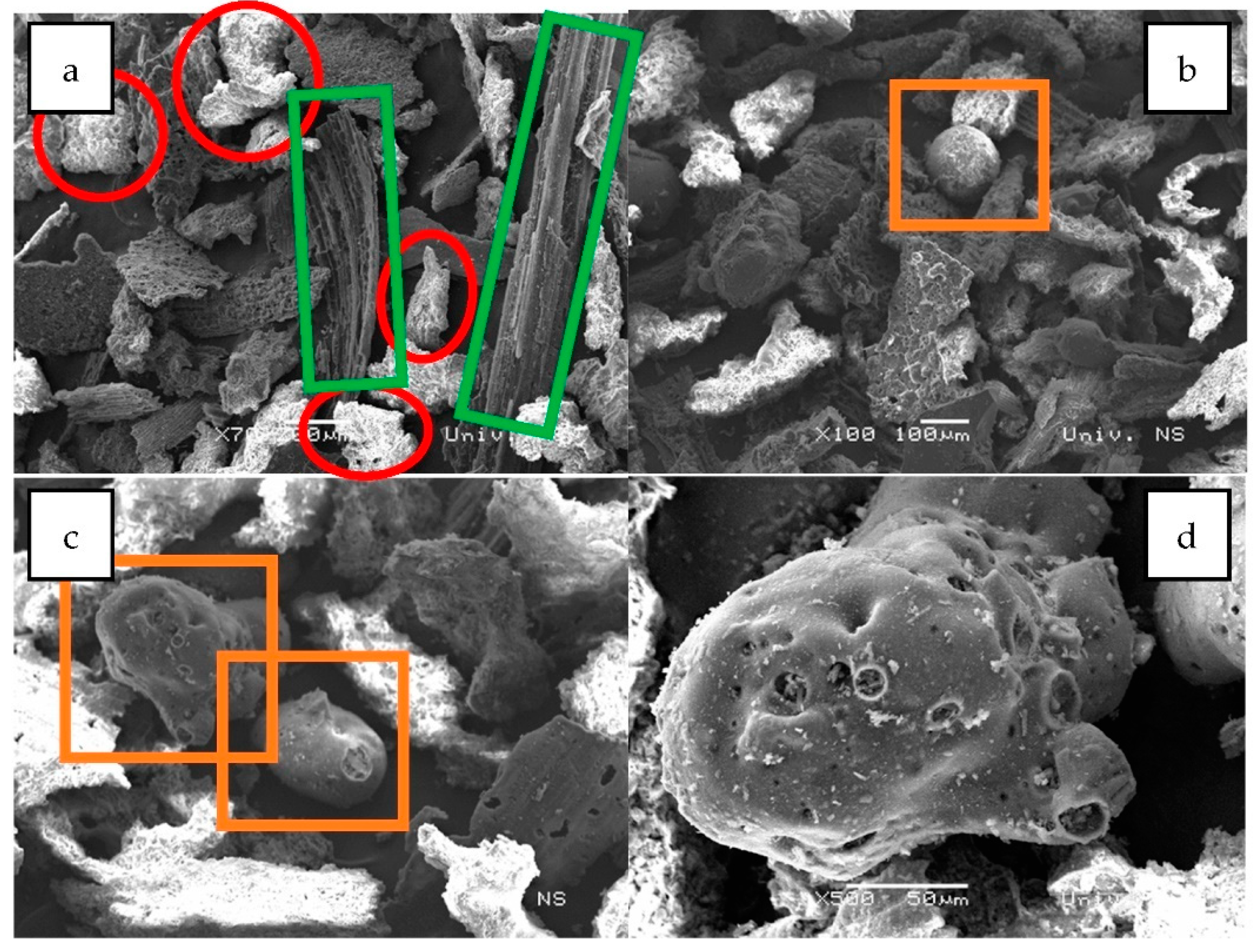
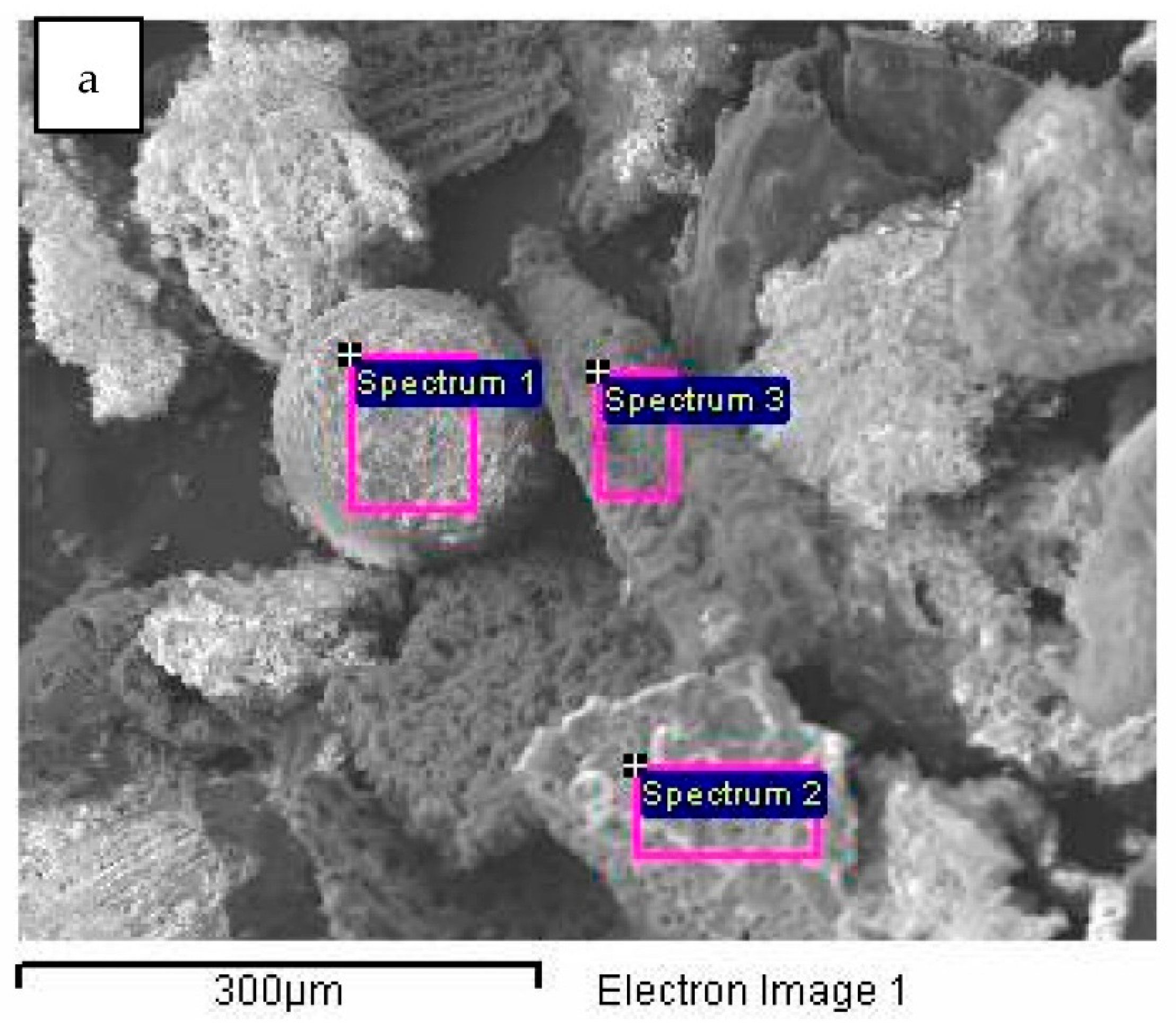
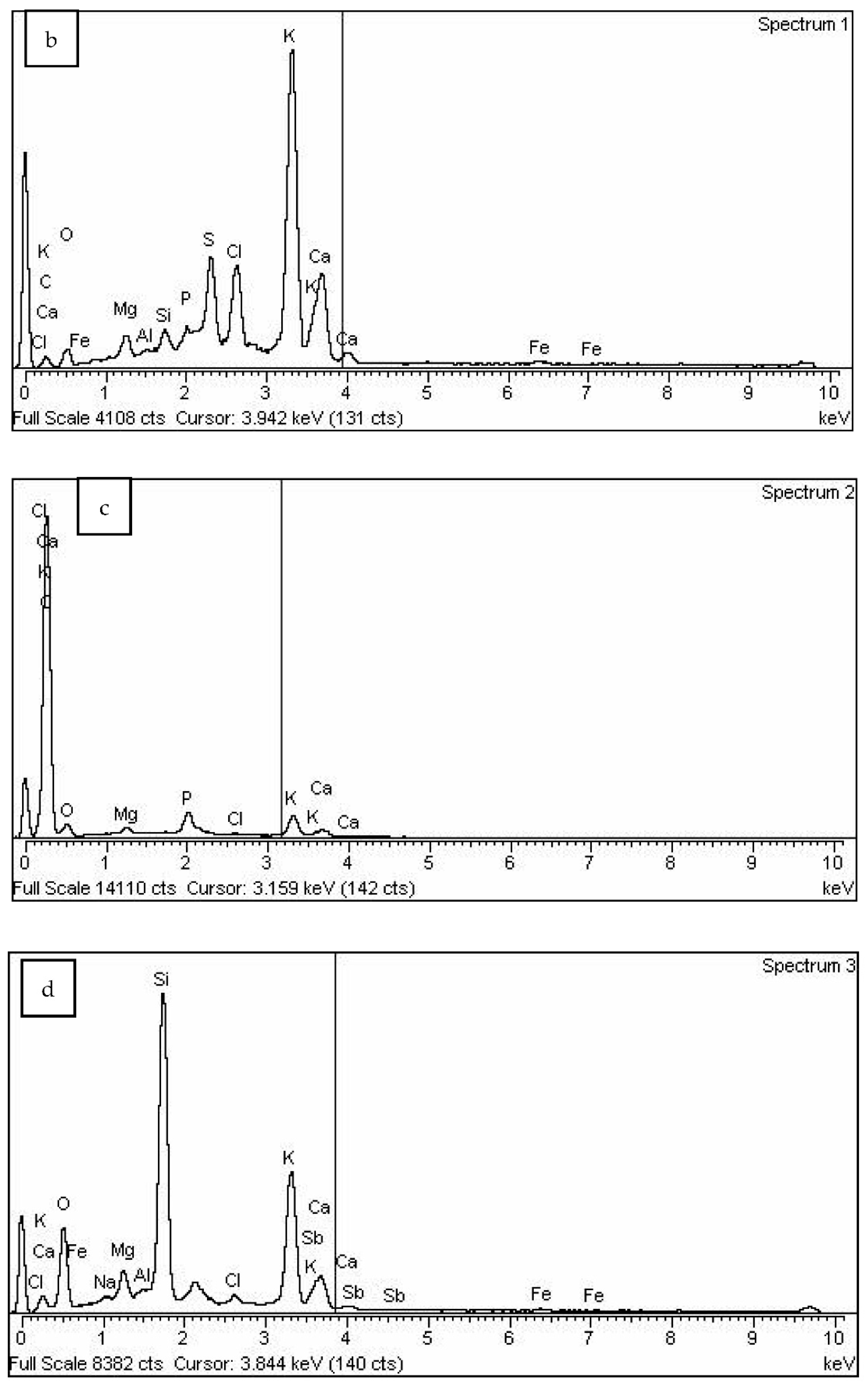
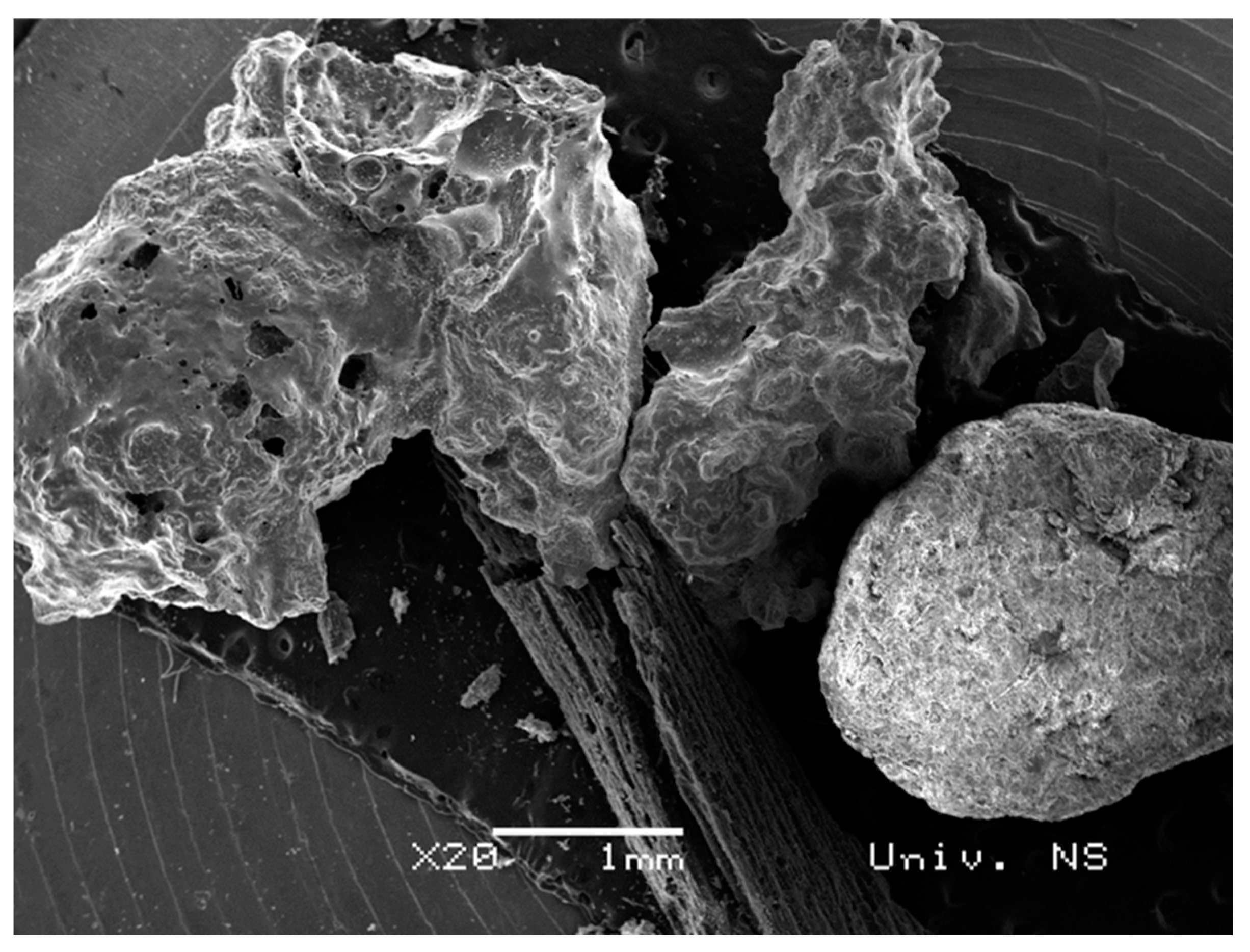
| Spectrum | C (%) | O (%) | Mg (%) | Al (%) | Si (%) | S (%) | Cl (%) | K (%) | Ca (%) | Fe (%) | Sb (%) | I (%) | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spectrum 1 | 0.00 | 42.62 | 7.20 | 0.00 | 23.20 | 0.00 | 0.00 | 14.29 | 10.05 | 0.00 | 1.90 | 0.73 | 100.00 |
| Spectrum 2 | 10.43 | 49.30 | 4.57 | 0.45 | 1.97 | 0.42 | 0.29 | 2.07 | 30.21 | 0.29 | 0.00 | 0.00 | 100.00 |
| Spectrum | C (%) | O (%) | Na (%) | Mg (%) | Al (%) | Si (%) | P (%) | S (%) | Cl (%) | K (%) | Ca (%) | Fe (%) | Sb (%) | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spectrum 1 | 16.48 | 21.68 | 0.00 | 2.04 | 0.25 | 1.36 | 1.34 | 7.45 | 7.73 | 30.98 | 9.97 | 0.72 | 0.00 | 100.00 |
| Spectrum 2 | 88.37 | 49.30 | 0.00 | 0.30 | 0.00 | 0.00 | 0.90 | 0.00 | 0.08 | 1.22 | 0.43 | 0.00 | 0.00 | 100.00 |
| Spectrum 3 | 0.00 | 53.21 | 0.55 | 2.59 | 0.38 | 22.25 | 0.00 | 0.00 | 0.99 | 15.04 | 3.62 | 0.52 | 0.58 | 100.00 |
| Parameter | Value |
|---|---|
| Residual cellulose content (g/L) | 2.8 |
| Residual total nitrogen content (g/L) | 0.27 |
| Biomass content (log(CFU/mL)) | 8.47 |
| pH value | 6.3 |
| Parameter | Concentration (mg/L) | Halo Zone Diameter (mm) | EAI1 | PSI2 | Positive/ Negative Test |
|---|---|---|---|---|---|
| Surfactin production | 1475 | / | / | / | / |
| IAA production | 15 | / | / | / | / |
| Cellulase activity | / | 54.50 ± 0.50 | 2.71 | / | / |
| Xylanase activity | / | 32.00 ± 0.50 | 2.85 | / | / |
| Pectinase activity | / | 21.00 ± 1.00 | 3.58 | / | / |
| Protease activity | / | 31.00 ± 0.00 | 1.55 | / | / |
| Phosphate solubilisation | / | 11.50 ± 0.50 | / | 2.03 | / |
| Ammonia production | / | / | / | / | + |
| ACC deaminase production | / | / | / | / | + |
| Number of Experiment | Incubation Time (h) | Biochar Amount (%, w/v) |
|---|---|---|
| 1 | 24 | 1 |
| 2 | 24 | 3 |
| 3 | 24 | 5 |
| 4 | 48 | 1 |
| 5 | 48 | 3 |
| 6 | 48 | 5 |
| 7 | 72 | 1 |
| 8 | 72 | 3 |
| 9 | 72 | 5 |
| Experiment | GP (%) | RL (mm) | SL (mm) | FSM (g) | DSM (g) | SVI | Desirability (%) |
|---|---|---|---|---|---|---|---|
| 2 | 100 c | 34.20 ± 25.46 a | 34.20 ± 12.92 bc | 0.43 ± 0.09 a | 0.12 ± 0.03 a | 6840 d | 0.7702 |
| 7 | 95 b | 32.00 ± 22.11 a | 33.85 ± 14.09 abc | 0.42 ± 0.07 a | 0.14 ± 0.03 abc | 6255 b | 0.7709 |
| 5 | 100 c | 42.70 ± 33.99 abc | 32.10 ± 13.10 ab | 0.43 ± 0.09 a | 0.13 ± 0.03 abc | 7480 f | 0.8131 |
| 1 | 100 c | 33.95 ± 18.70 a | 33.70 ± 11.95 abc | 0.45 ± 0.09 a | 0.15 ± 0.02 c | 6765 c | 0.8357 |
| 9 | 100 c | 37.30 ± 19.71 ab | 31.35 ± 9.21 ab | 0.45 ± 0.09 a | 0.15 ± 0.03 c | 6865 e | 0.8410 |
| 3 | 100 c | 57.10 ± 26.24 cde | 42.00 ± 12.44 c | 0.45 ± 0.08 a | 0.13 ± 0.03 ab | 9910 h | 0.9009 |
| 8 | 100 c | 71.60 ± 23.29 e | 34.25 ± 9.80 bc | 0.46 ± 0.08 a | 0.14 ± 0.03 abc | 10585 j | 0.9440 |
| 4 | 100 c | 53.00 ± 31.18 bcd | 39.00 ± 11.31 bc | 0.49 ± 0.10 a | 0.15 ± 0.04 c | 9200 g | 0.9470 |
| 6 | 100 c | 62.70 ± 33.96 de | 39.80 ± 12.22 bc | 0.49 ± 0.11 a | 0.15 ± 0.02 bc | 10250 i | 0.9795 |
| NC | 90 a | 28.05 ± 22.36 a | 25.45 ± 14.89 a | 0.426 ± 0.10 a | 0.138 ± 0.025 abc | 4815 a | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlajkov, V.; Pajčin, I.; Vučetić, S.; Anđelić, S.; Loc, M.; Grahovac, M.; Grahovac, J. Bacillus-Loaded Biochar as Soil Amendment for Improved Germination of Maize Seeds. Plants 2023, 12, 1024. https://doi.org/10.3390/plants12051024
Vlajkov V, Pajčin I, Vučetić S, Anđelić S, Loc M, Grahovac M, Grahovac J. Bacillus-Loaded Biochar as Soil Amendment for Improved Germination of Maize Seeds. Plants. 2023; 12(5):1024. https://doi.org/10.3390/plants12051024
Chicago/Turabian StyleVlajkov, Vanja, Ivana Pajčin, Snežana Vučetić, Stefan Anđelić, Marta Loc, Mila Grahovac, and Jovana Grahovac. 2023. "Bacillus-Loaded Biochar as Soil Amendment for Improved Germination of Maize Seeds" Plants 12, no. 5: 1024. https://doi.org/10.3390/plants12051024
APA StyleVlajkov, V., Pajčin, I., Vučetić, S., Anđelić, S., Loc, M., Grahovac, M., & Grahovac, J. (2023). Bacillus-Loaded Biochar as Soil Amendment for Improved Germination of Maize Seeds. Plants, 12(5), 1024. https://doi.org/10.3390/plants12051024










