Origanum heracleoticum Essential Oils: Chemical Composition, Phytotoxic and Alpha-Amylase Inhibitory Activities
Abstract
1. Introduction
2. Results and Discussion
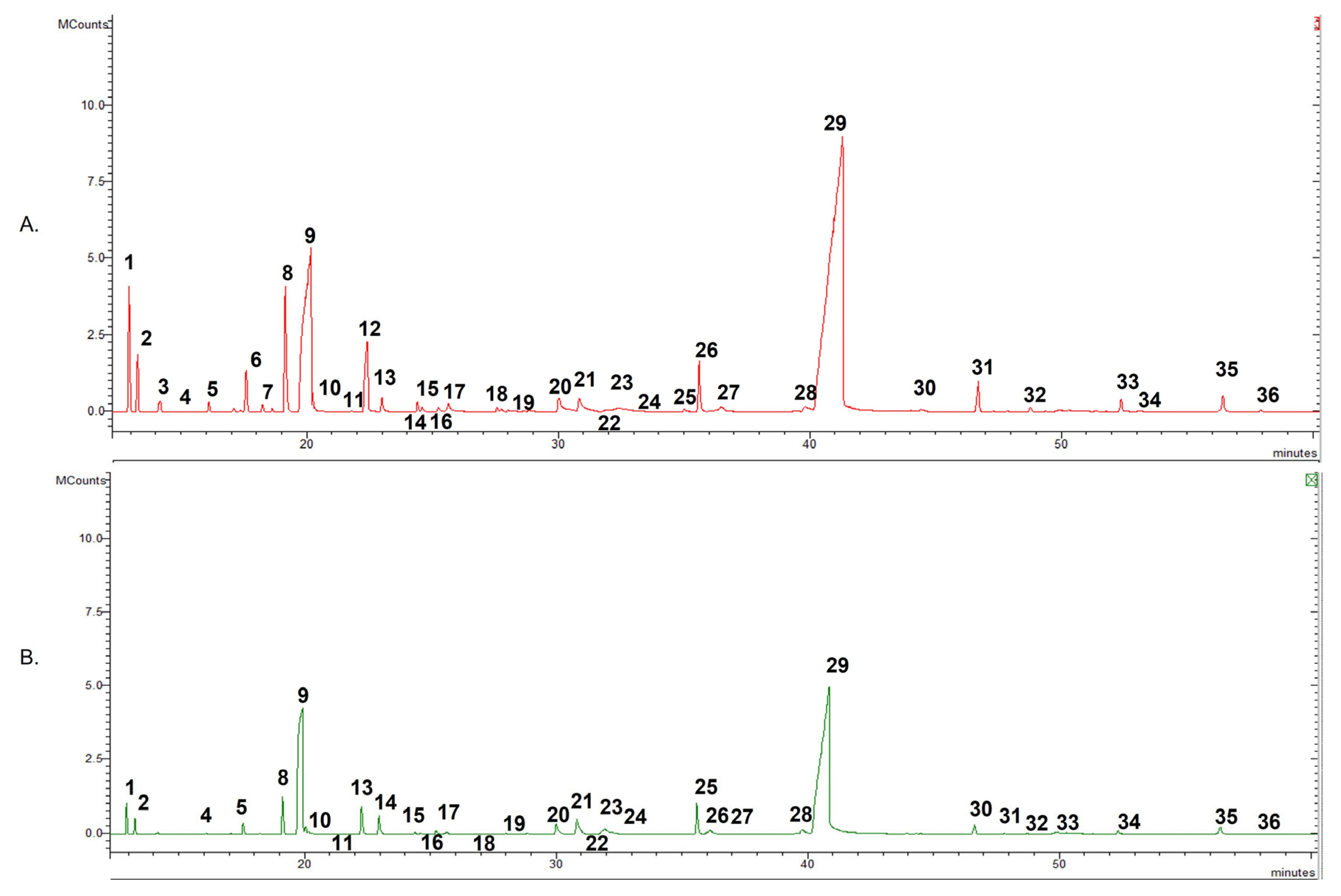
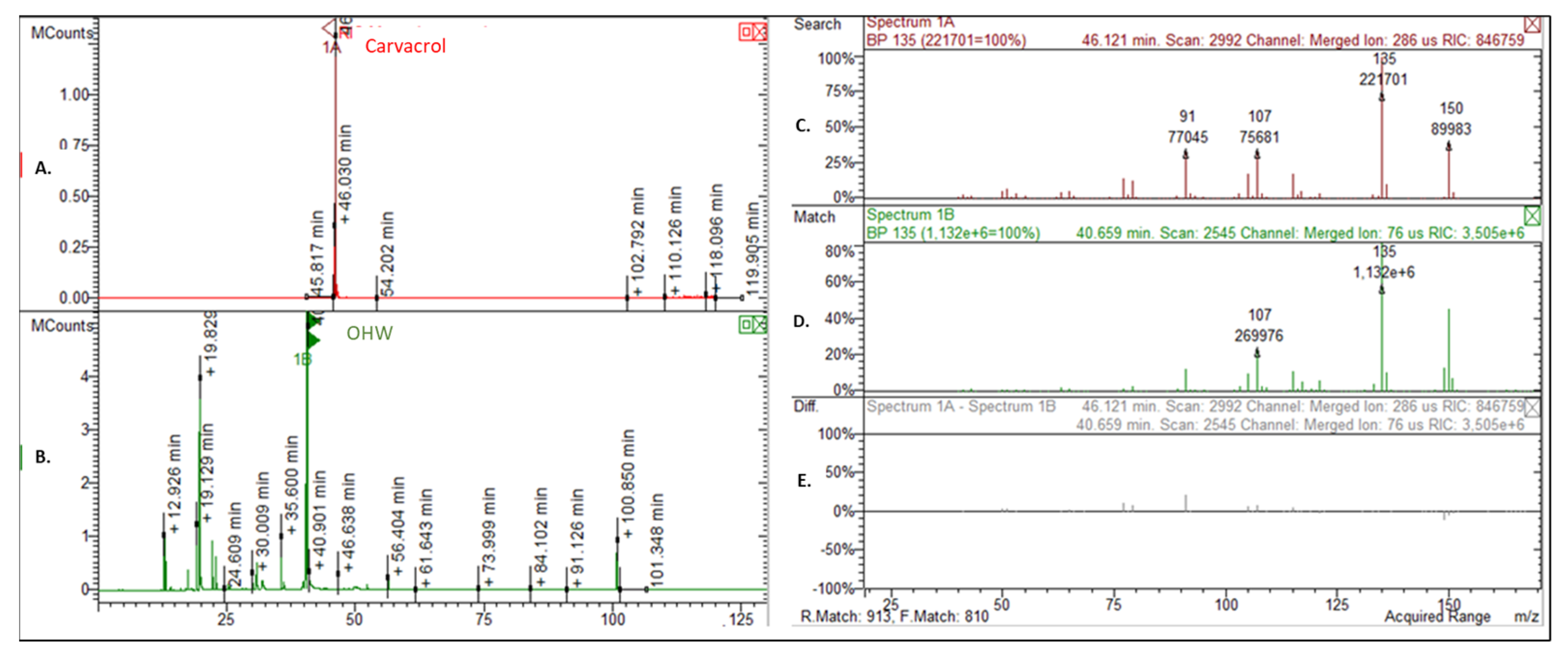
2.1. Chemical Composition
2.2. Phytotoxic Activity and α-Amylase Activity of Germinating Seeds
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Isolation of the Volatile Oil
3.4. Analysis of the Essential Oils
3.5. Phytotoxic Activity and Determination of α-Amylase Activity of Germinating Seeds
3.5.1. Phytotoxic Activity
3.5.2. Determination of α-Amylase Activity of Germinating Seeds
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Agrow. Agrow’s Top 20: 2007 Edition DS258; Informa Health Care: London, UK, 2007. [Google Scholar]
- Uremis, I.; Arslan, M.; Sangun, M.K. Herbicidal activity of essential oils on the germination of some problem weeds. Asian J. Chem. 2009, 21, 3199–3210. [Google Scholar]
- Jurado, A.S.; Fernandes, M.A.S.; Videira, R.A.; Peixoto, F.P.; Vicente, J.A.F. Herbicides: The Face and the Reverse of the Coin. An In Vitro Approach to the Toxicity of Herbicides in Non-Target Organisms. In Herbicides and Environment; InTech: Rijeka, Croatia, 2011; pp. 3–44. [Google Scholar]
- Dayan, F.E.; Owens, D.K.; Duke, S.O. Rationale for a natural products approach to herbicide discovery. Pest Manag. Sci. 2012, 68, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Zygadlo, J.A.; Grow, N.R. Comparative study of the antifungal activity of essential oils from aromatic plants growing wild in the central region of Argentina. Flavour Fragr. J. 1995, 10, 113–118. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Raja, R.R. Medicinally potential plants of labiatae (Lamiaceae) family: An overview. Res. J. Med. Plant Res. 2012, 6, 203–213. [Google Scholar] [CrossRef]
- Ietswaart, J.H. A Taxonomic Revision of the Genus Origanum (Labiatae); Leiden Botanical Series; Leiden University Press: Leiden, The Netherlands, 1980. [Google Scholar]
- Kozlowska, M.; Laudy, A.E.; Przybyl, J.; Ziarno, M.; Majewska, E. Chemical composition and antibacterial activity of some medicinal plants from Lamiaceae family. Acta Pol. Pharm. 2015, 72, 757–767. [Google Scholar]
- Salvo, A.; La Torre, G.L.; Rotondo, A.; Cicero, N.; Gargano, R.; Mangano, V.; Casale, K.E.; Dugo, G. Multiple analytical approaches for the organic and inorganic characterization of Origanum vulgare L. samples. Nat. Prod. Res. 2019, 33, 2815–2822. [Google Scholar] [CrossRef]
- Saric-Kundalic, B.; Dobes, C.; Klatte-Asselmeyer, V.; Saukel, J. Ethnobotanical study on medicinal use of wild and cultivated plants in middle, south and West Bosnia and Herzegovina. J. Ethnopharmacol. 2010, 131, 33–55. [Google Scholar] [CrossRef]
- Polat, R.; Satil, F. An ethnobotanical survey of medicinal plants in Edremit gulf (Balıkesir–Turkey). J. Ethnopharmacol. 2012, 139, 626–641. [Google Scholar] [CrossRef]
- Savikin, K.; Zdunic, G.; Menkovic, N.; Zivkovic, J.; Cujic, N.; Terescenko, M.; Bigovic, D. Ethnobotanical study on traditional use of medicinal plants in South-Western Serbia, Zlatibor district. J. Ethnopharmacol. 2013, 146, 803–810. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, L.F.R.; Frei, F.; Mancini, E.; De Martino, L.; De Feo, V. Phytotoxic activities of Mediterranean essential oils. Molecules 2010, 15, 4309–4323. [Google Scholar] [CrossRef] [PubMed]
- Kursat, M.; Emre, I.; Yilmaz, O.; Erecevit, P. Antioxidant and antimicrobial activity in the seeds of Origanum vulgare L. subsp. gracile (C. Koch) letswaart and Origanum acutidens (Hand. -Mazz.) letswaart from Turkey. Grasas Aceites 2011, 62, 410–417. [Google Scholar] [CrossRef]
- Della Pepa, T.; Elshafie, H.S.; Capasso, R.; De Feo, V.; Camele, I.; Nazzaro, F.; Scognamiglio, M.R.; Caputo, L. Antimicrobial and phytotoxic activity of Origanum heracleoticum and O. majorana essential oils growing in Cilento (Southern Italy). Molecules 2019, 24, 2576. [Google Scholar] [CrossRef]
- Grul’ová, D.; Pl’uchtová, M.; Fejér, J.; De Martino, L.; Caputo, L.; Sedlák, V.; De Feo, V. Influence of six essential oils on invasive Solidago canadensis L. seed germination. Nat. Prod. Res. 2020, 34, 3231–3233. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Berkay Yılmaz, Y.; Antika, G.; Salehi, B.; Tumer, T.B.; Kulandaisamy Venil, C.; Das, G.; Patra, J.K.; Karazhan, N.; Akram, M.; et al. Phytochemical constituents, biological activities, and health-promoting effects of the genus Origanum. Phytother. Res. 2021, 35, 95–121. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, M.; Mousa, A.A.; Mahmood, A.; Alkhathlan, H.Z. Chemical diversity in leaf and stem essential oils of Origanum vulgare L. and their effects on microbicidal activities. AMB Express 2019, 9, 176. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, N.A.; Mahmood, A.; Al-Kedhairy, A.A.; Alkhathlan, H.Z. The composition of the essential oil and aqueous distillate of Origanum vulgare L. growing in Saudi Arabia and evaluation of their antibacterial activity. Arab J. Chem. 2018, 11, 1189–1200. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef]
- Bounatirou, S.; Smiti, S.; Miguel, M.G.; Faleiro, L.; Rejeb, M.N.; Neffati, M.; Costa, M.M.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Chemical composition, antioxidant and antibacterial activities of the essential oils isolated from Tunisian Thymus capitatus Hoff. et Link. Food Chem. 2007, 105, 146–155. [Google Scholar] [CrossRef]
- Ilić, Z.; Stanojević, L.; Milenković, L.; Šunić, L.; Milenković, A.; Stanojević, J.; Cvetković, D. The Yield, Chemical Composition, and Antioxidant Activities of Essential Oils from Different Plant Parts of the Wild and Cultivated Oregano (Origanum vulgare L.). Horticulturae 2022, 8, 1042. [Google Scholar] [CrossRef]
- The Plant list. Available online: httpss://wfoplantlist.org/plant-list (accessed on 15 December 2022).
- Pieroni, A.; Quave, C.L. Traditional pharmacopoeias and medicines among Albanians and Italians in southern Italy: A comparison. J. Ethnopharmacol. 2005, 101, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Napoli, E.; Giovino, A.; Carrubba, A.; How Yuen Siong, V.; Rinoldo, C.; Nina, O.; Ruberto, G. Variations of Essential Oil Constituents in Oregano (Origanum vulgare subsp. viridulum (= O. heracleoticum) over Cultivation Cycles. Plants 2020, 9, 1174. [Google Scholar] [CrossRef] [PubMed]
- Aytaç, Z.; Gülbandılar, A.; Kürkçüoğlu, M. Humic Acid Improves Plant Yield, Antimicrobial Activity and Essential Oil Composition of Oregano (Origanum vulgare L. subsp. hirtum (Link.) Ietswaart). Agronomy 2022, 12, 2086. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Wasiela, M.; Głowacka, A. The antibacterial activity of oregano essential oil (Origanum heracleoticum L.) against clinical strains of Escherichia coli and Pseudomonas aeruginosa. Med. Dos. Mikrobiol. 2012, 64, 297–307. [Google Scholar]
- Yan, J.; Wu, H.; Chen, K.; Feng, J.; Zhang, Y. Antifungal Activities and Mode of Action of Cymbopogon citratus, Thymus vulgraris, and Origanum heracleoticum Essential Oil Vapours against Botrytis cinerea and Their Potential Application to Control Postharvest Strawberry Gray Mold. Foods 2021, 10, 2451. [Google Scholar] [CrossRef]
- Bendre, R.; Bagul, S.; Rajput, J. Carvacrol: An Excellent Natural Pest Control Agent. Nat. Prod. Chem. Res. 2018, 6, 349. [Google Scholar] [CrossRef]
- Werrie, P.Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.L. Phytotoxicity of Essential Oils: Opportunities and Constraints for the Development of Biopesticides. A Review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic Effects and Mechanism of Action of Essential Oils and Terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef]
- Caputo, L.; Cornara, L.; Raimondo, F.M.; De Feo, V.; Vanin, S.; Denaro, M.; Trombetta, D.; Smeriglio, A. Mentha pulegium L.: A Plant Underestimated for Its Toxicity to Be Recovered from the Perspective of the Circular Economy. Molecules 2021, 26, 2154. [Google Scholar] [CrossRef]
- Danna, C.; Cornara, L.; Smeriglio, A.; Trombetta, D.; Amato, G.; Aicardi, P.; De Martino, L.; De Feo, V.; Caputo, L. Eucalyptus gunnii and Eucalyptus pulverulenta ‘Baby Blue’ Essential Oils as Potential Natural Herbicides. Molecules 2021, 26, 6749. [Google Scholar] [CrossRef] [PubMed]
- Somala, N.; Laosinwattana, C.; Teerarak, M. Formulation process, physical stability, and herbicidal activities of Cymbopogon nardus essential oil-based nanoemulsion. Sci. Rep. 2022, 12, 10280. [Google Scholar] [CrossRef] [PubMed]
- Guglielminetti, L.; Busilacchi, H.A.; Alpi, A. Effect of anoxia on [alpha]-amylase induction in maize caryopsis. J. Plant Res. 2000, 113, 185. [Google Scholar] [CrossRef]
- Perata, P.; Guglielminetti, L.; Alpi, A. Mobilization of endosperm reserves in cereal seeds under anoxia. Ann. Bot. 1997, 79, 49–56. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007; pp. 1–811. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. The NIST Chemistry WebBook: A chemical data resource on the internet. J. Chem. Eng. Data 2001, 46, 1059–1063. [Google Scholar] [CrossRef]
- Russo, M.; Galletti, G.C.; Bocchini, P.; Carnacini, A. Essential oil chemical composition of wild populations of Italian oregano spice (Origanum vulgare ssp. hirtum (Link) Ietswaart): A preliminary evaluation of their use in chemotaxonomy by cluster analysis. 1. Inflorescences. J. Agric. Food Chem. 1998, 46, 3741–3746. [Google Scholar] [CrossRef]
- Vokou, D.; Kokkini, S.; Bessiere, J.M. Geographic variation of Greek oregano (Origanum vulgare ssp. hirtum) essential oils. Biochem. Syst. Ecol. 1993, 21, 287–295. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Hanlidou, E.; Lanaras, T. Essential oil composition of Greek (Origanum vulgare ssp. hirtum) and Turkish (O. onites) oregano: A tool for their distinction. J. Essent. Oil Res. 2004, 16, 334–338. [Google Scholar] [CrossRef]
- Dzamic, A.; Sokovic, M.; Ristic, M.S.; Grujic-Jovanovic, S.; Vukojevic, J.; Marin, P.D. Chemical composition and antifungal activity of Origanum heracleoticum essential oil. Chem. Nat. Compd. 2008, 44, 659–660. [Google Scholar] [CrossRef]
- Baycheva, S.K.; Dobreva, K.Z. Chemical composition of Bulgarian white oregano (Origanum heracleoticum L.) essential oils. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1031, 012107. [Google Scholar] [CrossRef]
- Poulose, A.J.; Croteau, R. Biosynthesis of aromatic monoterpenes: Conversion of γ-terpinene to p-cymene and thymol in Thymus vulgaris L. Arch. Biochem. Biophys. 1978, 187, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Jerković, I.; Mastelić, J.; Miloš, M. The impact of both the season of collection and drying on the volatile constituents of Origanum vulgare L. ssp. hirtum grown wild in Croatia. Int. J. Food Sci. Technol. 2001, 36, 649–654. [Google Scholar] [CrossRef]
- De Martino, L.; Mancini, E.; Marandino, A.; Rolim de Almeida, L.F.; De Feo, V. Chemistry and Antigerminative Activity of Essential Oils and Monoterpenoids from Mediterranean Plants. Curr. Bioact. Compds. 2012, 8, 13–49. [Google Scholar] [CrossRef]
- Mancini, E.; Camele, I.; Elshafie, H.S.; De Martino, L.; Pellegrino, C.; Grulova, D.; De Feo, V. Chemical composition and biological activity of the essential oil of Origanum vulgare ssp. hirtum from different areas in the Southern Apennines (Italy). Chem. Biodiver. 2014, 11, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Armentano, M.; Carmosino, M.; Bufo, S.; De Feo, V.; Camele, I. Cytotoxic activity of Origanum vulgare L. on hepatocellular carcinoma cell line HepG2 and evaluation of its biological activity. Molecules 2017, 22, 1435. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; Mancini, E.; Rolim de Almeida, L.F.; De Feo, V. The antigerminative activity of twenty-seven monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Phytotoxic effects of commercial essential oils on selected vegetable crops: Cucumber and tomato. Sustain. Chem. Pharm. 2020, 15, 100209. [Google Scholar] [CrossRef]
- Damaris, R.N.; Lin, Z.; Yang, P.; He, D. The Rice Alpha-Amylase, Conserved Regulator of Seed Maturation and Germination. Int. J. Mol. Sci. 2019, 20, 450. [Google Scholar] [CrossRef]
- Béjaoui, A.; Boulila, A.; Boussaid, M. Chemical composition and biological activities of essential oils and solvent extracts of Origanum vulgare subsp. glandulosum Desf. from Tunisia. J. Med. Plant Res. 2013, 7, 2429–2435. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal , S.; Ceylan , R.; Aktumsek , A. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp. vulgare and subsp. hirtum) essential oils. Ind. Crop. Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole: Bologna, Italy, 2017. [Google Scholar]
- Council of Europe. European Pharmacopeia, 10th ed.; Council of Europe: Strasbourg Cedex, France, 2020. [Google Scholar]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: San Francisco, CA, USA, 1980. [Google Scholar] [CrossRef]
- Davies, N. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Johnston, C. The Wiley/NBS Registry of Mass Spectral Data. J. Chem. Educ. 1989, 66, 256. [Google Scholar]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Elkhalifa, A.O.; Bernhardt, R. Influence of grain germination on functional properties of sorghum flour. Food Chem. 2010, 121, 387–392. [Google Scholar] [CrossRef]
- Bernfeld, P. Amylase a and b. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press Inc.: New York, 1955; pp. 149–158. [Google Scholar] [CrossRef]
| ||||||
|---|---|---|---|---|---|---|
| OHW | OHR | KI a | KI b | Identification e | ||
| 1 | α-Thujene | 1.4 | 3.1 | 924 | 930 | 1, 2, 3 |
| 2 | α-Pinene | 0.7 | 1.3 | 932 | 939 | 1, 2, 3 |
| 3 | Camphene | 0.2 | 0.4 | 949 | 954 | 1, 2, 3 |
| 4 | Sabinene | 0.1 | 0.2 | 971 | 975 | 1, 2, 3 |
| 5 | β-Pinene | 0.7 | 1.4 | 976 | 979 | 1, 2, 3 |
| 6 | α-Phellandrene | - | 0.2 | 1007 | 1002 | 1, 2, 3 |
| 7 | δ-3-Carene | - | 0.1 | 1009 | 1011 | 1, 2, 3 |
| 8 | α-Terpinene | 2.0 | 3.8 | 1014 | 1017 | 1, 2, 3 |
| 9 | p-Cymene | 22.0 | 7.0 | 1023 | 1024 | 1, 2, 3 |
| 10 | Limonene | 1.0 | 3.4 | 1028 | 1029 | 1, 2, 3 |
| 11 | 1,8-Cineole | t | t | 1031 | 1031 | 1, 2, 3 |
| 12 | (E)-β-Ocimene | - | 7.7 | 1043 | 1050 | 1, 2, 3 |
| 13 | γ- Terpinene | 3.5 | 3.4 | 1056 | 1059 | 1, 2, 3 |
| 14 | cis- Sabinene hydrate | 0.5 | 0.5 | 1065 | 1070 | 1, 2, 3 |
| 15 | Terpinolene | 0.2 | 0.2 | 1084 | 1088 | 1, 2 |
| 16 | trans-Sabinene hydrate | 0.2 | 0.2 | 1079 | 1098 | 1, 2, 3 |
| 17 | Linalool | 0.7 | 0.7 | 1099 | 1096 | 1, 2, 3 |
| 18 | allo-Ocimene | 0.1 | 0.1 | 1140 | 1132 | 1, 2, 3 |
| 19 | dehydro-Linalool | 0.5 | 0.5 | 1150 | 1135 | 1, 2 |
| 20 | Borneol | 1.1 | 1.1 | 1071 | 1169 | 1, 2, 3 |
| 21 | Terpinen-4-ol | 1.0 | 1.0 | 1180 | 1177 | 1, 2, 3 |
| 22 | meta-Cymen-8-ol | 0.3 | 0.3 | 1198 | 1179 | 1, 2, 3 |
| 23 | α-Terpineol | 0.5 | 0.5 | 1180 | 1188 | 1, 2, 3 |
| 24 | cis-Dihydrocarvone | 0.2 | 0.2 | 1190 | 1192 | 1, 2, 3 |
| 25 | Thymol methyl ether | 1.6 | 1.6 | 1240 | 1235 | 1, 2 |
| 26 | p- Cymenene | 0.2 | 0.2 | 1211 | 1250 | 1, 2, 3 |
| 27 | Thymoquinone | 0.5 | 0.5 | 1251 | 1252 | 1, 2 |
| 28 | Thymol | 0.5 | 0.5 | 1292 | 1290 | 1, 2, 3 |
| 29 | Carvacrol | 54.9 | 54.1 | 1301 | 1299 | 1, 2, 3 |
| 30 | (E)-Caryophyllene | 1.0 | 1.1 | 1415 | 1419 | 1, 2, 3 |
| 31 | Aromadendrene | 0.1 | t | 1420 | 1441 | 1, 2, 3 |
| 32 | α-Humulene | 0.1 | 0.2 | 1435 | 1454 | 1, 2, 3 |
| 33 | Acetovanillone | 1.5 | 0.2 | 1446 | 1482 | 1, 2 |
| 34 | Germacrene A | 0.4 | 0.9 | 1489 | 1509 | 1, 2 |
| 35 | Caryophyllene oxide | 0.1 | 0.9 | 1552 | 1583 | 1, 2, 3 |
| 36 | trans-Dihydrocarvone | 0.3 | 0.3 | 1606 | 1627 | 1, 2, 3 |
| 37 | α-Bisabolol | 0.3 | 0.3 | 1670 | 1685 | 1, 2, 3 |
| Total | 98.3 | 97.5 | ||||
| Monoterpene hydrocarbons | 32.3 | 32.6 | ||||
| Oxygenated monoterpenes | 62.6 | 61.3 | ||||
| Sesquiterpene hydrocarbons | 1.5 | 2.2 | ||||
| Oxygenated sesquiterpenes | 1.9 | 1.4 | ||||
| ||||||
| KI c | KI d | Identification e | ||||
| 1 | α-Pinene | 1015 | 1015 | 1, 2, 3 | ||
| 2 | α-Thujene | 1021 | 1221 | 1, 2, 3 | ||
| 3 | Camphene | 1052 | 1075 | 1, 2, 3 | ||
| 4 | β-Pinene | 1087 | 1087 | 1, 2, 3 | ||
| 5 | Sabinene | 1106 | 1112 | 1, 2, 3 | ||
| 6 | δ-3-Carene | 1132 | 1153 | 1, 2, 3 | ||
| 7 | α-Phellandrene | 1151 | 1160 | 1, 2, 3 | ||
| 8 | α-Terpinene | 1168 | 1166 | 1, 2, 3 | ||
| 9 | 1,8-Cineole | 1211 | 1210 | 1, 2, 3 | ||
| 10 | Limonene | 1212 | 1217 | 1, 2, 3 | ||
| 11 | (E)-β-Ocimene | 1235 | 1248 | 1, 2, 3 | ||
| 12 | γ- Terpinene | 1239 | 1253 | 1, 2, 3 | ||
| 13 | p-Cymene | 1263 | 1274 | 1, 2, 3 | ||
| 14 | Terpinolene | 1269 | 1278 | 1, 2 | ||
| 15 | allo-Ocimene | 1390 | 1382 | 1, 2, 3 | ||
| 16 | p- Cymenene | 1417 | 1414 | 1, 2, 3 | ||
| 17 | cis- Sabinene hydrate | 1500 | - | 1, 2, 3 | ||
| 18 | trans-Sabinene hydrate | 1547 | 1546 | 1, 2, 3 | ||
| 19 | Linalool | 1557 | 1551 | 1, 2, 3 | ||
| 20 | Terpinen-4-ol | 1595 | 1595 | 1, 2, 3 | ||
| 21 | Thymol methyl ether | 1610 | 1604 | 1, 2 | ||
| 22 | (E)-Caryophyllene | 1616 | 1612 | 1, 2, 3 | ||
| 23 | dehydro-Linalool | 1617 | 1617 | 1, 2 | ||
| 24 | cis-Dihydrocarvone | 1620 | - | 1, 2, 3 | ||
| 25 | trans-Dihydrocarvone | 1624 | 1627 | 1, 2, 3 | ||
| 26 | Aromadendrene | 1635 | 1637 | 1, 2, 3 | ||
| 27 | α-Terpineol | 1642 | 1662 | 1, 2, 3 | ||
| 28 | α-Humulene | 1679 | 1671 | 1, 2, 3 | ||
| 29 | Borneol | 1698 | 1715 | 1, 2, 3 | ||
| 30 | Germacrene A | 1712 | 1747 | 1, 2 | ||
| 31 | meta-Cymen-8-ol | 1845 | 1849 | 1, 2, 3 | ||
| 32 | α-Bisabolol | 2021 | 2232 | 1, 2, 3 | ||
| 33 | Thymoquinone | 2180 | - | 1, 2 | ||
| 34 | Thymol | 2200 | 2172 | 1, 2, 3 | ||
| 35 | Carvacrol | 2242 | 2225 | 1, 2, 3 | ||
| 36 | Caryophyllene oxide | 2500 | 1989 | 1, 2, 3 | ||
| 37 | Acetovanillone | 2667 | 2676 | 1, 2 | ||
| Number of Germinated Seeds | |||
|---|---|---|---|
| S. arvensis | R. sativus | L. multiflorum | |
| H2O | 9.3 ± 1.1 | 6.0 ± 1.7 | 7.3 ± 0.6 |
| OHW | |||
| 1000 µg/mL | 0.0 ± 0.0 **** | 0.0 ± 0.0 *** | 0.0 ± 0.0 **** |
| 500 µg/mL | 3.0 ± 1.4 **** | 2.0 ± 0.0 ** | 0.0 ± 0.0 **** |
| 250 µg/mL | 6.5 ± 0.7 * | 1.0 ± 0.0 *** | 0.0 ± 0.0 **** |
| 100 µg/mL | 10.0 ± 0.0 | 3.0 ± 1.0 * | 8.0 ± 1.7 |
| OHR | |||
| 1000 µg/mL | 0.0 ± 0.0 **** | 0.0 ± 0.0 ** | 0.0 ± 0.0 **** |
| 500 µg/mL | 0.5 ± 0.7 **** | 0.0 ± 0.0 ** | 0.0 ± 0.0 **** |
| 250 µg/mL | 1.0 ± 0.0 **** | 2.0 ± 2.8 | 0.0 ± 0.0 **** |
| 100 µg/mL | 8.7 ± 1.2 | 5.3 ± 1.5 | 8.0 ± 1.0 |
| Radicle Elongation (cm) | |||
|---|---|---|---|
| S. arvensis | R. sativus | L. multiflorum | |
| H2O | 1.9 ± 1.0 | 2.4 ± 1.0 | 4.9 ± 1.7 |
| OHW | |||
| 1000 µg/mL | 0.0 ± 0.0 * | 0.0 ± 0.0 *** | 0.0 ± 0.0 *** |
| 500 µg/mL | 0.3 ± 0.2 | 1.2 ± 0.2 * | 0.0 ± 0.0 *** |
| 250 µg/mL | 1.4 ± 0.6 | 1.5 ± 0.1 | 0.0 ± 0.0 *** |
| 100 µg/mL | 2.4 ± 1.2 | 1.8 ± 0.4 | 1.8 ± 0.7 |
| OHR | |||
| 1000 µg/mL | 0.0 ± 0.0 * | 0.0 ± 0.0 *** | 0.0 ± 0.0 *** |
| 500 µg/mL | 0.5 ± 0.1 | 0.0 ± 0.0 *** | 0.0 ± 0.0 *** |
| 250 µg/mL | 1.7 ± 1.4 | 1.4 ± 0.3 | 0.0 ± 0.0 *** |
| 100 µg/mL | 2.7 ± 1.5 | 2.2 ± 0.8 | 2.0 ± 0.7 |
| Maltose Equivalent (mg/g) | |||
|---|---|---|---|
| S. arvensis | R. sativus | L. multiflorum | |
| H2O | 1.44 ± 0.44 | 2.08 ± 0.78 | 10.75 ± 1.96 |
| OHW | |||
| 1000 µg/mL | 0.42 ± 0.06 ** | 1.22 ± 0.38 | 7.28 ± 0.02 ** |
| 500 µg/mL | 0.69 ± 0.10 * | 0.75 ± 0.50 | 8.05 ± 0.39 * |
| 250 µg/mL | 0.67 ± 0.20 * | 0.82 ± 0.54 | 9.64 ± 0.47 |
| 100 µg/mL | 0.87 ± 0.37 | 1.82 ± 0.89 | 9.14 ± 0.51 |
| OHR | |||
| 1000 µg/mL | 0.47 ± 0.02 * | 0.69± 0.17 ** | 7.68 ± 0.64 * |
| 500 µg/mL | 0.34 ± 0.20 * | 0.87 ± 0.38 * | 8.12 ± 0.21 |
| 250 µg/mL | 0.45 ± 0.40 * | 0.71 ± 0.49 ** | 8.08 ± 0.35 |
| 100 µg/mL | 2.28 ± 0.58 | 1.56 ± 0.07 | 8.69 ± 1.73 |
| α-Amylase Activity (µmol min−1 L−1) | |||
|---|---|---|---|
| S. arvensis | R. sativus | L. multiflorum | |
| H2O | 421.0 ± 7.8 | 608.1 ± 5.9 | 3143.2 ±59.7 |
| OHW | |||
| 1000 µg/mL | 122.8 ± 5.6 **** | 356.7 ± 5.2 **** | 212.8 ± 9.7 **** |
| 500 µg/mL | 201.7 ± 3.4 **** | 219.2 ± 6.7 **** | 235.4 ± 8.6 **** |
| 250 µg/mL | 195.9 ± 5.2 **** | 239.7 ± 7.9 **** | 281.8 ± 6.9 **** |
| 100 µg/mL | 254.3 ± 6.7 **** | 532.1 ± 5.1 **** | 267.2 ± 7.8 **** |
| OHR | |||
| 1000 µg/mL | 137.4 ± 3.2 **** | 201.7 ± 4.5 **** | 224.5 ± 9.7 **** |
| 500 µg/mL | 99.0 ± 8.9 **** | 254.3 ± 5.2 **** | 237.4 ± 8.4 **** |
| 250 µg/mL | 131.5 ± 3.2 **** | 207.6 ± 6.4 **** | 236.2 ± 7.3 **** |
| 100 µg/mL | 666.6 ±7.5 **** | 456.1 ± 7.3 **** | 254.1 ± 7.1 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amato, G.; Caputo, L.; Francolino, R.; Martino, M.; De Feo, V.; De Martino, L. Origanum heracleoticum Essential Oils: Chemical Composition, Phytotoxic and Alpha-Amylase Inhibitory Activities. Plants 2023, 12, 866. https://doi.org/10.3390/plants12040866
Amato G, Caputo L, Francolino R, Martino M, De Feo V, De Martino L. Origanum heracleoticum Essential Oils: Chemical Composition, Phytotoxic and Alpha-Amylase Inhibitory Activities. Plants. 2023; 12(4):866. https://doi.org/10.3390/plants12040866
Chicago/Turabian StyleAmato, Giuseppe, Lucia Caputo, Rosaria Francolino, Mara Martino, Vincenzo De Feo, and Laura De Martino. 2023. "Origanum heracleoticum Essential Oils: Chemical Composition, Phytotoxic and Alpha-Amylase Inhibitory Activities" Plants 12, no. 4: 866. https://doi.org/10.3390/plants12040866
APA StyleAmato, G., Caputo, L., Francolino, R., Martino, M., De Feo, V., & De Martino, L. (2023). Origanum heracleoticum Essential Oils: Chemical Composition, Phytotoxic and Alpha-Amylase Inhibitory Activities. Plants, 12(4), 866. https://doi.org/10.3390/plants12040866








