Investigation on Chemical Composition, Antioxidant, Antifungal and Herbicidal Activities of Volatile Constituents from Deverra tortuosa (Desf.)
Abstract
1. Introduction
2. Results and Discussion
2.1. Yield and Chemical Composition
2.2. Antioxidant Activity
2.3. Phytotoxic Activity
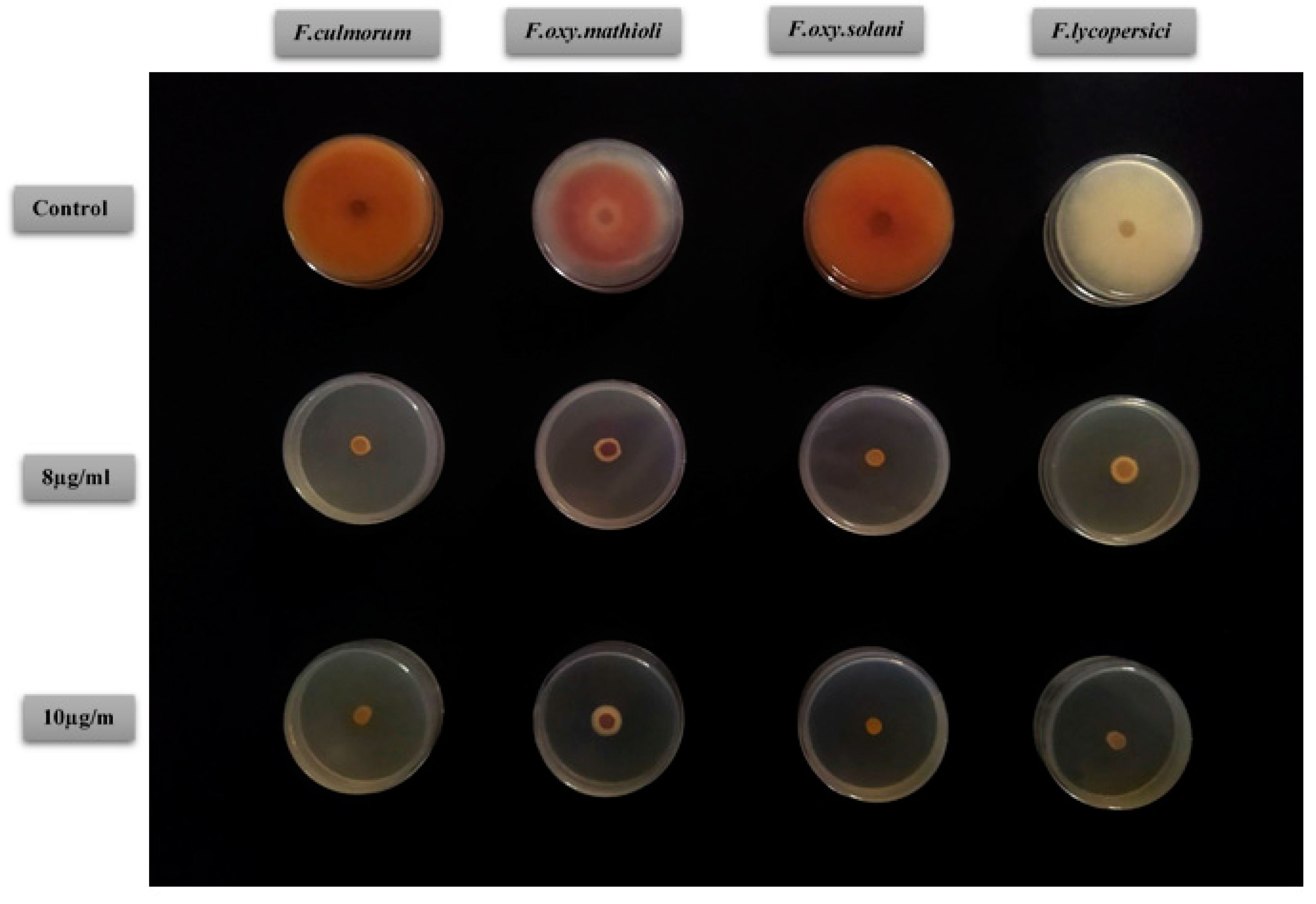
2.4. Antifungal Activity of D. tortuosa EOs
3. Materials and Methods
3.1. Plant Material
3.2. Extraction of the Essential Oils
3.3. Gas Chromatography Analysis with MS Detection
3.4. 2,2 -Diphenyl-1-picrylhydrazyl (DPPH) Assay
3.5. ABTS•+ Free Radical Scavenging Activity
3.6. Herbicidal Activity of Deverra tortuosa Eos
3.7. Antifungal Activity
3.7.1. Fungal Strains
3.7.2. In Vitro Antifungal Activities on Mycelial Growth
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-Derived Pesticides as an Alternative to Pest Management and Sustainable Agricultural Production: Prospects, Applications and Challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef] [PubMed]
- Copping, L.G.; Duke, S.O. Natural products that have been used commercially as crop protection agents. Pest Manag. Sci. 2007, 63, 524–554. [Google Scholar] [CrossRef] [PubMed]
- Kafle, S.; Vaidya, A.; Pradhan, B.; Jørs, E.; Onta, S. Factors Associated with Practice of Chemical Pesticide Use and Acute Poisoning Experienced by Farmers in Chitwan District, Nepal. Int. J. Environ. Res. Public Health 2021, 18, 4194. [Google Scholar] [CrossRef]
- Lamichhane, R.; Lama, N.; Subedi, S.; Singh, S.B.; Sah, R.B.; Yadav, B.K. Use of Pesticides and Health Risk among Farmers in Sunsari District, Nepal. J. Nepal Health Res. Counc. 2019, 17, 66–70. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Rosi, E.J.; Gessner, M.O. Synthetic chemicals as agents of global change. Front. Ecol. Environ. 2017, 15, 84–90. [Google Scholar] [CrossRef]
- Ahmed, H.F.A.; Seleiman, M.F.; Mohamed, I.A.A.; Taha, R.S.; Wasonga, D.O.; Battaglia, M.L. Activity of Essential Oils and Plant Extracts as Biofungicides for Suppression of Soil-Borne Fungi Associated with Root Rot and Wilt of Marigold (Calendula officinalis L.). Horticulturae 2023, 9, 222. [Google Scholar] [CrossRef]
- Wardle, D.A.; Nilsson, M.-C.; Gallet, C.; Zackrisson, O. An Ecosystem-Level Perspective of Allelopathy. Biol. Rev. 1998, 73, 305–319. [Google Scholar] [CrossRef]
- Macías, F.A.; Mejías, F.J.; Molinillo, J.M. Recent Advances in Allelopathy for Weed Control: From Knowledge to Applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef]
- Amri, I.; Mancini, E.; De Martino, L.; Hamrouni, L.; Hanana, M.; Jamoussi, B.; Gargouri, S.; Scognamiglio, M.; De Feo, V. Chemical Composition and Biological Activities of Tunisian Cupressus arizonica Greene Essential Oils. Chem. Biodivers. 2014, 11, 150–160. [Google Scholar] [CrossRef]
- Amri, I.; Lamia, H.; Mohsen, H.; Bassem, J.; Kaouthar, L. Essential oils as biological alternatives to protect date palm (Phoenix dactylifera L.) against Ectomyelois ceratoniae Zeller (Lepidoptera: Pyralidae). Chil. J. Agric. Res. 2014, 74, 273–279. [Google Scholar] [CrossRef]
- Khammassi, M.; Mighri, H.; Ben Mansour, M.; Amri, I.; Jamoussi, B.; Khaldi, A. Metabolite profiling and potential antioxidant activity of sixteen fennel (Foeniculum vulgare Mill.) populations wild-growing in Tunisia. South Afr. J. Bot. 2022, 148, 407–414. [Google Scholar] [CrossRef]
- Khammassi, M.; Khedhri, H.; Mighri, H.; Souihi, M.; Kochti, O.; Emine, S.; Amri, I.; Bassem, J.; Mabrouk, Y. Phytochemical Screening of Essential Oils and Methanol Extract Constituents of Wild Foeniculum vulgare Mill.: A Potential Natural Source for Bioactive Molecules. Chem. Afr. 2023, 6, 1227–1240. [Google Scholar] [CrossRef]
- Ahmad, B.S.; Talou, T.; Saad, Z.; Hijazi, A.; Ahmad, B.S.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O.; Apiaceae, T. The Apiaceae: Ethnomedicinal Family as Source for Industrial Uses. Ind. Crop. Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- Calvino, A.; Federico, E.T.; Downie, S.R. The role of the Southern Hemisphere in the evolutionary history of Apiaceae, a mostly north temperate plant family. J. Biogeogr. 2016, 43, 398–409. [Google Scholar] [CrossRef]
- Burtt, B.L. Umbelliferae of southern Africa: An introduction and annotated check-list. Edinb. J. Bot. 1991, 48, 133–282. [Google Scholar] [CrossRef]
- Van Wyk, B.E.; Tilney, P.M.; Magee, A.R. African Apiaceae: A Synopsis of the Apiaceae Umbelliferae of Sub-saharan Africa and Madagascar; Briza Academic Books: Pretoria, South Africa, 2013. [Google Scholar]
- Van Munster, S.; Magee, A.R.; Zietsman, P.C. Deverra rapaletsa (Apiaceae), a new limestone endemic species from the Ghaap Plateau, northern Cape, South Africa. South Afr. J. Bot. 2019, 121, 431–434. [Google Scholar] [CrossRef]
- Guetat, A. The Genus Deverra DC. (Syn. Pituranthos Viv.): A natural valuable source of bioactive phytochemicals: A review of traditional uses, phytochemistry and pharmacological properties. J. Ethnopharmacol. 2022, 284, 114447. [Google Scholar] [CrossRef]
- Assy, N.; Azazzy, M.; El-Alfy, T. Novel anatomy and DNA fingerprint of Deverra tortuosa (Desf) DC, syns: Pituranthos tortuosus Benth. Nov. Res. Sci. 2019, 1, 1–6. [Google Scholar] [CrossRef]
- El-Mokasabi, F.M. Floristic composition and traditional uses of plant species at Wadi Alkuf, Al-Jabal Al-Akhder, Libya. Am. Eurasian J. Agric. Environ. Sci. 2014, 14, 685–697. [Google Scholar] [CrossRef]
- Dahia, M. Medicinal and Aromatic Plants in the Regions of Djelfa, Boussada and Elmessaila. Study of Gozzah Plant (Pituranthos): Species Level, Phytochemistry and Biological Activities of Essential Oils of Stems. Ph.D. Thesis, Biological Department, Farhat Abbas University Steif, Sétif, Algeria, 2009. [Google Scholar]
- Mighri, H.; Sabri, K.; Eljeni, H.; Neffati, M.; Akrout, A. Chemical composition and antimicrobial activity of Pituranthos chloranthus (Benth.) Hook. and Pituranthos tortuosus (Coss.) Maire essential oils from southern Tunisia. Adv. Biol. Chem. 2015, 5, 273–278. [Google Scholar] [CrossRef]
- Abdel-Mogib, M.; Ayyad, S.N.; Metwally, M.A.; Dawidar, A.M. Lactones from Pituranthos tortusus. Pak. J. Sci. Ind. Res. 1992, 35, 93. [Google Scholar]
- Aloui, L.; Kossentini, M.; Geffroy-Rodier, C.; Guillard, J.; Zouari, S. Phytochemical investigation, isolation and characterization of coumarins from aerial parts and roots of Tunisian Pituranthos chloranthus (Apiaceae). Pharmacogn. Commun. 2015, 5, 237–243. [Google Scholar] [CrossRef]
- Karous, O.; Ben Haj Jilani, I.; Ghrabi-Gammar, Z. Ethnobotanical Study on Plant Used by Semi-Nomad Descendants’ Community in Ouled Dabbeb—Southern Tunisia. Plants 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Krifa, M.; Meshri, S.E.E.; Bentouati, N.; Pizzi, A.; Sick, E.; Chekir-Ghedira, L.; Ronde, P. In vitro and in vivo anti-melanoma effects of Pituranthos tortuosus essential oil via inhibition of FAK and Src activities. J. Cell. Biochem. 2016, 117, 1167–1175. [Google Scholar] [CrossRef]
- Guetat, A.; Boulila, A.; Boussaid, M. Phytochemical profile and biological activities of Deverra tortuosa (Desf.) DC.: A desert aromatic shrub widespread in Northern Region of Saudi Arabia. Nat. Prod. Res. 2019, 33, 2708–2713. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; Ezzat, S.M. Effect of the method of preparation on the composition and cytotoxic activity of the essential oil of Pituranthos tortuosus. Z. Naturforsch. C Biosci. 2011, 66, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Guesmi, F.; Ben Hadj Ahmed, S.; Landoulsi, A. Investigation of extracts from Tunisian ethnomedicinal plants as antioxidants, cytotoxins, and antimicrobials. Biomed. Environ. Sci. 2017, 30, 811–824. [Google Scholar] [CrossRef]
- Abd El-Moaty, H.I.; Nadia, A.S.; Rabab, S.H.; Eman, H.I.; Dina, Y.S.; Mostafa, M.H.K. Comparative therapeutic effects of Pituranthos tortuosus aqueous extract and phyto-synthesized gold nanoparticles on Helicobacter pylori, diabetic and cancer proliferation. South Afr. J. Bot. 2021, 139, 167–174. [Google Scholar] [CrossRef]
- Algandaby, M.M.; Salama, M. Management of the noxious weed, Medicago polymorpha L. via allelopathy of some medicinal plants from Taif region, Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 1339–1347. [Google Scholar] [CrossRef]
- Krifa, M.; Gharad, T.; Haouala, R. Biological activities of essential oil, aqueous and organic extracts of Pituranthos tortuosus (Coss.) Maire. Sci. Hortic. 2011, 128, 61–67. [Google Scholar] [CrossRef]
- Ashkenazy, D.; Eshel, A.; Kashman, Y.; Friedman, J. Morphological and chemical variations in natural populations of Pituranthos triradiatus in the Negev desert. Biochem. Syst. Ecol. 1987, 15, 453–458. [Google Scholar] [CrossRef]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Bouyahya, A. Health beneficial and pharmacological properties of p-cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef]
- Elshibani, F.; Alshalmani, S.; Mohammed, H.A. Pituranthos tortuosus Essential Oil from Libya: Season Effect on the Composition and Antioxidant Activity. J. Essent. Oil Bear. Plants 2020, 23, 1095–1104. [Google Scholar] [CrossRef]
- Aloui, L.; Zouari, S. Chemical Composition and Antioxidant Activities of Essential Oils from Different Organs of Tunisian Pituranthos chloranthus (Apiaceae). J. Essent. Oil Bear. Plants 2019, 22, 649–659. [Google Scholar] [CrossRef]
- Abdelwahed, A.; Hayder, N.; Kilani, S.; Mahmoud, A.; Chibani, J.; Hammami, M.; Chekir-Ghedira, L.; Ghedira, K. Chemical composition and antimicrobial activity of essential oils from Tunisian Pituranthos tortuosus (Coss.) Maire. Flavour Fragr. J. 2006, 21, 129–133. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Co.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Khan, M.; Abdullah, M.M.S.; Mousa, A.A.; Hamad, Z.A. Chemical composition of vegetative parts and flowers essential oils of wild Anvillea garcinii grown in Saudi Arabia. Rec. Nat. Prod. 2016, 10, 251. [Google Scholar]
- Khan, M.; Al-Saleem, M.S.; Alkhathlan, H.Z. A detailed study on chemical characterization of essential oil components of two Plectranthus species grown in Saudi Arabia. J. Saudi Chem. Soc. 2016, 20, 711–721. [Google Scholar] [CrossRef]
- Khan, M.; Al-Mansour, M.A.; Mousa, A.A.; Alkhathlan, H.Z. Compositional Characteristics of the Essential Oil of Myrtus communis Grown in the Central Part of Saudi Arabia. J. Essent. Oil Res. 2014, 26, 13–18. [Google Scholar] [CrossRef]
- Kummer, R.; Estevao-Silva, C.F.; Bastos, R.L.; Grespan, R.; De Souza, S.; Comar, F.M.; Spironello, R.A.; Rocha, B.A.; Silva, E.L.; Bersani-Amado, C.A.; et al. Effect of p-cymene on chemotaxis, phagocytosis and leukocyte behaviors. Int. J. Appl. Res. Nat. Prod. 2008, 8, 20–27. [Google Scholar]
- Chen, H.C.; Tsai, Y.J.; Lin, L.Y.; Wu, C.S.; Tai, S.P.; Chen, Y.C.; Chiang, H.M. Volatile Compounds from Roots, Stems and Leaves of Angelica Acutiloba Growing in Taiwan. Nat. Prod. Commun. 2014, 9, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-R.; Yu, Y.; Zulfajri, M.; Lin, P.-C.; Wang, C.C. Phthalide Derivatives from Angelica Sinensis Decrease Hemoglobin Oxygen Affinity: A New Allosteric-Modulating Mechanism and Potential Use as 2,3-BPG Functional Substitutes. Sci. Rep. 2017, 7, 5504. [Google Scholar] [CrossRef] [PubMed]
- Baananou, S.; Piras, A.; Marongiu, B.; Dessì, M.A.; Falconieri, D.; Porcedda, S.; Rosa, A.; Boughattas, N.A. Antiulcerogenic Activity of Apium Graveolens Seeds Oils Isolated by Supercritical CO2. Afr. J. Pharm. 2012, 6, 752–762. [Google Scholar] [CrossRef]
- Chae, S.-H.; Kim, S.-I.; Yeon, S.-H.; Lee, S.-W.; Ahn, Y.-J. Adulticidal Activity of Phthalides Identified in Cnidium Officinale Rhizome to B- and Q-Biotypes of Bemisia Tabaci. J. Agric. Food Chem. 2011, 59, 8193–8198. [Google Scholar] [CrossRef] [PubMed]
- Al-Gaby, A.M.; Allam, R.R. Chemical Analysis, Antimicrobial Activity, and the Essential Oils from Some Wild Herbs in Egypt. J. Herbs Spices Med. Plants 2000, 7, 15–23. [Google Scholar] [CrossRef]
- Junghyun, R.; Hyerim, L.; Seungwon, S. Biological Activities of the Essential Oil from Angelica acutiloba. Nat. Prod. Sci. 2012, 18, 244–249. [Google Scholar]
- Stanojević, J.; Ilić, Z.S.; Stanojević, L.; Milenković, L.; Kovač, R.; Lalević, D.; Šunić, L.; Milenković, A.; Cvetković, D. Essential Oil Yield, Composition, and Antioxidant Activity in Two Umbel Maturity Stages of Wild Carrot (Daucus carota L. ssp. carota) from Montenegro. Horticulturae 2023, 9, 328. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and Antioxidant Activity of Essential Oil Terpenes against Pathogenic and Spoilage-Forming Bacteria and Cell Structure-Activity Relationships Evaluated by SEM Microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef]
- Farukh, S.; Sharopova, M.; William, N.S. Radical Scavenging and Antioxidant Activities of Essential Oil Components—An Experimental and Computational Investigation. Nat. Prod. Commun. 2015, 10, 153–156. [Google Scholar] [CrossRef]
- De Martino, L.; Mancini, E.; Rolim de Almeida, L.F.; De Feo, V. The Antigerminative Activity of Twenty-Seven Monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef]
- Amri, I.; Khammassi, M.; Ben Ayed, R.; Khedhri, S.; Mansour, M.B.; Kochti, O.; Pieracci, Y.; Flamini, G.; Mabrouk, Y.; Gargouri, S.; et al. Essential Oils and Biological Activities of Eucalyptus falcata, E. sideroxylon and E. citriodora Growing in Tunisia. Plants 2023, 12, 816. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Arora, K.; Kohli, R.K. α–Pinene inhibits growth and induces oxidative stress in roots. Ann. Bot. 2006, 98, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Abrahim, D.; Braguini, W.L.; Kelmer–Bracht, A.M.; Ishii–Iwamoto, E.L. Effects of four monoterpenes on germination, primary root growth, and mitochondrial respiration of maize. J. Chem. Ecol. 2000, 26, 611–624. [Google Scholar] [CrossRef]
- Chowhan, N.; Singh, H.P.; Batish, D.R. Phytotoxic effects of β-pinene on early growth and associated biochemical changes in rice. Acta Physiol. Plant. 2011, 33, 2369–2376. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, C.; Zhang, C.; Han, C.; Kuchkarova, N.; Shao, H. Chemical Composition, Phytotoxic, Antimicrobial and Insecticidal Activity of the Essential Oils of Dracocephalum integrifolium. Toxins 2019, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Andrianjafinandrasana, S.N.; Andrianoelisoa, H.S.; Jeanson, M.L.; Ratsimiala, R.I.; Danthu, P. Allelopathic effects of volatile compounds of essential oil from Ravensara aromatica Sonnerat chemotypes. Allelopath. J. 2013, 31, 333–344. [Google Scholar]
- Park, B.I.; Kim, B.S.; Kim, K.J.; You, Y.O. Sabinene suppresses growth, biofilm formation, and adhesion of Streptococcus mutans by inhibiting cariogenic virulence factors. J. Oral Microbiol.. 2019, 11, 1632101. [Google Scholar] [CrossRef]
- Slim, Y.; Shin, S. Combinatorial Anti-Trichophyton Effects of Ligusticum Chuanxiong Essential Oil Components with Antibiotics. Arch. Pharm. Res. 2008, 31, 497–502. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, W.; Huang, X.; Hao, L.; Li, Y.; Sun, S. Antifungal Activity and Potential Mechanism of N-Butylphthalide Alone and in Combination With Fluconazole Against Candida Albicans. Front. Microbiol. 2019, 10, 1461. [Google Scholar] [CrossRef]
- Gach, J.; Olejniczak, T.; Krężel, P.; Boratyński, F. Microbial Synthesis and Evaluation of Fungistatic Activity of 3-Butyl-3-hydroxyphthalide, the Mammalian Metabolite of 3-n-Butylidenephthalide. Int. J. Mol. Sci. 2021, 22, 7600. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. NIST/EPA/NIH Mass Spectral Library; The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014.
- Ud-Daula, A.; Demirci, F.; Salim, K.A.; Demirci, B.; Lim, L.B.L.; Bber, K.H.; Ahmad, N. Chemical composition, antioxidant and antimicrobial activities of essential oils from leaves, aerial stems, basal stems, and rhizomes of Etlingera fimbriobracteata (K.Schum.) R.M.Sm. Ind. Crop. Prod. 2016, 84, 189–198. [Google Scholar] [CrossRef]
- Amri, I.; De Martino, L.; Marandino, A.; Hamrouni, L.; Hanana, M.; Scandolera, E.; De Feo, V.; Mancini, E. Chemical composition and biological activities of the essential oil from Artemisia herba-alba growing wild in Tunisia. Nat. Prod. Commun. 2013, 8, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Bouabidi, W.; Hanana, M.; Gargouri, S.; Amri, I.; Fezzani, T.; Ksontini, M.; Jamoussi, B.; Hamrouni, L. Chemical composition, phytotoxic and antifungal properties of Ruta chalepensis L. essential oils. Nat. Prod. Res. 2015, 29, 864–868. [Google Scholar] [CrossRef] [PubMed]

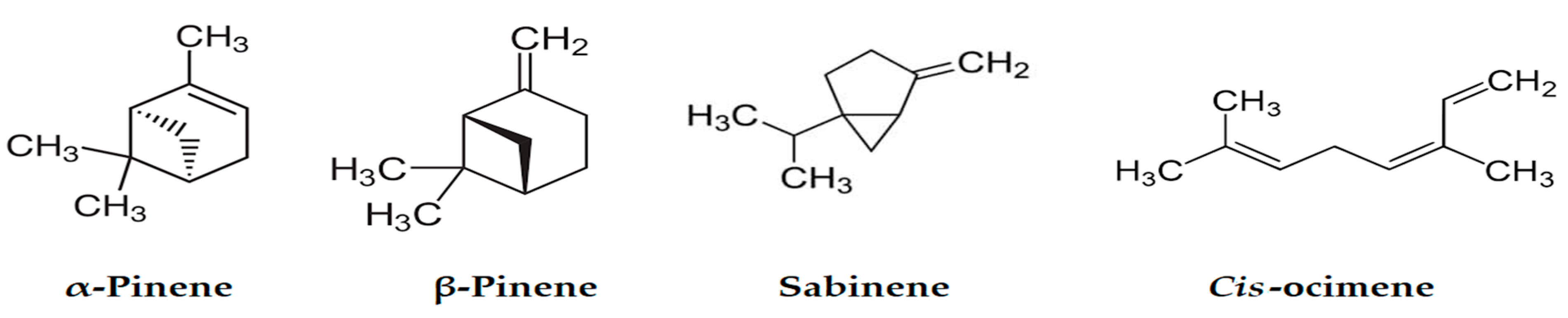
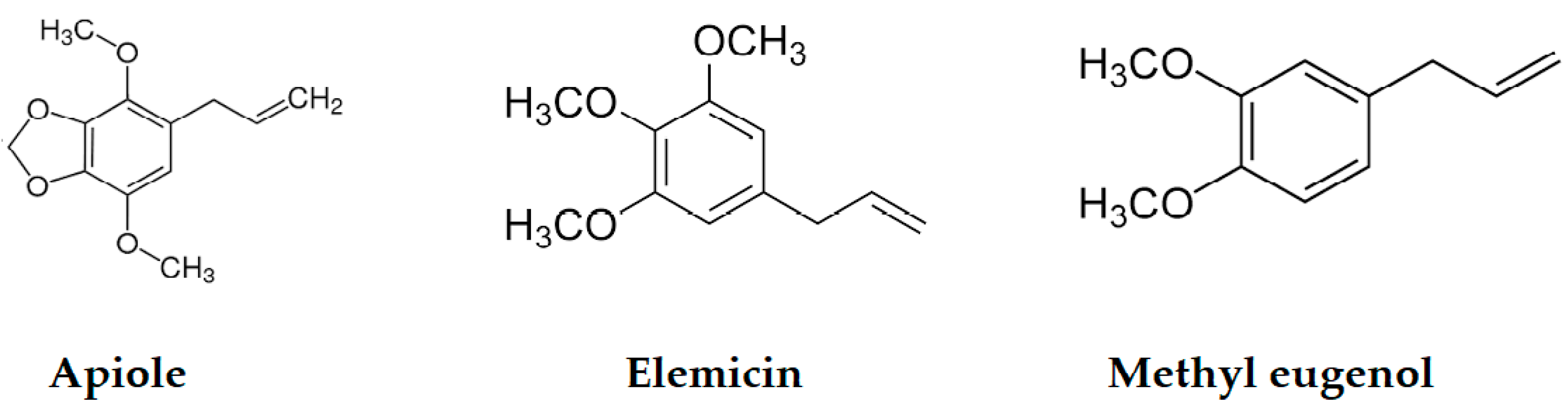
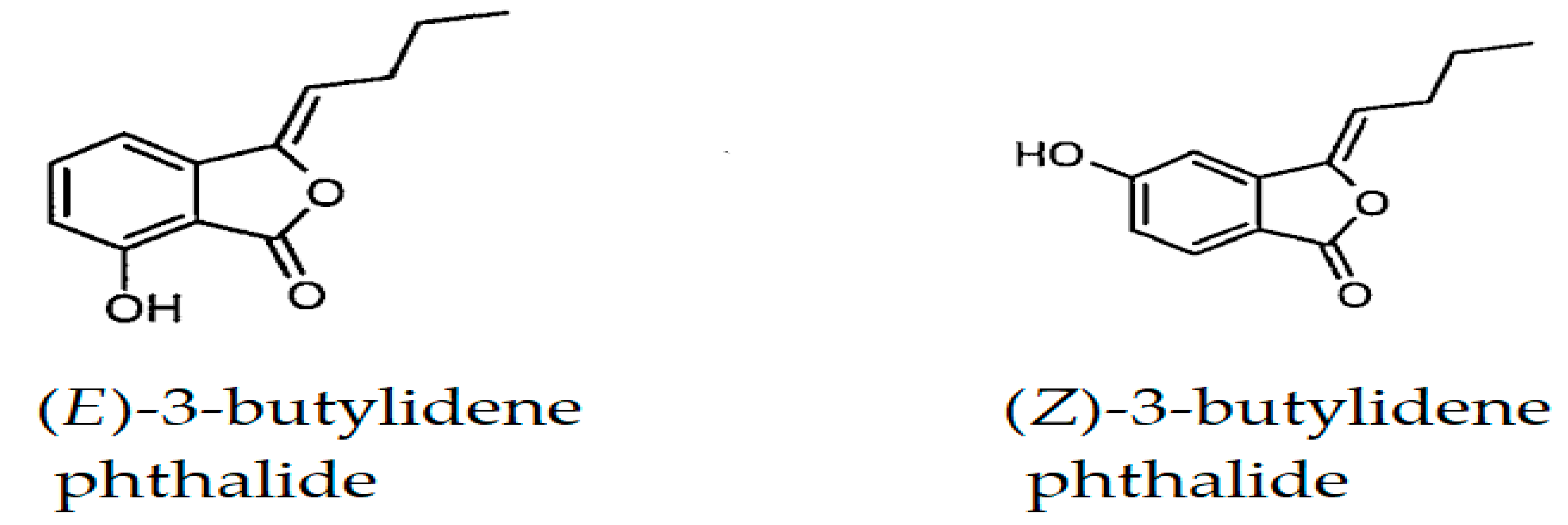



| Compound a | LRI Exp b | LRI Lit c [38,39,40,41] | Area% | Identification d | |
|---|---|---|---|---|---|
| Umbels | Stems | ||||
| α-Thujene | 926 | 930 | 4.20 | 2.37 | MS, RI |
| α-Pinene | 935 | 939 | 24.2 | 28.88 | MS, RI, Inj |
| Sabinene | 971 | 975 | 16.23 | 18.67 | MS, RI, Inj |
| β-Pinene | 976 | 979 | 5.10 | 6.20 | MS, RI |
| β-Myrcene | 992 | 990 | 2.64 | 2.50 | MS, RI |
| α-Phellandrene | 1003 | 1002 | 11.74 | 6.30 | MS, RI, Inj |
| α-Terpinene | 1014 | 1017 | 1.07 | 1.42 | MS, RI |
| p-Cymene | 1021 | 1024 | - | 6.77 | MS, RI |
| β-Phellandrene | 1027 | 1029 | 6.70 | - | MS, RI, Inj |
| cis-Ocimene | 1034 | 1037 | 7.85 | 5.28 | MS, RI |
| γ-Terpinene | 1057 | 1059 | 1.33 | 2.17 | MS, RI |
| cis-Sabinene hydrate | 1073 | 1070 | - | 0.27 | MS, RI |
| α-Terpinolene | 1085 | 1088 | 0.35 | 0.49 | MS, RI, Inj |
| neo-allo-Ocimene | 1146 | 1144 | 0.43 | - | MS, RI |
| trans-Verbenol | 1147 | 1144 | 0.31 | MS, RI | |
| Terpinen-4-ol | 1174 | 1177 | 3.25 | 6.74 | MS, RI |
| α-Copaene | 1372 | 1376 | 0.18 | - | MS, RI |
| Methyl eugenol | 1406 | 1403 | 0.55 | 0.81 | MS, RI, Inj |
| Germacrene D | 1480 | 1485 | 1.99 | 0.77 | MS, RI |
| Bicyclogermacrene | 1502 | 1500 | 1.72 | 0.58 | MS, RI |
| δ-Cadinene | 1525 | 1523 | 0.61 | 0.45 | MS, RI |
| Elemicin | 1554 | 1557 | 0.36 | - | MS, RI |
| Spathulenol | 1573 | 1578 | 2.56 | 3.74 | MS, RI |
| β-Eudesmol | 1651 | 1650 | 0.65 | 0.73 | MS, RI |
| α-Vadinol | 1656 | 1654 | 0.96 | 1.14 | MS, RI |
| (E)-3-Butylidene phthalide | 1675 | 1672 | 0.29 | - | MS, RI |
| Apiole | 1680 | 1678 | 0.55 | - | MS, RI |
| (Z)-3-Butylidene phthalide | 1721 | 1718 | 3.67 | 0.86 | MS, RI |
| Total | 99.49 | 97.14 | |||
| Monoterpene hydrocarbons | 81.84 | 81.05 | |||
| Oxygenated monoterpenes | 3.56 | 7.01 | |||
| Sesquiterpene hydrocarbons | 4.5 | 1.8 | |||
| Oxygenated sesquiterpenes | 4.17 | 5.61 | |||
| Phenylpropanoids | 1.46 | 0.81 | |||
| Phthalide derivatives | 3.96 | 0.86 | |||
| Tunisia [22] | Tunisia [37] | Tunisia [36] | Lybia [35] | Algeria [21] | Egypt [34] | Egypt [47] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh Plant | Dry Plant | November | April | Stems | Seeds | Summer | Spring | Aerial Parts | Aerial Parts | Aerial Parts | |||
| Origin 1 | Origin 2 | Origin 1 | Origin 2 | ||||||||||
| α-Thujene | - | - | 1.2 | 0.6 | 2.8 | 1.5 | 2.1 | 1.9 | 6.64 | 21.22 | 1.2 | 0.44 | |
| α-Pinene | 6.2 | 5.1 | 5.5 | 1 | 11.0 | 8.9 | 32.2 | 37.7 | - | 3.30 | 11.4 | - | 15 |
| α-Fenchene | - | - | 0.06 | - | - | - | - | - | - | 8.45 | - | - | |
| Camphene | - | - | 0.06 | - | - | 0.2 | 0.3 | 0.5 | - | - | 0.2 | - | 33 |
| Sabinene | 38.9 | 35.8 | 11.0 | - | - | 2.4 | 4.1 | 3.2 | - | 2.04 | 6.4 | 18.49 | |
| β-Pinene | 1.8 | 2.1 | 1.7 | - | - | 6.5 | 27.8 | 30.7 | - | - | 3.9 | - | |
| β-Myrcene | 8.9 | 12.5 | 1.1 | - | - | 6.9 | 3.4 | 3.2 | - | 1.30 | 1 | 18.81 | |
| 1,8-Cineole | - | - | - | - | - | - | - | - | 13.43 | 4.42 | - | - | 15.2 |
| Linalool | - | - | - | - | - | 0.2 | - | 0.3 | - | 27.9 | - | - | |
| α -Phellandrene | 0.8 | 1.8 | 0.9 | - | - | 7.2 | - | 0.6 | - | - | 8.3 | - | |
| Δ-3-carene | 2.9 | 1.9 | 0.2 | 0.08 | - | - | 7.8 | 3.0 | - | - | 0.1 | - | |
| α-Terpinene | - | - | 0.7 | 0.6 | 0.7 | 0.3 | 5.9 | 0.3 | - | - | 0.2 | - | |
| p-Cymene | 4.9 | 8.8 | 7.0 | 2.2 | 5.8 | 8.7 | 0.9 | 1.3 | - | - | 2.6 | 1.17 | |
| Limonene | 4.3 | 5.0 | 10.9 | 4.1 | - | - | - | - | - | 15.8 | - | ||
| β-Phellandrene | 1.3 | 1 | - | - | 17.2 | - | - | - | - | - | 3.2 | - | |
| cis-Ocimene | 5.6 | 6.6 | 1.9 | 0.7 | 0.7 | 0.6 | 0.8 | 1.4 | - | - | 0.4 | 1.53 | |
| Borneol | - | 16.6 | |||||||||||
| Myrtenol | - | - | 26.2 | 0.3 | - | - | - | - | - | 0.38 | - | - | |
| Terpinen-4-ol | - | - | 1.8 | 39.6 | 1.7 | 1.1 | 0.2 | 1.2 | - | - | 0.9 | 8.09 | |
| Cubebene | - | - | - | - | - | - | - | - | 2.62 | 10.9 | 0.1 | - | |
| Aromadendrene | - | - | 0.2 | 0.2 | - | - | - | - | 6.25 | 8.41 | 0.1 | - | |
| Myristicin | 27.4 | 0.89 | |||||||||||
| β-Caryophyllene | - | - | - | - | - | - | - | - | 11.62 | - | - | ||
| Elemicin | - | - | - | - | - | - | - | - | - | - | - | 12.9 | |
| α.-Cadinol | tr | 0.7 | - | - | - | - | - | - | 21.51 | - | 0.2 | - | |
| (Z)-3-Butylidene phthalide | 5.0 | 1.5 | 2.4 | 4.1 | - | - | - | - | - | - | - | - | |
| (E)-3-Butylidene phthalide | - | - | 5.9 | 11.4 | - | - | - | - | - | - | - | - | |
| Doses | % Germination | |||
|---|---|---|---|---|
| (mg/mL) | Stem EOs | Umbel EOs | Glyphosate | |
| T. campestre | 0 | 100 ± 0 A | 100 ± 0 A | 100 ± 0 A |
| 1 | 100 ± 0 Aa | 100 ± 0 Aa | 100 ± 0 Aa | |
| 2 | 66.66 ± 15.27 Ba | 73.33 ± 5.77 Ba | 63.33 ± 5.77 Bb | |
| 3 | 0 ± 0 Cc | 33.33 ± 5.77 Cb | 43.33 ± 5.77 Ca | |
| 4 | 0 ± 0 Cb | 20 ± 0 Da | 23.33 ± 5.77 Da | |
| L. sativum | 0 | 100 ± 0 A | 100 ± 0 A | 100 ± 0 A |
| 1 | 90 ± 10 Ba | 96.66 ± 5.77 Ba | 90 ± 0 Ba | |
| 2 | 53.33 ± 5.77 Cb | 23.33 ± 5.77 Cc | 80 ± 10 Ca | |
| 3 | 0 ± 0 Db | 0 ± 0 Db | 33.33 ± 10 Da | |
| 4 | 0 ± 0 Da | 0 ± 0 Da | 0 ± 0 Ea | |
| L. rigidum | 0 | 96.66 ± 5.77 A | 96.66 ± 5.77 A | 96.66 ± 5.77 A |
| 1 | 50 ± 10 Ba | 33.33 ± 5.77 Bb | 13.33 ± 5.77 Bc | |
| 2 | 0 ± 0 Ca | 0 ± 0 Ca | 0 ± 0 Ca | |
| 3 | 0 ± 0 Ca | 0 ± 0 Ca | 0 ± 0 Ca | |
| 4 | 0 ± 0 Ca | 0 ± 0 Ca | 0 ± 0 Ca | |
| S. arvensis | 0 | 86.66 ± 15.27 A | 86.66 ± 15.27 A | 86.66 ± 15.27 A |
| 1 | 30 ± 10 Ba | 23.33 ± 5.77 Bb | 13.33 ± 5.77 Bc | |
| 2 | 0 ± 0 Ca | 0 ± 0 Ca | 0 ± 0 Ca | |
| 3 | 0 ± 0 Ca | 0 ± 0 Ca | 0 ± 0 Ca | |
| 4 | 0 ± 0 Ca | 0 ± 0 Ca | 0 ± 0 Ca | |
| Herbs | Doses (mg/mL) | Shoot Length (cm) | ||
|---|---|---|---|---|
| Stem EOs | Umbel EOs | Glyphosate | ||
| L. sativum | 0 | 2.08 ± 0.07 Aa | 2.08 ± 0.07 Aa | 2.08 ± 0.07 Aa |
| 1 | 1.19 ± 0.09 ABa | 1.25 ± 0.06 ABa | 2.29 ± 0.11 Ab | |
| 2 | 0.8 ± 0.47 ABa | 0.58 ± 0.07 ABa | 0.26 ± 0.02 Ba | |
| 3 | 0 ± 0 Ba | 0 ± 0 Ba | 0 ± 0 Ba | |
| 4 | 0 ± 0 Ba | 0 ± 0 Ba | 0 ± 0 Ba | |
| L. rigidum | 0 | 3.76 ± 0.9 Aa | 3.76 ± 0.9 Aa | 3.76 ± 0.9 Aa |
| 1 | 1.02 ± 0.07 Ba | 0.86 ± 0.11 Bab | 0.76 ± 0.11 Bb | |
| 2 | 0 ± 0 Ba | 0 ± 0 Ba | 0 ± 0 Ba | |
| 3 | 0 ± 0 Ba | 0 ± 0 Ba | 0 ± 0 Ba | |
| 4 | 0 ± 0 Ba | 0 ± 0 Ba | 0 ± 0 Ba | |
| S. arvensis | 0 | 1.72 ± 0.28 Aa | 1.72 ± 0.28 Aa | 1.72 ± 0.28 Aa |
| 1 | 0 ± 0 Ba | 0 ± 0 Ba | 1.7 ± 0.05 Ab | |
| 2 | 0 ± 0 Ba | 0 ± 0 Ba | 0.28 ± 0.01 Bb | |
| 3 | 0 ± 0 Ba | 0 ± 0 Ba | 0 ± 0 Ba | |
| 4 | 0 ± 0 Ba | 0 ± 0 Ba | 0 ± 0 Ba | |
| T. campestre | 0 | 1.4 ± 0.93 Aa | 1.4 ± 0.93 Aa | 1.4 ± 0.93 Aa |
| 1 | 0.7 ± 0.08 ABa | 0.77 ± 0.07 ABa | 1.3 ± 0.08 Ab | |
| 2 | 0.54 ± 0.05 ABa | 0.52 ± 0.1 ABa | 0.31 ± 0.1 Ba | |
| 3 | 0 ± 0 Ba | 0.5 ± 0 ABb | 0.28 ± 0.01 Bc | |
| 4 | 0 ± 0 Ba | 0.25 ± 0.08 Ba | 0 ± 0 Ba | |
| Herbs | Doses (mg/mL) | Roots Length in cm | ||
|---|---|---|---|---|
| Stem EOs | Umbel EOs | Glyphosate | ||
| L.sativum | 0 | 5.31 ± 1.08 Aa | 5.31 ± 1.08 Aa | 5.31 ± 1.08 Aa |
| 1 | 3.7 ± 0.3 ABa | 1.25 ± 0.23 ABc | 2.57 ± 0.41 ABb | |
| 2 | 0.95 ± 0.14 Ba | 0.61 ± 0.12 Bb | 0.66 ± 0.02 Bb | |
| 3 | 0 ± 0 Bb | 0 ± 0 Bb | 0.35 ± 0.06 Ba | |
| 4 | 0 ± 0 Ba | 0 ± 0 Ba | 0 ± 0 Ba | |
| L.rigidum | 0 | 3.49 ± 1.6 Aa | 3.49 ± 1.6 Aa | 3.49 ± 1.6 Aa |
| 1 | 1.3 ± 0.2 Bbc | 0.94 ± 0.10 Ba | 1.68 ± 0.43 Bc | |
| 2 | 0 ± 0 Ba | 0 ± 0 Ba | 0 ± 0 Ba | |
| 3 | 0 ± 0 Ba | 0 ± 0 Ba | 0 ± 0 Ba | |
| 4 | 0 ± 0 Ba | 0 ± 0 Ba | 0 ± 0 Ba | |
| S. arvensis | 0 | 2.83 ± 1.19 Aa | 2.83 ± 1.19 Aa | 2.83 ± 1.19 Aa |
| 1 | 0 ± 0 Bb | 0 ± 0 Bb | 0.43 ± 0.06 Ba | |
| 2 | 0 ± 0 Bb | 0 ± 0 Bb | 0.29 ± 0.01 Ba | |
| 3 | 0 ± 0 Ba | 0 ± 0 Ba | 0 ± 0 Ba | |
| 4 | 0 ± 0 Ba | 0 ± 0 Ba | 0 ± 0 Ba | |
| T. campestre | 0 | 4.25 ± 0.64 Aa | 4.25 ± 0.64 Aa | 4.25 ± 0.64 Aa |
| 1 | 1.32 ± 0.08 ABb | 0.94 ± 0.07 Bc | 2.85 ± 0.08 Aa | |
| 2 | 0.49 ± 0.7 Ba | 0.45 ± 0.18 Ba | 0.54 ± 0.11 Ba | |
| 3 | 0 ± 0 Bb | 0.5 ± 0 Ba | 0.38 ± 0.02 Ba | |
| 4 | 0 ± 0 Ba | 0.33 ± 0.08 Ba | 0 ± 0 Ba | |
| Fungal Strains Growth Inhibition (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| F. culmorum | F. oxysporum mathioli | F. oxysporum solani | F. lycopersici | |||||
| Doses | Umbels | Stems | Umbels | Stems | Umbels | Stems | Umbels | Stems |
| 6 mg/mL | 94.55 a ± 2.35 | 71.42 a ± 7.06 | 86.99 a ± 2.81 | 43.08 b ± 10.15 | 91.83 b ± 4.08 | 75.51 b ± 4.08 | 82.22 c ± 4.44 | 49.62 b ± 2.56 |
| 8 mg/mL | 95.91 a ± 4.08 | 76.87 a ± 2.35 | 91.86 a ± 2.81 | 57.72 ab ± 7.45 | 100 ± 0.0 a | 83.67 a ± 4.08 | 92.59 b ± 2.56 | 55.55 ab ± 4.44 |
| 10 mg/mL | 100 ± 0.0 a | 83.67 a ± 4.08 | 95.12 a ± 4.87 | 62.6 a ± 2.81 | 100 ± 0.0 a | 87.75 a ± 4.08 | 100 ± 0.0 a | 60 a ± 4.44 |
| MIC (mg/mL) | 10 | >10 | >10 | >10 | 8 | >10 | 10 | >10 |
| Collected Species | Used Parts | Preserved Specimens | Date of Harvest | Origins |
|---|---|---|---|---|
| Deverra tortuosa | Umbels | DIJ0122 | September 2022 | Djerissa, Kef |
| Stems | DSJ0122 | September 2022 | Djerissa, Kef | |
| Lepidium sativum L. | Seeds | LS22 | June 2022 | Kalâat el-Andalous, Ariana |
| Lolium rigidum Gaudin | LR22 | July 2022 | Sidi ismail, Beja | |
| Sinapis arvensis L. | SA22 | July 2022 | Sidi ismail, Beja | |
| Trifolium campestre Schreb. | TC22 | July 2022 | Sidi ismail, Beja |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khammassi, M.; Polito, F.; Kochti, O.; Kouki, H.; Souihi, M.; Khedhri, S.; Hamrouni, L.; Mabrouk, Y.; Amri, I.; De Feo, V. Investigation on Chemical Composition, Antioxidant, Antifungal and Herbicidal Activities of Volatile Constituents from Deverra tortuosa (Desf.). Plants 2023, 12, 2556. https://doi.org/10.3390/plants12132556
Khammassi M, Polito F, Kochti O, Kouki H, Souihi M, Khedhri S, Hamrouni L, Mabrouk Y, Amri I, De Feo V. Investigation on Chemical Composition, Antioxidant, Antifungal and Herbicidal Activities of Volatile Constituents from Deverra tortuosa (Desf.). Plants. 2023; 12(13):2556. https://doi.org/10.3390/plants12132556
Chicago/Turabian StyleKhammassi, Marwa, Flavio Polito, Oumayma Kochti, Habiba Kouki, Mouna Souihi, Sana Khedhri, Lamia Hamrouni, Yassine Mabrouk, Ismail Amri, and Vincenzo De Feo. 2023. "Investigation on Chemical Composition, Antioxidant, Antifungal and Herbicidal Activities of Volatile Constituents from Deverra tortuosa (Desf.)" Plants 12, no. 13: 2556. https://doi.org/10.3390/plants12132556
APA StyleKhammassi, M., Polito, F., Kochti, O., Kouki, H., Souihi, M., Khedhri, S., Hamrouni, L., Mabrouk, Y., Amri, I., & De Feo, V. (2023). Investigation on Chemical Composition, Antioxidant, Antifungal and Herbicidal Activities of Volatile Constituents from Deverra tortuosa (Desf.). Plants, 12(13), 2556. https://doi.org/10.3390/plants12132556








