Influence of Drought and Heat Stress on Mineral Content, Antioxidant Activity and Bioactive Compound Accumulation in Four African Amaranthus Species
Abstract
1. Introduction
2. Results
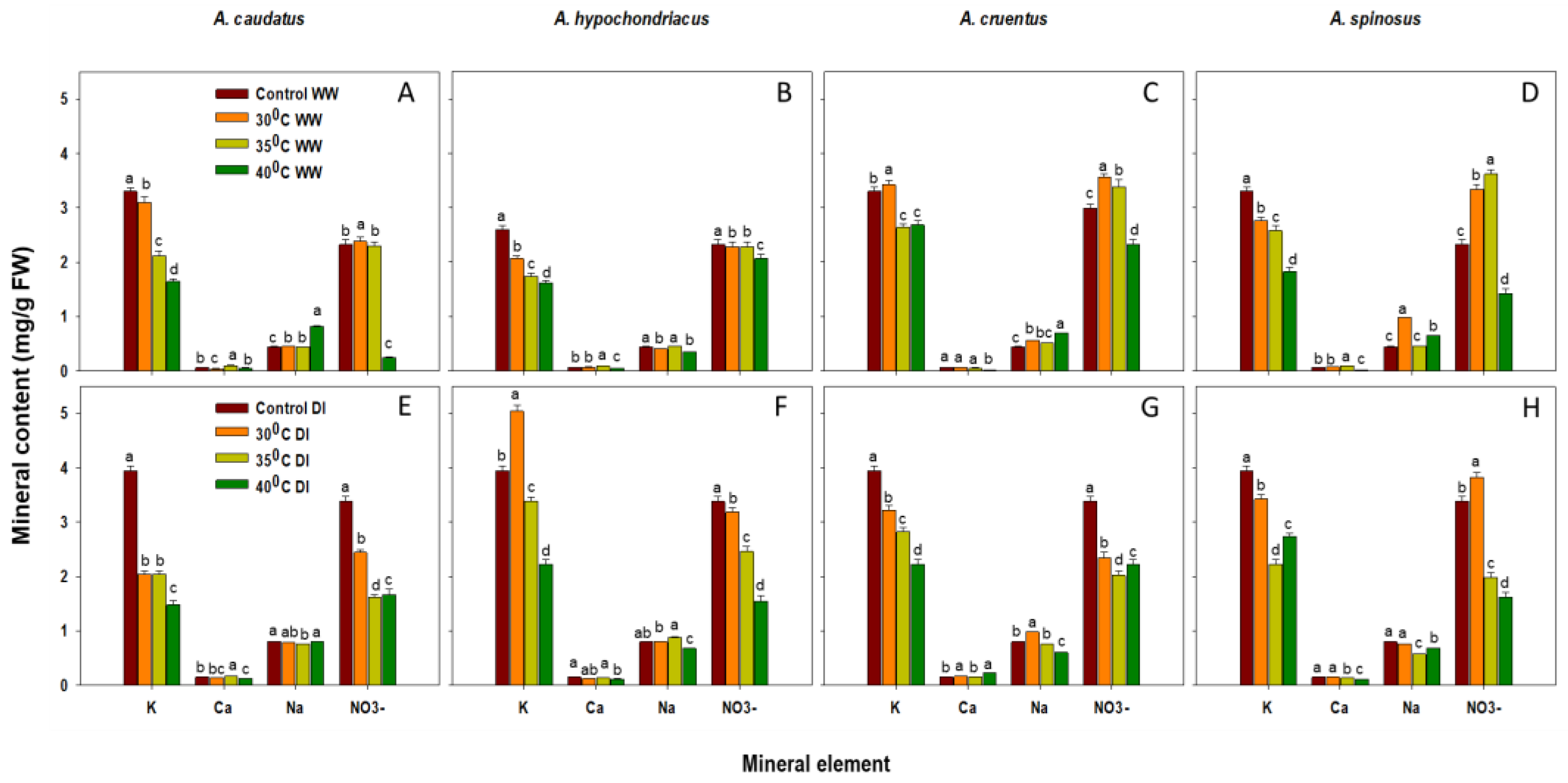
2.1. Effect of Drought and Heat Stress on Mineral Content
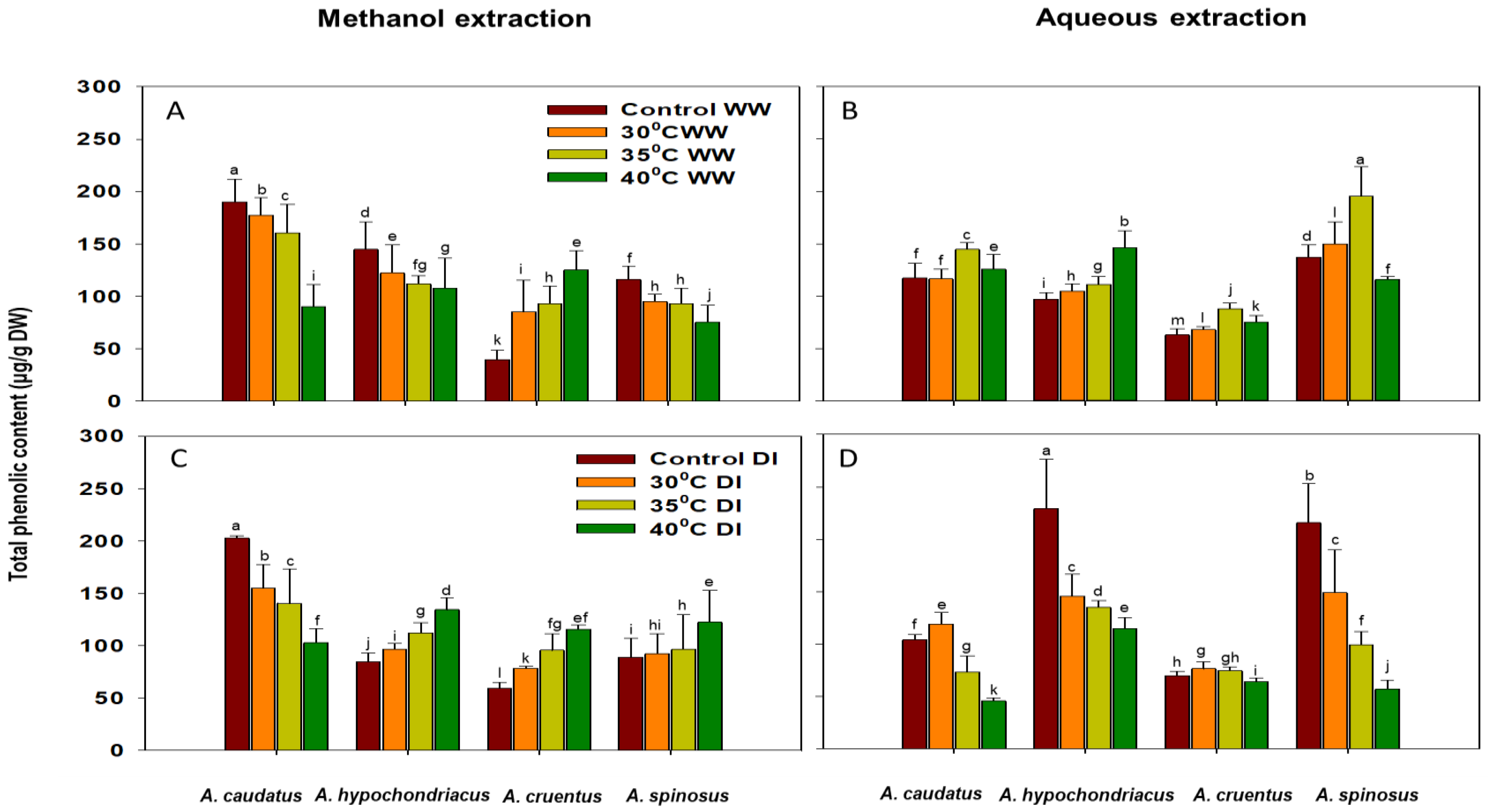
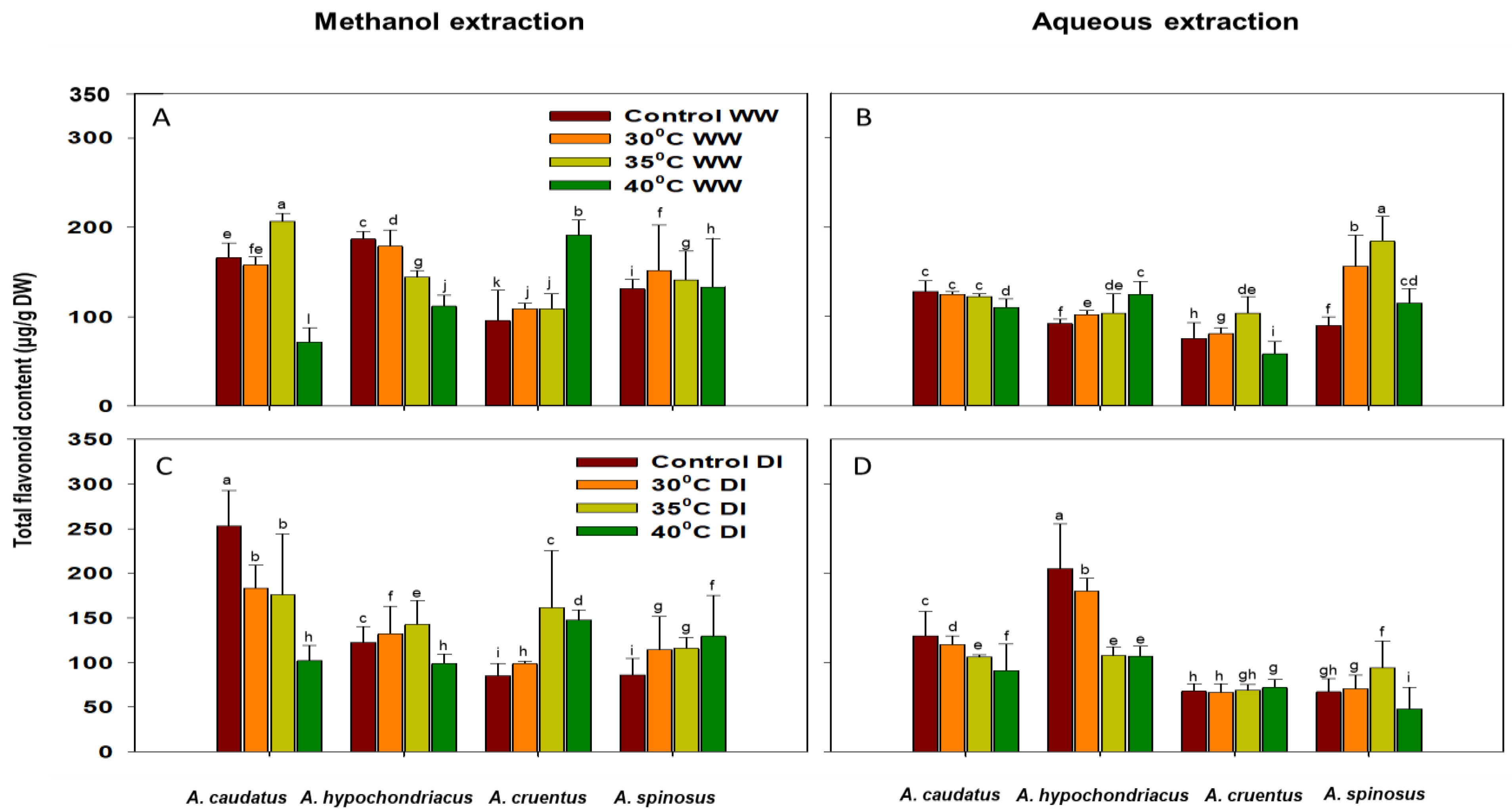
2.2. Effect of Drought and Heat Stress on Total Antioxidant Capacity (TAC), Phenolic (TPC) and Flavonoid (TFC) Content Accumulation
2.3. Influence of Drought and Heat Stress on Organic Acids and Flavonoids Compound Accumulation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reference Standards
4.2. Plant Material and Growth Conditions
4.3. Determination of Mineral Content
4.4. Determination of Total Phenolic Content, Total Flavonoid Content and Antioxidant Activity
4.4.1. Sample Preparation
4.4.2. Extraction of Flavonoids and Phenolic Compounds
4.4.3. Antioxidant Activity
4.5. Determination of Total Phenolic Content
4.6. Determination of Total Flavonoid Content
4.7. Sample Analysis by HPLC and LC-MS
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Romani, A.; Pinelli, P.; Galardi, C.; Sani, G.; Cimato, A.; Heimler, D. Polyphenols in greenhouse and open-air-grown lettuce. Food Chem. 2002, 79, 337–342. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Barba de la Rosa, A.P.; de León-Rodríguez, A.; Laursen, B.; Fomsgaard, I.S. Influence of the growing conditions on the flavonoids and phenolic acids accumulation in amaranth (Amaranthus hypochondriacus L.) leaves. Terra Latinoam. 2019, 37, 449–457. [Google Scholar] [CrossRef]
- Shawon, R.A.; Kang, B.S.; Lee, S.G.; Kim, S.K.; Lee, H.J.; Katrich, E.; Gorinstein, S.; Ku, Y.G. Influence of drought stress on bioactive compounds, antioxidant enzymes and glucosinolate contents of Chinese cabbage (Brassica rapa). Food Chem. 2020, 308, 125–657. [Google Scholar] [CrossRef]
- Vogel, C.; van Zyl, K. Drought: In search of sustainable solutions to a persistent, ‘wicked’ problem in South Africa. In Climate Change Adaptation Strategies—An Upstream-Downstream Perspective; Salzmann, N., Huggel, C., Nussbaumer, S.U., Ziervogel, G., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 195–211. [Google Scholar]
- Baudoin, M.A.; Vogel, C.; Nortje, K.; Naik, M. Living with drought in South Africa: Lessons learnt from the recent El Niño drought period. Int. J. Disaster Risk Reduct. 2017, 23, 128–137. [Google Scholar] [CrossRef]
- Amin, I.; Norazaidah, Y.; Emmy Hainida, K.I. Antioxidant activity and phenolic content of raw and blanched Amaranthus species. Food Chem. 2006, 94, 47–52. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.-G. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ. Rev. 2010, 18, 309–319. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S. Bioactive substances in leaves of two amaranth species, Amaranthus tricolor and A. hypochondriacus. Can. J. Plant Sci. 2013, 93, 47–58. [Google Scholar] [CrossRef]
- Sarker, U.; Islam, M.T.; Rabbani, M.G.; Oba, S. Genotypic diversity in vegetable amaranth for antioxidant, nutrient and agronomic traits. Indian J. Genet. Plant Breed. 2017, 77, 173–176. [Google Scholar] [CrossRef]
- Al-Huqail, A.; El-Dakak, R.M.; Sanad, M.N.; Badr, R.H.; Ibrahim, M.M.; Soliman, D.; Khan, F. Effects of climate temperature and water stress on plant growth and accumulation of antioxidant compounds in sweet basil (Ocimum basilicum L.) Leafy vegetable. Scientifica 2020, 2020, 3808909. [Google Scholar] [CrossRef] [PubMed]
- Soliva-Fortuny, R.; Ricart-Coll, M.; Elez-Martinez, P.; MartinBelloso, O. Internal atmosphere, quality attributes and sensory evaluation of MAP packaged fresh-cut conference pears. Int. J. Food Sci. Technol. 2007, 42, 208–213. [Google Scholar] [CrossRef]
- Ramallo, L.A.; Lovera, N.; Schmalko, M.E. Effect of the application of intermittent drying on Ilex paraguariensis quality and drying kinetics. J. Food Eng. 2010, 97, 188–193. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J. Funct. Foods. 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.; Soliman, M.; Ameta, K.L.; El-Esawi, M.A.; Elkelish, A. Changes in ecophysiology, osmolytes, and secondary metabolites of medicinal plants of Mentha piperita and Catharanthus roseus subjected to drought and heat stress. Biomolecules 2020, 10, 43. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei BE, S.; Saeidi, G.; Goli SA, H. Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of Achillea species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef]
- Siracusa, L.; Gresta, F.; Sperlinga, E.; Ruberto, G. Effect of sowing time and soil water content on grain yield and phenolic profile of four buckwheat (Fagopyrum esculentum Moench.) varieties in a Mediterranean environment. J. Food Compos. Anal. 2017, 62, 1–7. [Google Scholar] [CrossRef]
- El-Gendy AN, G.; Tavarini, S.; Conte, G.; Pistelli, L.; Hendawy, S.F.; Omer, E.A.; Angelini, L.G. Yield and qualitative characterisation of seeds of Amaranthus hypochondriacus L. and Amaranthus cruentus L. grown in central Italy. Ital. J. Agron. 2018, 13, 993. [Google Scholar] [CrossRef]
- Karamać, M.; Gai, F.; Longato, E.; Meineri, G.; Janiak, M.A.; Amarowicz, R.; Peiretti, P.G. Antioxidant activity and phenolic composition of amaranth (Amaranthus caudatus) during plant growth. Antioxidants 2019, 8, 173. [Google Scholar] [CrossRef]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress ofcaffeic acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Chaudhary, H.K. Nutraceutical Crop Buckwheat: A Concealed Wealth in the Lap of Himalayas. Crit. Rev. Biotechnol. 2020, 40, 539–554. Available online: https://www.tandfonline.com/doi/abs/10.1080/07388551.2020.1747387 (accessed on 20 August 2022). [CrossRef] [PubMed]
- Farha, A.K.; Gan, R.Y.; Li, H.B.; Wu, D.T.; Atanasov, A.G.; Gul, K.; Zhang, J.R.; Yang, Q.Q.; Corke, H. The anticancer potential of the dietary polyphenol rutin: Current status, challenges, and perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 832–859. Available online: https://www.tandfonline.com/doi/abs/10.1080/10408398.2020.1829541 (accessed on 2 December 2022). [CrossRef] [PubMed]
- Brownmiller, C.; Howard, L.R.; Prior, R.L. Processing and storage effects on monomeric anthocyanins, percent polymeric colour, and antioxidant capacity of processed blueberry products. J. Food Sci. 2008, 73, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Viskelis, P.; Runinskienê, M.; Jasutienê, I.; Šarkinas, A.; Daubaras, R.; Česonienê, L. Anthocyanins, antioxidative, and antimicrobial properties of American cranberry (Vaccinium macrocarpon Ait.) and their press cakes. J. Food Sci. 2009, 74, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Paśko, P.; Sajewicz, M.; Gorinstein, S.; Zachwieja, Z. Analysis of selected phenolic acids and flavonoids in Amaranthus cruentus and Chenopodium quinoa seeds and sprouts by HPLC. Acta Chromatogr. 2008, 20, 661–672. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Liu, R.; Young, J.C.; Zhu, H.; Loewen, S.; Tsao, R. Characterization of phytochemicals and antioxidant activities of a purple tomato (Solanum lycopersicum L.). J. Agric. Food Chem. 2011, 59, 11803–11811. [Google Scholar] [CrossRef]
- Oloyede, F.M.; Obyede, F.A.; Obuotor, E.M. Effects of plant maturity of the antioxidant profile of Amaranthus cruentus L. and Celosia argentea L. Bull. Environ. Pharmacol. Life Sci. 2013, 2, 18–21. [Google Scholar]
- Chivenge, P.; Mabhaudhi, T.; Modi, A.T.; Mafongoya, P. The potential role of neglected and underutilised crop species as future crops under water scarce conditions in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2015, 12, 5685–5711. [Google Scholar] [CrossRef]
- Hanson, P.; Yang, R.Y.; Chang, L.C.; Ledesma, L.; Ledesma, D. Carotenoids, vitamin C, minerals, and total glucosinolates in choysum (Brassica rapa cv g. parachinensis) and kailaan (B. oleracea Alboglabra group) as affected by variety and wet and dry season production. J. Food Compos. Anal. 2011, 24, 950–962. [Google Scholar] [CrossRef]
- Hniličková, H.; Hnilička, F.; Orsák, M.; Hejnák, V. Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ. 2019, 65, 82–89. [Google Scholar] [CrossRef]
- Netshimbupfe, M.H.; Berner, J.; Gouws, C. The interactive effects of drought and heat stress on photosynthetic efficiency and biochemical defense mechanisms of Amaranthus species. Plant-Environ. Interact. 2022, 3, 212–225. [Google Scholar] [CrossRef]
- Earnshaw, M.J.; Hendrey, G. Stress indicators: Electrolyte leakage. In Methods in Comparative Plant Ecology; Chapman and Hall: Landon, UK, 1993; pp. 152–154. [Google Scholar]
- Conde, A.; Chaves, M.M.; Gerós, H. Membrane transport, sensing and signalling in plant adaptation to environmental stress. Plant Cell Physiol. 2011, 52, 1583–1602. [Google Scholar] [CrossRef] [PubMed]
- Farahmandfar, R.; Kenari, R.E.; Asnaashari, M.; Shahrampour, D.; Bakhshandeh, T. Bioactive compounds, antioxidant and antimicrobial activities of Arum maculatum leaves extracts as affected by various solvents and extraction methods. Food Sci. Nutr. 2019, 7, 465–475. [Google Scholar] [CrossRef]
- Yoon, H.I.; Zhang, W.; Son, J.E. Optimal duration of drought stress Near harvest for promoting bioactive compounds and antioxidant capacity in kale with or without UV-B radiation in plant factories. Plants 2020, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of preharvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Front. Plant Sci. 2019, 10, 1212. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Liu, R.; Zhu, H.; Draves, J.; Marcone, M.; Sun, Y.; Tsao, R. Characterization of phenolics, betacyanins and antioxidant activities of the seed, leaf, sprout, flower and stalk extracts of three Amaranthus species. J. Food Compos. Anal. 2015, 37, 75–81. [Google Scholar] [CrossRef]
- López-Mejía, O.A.; López-Malo, A.; Palou, E. Antioxidant capacity of extracts from amaranth (Amaranthus hypochondriacus L.) seeds or leaves. Ind. Crops Prod. 2014, 53, 55–59. [Google Scholar] [CrossRef]
- Niveyro, S.L.; Mortensen, A.G.; Fomsgaard, I.S.; Salvo, A. Differences among five amaranth varieties (Amaranthus spp.) regarding secondary metabolites and foliar herbivory by chewing insects in the field. Arthropod-Plant Interact. 2013, 7, 235–245. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Kammerer, D.; Schieber, A.; Adama, H.; Nacoulma, O.G.; Carle, R. Betacyanins and phenolic compounds from Amaranthus spimnosus L. and Boerhavia erecta L. Z. Für Nat. C 2004, 59, 1–8. [Google Scholar] [CrossRef]
- Ambaye, T.G. Evaluation of physiochemical, phytochemical, antioxidant and antimicrobial screening parameters of Amaranthus spinosus leaves. Nat. Prod. Chem. Res. 2015, 4, 199. [Google Scholar] [CrossRef]
- Mufunanya AA, J.; Ebigwai, J.K.; Bello, O.S.; Egbe, A.O. Comparative study of the effects of organic and inorganic fertilizer on nutritional composition of Amaranthus spinosus. Asian J. Plant Sci. 2015, 14, 34–39. [Google Scholar] [CrossRef]
- Neugart, S.; Baldermann, S.; Ngwene, B.; Wesong, J.; Schreiner, M. Indigenous leafy vegetables of Eastern Africa—A source of extraordinary secondary plant metabolites. Food Res. Int. 2017, 100, 411–422. [Google Scholar] [CrossRef]
- Paucar-Menacho, M.; Dueñas, M.; Peñas, E.; Frias, J.; Martínez-Villaluenga, C. Effect of dry heat puffing on nutritional composition, fatty acid, amino acid and phenolic profiles of pseudocereals grains. Pol. J. Food Nutr. Sci. 2018, 68, 289–297. [Google Scholar] [CrossRef]
- Kalinova, J.; Dadakova, E. Rutin and total quercetin content in amaranth (Amaranthus spp.). Plant Foods Hum. Nutr. 2009, 64, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, S.K.; Pedersen, H.A.; Labouriau, R.; Mortensen, A.G.; Laursen, B.; de Troiani, R.M.; Noellemeyer, E.J.; Janovska, D.; Stavelikova, H.; Taberner, A.; et al. Variation of polyphenols and betaines in aerial parts of young, field-grown Amaranthus genotypes. J. Agric. Food Chem. 2011, 59, 12073–12082. [Google Scholar] [CrossRef] [PubMed]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxidative medicine and cellular longevity. Oxidative Med. Cell. Longev. 2018, 1, 6241017. [Google Scholar] [CrossRef] [PubMed]
- Mingnan, Q.U.; Bunce, J.A.; Sicher, R.C.; Zhu, X.; Gao, B.; Chen, G. An attempt to interpret a biochemical mechanism of C4 photosynthetic thermo-tolerance under sudden heat shock on detached leaf in elevated CO2 grown maize. PLoS ONE 2017, 12, e0187437. [Google Scholar]
- Ali, M.B.; Khandaker, L.; Oba, S. Comparative study on functional components, antioxidant activity and colour parameters of selected coloured leafy vegetables as affected by photoperiods. J. Food Agric. Environ. 2009, 7, 392–398. [Google Scholar]
- Cai, Y.; Sun, M.; Corke, H. Identification and distribution of simple and acylated betacyanins in the Amaranthaceae. J. Agric. Food Chem. 2001, 49, 1971–1978. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Quantification of Tannins in Tree Foliage: A Laboratory Manual for the FAO/IAEA Co-Ordinate Research Project on Use of Nuclear and Related Techniques to Develop Simple Tannin Assay for Predicting and Improving the Safety and Efficiency of Feeding Ruminants on the Tanniniferous Tree Foliage; Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture: Vienna, Austria, 2000. [Google Scholar]
- Kim, D.K.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Muanda, N.F.; Kon’e, D.; Dicko, A.; Soulimani, R.; Younos, C. Phytochemical composition and antioxidant capacity of three Malian medicinal plant parts. Evid. Based Complement Altern. Med. 2011, 2011, 674320. [Google Scholar] [CrossRef] [PubMed]

| Compound | A. caudatus | A. hypochondriacus | A. cruentus | A. spinosus |
|---|---|---|---|---|
| Catechin | 28.80 ± 0.04 b | 28.81 ± 0.03 bc | 28.81 ± 0.02 c | 28.80 ± 0.08 a |
| P-hydrobenzoic acid | 33.38 ± 7.01 a | ND | ND | ND |
| Caffeic acid | 109.91 ± 8.16 b | 1318.80 ± 12.53 a | ND | ND |
| Vanillic acid | 139.73 ± 24.32 a | 14.16 ± 2.50 b | ND | ND |
| Rutin | 550.61 ± 26.73 a | 449.85 ± 24.30 b | 363.29 ± 11.78 c | 397.39 ± 78.42 b |
| P-coumaric acid | 315.97 ± 40.28 a | 57.75 ± 4.95 b | ND | ND |
| Isoquercetin | 223.10 ± 17.15 a | 106.20 ± 8.58 b | ND | ND |
| Quercetin | 437.90 ± 26.31 a | 412.30 ± 51.19 a | 204.76 ± 33.67 b | 88.11 ± 2.54 c |
| Kampferol-3-O-rutinoside | 22.23 ± 2.66 d | 47.30 ± 7.32 b | 37.24 ± 6.15 c | 65.08 ± 10.34 a |
| Sinapic acid | 26.51 ± 5.90 a | ND | ND | ND |
| Ferulic acid | 341.14 ± 30.30 a | 154.20 ± 25.44 b | 58.95 ± 9.39 c | 35.14 ± 4.12 d |
| Gallic acid | ND | ND | ND | ND |
| Coumarin | ND | ND | ND | ND |
| Ellagic acid | ND | ND | ND | ND |
| Compound | A. caudatus | A. hypochondriacus | A. cruentus | A. spinosus | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control WW | 30° WW | 35° WW | 40° WW | Control WW | 30° WW | 35° WW | 40° WW | Control WW | 30° WW | 35° WW | 40° WW | Control WW | 30° WW | 35° WW | 40° WW | |
| Catechin | 1.8 ± 0.0040 a | 1.8 ± 0.0014 b | 1.8 ± 0.00061 b | 1.8 ± 0.00040 b | 1.8 ± 0.00024 b | 1.8 ± 0.0040 a | 1.8 ± 0.0033 a | 1.8 ± 0.0041 a | 1.8 ± 0.0026 a | 1.8 ± 0.0023 a | 1.8 ± 0.00023 b | 1.8 ± 0.0030 a | 1.8 ± 0.0069 a | 1.8 ± 0.056 a | 1.8 ± 0.0082 a | 1.8 ± 0.0017 a |
| P-hydrobenzoic acid | 14.3 ± 2.89 a | 14.3 ± 2.85 a | 2.0 ± 0.35 b | 2.8 ± 0.92 b | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Caffeic acid | 11.3 ± 0.62 a | 11.3 ± 0.68 a | 6.6 ± 0.83 b | 2.6 ± 0.75 c | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Vanillic acid | 12.3 ± 0.924 a | 12.2 ± 0.902 a | 8.1 ± 0.102 b | 4.3 ± 1.13 c | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Rutin | 41.0 ± 2.04 f | 38.5 ± 5.4 f | 48.6 ± 1.4 d | 51.0 ± 1.61 c | 51.5 ± 0.29 cd | 51.5 ± 0.27 cd | 36.9 ± 0.83 g | 120.0 ± 12.9 a | 26.9 ± 0.094 h | 26.8 ± 0.13 h | 44.7 ± 0.058 f | 44.7 ± 0.058 f | 15.1 ± 0.96 j | 15.1 ± 0.96 j | 17.5 ± 0.25 i | 92.1 ± 5.47 b |
| P-coumaric acid | 9.8 ± 1.67 a | 10.1 ± 1.99 a | 8.3 ± 0.84 b | 3.8 ± 1.81 c | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Isoquercetin | 13.1 ± 1.68 bd | 13.2 ± 1.73 d | 16.4 ± 1.72 c | 19.4 ±2.01 b | 12.7 ± 0.89 ae | 12.7 ± 0.91 e | 12.6 ± 0.35 f | 68.2 ± 6.44 a | ND | ND | ND | ND | ND | ND | ND | ND |
| Quercetin | 33.6 ± 1.49 d | 33.7 ± 1.53 d | 40.7 ± 2.11 c | 43.5 ± 2.38 c | 39.1 ± 8.03 b | 39.2 ± 80 b | 32.2 ± 1.44 e | 114.0 ± 7.85 a | 12.4 ± 1.18 h | 12.5 ± 1.16 h | 24.6 ± 3.34 f | 19.9 ± 5.15 g | ND | ND | ND | ND |
| Kampferol-3-O-rutinoside | ND | ND | ND | ND | 3.5 ± 0.71 de | 3.7 ± 0.65 d | 1.2 ± 1.41 g | 14.4 ± 2.34 a | 1.6 ± 0.49 f | 1.8 ± 0.47 f | 2.8 ± 0.96 e | 8.49 ± 0.29 c | 9.3 ± 4.09 b | 9.1 ± 4.13 b | 8.4 ± 0.60 c | 9.39 ± 0.30 b |
| Sinapic acid | 5.8 ± 10 a | 5.5 ± 1.31 a | 1.7 ± 0.43 b | 2.26 ± 1.19 b | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Ferulic acid | 7.1 ± 1.98 ba | 7.3 ± 1.80 a | 5.0 ± 0.27 b | 2.0 ± 0.92 c | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Gallic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Coumarin | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Ellagic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Compound | A. caudatus | A. hypochondriacus | A. cruentus | A. spinosus | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control DI | 30° DI | 35° DI | 40° DI | Control DI | 30° DI | 35° DI | 40° DI | Control DI | 30° DI | 35° DI | 40° DI | Control DI | 30° DI | 35° DI | 40° DI | |
| Catechin | 1.8 ± 6.3 × 10−6 f | 1.8 ± 0.012 c | 1.8 ± 0.0071 b | 1.8 ± 0.00887 b | 1.8 ± 0.00051 d | 1.8 ± 0.00051 d | 1.8 ± 0.0024 b | 1.8 ± 0.001 c | 1.8 ± 0.001 c | 1.81 ± 0.0014 a | 1.8 ± 0.0068 b | 1.8 ± 0.0015 c | 1.8 ± 0.00022 e | 1.8 ± 0.0015 c | 1.8 ± 0.00047 d | 1.8 ± 0.00057 de |
| P-hydrobenzoic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Caffeic acid | 4.25 ± 0 c | 4.28 ± 0.043 bc | 7.05 ± 0.12 a | 4.54 ± 0.27 b | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Vanillic acid | 1.96 ± 0.84 c | 3.04 ± 0.22 b | 3.61 ± 0.053 b | 9.16 ± 4.11 a | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Rutin | 79.5 ± 4.64 c | 79.7 ± 4.6 c | 106 ± 5.33 a | 87.9 ± 1.11 c | 73.9 ± 2.57 d | 74.2 ± 2.13 d | 73.3 ± 1.3 gd | 46 ± 3.26 g | 51.6 ± 4.63 f | 51.6 ± 4.56 f | 67.2 ± 0.53 e | 35.6 ± 0.90 i | 71.1 ± 28.4 b | 71.2 ± 28.40 b | 40.2 ± 1.14 h | 33 ± 3.06 i |
| P-coumaric acid | 1.66 ± 1.41 b | 1.7 ± 1.38 b | 3.01 ± 2.52 a | 1.75 ± 0.058 c | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Isoquercetin | 35.8 ± 3.21 b | 35.8 ± 3.24 b | 49.8 ± 3.42 a | 39.6 ± 0.14 b | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Quercetin | 64 ± 5.30 b | 64 ± 5.32 b | 87.9 ± 7.77 a | 70.5 ± 0.41 b | 51.5 ± 9.93 c | 51.5 ± 9.88 c | 56.1 ± 2.01 c | 28.7 ± 4.05 f | 32.8 ± 6.64 e | 32.9 ± 6.67 e | 39.3 ± 7.88 d | 16.4 ± 0.218 g | 5.79 ± 0.68 i | 5.82 ± 0.68 i | 12.2 ± 0.073 h | 12.4 ± 0.20 h |
| Kampferol-3-O-rutinoside | 5.02 ± 0.68 e | 5.27 ± 1.01 e | 9.49 ± 0.67 b | 2.45 ± 0.31 g | 7.36 ± 0.42 c | 7.61 ± 0.18 c | 8.09 ± 0.74 b | 1.53 ± 0.89 h | 2.26 ± 1.69 fg | 2.51 ± 2.17 ef | 12 ± 0.0056 a | 5.95 ± 0.073 d | 2.6 ± 0.23 f | 2.85 ± 0.22 f | 5.12 ± 0.17 e | 1.97 ± 0.25 h |
| Sinapic acid | 3.58 ± 0.018 a | 3.83 ± 0.49 a | 1.28 ± 0.39 c | 2.62 ± 1.07 b | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Ferulic acid | 6.95 ± 1.14 a | 7.45 ± 0.39 a | 6.22 ± 2.59 b | 4.41 ± 1.14 d | ND | ND | ND | ND | 2.5 ± 0.26 e | 2.52 ± 0.26 e | 5.92 ± 0.35 c | 5.36 ± 0.32 cd | ND | ND | ND | ND |
| Gallic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Coumarin | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Ellagic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Compound | A. caudatus | A. hypochondriacus | A. cruentus | A. spinosus | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control WW | 30° WW | 35° WW | 40° WW | Control WW | 30° WW | 35° WW | 40° WW | Control WW | 30° WW | 35° WW | 40° WW | Control WW | 30° WW | 35° WW | 40° WW | |
| Catechin | 1.8 ± 0.0012 a | 1.8 ± 0.0012 a | 1.8 ± 9.2 × 10−6 e | 1.8 ± 0.00042 b | 1.8 ± 0.000012 d | 1.8 ± 0.00012 c | 1.8 ± 0.00020 b | 1.8 ± 0.0022 a | 1.8 ± 0.00025 b | 1.8 ± 0.000091 cd | 1.8 ± 0.00017 b | 1.8 ± 0.000067 bc | 1.8 ± 0.00025 a | 1.8 ± 0.0016 b | 1.8 ± 0.000086 cd | 1.8 ± 0.00011 c |
| P-hydrobenzoic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Caffeic acid | 7.68 ± 1.39 e | 7.71 ± 1.43 e | 6.26 ± 0.0093 d | 5.87 ± 1.38 c | 1.39 ± 0.82 a | 1.4 ± 0.82 a | 3.6 ± 10 b | 248 ± 1.68 f | ND | ND | ND | ND | ND | ND | ND | ND |
| Vanillic acid | 9.16 ± 4.11 b | 9.1 ± 4.11 b | 18.2 ± 6.84 a | 11.6 ± 0.57 b | 1.56 ± 0.12 e | 1.51 ± 0.11 f | 1.98 ± 0.52 d | 3.02 ± 0.50 c | ND | ND | ND | ND | ND | ND | ND | ND |
| Rutin | 3 ± 0.022 bc | 3.25 ± 0.49 b | 1.75 ± 0.018 f | 2.18 ± 0.046 d | 2.02 ± 0.081 d | 2.07 ± 0.13 d | 1.6 ± 0.020 g | 2.38 ± 0.037 c | 1.84 ± 0.24 e | 1.87 ± 0.19 e | 1.8 ± 0.15 ef | 1.86 ± 0.14 e | 11.5 ± 0.014 a | 12 ± 0.015 a | 1.84 ± 0.13 e | 1.67 ± 0.14 f |
| P-coumaric acid | 34.5 ± 2.55 b | 34.6 ± 2.57 b | 43.9 ± 9.74 a | 44 ± 7.36 a | 13.9 ± 0.082 c | 13.9 ± 0.14 c | 3.76 ± 0.49 d | 1.73 ± 1.28 d | ND | ND | ND | ND | ND | ND | ND | ND |
| Isoquercetin | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Quercetin | ND | ND | ND | ND | ND | ND | ND | ND | 3.1 ± 0 d | 3.12 ± 0.02 d | 3.62 ± 0.59 c | 4.12 ± 0.82 b | 13 ± 0.15 a | 12.9 ± 0.19 a | 13 ± 0.13 a | 13 ± 0.45 a |
| Kampferol-3-O-rutinoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 6.93 ± 0.15 a | 6.91 ± 0.19 a | 1.128 ± 0.0021 b | 1.448 ± 0.0021 b |
| Sinapic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Ferulic acid | 50.5 ± 0.21 a | 48 ± 4.9 a | 45 ± 2.65 a | 30 ± 6.05 c | 20.3 ± 8.5 d | 21.6 ± 7.63 d | 15.9 ± 0.47 e | 14.3 ± 2.90 e | 12.1 ± 2.35 f | 12.2 ± 2.30 f | 11.5 ± 2.33 g | 6.85 ± 1.22 h | 4.71 ± 0.43 j | 4.96 ± 0.80 j | 5.33 ± 0.34 i | 4.55 ± 0.30 j |
| Gallic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Coumarin | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Ellagic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Compound | A. caudatus | A. hypochondriacus | A. cruentus | A. spinosus | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control DI | 30° DI | 35° DI | 40° DI | Control DI | 30° DI | 35° DI | 40° DI | Control DI | 30° DI | 35° DI | 40° DI | Control DI | 30° DI | 35° DI | 40° DI | |
| Catechin | 1.8 ± 0.0011 c | 1.8 ± 0.0021 b | 1.8 ± 0.00029 e | 1.8 ± 0.00029 e | 1.8 ± 0.00035 e | 1.8 ± 0.00012 f | 1.8 ± 0.0011 c | 1.81 ± 0.0078 a | 1.8 ± 0.0014 bc | 1.8 ± 0.0015 bc | 1.8 ± 0.00046 de | 1.8 ± 0.00047 de | 1.8 ± 0.00068 d | 1.8 ± 0.00077 d | 1.8 ± 0.00011 f | 1.8 ± 0.00004 g |
| P-hydrobenzoic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Caffeic acid | 10.7 ± 0.058 c | 10.8 ± 0.058 c | 4.7 ± 0.53 d | 4.25 ± 0 e | 528 ± 3.63 b | 532 ± 3.66 a | 3.21 ± 0.18 f | 1.2 ± 0.74 g | ND | ND | ND | ND | ND | ND | ND | ND |
| Vanillic acid | 13.5 ± 0.091 a | 13.5 ± 0.082 a | 2.82 ± 0.23 c | 7.19 ± 0 b | 1.34 ± 0.099 f | 1.34 ± 0.098 f | 1.23 ± 0.58 e | 2.18 ± 0.49 d | ND | ND | ND | ND | ND | ND | ND | ND |
| Rutin | 2.23 ± 0.0019 d | 2.23 ± 0.0067 d | 1.73 ± 0.018 bf | 2.04 ± 0 e | 4.78 ± 0.22 b | 4.8 ± 0.19 b | 2.43 ± 0.0086 c | 2.47 ± 0.051 c | 1.71 ± 0.03 g | 1.75 ± 0.063 f | 1.71 ± 0.010 g | 1.65 ± 0.013 gh | 1.52 ± 0.021 h | 1.53 ± 0.024 h | 7.29 ± 0.40 a | 4.74 ± 0.44 b |
| P-coumaric acid | 54.9 ± 2.92 a | 55 ± 2.91 a | 4.3 ± 0.55 d | 4.67 ± 0 d | 5.6 ± 0.50 cd | 5.61 ± 0.51 c | 6.33 ± 1.94 b | 6.92 ± 0.0089 bc | ND | ND | ND | ND | ND | ND | ND | ND |
| Isoquercetin | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Quercetin | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Kampferol-3-O-rutinoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Sinapic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Ferulic acid | 53.1 ± 1.32 b | 55.6 ± 4.53 a | 9.72 ± 0.41 e | 2.89 ± 0 g | 24 ± 1.35 c | 24.6 ± 1.26 c | 16.5 ± 1.15 d | 17 ± 2.18 d | ND | ND | ND | ND | 5.07 ± 0.66 f | 5.32 ± 0.83 f | 4.34 ± 0.68 f | 0.861 ± 0.078 h |
| Gallic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Coumarin | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Ellagic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Netshimbupfe, M.H.; Berner, J.; Van Der Kooy, F.; Oladimeji, O.; Gouws, C. Influence of Drought and Heat Stress on Mineral Content, Antioxidant Activity and Bioactive Compound Accumulation in Four African Amaranthus Species. Plants 2023, 12, 953. https://doi.org/10.3390/plants12040953
Netshimbupfe MH, Berner J, Van Der Kooy F, Oladimeji O, Gouws C. Influence of Drought and Heat Stress on Mineral Content, Antioxidant Activity and Bioactive Compound Accumulation in Four African Amaranthus Species. Plants. 2023; 12(4):953. https://doi.org/10.3390/plants12040953
Chicago/Turabian StyleNetshimbupfe, Mmbulaheni Happiness, Jacques Berner, Frank Van Der Kooy, Olakunle Oladimeji, and Chrisna Gouws. 2023. "Influence of Drought and Heat Stress on Mineral Content, Antioxidant Activity and Bioactive Compound Accumulation in Four African Amaranthus Species" Plants 12, no. 4: 953. https://doi.org/10.3390/plants12040953
APA StyleNetshimbupfe, M. H., Berner, J., Van Der Kooy, F., Oladimeji, O., & Gouws, C. (2023). Influence of Drought and Heat Stress on Mineral Content, Antioxidant Activity and Bioactive Compound Accumulation in Four African Amaranthus Species. Plants, 12(4), 953. https://doi.org/10.3390/plants12040953








