Leaf Functional Traits in Relation to Species Composition in an Arctic–Alpine Tundra Grassland
Abstract
1. Introduction
2. Results
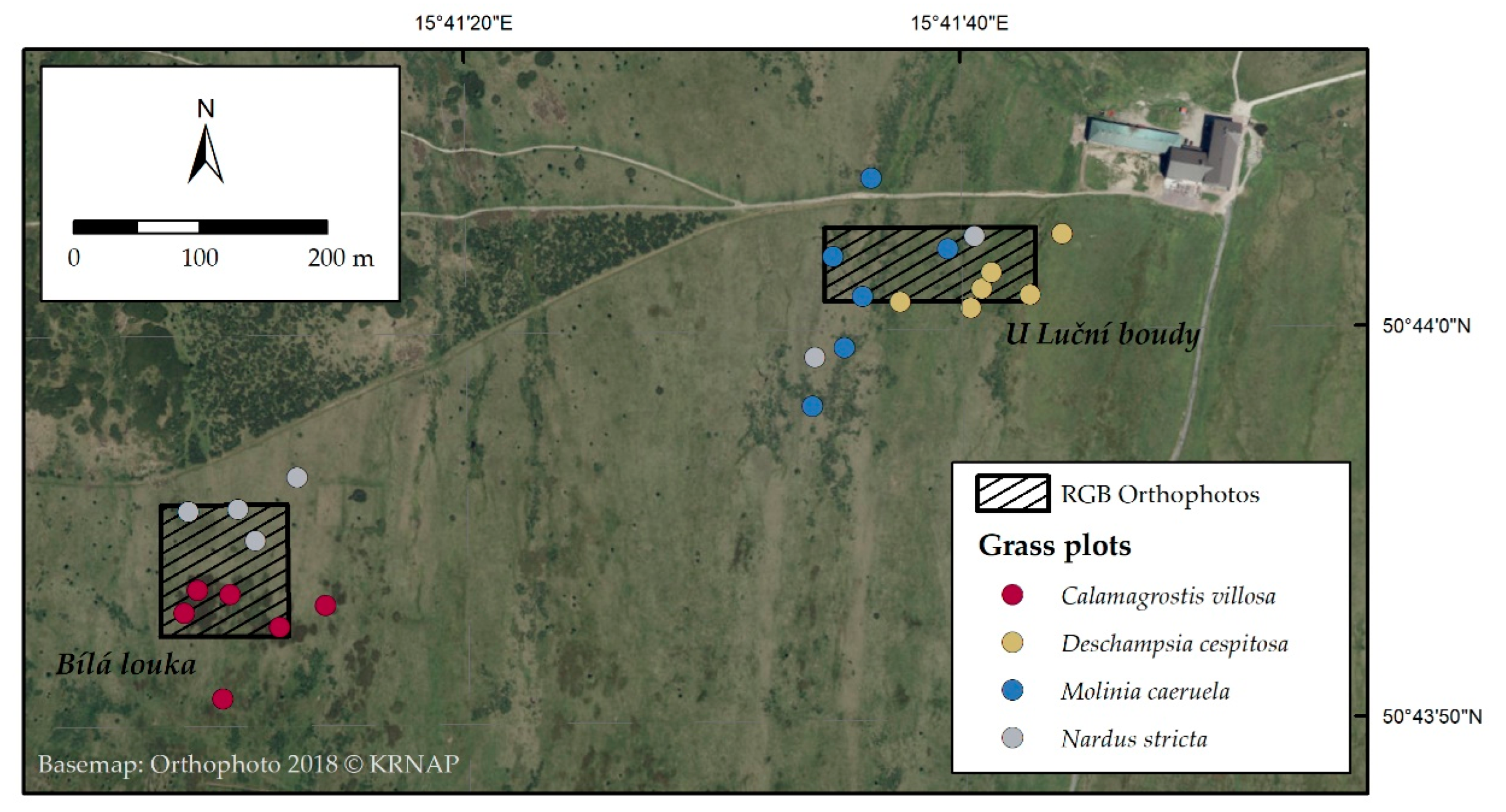
2.1. Orthophotos Classifications
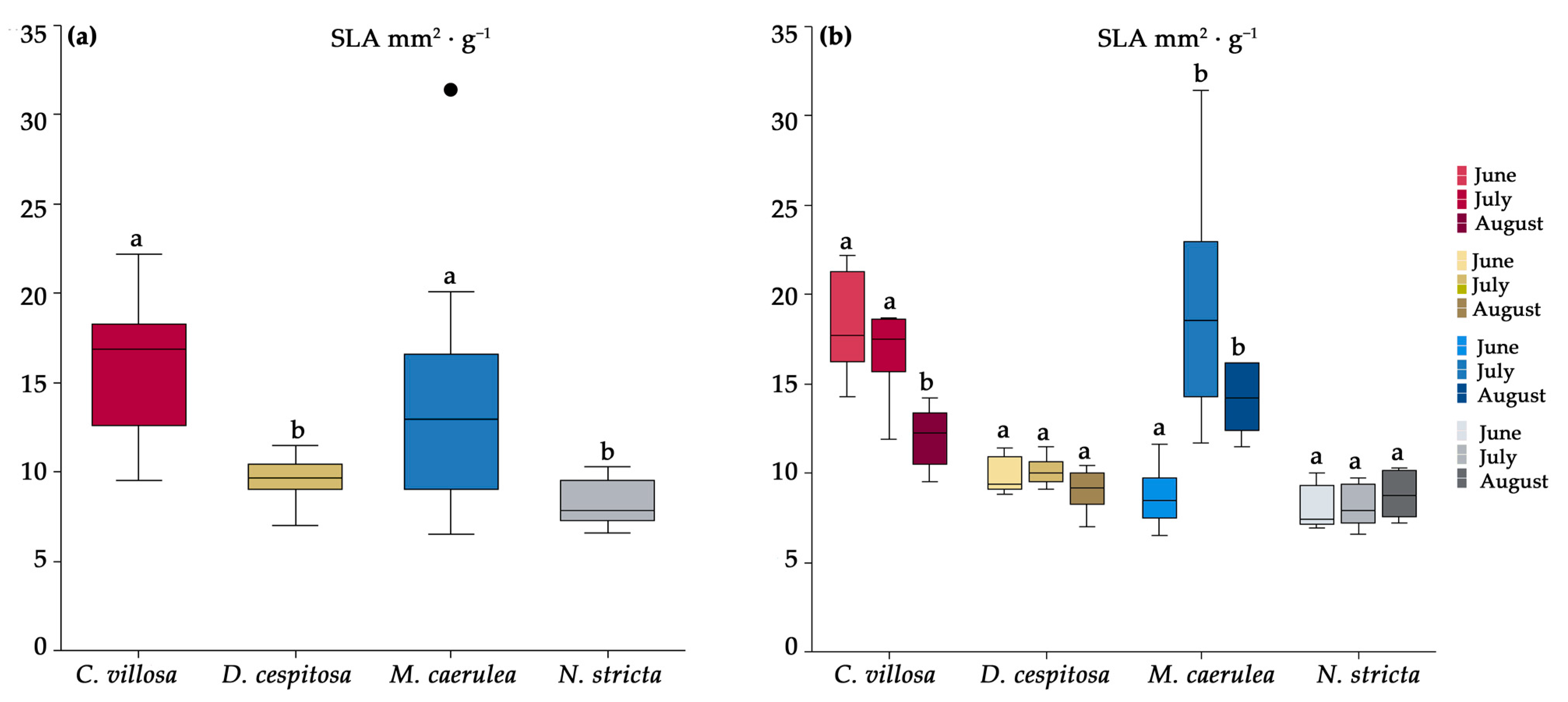
2.2. Morphological Characteristics
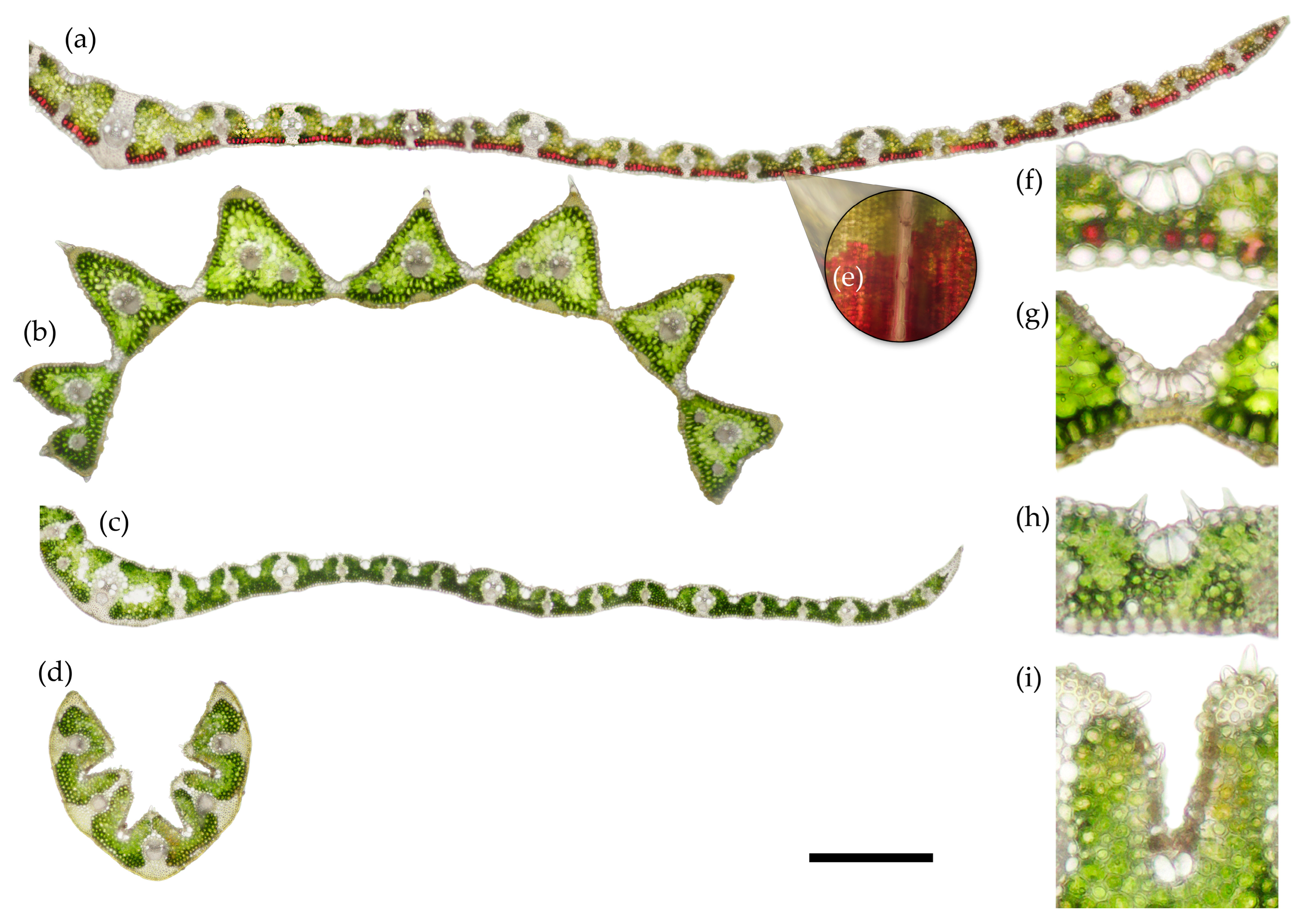
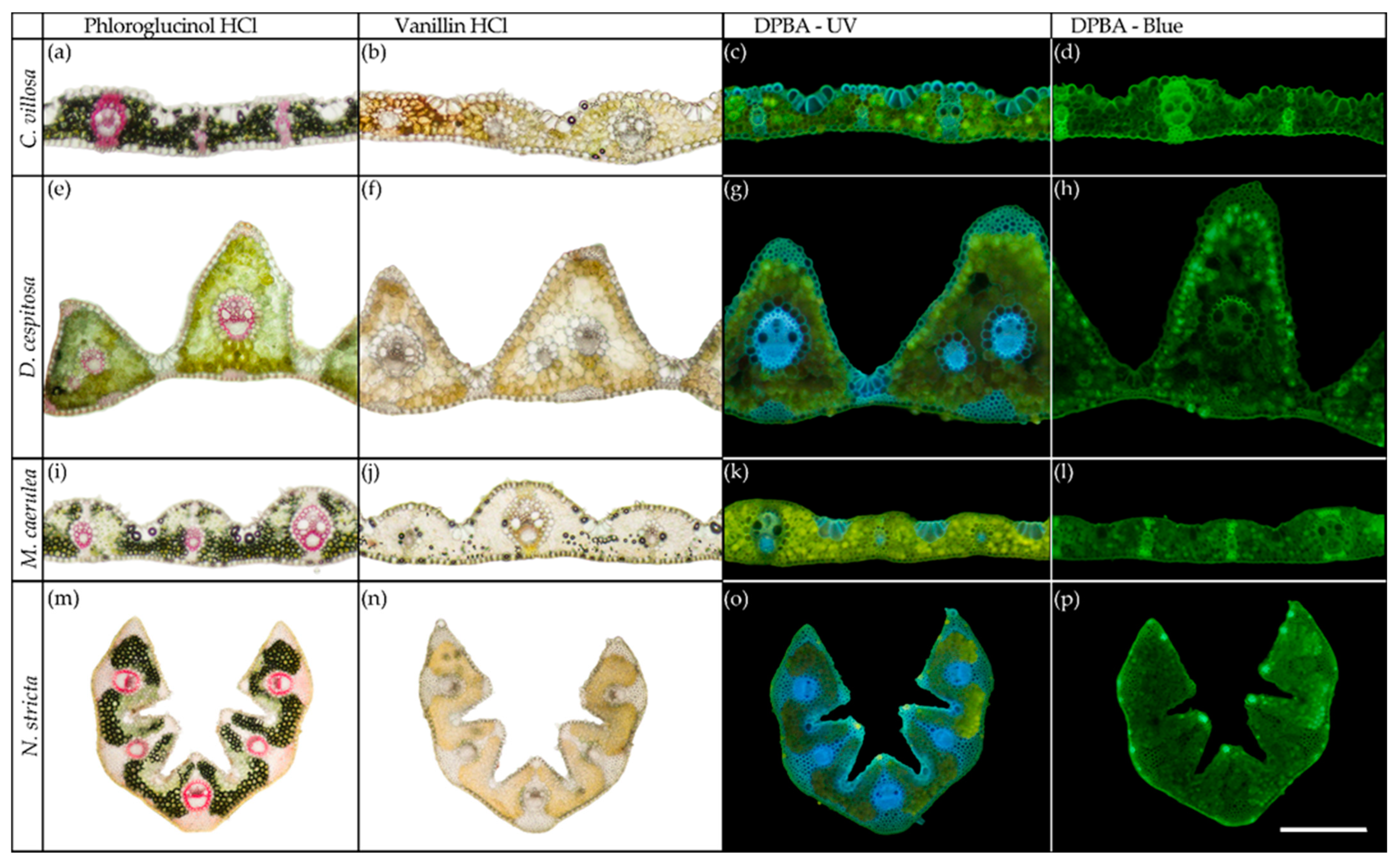
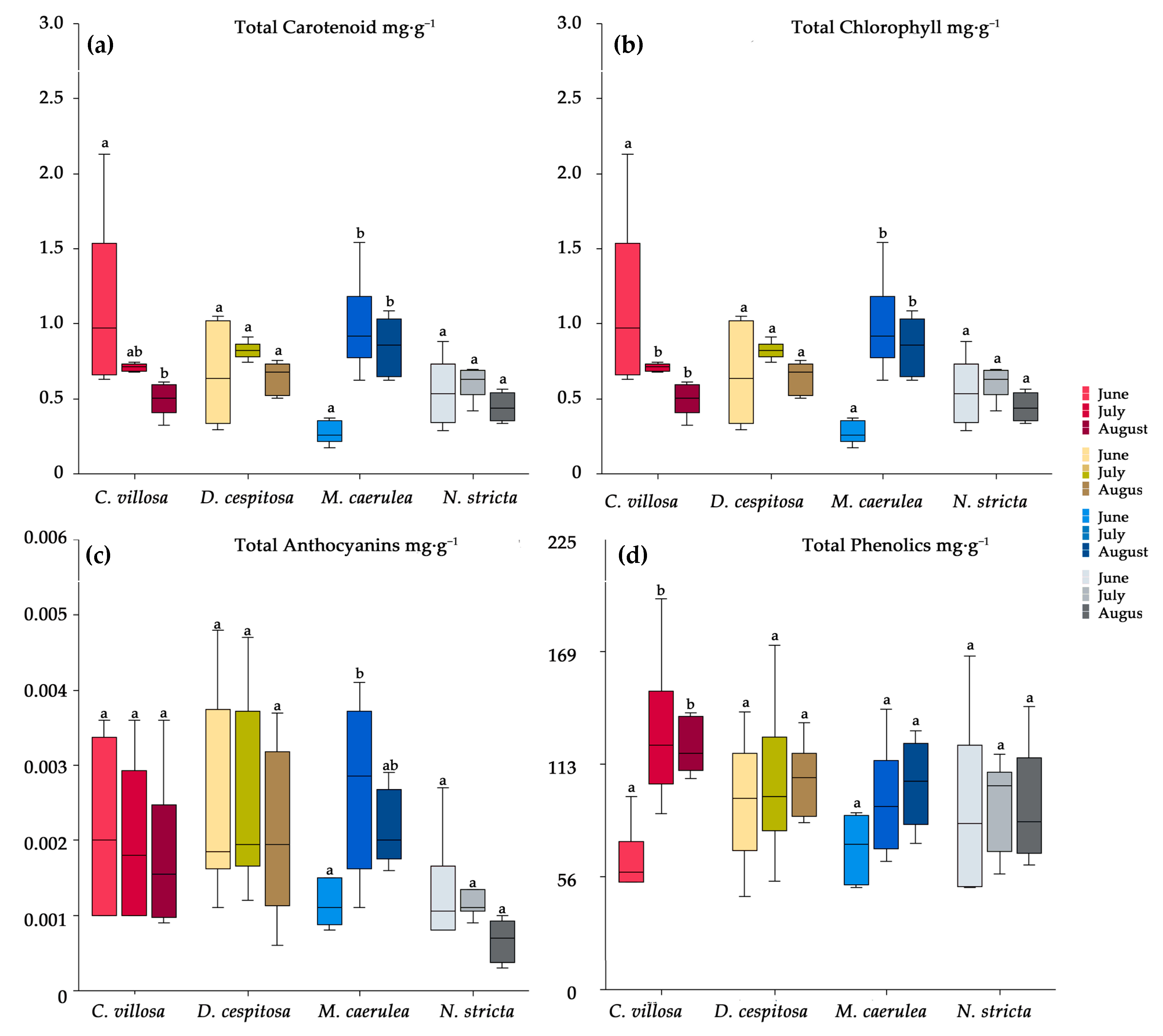
2.3. Histochemistry
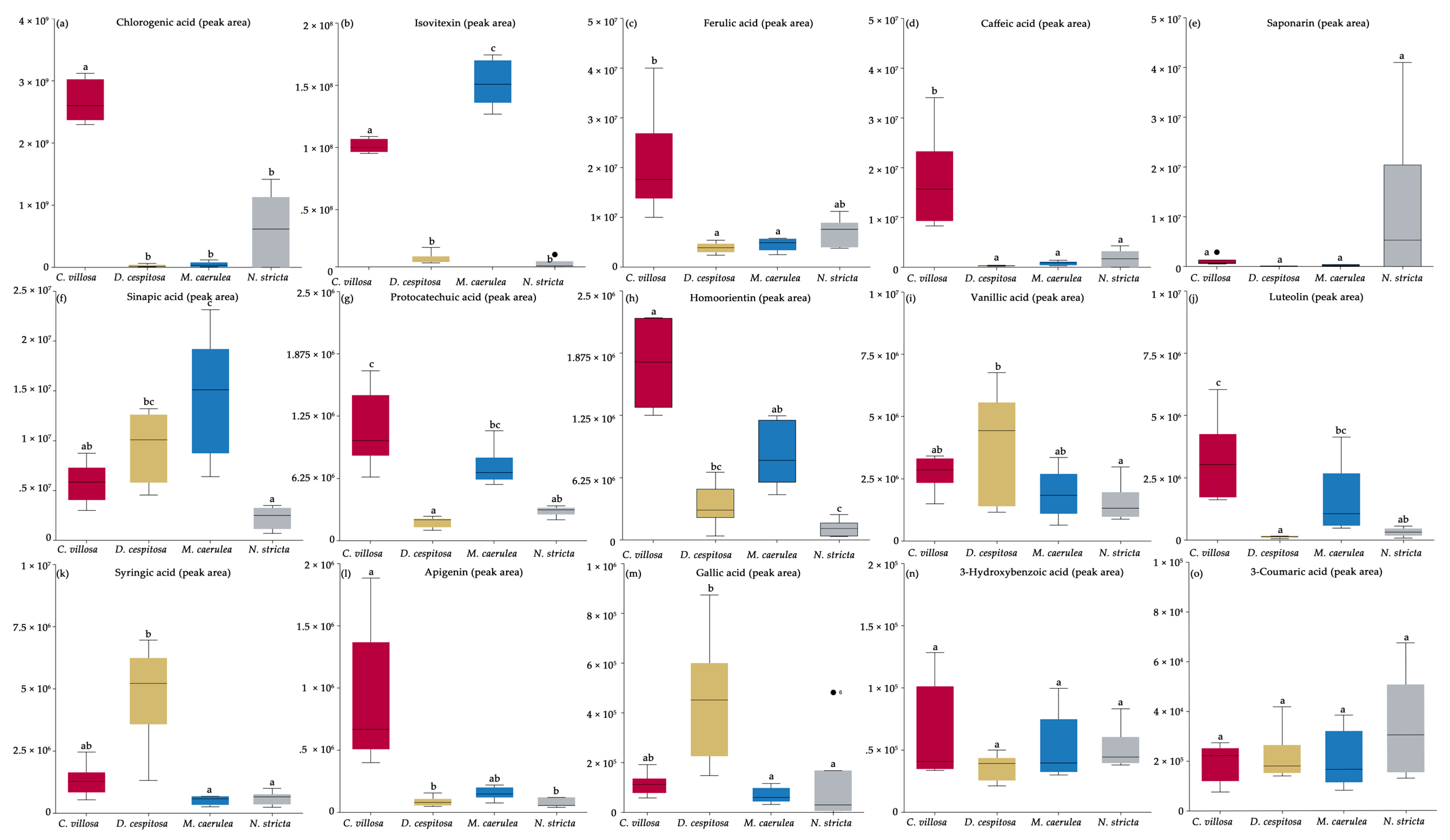
2.4. Biochemical Profiles
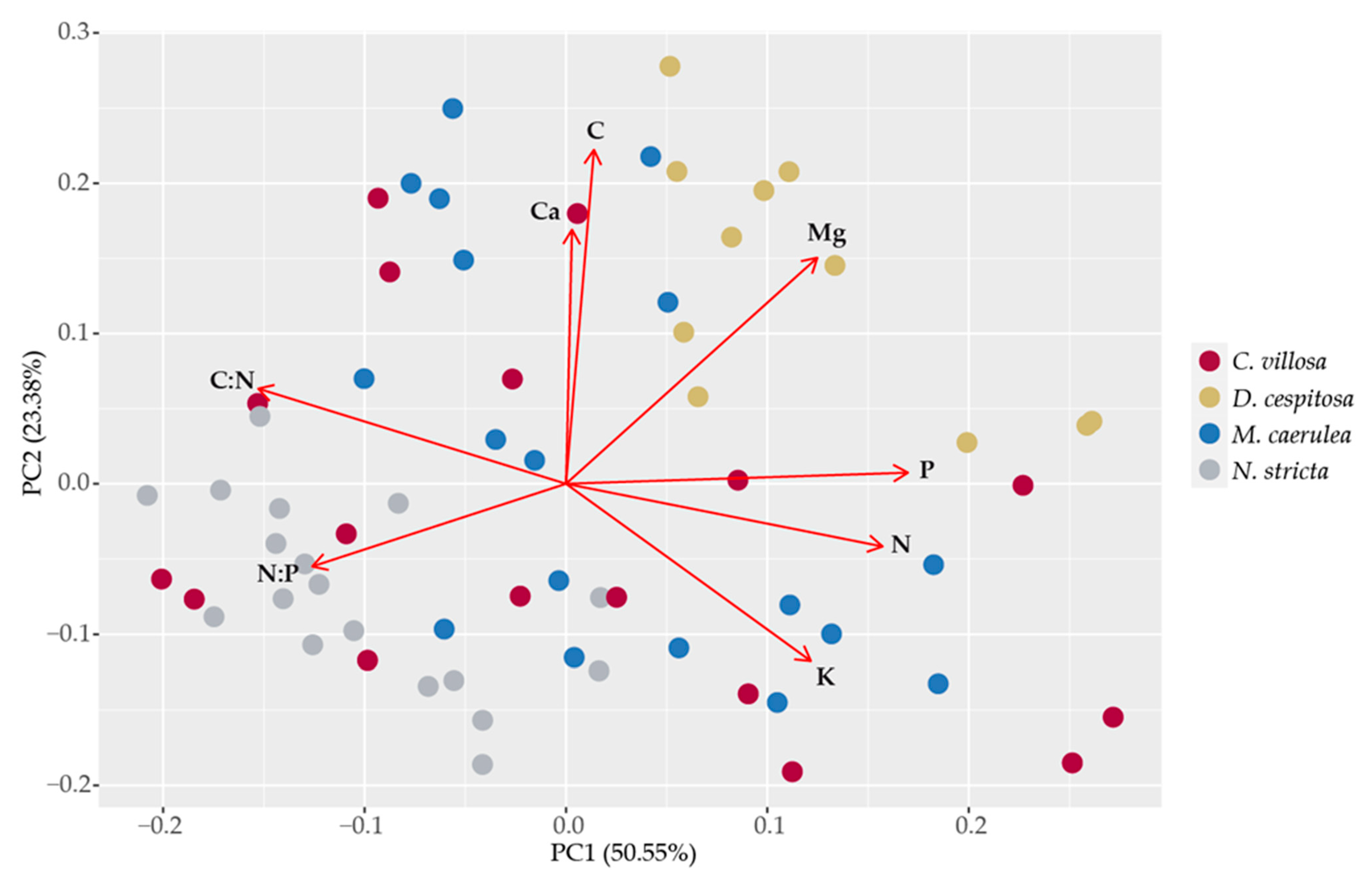
2.5. Leaf Element Composition
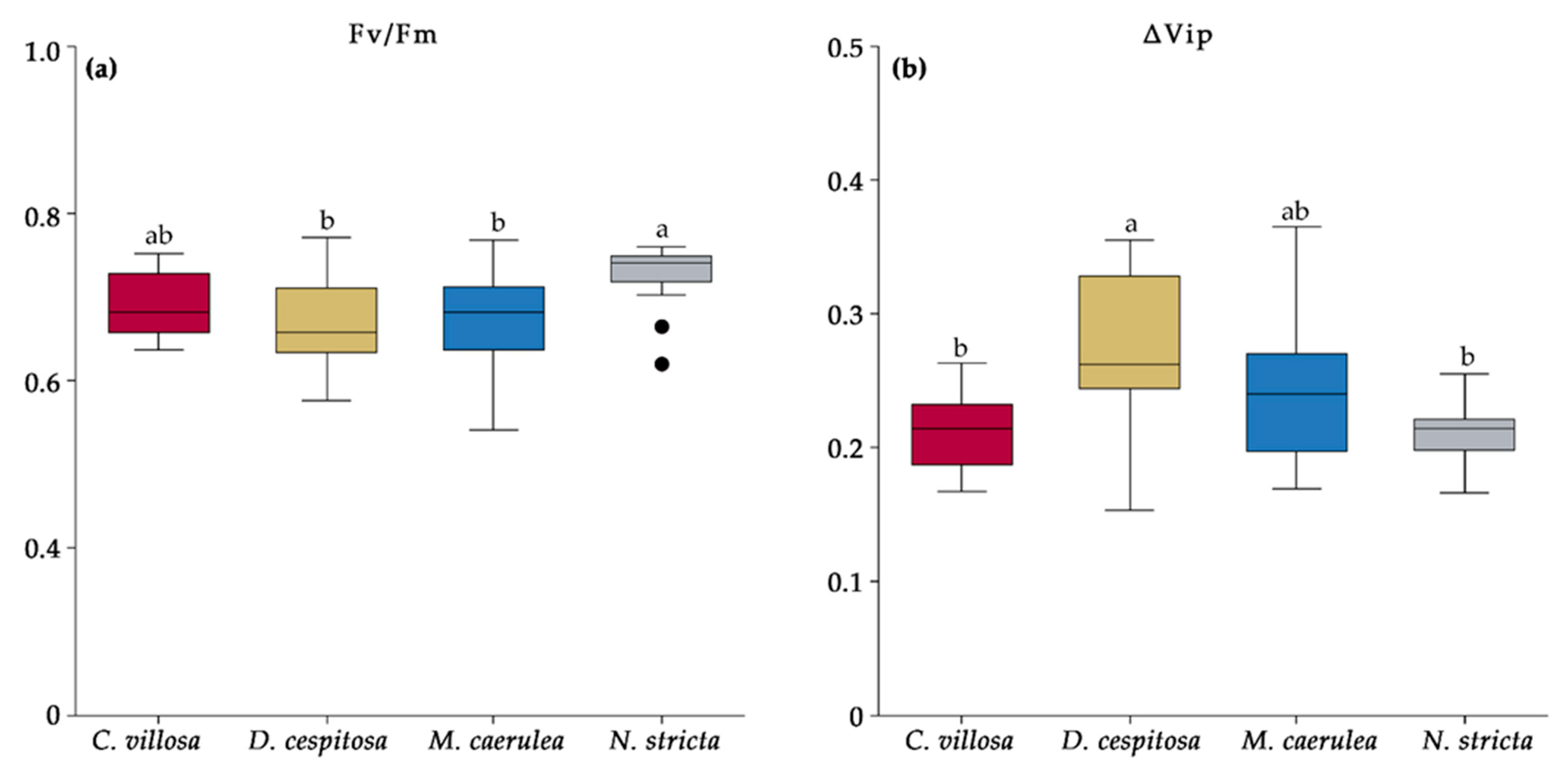
2.6. Chlorophyll Fluorescence
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Morphology and Biochemical Analysis
4.3. Histochemistry and Anatomy
4.4. Specific Phenolic Compounds Identified via HPLC-HRMS
4.5. Chlorophyll Fluorescence
4.6. Element Analysis
4.7. Statistical Analyses
4.8. Analysis of Change in Grass Species Cover Using Remote Sensing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soukupová, L.; Krociánová, M.; Jeník, J.; Sekzra, J. Artic-alpine tundra in the Krkonoše, the Sudetes. Opera Corcon. 1995, 32, 5–88. [Google Scholar]
- Galvánek, D.; Janák, M. Management of Natura 2000 Habitats. 6230 *Species-Rich Nardus Grasslands; European Commission Natura 2000 Technichal Report. 2008. Available online: https://ec.europa.eu/environment/nature/natura2000/management/habitats/pdf/6230_Nardus_grasslands.pdf (accessed on 11 November 2022).
- Štursa, J. Research and management of the giant mountains’arctic-alpine tundra (Czech Republic). AMBIO J. Hum. Environ. 1998, 27, 358–360. Available online: https://www.semanticscholar.org/paper/RESEARCH-AND-MANAGEMENT-OF-THE-GIANT-TUNDRA-%C5%A0tursa/9bb9e54762754b8f2002c7decbd127fe27755443 (accessed on 16 January 2023).
- Lokvenc, T. Analysis of anthropogenic changes of woody plant stands above the alpine timber line in the Krkonose Mts. Opera Corcon. 1995, 32, 99–114. [Google Scholar]
- Novák, J.; Petr, L.; Treml, V. Late-Holocene human-induced changes to the extent of alpine areas in the East Sudetes, Central Europe. Holocene 2010, 20, 895–905. [Google Scholar] [CrossRef]
- Brechtel, H.M. Impact of Acid Deposition Caused by Air Pollution in Central Europe. In Responses of Forest Ecosystems to Environmental Changes; Teller, A., Mathy, P., Jeffers, J.N.R., Eds.; Springer: Dordrecht, The Netherlands, 1992; pp. 594–595. ISBN 978-94-011-2866-7. [Google Scholar]
- Rusek, J. Air-Pollution-Mediated Changes in Alpine Ecosystems and Ecotones. Ecol. Appl. 1993, 3, 409–416. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Forbes, B.C.; Wilmking, M.; Hallinger, M.; Lantz, T.; Blok, D.; Tape, K.D.; Macias-Fauria, M.; Sass-Klaassen, U.; Lévesque, E.; et al. Shrub expansion in tundra ecosystems: Dynamics, impacts and research priorities. Environ. Res. Lett. 2011, 6, 045509. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Hik, D.S. Climate warming as a driver of tundra shrubline advance. J. Ecol. 2018, 106, 547–560. [Google Scholar] [CrossRef]
- Müllerová, J. Anthropogenous Vegetation Changes in Alpine Tundra, a Remote Sensing Study from the Krkonoše Mountains, Czech Republic. Preliminary Results. In Proceedings of the EUROMAB-Symposium, Austrian Academy of Sciences, Vienna, Austria, 15–19 September 1999; pp. 39–41. [Google Scholar]
- Málková, J. Kůlová Impact of dolomitic limestone on changes of species diversity along roads of the eastern Krkonoše Mts. Opera Corcon. 1995, 32, 11–130. [Google Scholar]
- Husáková, J. Subalpine turf communities with Deschampsia cespitosa along the tracks and paths in the Krkonoše (-Giant Mountains) National Park. Preslia 1986, 58, 231–246. [Google Scholar]
- Müllerová, J.; Vítková, M. Long-term human impact on Alpine Tundra—25 years of changes assessed by aerial photography. South-East. Eur. J. Earth Obs. Geomat. 2014, 3, 483–487. [Google Scholar]
- Harčarik, J. Management of the dwarf pine plantations on the naturally valuable localities in the Giant Mountains. Opera Corcon. 2007, 36, 363–369. [Google Scholar]
- Hejcman, M.; Klaudisová, M.; Hejcmanová, P.; Pavlů, V.; Jones, M. Expansion of Calamagrostis villosa in sub-alpine Nardus stricta grassland: Cessation of cutting management or high nitrogen deposition? Agric. Ecosyst. Environ. 2009, 129, 91–96. [Google Scholar] [CrossRef]
- Vacek, S.; Bastl, M.; Lepš, J. Vegetation changes in forests of the Krkonoše Mts. over a period of air pollution stress (1980–1995). Plant Ecol. 1999, 143, 1–11. [Google Scholar] [CrossRef]
- Chambers, F.M.; Mauquoy, D.; Todd, P.A. Recent rise to dominance of Molinia caerulea in environmentally sensitive areas: New perspectives from palaeoecological data. J. Appl. Ecol. 1999, 36, 719–733. [Google Scholar] [CrossRef]
- Pyšek, P. What do we know about Calamagrostis villosa?—A review of the species behaviour in secondary habitats. Preslia 1993, 64, 284–289. [Google Scholar]
- Hejcman, M.; Pavlu, V.; Peterova, J.; Ricarova, P. The expansion of Calamagrostis villosa in the Giant Mountains—Preliminary results. Acta Agrar. Et Silvestria Ser. Agrar. 2003. Available online: https://www.infona.pl//resource/bwmeta1.element.agro-article-728d3f02-df5b-4b54-8b6d-25603886a947 (accessed on 16 November 2022).
- Lokvenc, T.; Vacek, S.; Štursa, J. Vývoj zdravotního stavu a plodivosti kleče horské v Krkonoších. In Výzkum a Management Ekosystémů na území KRNAP; VÚLHM: Opočno, Czech Republic, 1996; pp. 224–228. [Google Scholar]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Colin Prentice, I.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.E.; Williams, L.; Wright, J. Revisiting the Holy Grail: Using plant functional traits to understand ecological processes. Biol. Rev. Camb. Philos. Soc. 2017, 92, 1156–1173. [Google Scholar] [CrossRef]
- Palta, J.P. Leaf chlorophyll content. Remote Sens. Rev. 1990, 5, 207–213. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Gilmore, A.M.; Iii, W.W.A. In vivo functions of carotenoids in higher plants. FASEB J. 1996, 10, 403–412. [Google Scholar] [CrossRef]
- Grace, S. Phenolics as antioxidants. In Antioxidants and Reactive Oxygen Species in Plants; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-1-4051-7146-5. [Google Scholar]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Dixon, R.; Paiva, N. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Gould, K.S. Nature’s Swiss Army Knife: The Diverse Protective Roles of Anthocyanins in Leaves. J. Biomed. Biotechnol. 2004, 2004, 314–320. [Google Scholar] [CrossRef]
- Jansen, M.A.K.; Hectors, K.; O’Brien, N.M.; Guisez, Y.; Potters, G. Plant stress and human health: Do human consumers benefit from UV-B acclimated crops? Plant Sci. 2008, 175, 449–458. [Google Scholar] [CrossRef]
- Míka, V.; Kubáň, V.; Klejdus, B.; Odstrčilová, V.; Nerušil, P. Phenolic compounds as chemical markers of low taxonomic levels in the family Poaceae. Plant Soil Environ. 2005, 51, 506. [Google Scholar] [CrossRef]
- Venn, S.E.; Green, K.; Pickering, C.M.; Morgan, J.W. Using plant functional traits to explain community composition across a strong environmental filter in Australian alpine snowpatches. Plant Ecol. 2011, 212, 1491–1499. [Google Scholar] [CrossRef]
- Kupková, L.; Červená, L.; Suchá, R.; Jakešová, L.; Zagajewski, B.; Březina, S.; Albrechtová, J. Classification of Tundra Vegetation in the Krkonoše Mts. National Park Using APEX, AISA Dual and Sentinel-2A Data. Eur. J. Remote Sens. 2017, 50, 29–46. [Google Scholar] [CrossRef]
- Müllerová, J. Use of digital aerial photography for sub-alpine vegetation mapping: A case study from the Krkonoše Mts., Czech Republic. Plant Ecol. 2005, 175, 259–272. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Lillesand, T.M.; Kiefer, R.W.; Chipman, J. Remote Sensing and Image Interpretation, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-470-05245-7. [Google Scholar]
- Hejcman, M.; Češková, M.; Pavlů, V. Control of Molinia caerulea by cutting management on sub-alpine grassland. Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 577–582. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins | SpringerLink. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]
- Johnson, G.N.; Young, A.J.; Scholes, J.D.; Horton, P. The dissipation of excess excitation energy in British plant species. Plant Cell Environ. 1993, 16, 673–679. [Google Scholar] [CrossRef]
- Lawson, T.; Vialet-Chabrand, S. Chlorophyll Fluorescence Imaging. In Photosynthesis; Covshoff, S., Ed.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1770, pp. 121–140. [Google Scholar] [CrossRef]
- Bantis, F.; Früchtenicht, E.; Graap, J.; Ströll, S.; Reininger, N.; Schäfer, L.; Pollastrini, M.; Holland, V.; Bussotti, F.; Radoglou, K.; et al. Special issue in honour of Prof. Reto J. Strasser—The JIP-test as a tool for forestry in times of climate change. Photosynthetica 2020, 58, 409–421. [Google Scholar] [CrossRef]
- Ceppi, M.G.; Oukarroum, A.; Çiçek, N.; Strasser, R.J.; Schansker, G. The IP amplitude of the fluorescence rise OJIP is sensitive to changes in the photosystem I content of leaves: A study on plants exposed to magnesium and sulfate deficiencies, drought stress and salt stress. Physiol. Plant. 2012, 144, 277–288. [Google Scholar] [CrossRef]
- Çiçek, N.; Oukarroum, A.; Strasser, R.; Schansker, G. Salt stress effects on the photosynthetic electron transport chain in two chickpea lines differing in their salt stress tolerance. Photosynth. Res. 2018, 136, 291–301. [Google Scholar] [CrossRef]
- Oukarroum, A.; Schansker, G.; Strasser, R. Drought stress effects on photosystem I content and photosystem II thermotolerance analyzed using Chl a fluorescence kinetics in barley varieties differing in their drought tolerance. Physiol. Plant. 2009, 137, 188–199. [Google Scholar] [CrossRef]
- Çiçek, N.; Kalaji, H.M.; Ekmekçi, Y. Probing the photosynthetic efficiency of some European and Anatolian Scots pine populations under UV-B radiation using polyphasic chlorophyll a fluorescence transient. Photosynthetica 2020, 58, 468–478. [Google Scholar] [CrossRef]
- Armstrong, R.H.; Common, T.G.; Davies, G.J. The prediction of the in vivodigestibility of the diet of sheep and cattle grazing indigenous hill plant communities by in vitrodigestion, faecal nitrogen concentration or indigestible’ acid-detergent fibre—ARMSTRONG—1989—Grass and Forage Science—Wiley Online Library. Grass Forage Sci. 1989, 44, 303–313. [Google Scholar]
- Leuschner, C.; Ellenberg, H. Ecology of Central European Non-Forest Vegetation: Coastal to Alpine, Natural to Man-Made Habitats: Vegetation Ecology of Central Europe, Volume II; Springer: Berlin/Heidelberg, Germany, 2017; Volume 2, ISBN 978-3-319-43048-5. [Google Scholar]
- Ceulemans, T.; Merckx, R.; Hens, M.; Honnay, O. Plant species loss from European semi-natural grasslands following nutrient enrichment—Is it nitrogen or is it phosphorus? Glob. Ecol. Biogeogr. 2013, 22, 73–82. [Google Scholar] [CrossRef]
- CHAPIN, F.S. Effects of Plant Traits on Ecosystem and Regional Processes: A Conceptual Framework for Predicting the Consequences of Global Change. Ann. Bot. 2003, 91, 455–463. [Google Scholar] [CrossRef]
- Jin, Y.; Lai, S.; Chen, Z.; Jian, C.; Zhou, J.; Niu, F.; Xu, B. Leaf Photosynthetic and Functional Traits of Grassland Dominant Species in Response to Nutrient Addition on the Chinese Loess Plateau. Plants 2022, 11, 2921. [Google Scholar] [CrossRef]
- Zorić, N. Level and distribution of genetic diversity in the European species Nardus stricta L. (Poaceae) inferred from chloroplast DNA and nuclear amplified fragment length polymorphism markers. Master’s Thesis, Norwegian University of Life Sciences, Ås, Norway, 2013. Available online: https://nmbu.brage.unit.no/nmbu-xmlui/handle/11250/189603 (accessed on 10 November 2022).
- Ehleringer, J.R.; Comstock, J. Leaf absorptance and leaf angle: Mechanisms for stress avoidance. In Plant Response to Stress; Tenhunen, J.D., Catarino, F.M., Lange, O.L., Oechel, W.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1987; pp. 55–76. [Google Scholar]
- Lloyd, K.M.; Pollock, M.L.; Mason, N.W.H.; Lee, W.G. Leaf trait-palatability relationships differ between ungulate species: Evidence from cafeteria experiments using naïve tussock grasses. N. Z. J. Ecol. 2010, 34, 219–226. [Google Scholar]
- Smith, W.K.; Vogelmann, T.C.; DeLucia, E.H.; Bell, D.T.; Shepherd, K.A. Leaf Form and Photosynthesis. BioScience 1997, 47, 785–793. [Google Scholar] [CrossRef]
- Hunt, L.; Klem, K.; Lhotáková, Z.; Vosolsobě, S.; Oravec, M.; Urban, O.; Špunda, V.; Albrechtová, J. Light and CO2 Modulate the Accumulation and Localization of Phenolic Compounds in Barley Leaves. Antioxidants 2021, 10, 385. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. Int. J. Exp. Plant Biol. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Ryan, K.G.; Swinny, E.E.; Markham, K.R.; Winefield, C. Flavonoid gene expression and UV photoprotection in transgenic and mutant Petunia leaves. Phytochemistry 2002, 59, 23–32. [Google Scholar] [CrossRef]
- Agati, G.; Stefano, G.; Biricolti, S.; Tattini, M. Mesophyll distribution of ‘antioxidant’ flavonoid glycosides in Ligustrum vulgare leaves under contrasting sunlight irradiance. Ann. Bot. 2009, 104, 853–861. [Google Scholar] [CrossRef]
- Klimeš, L.; Klimešová, J. The effects of mowing and fertilization on carbohydrate reserves and regrowth of grasses: Do they promote plant coexistence in species-rich meadows? Evol. Ecol. 2002, 15, 363–382. [Google Scholar] [CrossRef]
- Clayton, W.D.; Vorontsova, M.S.; Harman, K.T.; Williamson, H. GrassBase—The Online World Grass Flora. 2006. Available online: http://www.kew.org/data/grasses-db.html (accessed on 24 October 2022).
- Grant, S.A.; Torvell, L.; Sim, E.M.; Small, J.L.; Armstrong, R.H. Controlled Grazing Studies on Nardus Grassland: Effects of Between-Tussock Sward Height and Species of Grazer on Nardus utilization and Floristic Composition in Two Fields in Scotland. J. Appl. Ecol. 1996, 33, 1053–1064. [Google Scholar] [CrossRef]
- Jones, L.I. Studies on Hill Land in Wales; Welsh Plant Breeding Station: Aberystwyth, UK, 1967. [Google Scholar]
- Krahulec, F.; Skálová, H.; Herben, T.; Hadincová, V.; Wildová, R.; Pecháčková, S. Vegetation changes following sheep grazing in abandoned mountain meadows. Appl. Veg. Sci. 2001, 4, 97–102. [Google Scholar] [CrossRef]
- Mašková, Z.; Doležal, J.; Květ, J.; Zemek, F. Long-term functioning of a species-rich mountain meadow under different management regimes. Agric. Ecosyst. Environ. 2009, 132, 192–202. [Google Scholar] [CrossRef]
- Ma, Y.-Z.; Holt, N.E.; Li, X.-P.; Niyogi, K.K.; Fleming, G.R. Evidence for direct carotenoid involvement in the regulation of photosynthetic light harvesting. Proc. Natl. Acad. Sci. USA 2003, 100, 4377–4382. [Google Scholar] [CrossRef]
- Barrere, J.; Collet, C.; Saïd, S.; Bastianelli, D.; Verheyden, H.; Courtines, H.; Bonnet, A.; Segrestin, J.; Boulanger, V. Do trait responses to simulated browsing in Quercus robur saplings affect their attractiveness to Capreolus capreolus the following year? Environ. Exp. Bot. 2022, 194, 104743. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Quested, H.M.; Gwynn-Jones, D.; Van Logtestijn, R.S.P.; De Beus, M.A.H.; Kondratchuk, A.; Callaghan, T.V.; Aerts, R. Leaf digestibility and litter decomposability are related in a wide range of subarctic plant species and types. Funct. Ecol. 2004, 18, 779–786. [Google Scholar] [CrossRef]
- Lee, D.W.; Lowry, J.B.; Stone, B.C. Abaxial Anthocyanin Layer in Leaves of Tropical Rain Forest Plants: Enhancer of Light Capture in Deep Shade. Biotropica 1979, 11, 70–77. [Google Scholar] [CrossRef]
- Hughes, N.M.; Vogelmann, T.C.; Smith, W.K. Optical effects of abaxial anthocyanin on absorption of red wavelengths by understorey species: Revisiting the back-scatter hypothesis. J. Exp. Bot. 2008, 59, 3435–3442. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef]
- Sun, J.; Nishio, J.N.; Vogelmann, T.C. High-light effects on CO2 fixation gradients across leaves. Plant Cell Environ. 1996, 19, 1261–1271. [Google Scholar] [CrossRef]
- Kovinich, N.; Kayanja, G.; Chanoca, A.; Otegui, M.S.; Grotewold, E. Abiotic stresses induce different localizations of anthocyanins in Arabidopsis. Plant Signal. Behav. 2015, 10, e1027850. [Google Scholar] [CrossRef]
- Cobbina, J.; Miller, M.H. Purpling in Maize Hybrids as Influenced by Temperature and Soil Phosphorus. Agron. J. 1987, 79, 576–582. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Do Anthocyanins Function of Osmoregulators in Leaf Tissues? Adv. Bot. Res. 2002, 37, 103–127. [Google Scholar]
- Veresoglou, D.S.; Fitter, A.H. Spatial and Temporal Patterns of Growth and Nutrient Uptake of Five Co-Existing Grasses. J. Ecol. 1984, 72, 259. [Google Scholar] [CrossRef]
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef]
- Hunt, L.; Fuksa, M.; Klem, K.; Lhotáková, Z.; Oravec, M.; Urban, O.; Albrechtová, J. Barley Genotypes Vary in Stomatal Responsiveness to Light and CO2 Conditions. Plants 2021, 10, 2533. [Google Scholar] [CrossRef]
- Lin, C.-M.; Chen, C.-T.; Lee, H.-H.; Lin, J.-K. Prevention of Cellular ROS Damage by Isovitexin and Related Flavonoids. Planta Med. 2002, 68, 365–367. [Google Scholar] [CrossRef]
- Ibaraki, Y.; Murakami, J. Distribution of chlorophyll fluorescence parameter fv/fm within individual plants under various stress conditions. Acta Hortic. 2007, 761, 255–260. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.-O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Rizza, F.; Pagani, D.; Stanca, A.M.; Cattivelli, L. Use of chlorophyll fluorescence to evaluate the cold acclimation and freezing tolerance of winter and spring oats. Plant Breed. 2001, 120, 389–396. [Google Scholar] [CrossRef]
- Dias, M.C.; Correia, S.; Serôdio, J.; Silva, A.M.S.; Freitas, H.; Santos, C. Chlorophyll fluorescence and oxidative stress endpoints to discriminate olive cultivars tolerance to drought and heat episodes. Sci. Hortic. 2018, 231, 31–35. [Google Scholar] [CrossRef]
- Schansker, G.; Tóth, S.Z.; Strasser, R.J. Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim. Biophys. Acta BBA-Bioenerg. 2005, 1706, 250–261. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Semelová, V.; Hejcman, M.; Pavlů, V.; Vacek, S.; Podrázský, V. The Grass Garden in the Giant Mts. (Czech Republic): Residual effect of long-term fertilization after 62 years. Agric. Ecosyst. Environ. 2008, 123, 337–342. [Google Scholar] [CrossRef]
- Chadwick, M.J. Nardus stricta L. J. Ecol. 1960, 48, 255–267. [Google Scholar] [CrossRef]
- Peñuelas, J.; Fernández-Martínez, M.; Ciais, P.; Jou, D.; Piao, S.; Obersteiner, M.; Vicca, S.; Janssens, I.A.; Sardans, J. The bioelements, the elementome, and the biogeochemical niche. Ecology 2019, 100, e02652. [Google Scholar] [CrossRef]
- Ahmad-Ramli, M.F.; Cornulier, T.; Johnson, D. Partitioning of soil phosphorus regulates competition between Vaccinium vitis-idaea and Deschampsia cespitosa. Ecol. Evol. 2013, 3, 4243–4252. [Google Scholar] [CrossRef]
- Alonso, I.; Hartley, S.E.; Thurlow, M. Competition between heather and grasses on Scottish moorlands: Interacting effects of nutrient enrichment and grazing regime. J. Veg. Sci. 2001, 12, 249–260. [Google Scholar] [CrossRef]
- Schelfhout, S.; Wasof, S.; Mertens, J.; Vanhellemont, M.; Demey, A.; Haegeman, A.; DeCock, E.; Moeneclaey, I.; Vangansbeke, P.; Viaene, N.; et al. Effects of bioavailable phosphorus and soil biota on typical Nardus grassland species in competition with fast-growing plant species. Ecol. Indic. 2021, 120, 106880. [Google Scholar] [CrossRef]
- Lepš, J.J.P.; Grime, J.G. Hodgson and R. Hunt Comparative plant ecology. Folia Geobot. Phytotaxon. 1990, 25, 216. [Google Scholar] [CrossRef]
- Sochorová, L.; Jansa, J.; Verbruggen, E.; Hejcman, M.; Schellberg, J.; Kiers, E.T.; Johnson, N.C. Long-term agricultural management maximizing hay production can significantly reduce belowground C storage. Agric. Ecosyst. Environ. 2016, 220, 104–114. [Google Scholar] [CrossRef]
- Clein, J.S.; Schimel, J.P. Nitrogen turnover and availability during succession from alder to poplar in Alaskan taiga forests. Soil Biol. Biochem. 1995, 27, 743–752. [Google Scholar] [CrossRef]
- Meier, C.L.; Bowman, W.D. Phenolic-rich leaf carbon fractions differentially influence microbial respiration and plant growth. Oecologia 2008, 158, 95–107. [Google Scholar] [CrossRef]
- Beamish, A.; Raynolds, M.K.; Epstein, H.; Frost, G.V.; Macander, M.J.; Bergstedt, H.; Bartsch, A.; Kruse, S.; Miles, V.; Tanis, C.M.; et al. Recent trends and remaining challenges for optical remote sensing of Arctic tundra vegetation: A review and outlook. Remote Sens. Environ. 2020, 246, 111872. [Google Scholar] [CrossRef]
- Heijmans, M.M.P.D.; Magnússon, R.; Lara, M.J.; Frost, G.V.; Myers-Smith, I.H.; van Huissteden, J.; Jorgenson, M.T.; Fedorov, A.N.; Epstein, H.E.; Lawrence, D.M.; et al. Tundra vegetation change and impacts on permafrost. Nat. Rev. Earth Environ. 2022, 3, 68–84. [Google Scholar] [CrossRef]
- Franke, A.K.; Feilhauer, H.; Bräuning, A.; Rautio, P.; Braun, M. Remotely sensed estimation of vegetation shifts in the polar and alpine tree-line ecotone in Finnish Lapland during the last three decades. For. Ecol. Manag. 2019, 454, 117668. [Google Scholar] [CrossRef]
- Meng, B.; Ge, J.; Liang, T.; Yang, S.; Gao, J.; Feng, Q.; Cui, X.; Huang, X.; Xie, H. Evaluation of Remote Sensing Inversion Error for the Above-Ground Biomass of Alpine Meadow Grassland Based on Multi-Source Satellite Data. Remote Sens. 2017, 9, 372. [Google Scholar] [CrossRef]
- Červená, L.; Pinlová, G.; Lhotáková, Z.; Neuwirthová, E.; Kupková, L.; Potůčková, M.; Lysák, J.; Campbell, P.; Albrechtová, J. Determination of Chlorophyll Content in Selected Grass Communities of Krkonoše Mts. Tundra Based on Laboratory Spectroscopy and Aerial Hyperspectral data. In Proceedings of the International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences, Volume XLIII-B3-2022 XXIV ISPRS Congress (2022 edition), Nice, France, 6–11 June 2022; pp. 381–388. [Google Scholar]
- Chen, J.M.; Black, T.A. Defining leaf area index for non-flat leaves. Plant Cell Environ. 1992, 15, 421–429. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Retrieval of foliar information about plant pigment systems from high resolution spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Chivkunova, O.B.; Merzlyak, M.N. Nondestructive estimation of anthocyanins and chlorophylls in anthocyanic leaves. Am. J. Bot. 2009, 96, 1861–1868. [Google Scholar] [CrossRef]
- Chlus, A.; Townsend, P.A. Characterizing seasonal variation in foliar biochemistry with airborne imaging spectroscopy. Remote Sens. Environ. 2022, 275, 113023. [Google Scholar] [CrossRef]
- Bussotti, F.; Gerosa, G.; Digrado, A.; Pollastrini, M. Selection of chlorophyll fluorescence parameters as indicators of photosynthetic efficiency in large scale plant ecological studies. Ecol. Indic. 2020, 108, 105686. [Google Scholar] [CrossRef]
- Mancinelli, A.L.; Yang, C.-P.H.; Lindquist, P.; Anderson, O.R.; Rabino, I. Photocontrol of Anthocyanin Synthesis: III. The Action of Streptomycin on the Synthesis of Chlorophyll and Anthocyanin 1. Plant Physiol. 1975, 55, 251–257. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Chivkunova, O.B.; Solovchenko, A.E.; Naqvi, K.R. Light absorption by anthocyanins in juvenile, stressed, and senescing leaves. J. Exp. Bot. 2008, 59, 3903–3911. [Google Scholar] [CrossRef]
- Fossen, T.; Slimestad, R.; Øvstedal, D.O.; Andersen, Ø.M. Anthocyanins of grasses. Biochem. Syst. Ecol. 2002, 30, 855. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Porra, R.; Thompson, W.; Kriedemann, P. Determination of Accurate Extinction Coefficients and Simultaneous-Equations for Assaying Chlorophyll-a and Chlorophyll-B Extracted with 4 Different Solvents—Verification of the Concentration. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Brauns, F.E. The Chemistry of Lignin; Academic Press Inc.: New York, NY, USA, 1952. [Google Scholar]
- Gardner, R.O. Vanillin-Hydrochloric Acid as a Histochemical Test for Tannin. Stain Technol. 1975, 50, 315–317. [Google Scholar] [CrossRef]
- Hutzler, P.; Fischbach, R.; Heller, W.; Jungblut, T.P.; Reuber, S.; Schmitz, R.; Veit, M.; Weissenböck, G.; Schnitzler, J.-P. Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. J. Exp. Bot. 1998, 49, 953–965. [Google Scholar] [CrossRef]
- Tattini, M.; Guidi, L.; Morassi-Bonzi, L.; Pinelli, P.; Remorini, D.; Degl’Innocenti, E.; Giordano, C.; Massai, R.; Agati, G. On the role of flavonoids in the integrated mechanisms of response of Ligustrum vulgare and Phillyrea latifolia to high solar radiation. New Phytol. 2005, 167, 457–470. [Google Scholar] [CrossRef]
- Tang, Y.; Horikoshi, M.; Li, W. ggfortify: Unified Interface to Visualize Statistical Results of Popular R Packages. R J. 2016, 8, 474–485. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 11 November 2022).
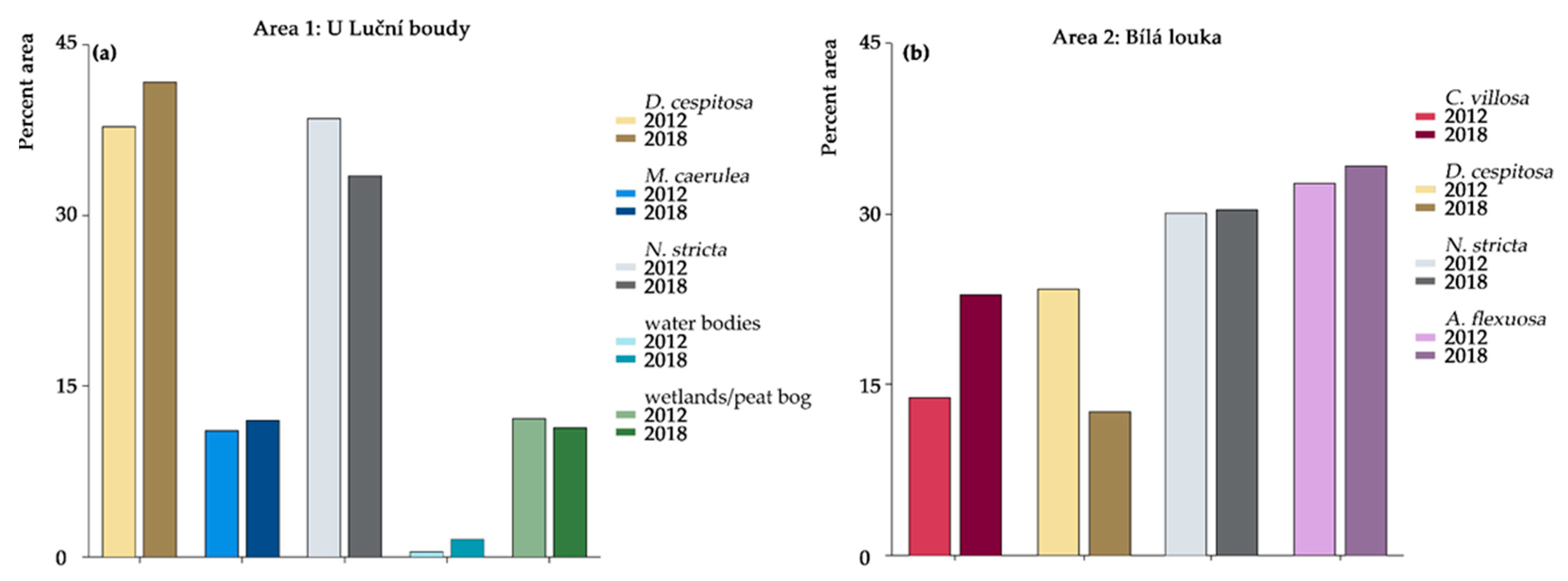


| Area 1: U Luční Boudy | ||||||
|---|---|---|---|---|---|---|
| class | Accuracies | Area in m2 | ||||
| 2012 | 2018 | 2012 | 2018 | |||
| PA | UA | PA | UA | |||
| D. cespitosa | 79.05 | 90.84 | 75.12 | 85.89 | 4571.6 | 5042.9 |
| M. caerulea | 68.02 | 90.36 | 82.78 | 92.67 | 1341.9 | 1445.9 |
| N. stricta | 92.83 | 77.61 | 93.67 | 89.89 | 4660.8 | 4047.4 |
| water bodies | 93.44 | 64.77 | 70.59 | 34.53 | 58.1 | 183.4 |
| wetlands and peat bogs | 73.22 | 31.02 | 60.56 | 26.79 | 1466.0 | 1369.6 |
| Area 2: Bílá Louka | ||||||
| class | Accuracies | Area in m2 | ||||
| 2012 | 2018 | 2012 | 2018 | |||
| PA | UA | PA | UA | |||
| C. villosa | 81.07 | 98.03 | 92.98 | 99.25 | 1628.3 | 2677.4 |
| D. cespitosa | 79.74 | 23.84 | 55.7 | 36.26 | 2751.9 | 1470.6 |
| N. stricta | 75.59 | 97.67 | 78.94 | 96.95 | 3529.0 | 3546.2 |
| A. flexuosa | 71.5 | 35.03 | 77.48 | 37.14 | 3845.2 | 4006.3 |
| Species | Mesophyll % | Epidermis % | Vascular Bundles Including Sclerenchyma % |
|---|---|---|---|
| C. villosa | 46.6 (7.6) b | 35.0 (4.9) a | 17.9 (4.4) a |
| D. cespitosa | 62.0 (4.3) a | 30.8 (3.5) a | 7.0 (0.6) b |
| M. caerulea | 44.0 (3.9) b | 33.6 (1.1) a | 22.2 (3.3) a |
| N. stricta | 60.0 (2.7) a | 22.2 (2.2) b | 17.5 (1.4) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunt, L.; Lhotáková, Z.; Neuwirthová, E.; Klem, K.; Oravec, M.; Kupková, L.; Červená, L.; Epstein, H.E.; Campbell, P.; Albrechtová, J. Leaf Functional Traits in Relation to Species Composition in an Arctic–Alpine Tundra Grassland. Plants 2023, 12, 1001. https://doi.org/10.3390/plants12051001
Hunt L, Lhotáková Z, Neuwirthová E, Klem K, Oravec M, Kupková L, Červená L, Epstein HE, Campbell P, Albrechtová J. Leaf Functional Traits in Relation to Species Composition in an Arctic–Alpine Tundra Grassland. Plants. 2023; 12(5):1001. https://doi.org/10.3390/plants12051001
Chicago/Turabian StyleHunt, Lena, Zuzana Lhotáková, Eva Neuwirthová, Karel Klem, Michal Oravec, Lucie Kupková, Lucie Červená, Howard E. Epstein, Petya Campbell, and Jana Albrechtová. 2023. "Leaf Functional Traits in Relation to Species Composition in an Arctic–Alpine Tundra Grassland" Plants 12, no. 5: 1001. https://doi.org/10.3390/plants12051001
APA StyleHunt, L., Lhotáková, Z., Neuwirthová, E., Klem, K., Oravec, M., Kupková, L., Červená, L., Epstein, H. E., Campbell, P., & Albrechtová, J. (2023). Leaf Functional Traits in Relation to Species Composition in an Arctic–Alpine Tundra Grassland. Plants, 12(5), 1001. https://doi.org/10.3390/plants12051001








