Nocturnal Transpiration May Be Associated with Foliar Nutrient Uptake
Abstract
1. Introduction
2. Results
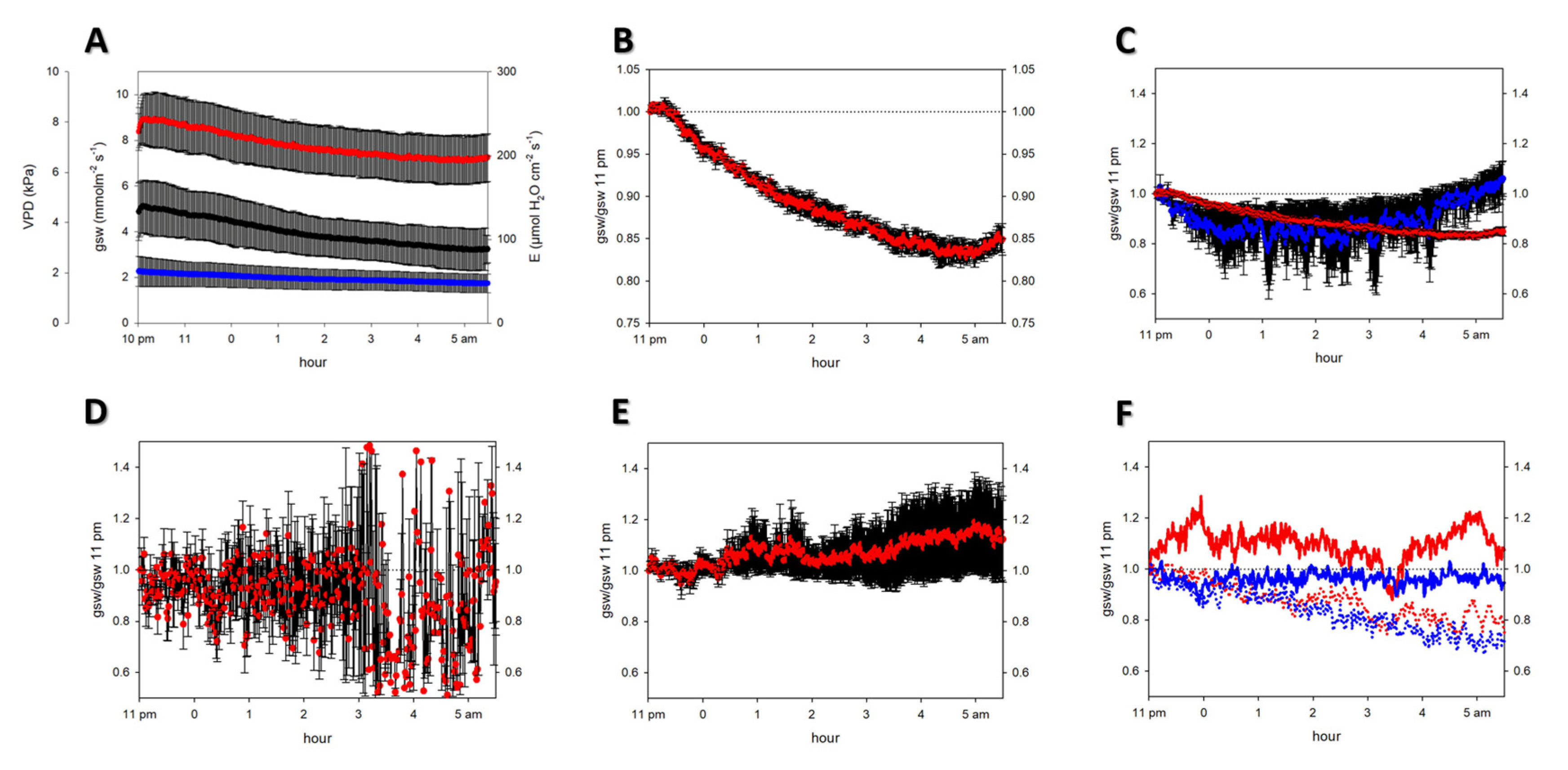
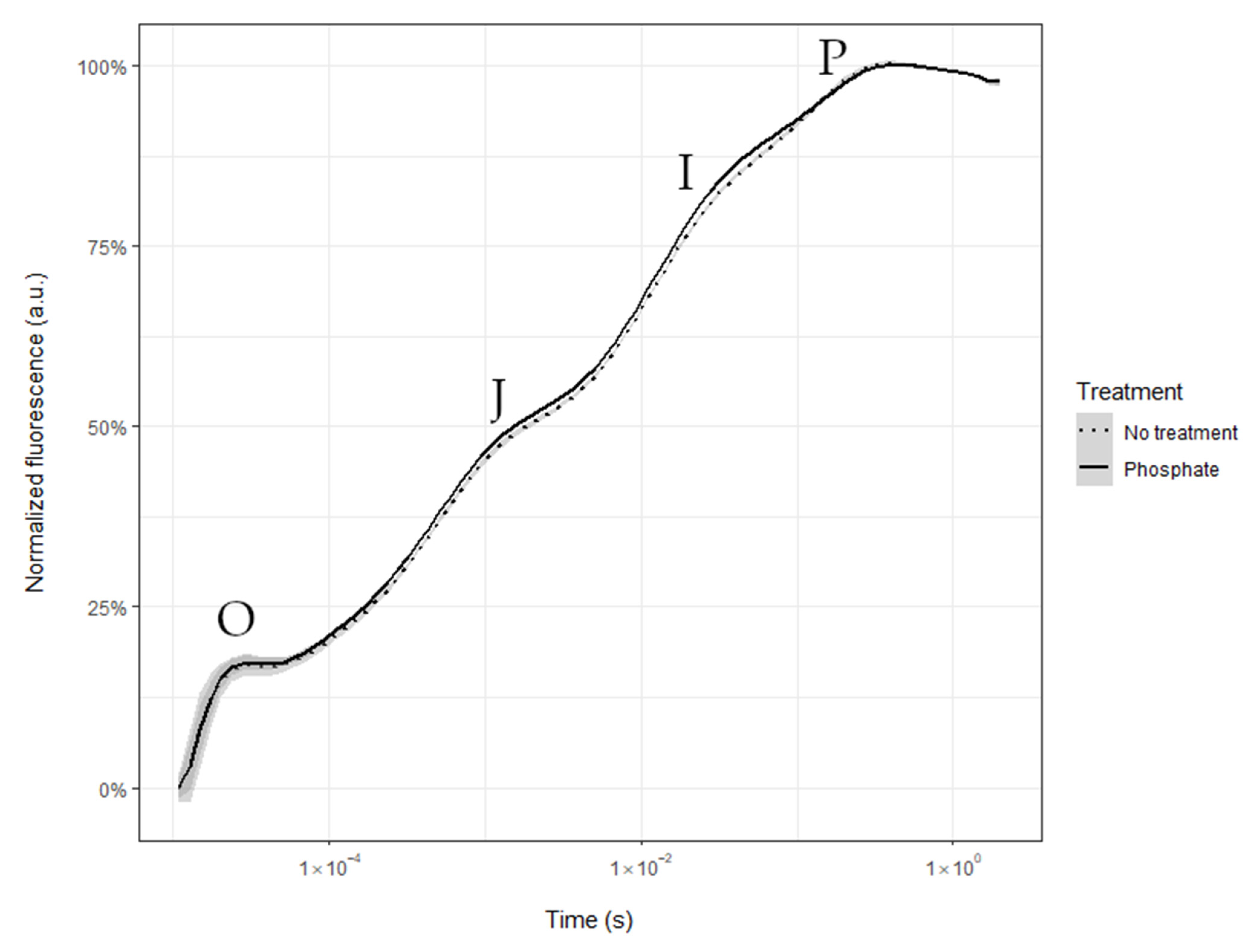
2.1. Nocturnal Transpiration
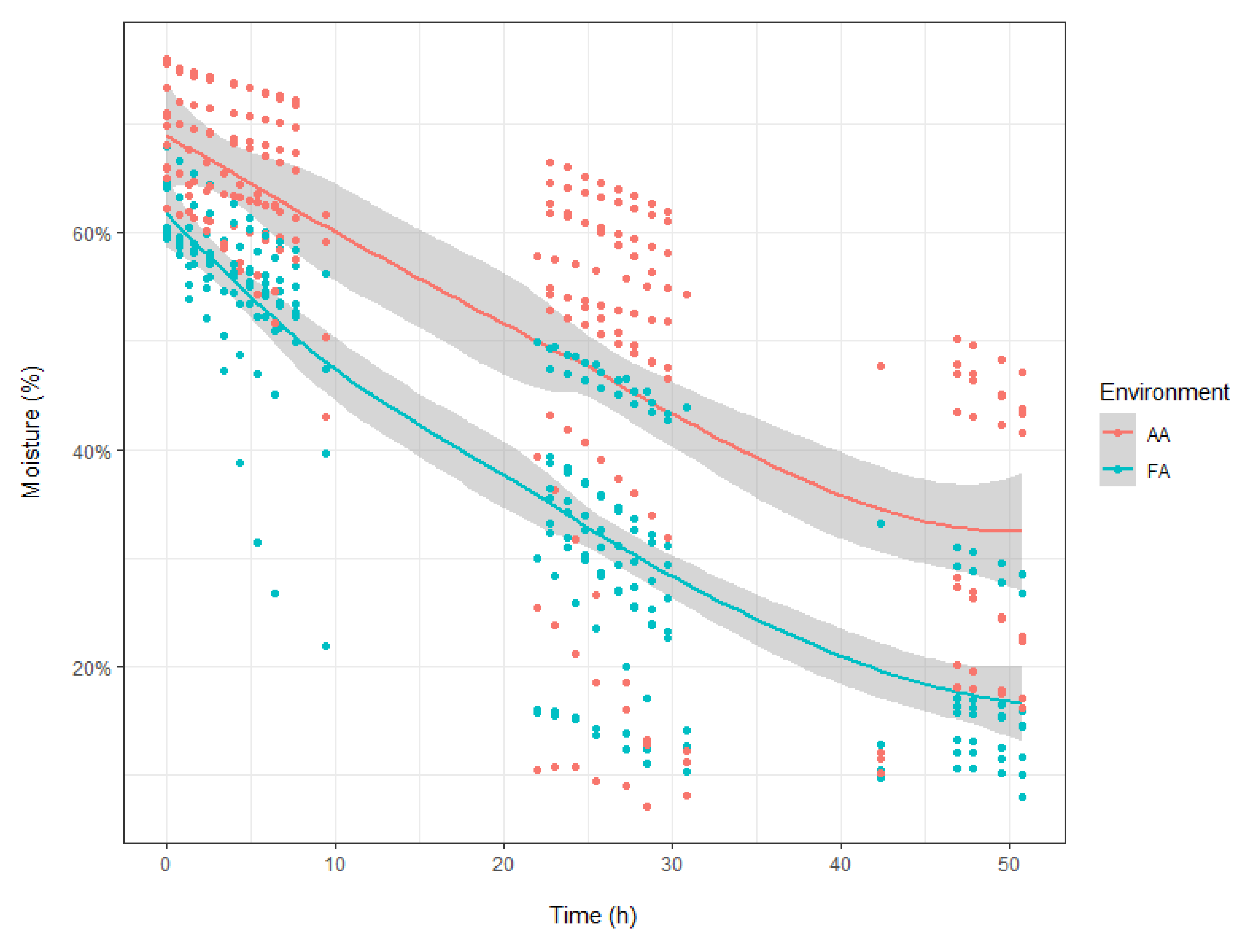
2.2. Physiological Parameters
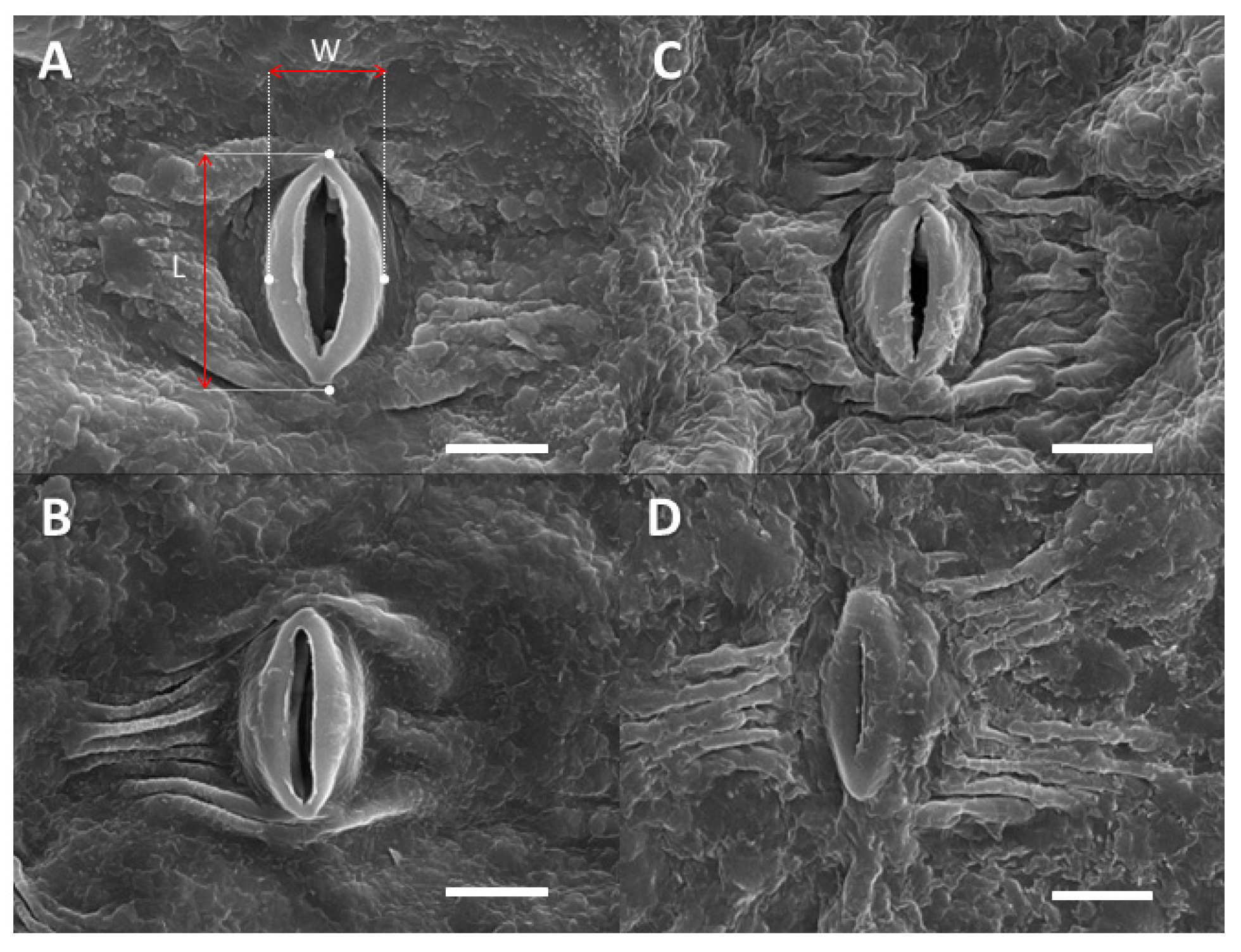
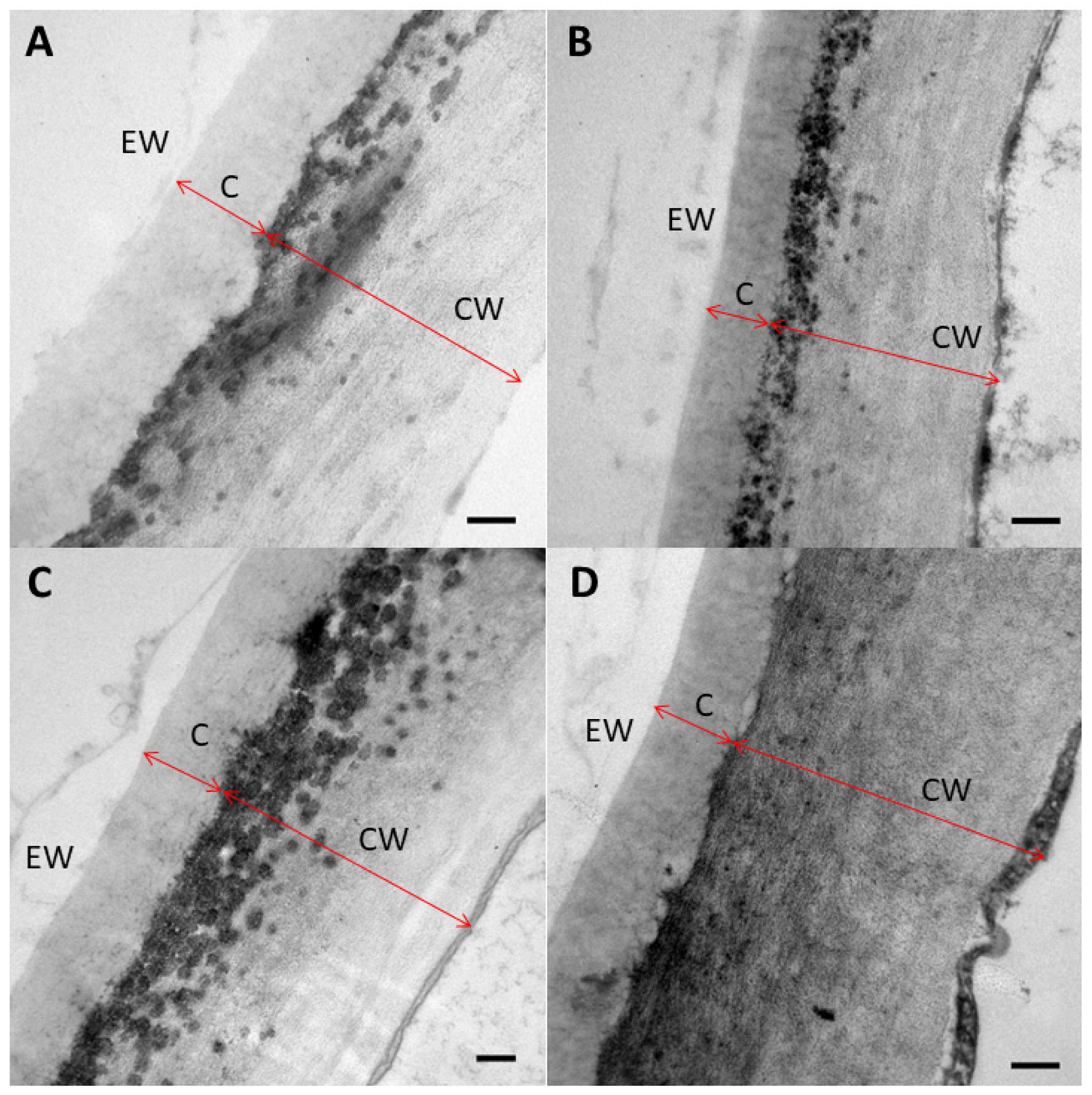
2.3. Leaf Surface-Related Parameters
2.4. Leaf Mineral Element Concentration
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Conditions
4.2. Nocturnal Transpiration
4.3. Water Loss and Minimum Leaf Conductance
4.4. Leaf Turgor Loss Point
4.5. Carbon Isotope Ratios
4.6. Leaf Anatomy
4.7. Cuticular and Stomatal Characteristics
4.7.1. Contact Angle Measurements
4.7.2. Electron Microscopy
4.8. Leaf Tissue Mineral Analysis
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baron, P.A.; Willeke, K. Aerosol Measurement: Principles, Techniques, and Applications, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2001. [Google Scholar]
- Burkhardt, J.; Grantz, D.A. Plants and atmospheric aerosols. In Progress in Botany; Springer: Cham, Switzerland; New York, NY, USA, 2017; Volume 78, pp. 369–406. [Google Scholar]
- Boucher, O. Atmospheric aerosols. In Atmospheric Aerosols; Springer: Dordrecht, The Nederlands, 2015; pp. 9–24. [Google Scholar]
- Chadwick, O.A.; Derry, L.A.; Vitousek, P.M.; Huebert, B.J.; Hedin, L.O. Changing sources of nutrients during four million years of ecosystem development. Nature 1999, 397, 491–497. [Google Scholar] [CrossRef]
- Pariyar, S.; Noga, G. Rainfall does not impair particulate matter accumulation on peri-urban field crops but improves photosynthetic activity at UV exposure. Environ. Exp. Bot. 2018, 156, 288–297. [Google Scholar] [CrossRef]
- Mooney, H.A.; Gulmon, S.L.; Ehleringer, J.; Rundel, P.W. Atmospheric water uptake by an Atacama Desert shrub. Science 1980, 209, 693–694. [Google Scholar] [CrossRef]
- Coopman, R.E.; Nguyen, H.T.; Mencuccini, M.; Oliveira, R.S.; Sack, L.; Lovelock, C.E.; Ball, M.C. Harvesting water from unsaturated atmospheres: Deliquescence of salt secreted onto leaf surfaces drives reverse sap flow in a dominant arid climate mangrove, Avicennia marina. New Phytol. 2021, 231, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Tredenick, E.C.; Stuart-Williams, H.; Enge, T.G. Materials on plant leaf surfaces are deliquescent in a variety of environments. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Putaud, J.P.; Van Dingenen, R.; Alastuey, A.; Bauer, H.; Birmili, W.; Cyrys, J.; Flentje, H.; Fuzzi, S.; Gehrig, R.; Hansson, H.C.; et al. A European aerosol phenomenology-3: Physical and chemical characteristics of particulate matter from 60 rural, urban, and kerbside sites across Europe. Atmos. Environ. 2010, 44, 1308–1320. [Google Scholar] [CrossRef]
- Huang, J.; Lin, B.; Minnis, P.; Wang, T.; Wang, X.; Hu, Y.; Yi, Y.; Ayers, J.K. Satellite-based assessment of possible dust aerosols semi-direct effect on cloud water path over East Asia. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Penner, J.E.; Dickinson, R.E.; O’Neill, C.A. Effects of aerosol from biomass burning on the global radiation budget. Science 1992, 256, 1432–1434. [Google Scholar] [CrossRef]
- Mahowald, N.; Scanza, R.; Brahney, J.; Goodale, C.L.; Hess, P.G.; Moore, J.K.; Neff, J. Aerosol deposition impacts on land and ocean carbon cycles. Curr. Clim. Chang. Rep. 2017, 3, 16–31. [Google Scholar] [CrossRef]
- Burkhardt, J.; Pariyar, S. Particulate pollutants are capable to ‘degrade’ epicuticular waxes and to decrease the drought tolerance of Scots pine (Pinus sylvestris L.). Environ. Pollut. 2014, 184, 659–667. [Google Scholar] [CrossRef]
- Chi, C.J.E.; Zinsmeister, D.; Lai, I.L.; Chang, S.C.; Kuo, Y.L.; Burkhardt, J. Aerosol Impacts on Water Relations of Camphor (Cinnamomum camphora). Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Burkhardt, J. Hygroscopic particles on leaves: Nutrients or desiccants? Ecol. Monogr. 2010, 80, 369–399. [Google Scholar] [CrossRef]
- Pariyar, S.; Eichert, T.; Goldbach, H.E.; Hunsche, M.; Burkhardt, J. The exclusion of ambient aerosols changes the water relations of sunflower (Helianthus annuus) and bean (Vicia faba) plants. Environ. Exp. Bot. 2013, 88, 43–52. [Google Scholar] [CrossRef]
- Daley, M.J.; Phillips, N.G. Interspecific variation in nighttime transpiration and stomatal conductance in a mixed New England deciduous forest. Tree Physiol. 2006, 26, 411–419. [Google Scholar] [CrossRef]
- Kavanagh, K.L.; Pangle, R.; Schotzko, A.D. Nocturnal transpiration causing disequilibrium between soil and stem predawn water potential in mixed conifer forests of Idaho. Tree Physiol. 2007, 27, 621–629. [Google Scholar] [CrossRef]
- Kangur, O.; Steppe, K.; Schreel, J.D.; Jonas, S.; Sellin, A. Variation in nocturnal stomatal conductance and development of predawn disequilibrium between soil and leaf water potentials in nine temperate deciduous tree species. Funct. Plant Biol. 2021, 48, 483–492. [Google Scholar] [CrossRef]
- Dayer, S.; Herrera, J.C.; Dai, Z.W.; Burlett, R.; Lamarque, L.J.; Delzon, S.; Bortolami, G.; Cochard, H.; Gambetta, G.A. Nighttime transpiration represents a negligible part of water loss and does not increase the risk of water stress in grapevine. Plant Cell Environ. 2021, 44, 387–398. [Google Scholar] [CrossRef]
- Even, M.; Sabo, M.; Meng, D.; Kreszies, T.; Schreiber, L.; Fricke, W. Night-time transpiration in barley (Hordeum vulgare) facilitates respiratory carbon dioxide release and is regulated during salt stress. Ann. Bot. 2018, 122, 569–582. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Clarke, S.J. Nocturnal and daytime stomatal conductance respond to root-zone temperature in ‘Shiraz’grapevines. Ann. Bot. 2013, 111, 433–444. [Google Scholar] [CrossRef]
- Siddiq, Z.; Cao, K.F. Nocturnal transpiration in 18 broadleaf timber species under a tropical seasonal climate. For. Ecol. Manag. 2018, 418, 47–54. [Google Scholar] [CrossRef]
- Fricke, W. Night-time transpiration–favouring growth? Trends Plant Sci. 2019, 24, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Resco de Dios, V.; Roy, J.; Ferrio, J.P.; Alday, J.G.; Landais, D.; Milcu, A.; Gessler, A. Processes driving nocturnal transpiration and implications for estimating land evapotranspiration. Sci. Rep. 2015, 5, 10975. [Google Scholar] [CrossRef] [PubMed]
- Gansert, D. Xylem sap flow as a major pathway for oxygen supply to the sapwood of birch (Betula pubescens Ehr.). Plant Cell Environ. 2003, 26, 1803–1814. [Google Scholar] [CrossRef]
- Ludwig, F.; Jewitt, R.A.; Donovan, L.A. Nutrient and water addition effects on day-and night-time conductance and transpiration in a C3 desert annual. Oecologia 2006, 148, 219–225. [Google Scholar] [CrossRef]
- Sadok, W.; Tamang, B.G. Diversity in daytime and night-time transpiration dynamics in barley indicates adaptation to drought regimes across the Middle-East. J. Agron. Crop Sci. 2019, 205, 372–384. [Google Scholar] [CrossRef]
- Schoppach, R.; Sinclair, T.R.; Sadok, W. Sleep tight and wake-up early: Nocturnal transpiration traits to increase wheat drought tolerance in a Mediterranean environment. Funct. Plant Biol. 2020, 47, 1117–1127. [Google Scholar] [CrossRef]
- Kupper, P.; Rohula, G.; Saksing, L.; Sellin, A.; Lõhmus, K.; Ostonen, I.; Helmisaari, H.S.; Sõber, A. Does soil nutrient availability influence night-time water flux of aspen saplings? Environ. Exp. Bot. 2012, 82, 37–42. [Google Scholar] [CrossRef]
- Rohula, G.; Kupper, P.; Räim, O.; Sellin, A.; Sõber, A. Patterns of night-time water use are interrelated with leaf nitrogen concentration in shoots of 16 deciduous woody species. Environ. Exp. Bot. 2014, 99, 180–188. [Google Scholar] [CrossRef]
- Snyder, K.A.; James, J.J.; Richards, J.H.; Donovan, L.A. Does hydraulic lift or nighttime transpiration facilitate nitrogen acquisition? Plant Soil 2008, 306, 159–166. [Google Scholar] [CrossRef]
- Eller, F.; Jensen, K.; Reisdorff, C. Nighttime stomatal conductance differs with nutrient availability in two temperate floodplain tree species. Tree Physiol. 2017, 37, 428–440. [Google Scholar] [CrossRef]
- Scholz, F.G.; Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Franco, A.C.; Miralles-Wilhelm, F. Removal of nutrient limitations by long-term fertilization decreases nocturnal water loss in savanna trees. Tree Physiol. 2007, 27, 551–559. [Google Scholar] [CrossRef]
- Howard, A.R.; Donovan, L.A. Helianthus nighttime conductance and transpiration respond to soil water but not nutrient availability. Plant Physiol. 2007, 143, 145–155. [Google Scholar] [CrossRef]
- Mahowald, N. Aerosol indirect effect on biogeochemical cycles and climate. Science 2011, 334, 794–796. [Google Scholar] [CrossRef]
- Newman, E.I. Phosphorus inputs to terrestrial ecosystems. J. Ecol. 1995, 83, 713–726. [Google Scholar] [CrossRef]
- Anderson, L.D.; Faul, K.L.; Paytan, A. Phosphorus associations in aerosols: What can they tell us about P bioavailability? Mar. Chem. 2010, 120, 44–56. [Google Scholar] [CrossRef]
- Maréchaux, I.; Bartlett, M.K.; Sack, L.; Baraloto, C.; Engel, J.; Joetzjer, E.; Chave, J. Drought tolerance as predicted by leaf water potential at turgor loss point varies strongly across species within an Amazonian forest. Funct. Ecol. 2015, 29, 1268–1277. [Google Scholar] [CrossRef]
- Duursma, R.A.; Blackman, C.J.; Lopéz, R.; Martin-StPaul, N.K.; Cochard, H.; Medlyn, B.E. On the minimum leaf conductance: Its role in models of plant water use, and ecological and environmental controls. New Phytol. 2018, 221, 693–705. [Google Scholar] [CrossRef]
- Zhu, S.D.; Chen, Y.J.; Ye, Q.; He, P.C.; Liu, H.; Li, R.H.; Fu, P.L.; Jiang, G.F.; Cao, K.F. Leaf turgor loss point is correlated with drought tolerance and leaf carbon economics traits. Tree Physiol. 2018, 38, 658–663. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Richards, R.A. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Funct. Plant Biol. 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Seibt, U.; Rajabi, A.; Griffiths, H.; Berry, J.A. Carbon isotopes and water use efficiency: Sense and sensitivity. Oecologia 2008, 155, 441–454. [Google Scholar] [CrossRef]
- Gostin, I.N. Air pollution effects on the leaf structure of some Fabaceae species. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 57–63. [Google Scholar]
- Pourkhabbaz, A.; Rastin, N.; Olbrich, A.; Langenfeld-Heyser, R.; Polle, A. Influence of environmental pollution on leaf properties of urban plane trees, Platanus orientalis L. Bull. Environ. Contam. Toxicol. 2010, 85, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Grantz, D.A.; Karr, M.; Burkhardt, J. Heterogeneity of stomatal pore area is suppressed by ambient aerosol in the homobaric species, vicia faba. Front. Plant Sci. 2020, 11, 897. [Google Scholar] [CrossRef] [PubMed]
- Kardel, F.; Wuyts, K.; Babanezhad, M.; Wuytack, T.; Potters, G.; Samson, R. Assessing urban habitat quality based on specific leaf area and stomatal characteristics of Plantago lanceolata L. Environ. Pollut. 2010, 158, 788–794. [Google Scholar] [CrossRef]
- Kováts, N.; Horváth, E.; Eck-Varanka, B.; Csajbók, E.; Hoffer, A. Adapting the vegetative vigour terrestrial plant test for assessing ecotoxicity of aerosol samples. Environ. Sci. Pollut. Res. 2017, 24, 15291–15298. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Alves, E.S.; Moura, B.B.; Domingos, M. Structural analysis of Tillandsia usneoides L. exposed to air pollutants in São Paulo City–Brazil. Water Air Soil Pollut. 2008, 189, 61–68. [Google Scholar] [CrossRef]
- Christman, M.A.; Richards, J.H.; McKay, J.K.; Stahl, E.A.; Juenger, T.E.; Donovan, L.A. Genetic variation in Arabidopsis thaliana for night-time leaf conductance. Plant Cell Environ. 2008, 31, 1170–1178. [Google Scholar] [CrossRef]
- Barbes, L.; Barbulescu, A.; Radulescu, C.; Stihi, C.; Chelarescu, E.D. Determination of heavy metals in leaves and bark of Populus nigra L. by atomic absorption spectrometry. Rom. Rep. Phys. 2014, 66, 877–886. [Google Scholar]
- Yalaltdinova, A.; Kim, J.; Baranovskaya, N.; Rikhvanov, L. Populus nigra L. as a bioindicator of atmospheric trace element pollution and potential toxic impacts on human and ecosystem. Ecol. Indic. 2018, 95, 974–983. [Google Scholar] [CrossRef]
- Górka, M.; Bartz, W.; Skuridina, A.; Potysz, A. Populus nigra italica leaves as a valuable tool for mineralogical and geochemical interpretation of inorganic atmospheric aerosols’ genesis. Atmosphere 2020, 11, 1126. [Google Scholar] [CrossRef]
- Guzmán, P.; Fernández, V.; García, M.L.; Khayet, M.; Fernández, A.; Gil, L. Localization of polysaccharides in isolated and intact cuticles of eucalypt, poplar and pear leaves by enzyme-gold labelling. Plant Physiol. Biochem. 2014, 76, 1–6. [Google Scholar] [CrossRef]
- van den Burg, J. Foliar Analysis for Determination of Tree Nutrient Status: A Compilation of Literature Data; “De Dorschkamp” Institute for Forestry and Urban Ecology, Rapport Nr. 414: Wageningen, The Netherlands, 1985. [Google Scholar]
- van den Burg, J. Foliar Analysis for Determination of Tree Nutrient Status: A Compilation of Literature Data; “De Dorschkamp” Institute for Forestry and Urban Ecology, Rapport Nr. 591: Wageningen, The Netherlands, 1990. [Google Scholar]
- Carstensen, A.; Herdean, A.; Schmidt, S.B.; Sharma, A.; Spetea, C.; Pribil, M.; Husted, S. The impacts of phosphorus deficiency on the photosynthetic electron transport chain. Plant Physiol. 2018, 177, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Frydenvang, J.; van Maarschalkerweerd, M.; Carstensen, A.; Mundus, S.; Schmidt, S.B.; Pedas, P.R.; Laursen, K.H.; Schjoerring, J.K.; Husted, S. Sensitive detection of phosphorus deficiency in plants using chlorophyll a fluorescence. Plant Physiol. 2015, 169, 353–361. [Google Scholar] [CrossRef]
- Burkhardt, J.; Pariyar, S. How does the VPD response of isohydric and anisohydric plants depend on leaf surface particles? Plant Biol. 2016, 18, 91–100. [Google Scholar] [CrossRef]
- Burkhardt, J.; Basi, S.; Pariyar, S.; Hunsche, M. Stomatal penetration by aqueous solutions—An update involving leaf surface particles. New Phytol. 2012, 196, 774–787. [Google Scholar] [CrossRef]
- Qazi, M.J.; Salim, H.; Doorman, C.A.W.; Jambon-Puillet, E.; Shahidzadeh, N. Salt creeping as a self-amplifying crystallization process. Sci. Adv. 2019, 5, eaax1853. [Google Scholar] [CrossRef]
- Burkhardt, J.; Zinsmeister, D.; Grantz, D.A.; Vidic, S.; Sutton, M.A.; Hunsche, M.; Shyam, P. Camouflaged as degraded wax: Hygroscopic aerosols contribute to leaf desiccation, tree mortality, and forest decline. Environ. Res. Lett. 2018, 13, 085001. [Google Scholar] [CrossRef]
- Bosabalidis, A.M.; Kofidis, G. Comparative effects of drought stress on leaf anatomy of two olive cultivars. Plant Sci. 2002, 163, 375–379. [Google Scholar] [CrossRef]
- Rauret, G.; Llauradó, M.; Tent, J.; Rigol, A.; Alegre, L.H.; Utrillas, M.J. Deposition on holm oak leaf surfaces of accidentally released radionuclides. Sci. Total Environ. 1994, 157, 7–16. [Google Scholar] [CrossRef]
- Houshmandfar, A.; Fitzgerald, G.J.; O’Leary, G.; Tausz-Posch, S.; Fletcher, A.; Tausz, M. The relationship between transpiration and nutrient uptake in wheat changes under elevated atmospheric CO2. Physiol. Plant. 2018, 163, 516–529. [Google Scholar] [CrossRef]
- Tang, J.; Riley, W.J. On the modeling paradigm of plant root nutrient acquisition. Plant Soil 2021, 459, 441–451. [Google Scholar] [CrossRef]
- Okuyama, Y.; Ozawa, K.; Takagaki, M. Diurnal changes in nitrogen and potassium absorption rates of plants grown in a greenhouse. J. Agric. Meteorol. 2015, 71, 256–262. [Google Scholar] [CrossRef]
- Albornoz, F.; Lieth, J.H. Diurnal macronutrients uptake patterns by lettuce roots under various light and temperature levels. J. Plant Nutr. 2015, 38, 2028–2043. [Google Scholar] [CrossRef]
- Howard, A.R.; Donovan, L.A. Soil nitrogen limitation does not impact nighttime water loss in Populus. Tree Physiol. 2010, 30, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.K.; Scoffoni, C.; Ardy, R.; Zhang, Y.; Sun, S.; Cao, K.; Sack, L. Rapid determination of comparative drought tolerance traits: Using an osmometer to predict turgor loss point. Methods Ecol. Evol. 2012, 3, 880–888. [Google Scholar] [CrossRef]
- Mart, K.B.; Veneklaas, E.J.; Bramley, H. Osmotic potential at full turgor: An easily measurable trait to help breeders select for drought tolerance in wheat. Plant Breed. 2016, 135, 279–285. [Google Scholar] [CrossRef]
- Farrell, C.; Szota, C.; Arndt, S.K. Does the turgor loss point characterize drought response in dryland plants? Plant Cell Environ. 2017, 40, 1500–1511. [Google Scholar] [CrossRef]
- Banks, J.M.; Hirons, A.D. Alternative methods of estimating the water potential at turgor loss point in Acer genotypes. Plant Methods 2019, 15, 34. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Holbrook, N.M.; Edwards, E.J.; Gutiérrez, M.V. Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant Cell Environ. 2003, 26, 443–450. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Scoffoni, C.; Sack, L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: A global meta-analysis. Ecol. Lett. 2012, 15, 393–405. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Rodríguez, P.; Olmos, E.; Morales, M.A.; Torrecillas, A. Differences in the Effects of Simulated Sea Aerosol on Water Relations, Salt Content, and Leaf Ultrastructure of Rock-Rose Plants. J. Environ. Qual. 2004, 33, 1369–1375. [Google Scholar] [CrossRef]
- Dluzniewska, P.; Gessler, A.; Dietrich, H.; Schnitzler, J.-P.; Teuber, M.; Rennenberg, H. Nitrogen uptake and metabolism in Populus × canescens as affected by salinity. New Phytol. 2007, 173, 279–293. [Google Scholar] [CrossRef]
- Al-Abadleh, H.A. Aging of atmospheric aerosols and the role of iron in catalyzing brown carbon formation. Environ. Sci. Atmos. 2021, 1, 297–345. [Google Scholar] [CrossRef]
- Lehndorff, E.; Schwark, L. Biomonitoring of air quality in the Cologne Conurbation using pine needles as a passive sampler–Part III: Major and trace elements. Atmos. Environ. 2010, 44, 2822–2829. [Google Scholar] [CrossRef]
- Weckwerth, G. Verification of traffic emitted aerosol components in the ambient air of Cologne (Germany). Atmos. Environ. 2001, 35, 5525–5536. [Google Scholar] [CrossRef]
- Räsch, A.; Hunsche, M.; Mail, M.; Burkhardt, J.; Noga, G.; Pariyar, S. Agricultural adjuvants may impair leaf transpiration and photosynthetic activity. Plant Physiol. Biochem. 2018, 132, 229–237. [Google Scholar] [CrossRef]
- Motai, A.; Yamazaki, M.; Muramatsu, N.; Watanabe, M.; Izuta, T. Submicron ammonium sulfate particles deposited on leaf surfaces of a leafy vegetable (Komatsuna, Brassica rapa L. var. perviridis) are taken up by leaf and enhance nocturnal leaf conductance. Atmos. Environ. 2018, 187, 155–162. [Google Scholar]
- Goodwin, S.M.; Jenks, M.A. Plant cuticle function as a barrier to water loss. In Plant Abiotic Stress; Blackwell Publishing Ltd.: Oxford, England, 2005; pp. 14–36. [Google Scholar]
- Zeppel, M.J.; Lewis, J.D.; Chaszar, B.; Smith, R.A.; Medlyn, B.E.; Huxman, T.E.; Tissue, D.T. Nocturnal stomatal conductance responses to rising [CO2], temperature and drought. New Phytol. 2012, 193, 929–938. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Geissler, P.R. Hygroscopicity of complex fertilizers. Determination of critical relative humidity of multicomponent systems. J. Agric. Food Chem. 1968, 16, 378–383. [Google Scholar] [CrossRef]
- Vega, C.; Valbuena-Carabaña, M.; Gil, L.; Fernández, V. Water sorption and desorption of isolated cuticles from three woody species with focus on Ilex aquifolium. Front. Plant Sci. 2021, 1973. [Google Scholar] [CrossRef]
- Callister, A.N.; Arndt, S.K.; Adams, M.A. Comparison of four methods for measuring osmotic potential of tree leaves. Physiol. Plant. 2006, 127, 383–392. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Improving intrinsic water-use efficiency and crop yield. Crop Sci. 2002, 42, 122–131. [Google Scholar] [PubMed]
- Bahamonde, H.A.; Fernandez, V.; Gil, L. Surface properties and permeability to calcium chloride of Fagus sylvatica and Quercus petraea leaves of different canopy heights. Front. Plant Sci. 2018, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Arsic, M.; Le Tougaard, S.; Persson, D.P.; Martens, H.J.; Doolette, C.L.; Lombi, E.; Schjoerring, J.K.; Husted, S. Bioimaging techniques reveal foliar phosphate uptake pathways and leaf phosphorus status. Plant Physiol. 2020, 183, 1472–1483. [Google Scholar] [CrossRef]
- Razali, N.M.; Wah, Y.B. Power comparisons of shapiro-wilk, kolmogorov-smirnov, lilliefors and anderson-darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- Conover, W.J.; Johnson, M.E.; Johnson, M.M. A comparative study of tests for homogeneity of variances, with applications to the outer continental shelf bidding data. Technometrics 1981, 23, 351–361. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Woolson, R.F. Wilcoxon signed-rank test. In Wiley Encyclopedia of Clinical Trials; John Wiley and Sons: New York, NY, USA, 2007; pp. 1–3. [Google Scholar]
- Yuen, K.K. The two-sample trimmed t for unequal population variances. Biometrika 1974, 61, 165–170. [Google Scholar] [CrossRef]
| Environment | Beta (β) | tseca (h) | Moisture (%)dry | gmin (mmol m−2 s−1) | Turgor Loss Point πtlp (MPa) | δ13C (‰) | SLA (m2/kg) |
|---|---|---|---|---|---|---|---|
| FA | −6.60 ± 0.59 | 29.3 ± 4.8 | 182.9 ± 1.6 | 2.54 ± 0.9 | −2.50 ± 0.10 | −29.2 ± 0.8 | 13.1 ± 0.3 |
| AA | −7.18 ± 0.43 | 30.4 ± 4.3 | 206.3 ± 10.1 | 1.61 ± 0.3 | −2.85 ± 0.07 | −29.8 ± 0.6 | 14.4 ± 0.8 |
| Differences | * | * |
| Environment | Leaf Side | St. Length (µm) | St. Width (µm) | St. Area (µm2) | Stomatal Density (stomata/mm2) | θw (°) |
|---|---|---|---|---|---|---|
| FA | Adaxial | 23.9 ± 0.4 a | 9.97 ± 0.29 a | 187.8 ± 7.4 a | 34.8 ± 6.1 a | 94.4 ± 2.1 |
| Abaxial | 19.7 ± 0.6 b | 7.92 ± 0.24 b | 123.0 ± 6.0 b | 246.7 ± 16.7 b | 103.21 ± 1.7 | |
| AA | Adaxial | 17.2 ± 0.5 c | 8.92 ± 0.39 ab | 122.2 ± 8.1 b | 49.1 ± 6.9 a | 88.7 ± 2.0 |
| Abaxial | 19.2 ± 0.3 b | 7.66 ± 0.31 b | 116.2 ± 6.1 b | 300.5 ± 28.5 b | 90.0 ± 2.7 | |
| Environment | *** | *** | ** | |||
| Differences | Leaf side | * | *** | ** | *** | * |
| Interaction between factors | *** | *** | *** | ** |
| Macronutrients (g 100g−1) | Micronutrients (mg kg−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Environment | N | P | S | Mg | K | Ca | Fe | Zn | Cu |
| FA | 1.44 ± 0.16 | 0.10 ± 0.01 | 0.20 ± 0.02 | 0.29 ± 0.03 | 1.00 ± 0.07 | 1.15 ± 0.12 | 41.3 ± 5.2 | 17.2 ± 1.1 | 3.6 ± 0.4 |
| AA | 1.70 ± 0.10 | 0.09 ± 0.01 | 0.24 ± 0.02 | 0.34 ± 0.03 | 1.07 ± 0.13 | 1.05 ± 0.15 | 74.9 ± 5.8 | 80.8 ± 15.3 | 4.2 ± 0.2 |
| Differences | ** | * | * | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega, C.; Chi, C.-J.E.; Fernández, V.; Burkhardt, J. Nocturnal Transpiration May Be Associated with Foliar Nutrient Uptake. Plants 2023, 12, 531. https://doi.org/10.3390/plants12030531
Vega C, Chi C-JE, Fernández V, Burkhardt J. Nocturnal Transpiration May Be Associated with Foliar Nutrient Uptake. Plants. 2023; 12(3):531. https://doi.org/10.3390/plants12030531
Chicago/Turabian StyleVega, Clara, Chia-Ju Ellen Chi, Victoria Fernández, and Juergen Burkhardt. 2023. "Nocturnal Transpiration May Be Associated with Foliar Nutrient Uptake" Plants 12, no. 3: 531. https://doi.org/10.3390/plants12030531
APA StyleVega, C., Chi, C.-J. E., Fernández, V., & Burkhardt, J. (2023). Nocturnal Transpiration May Be Associated with Foliar Nutrient Uptake. Plants, 12(3), 531. https://doi.org/10.3390/plants12030531








