Effects of Biochar on the Fluorescence Spectra of Water-Soluble Organic Matter in Black Soil Profile after Application for Six Years
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Design Patterns
2.3. Measurements
2.3.1. DOM Extraction
2.3.2. Ultraviolet-Visible (UV-Vis) Spectroscopy and Fluorescence Spectroscopy
2.3.3. Parallel Factors (PARAFAC) Modeling
2.3.4. Statistical Analysis
3. Results
3.1. The Fluorescence Properties (2D) of WSOM at Different Soil Depths
3.2. Excitation–Emission Matrices (EEMs) and Fluorescence Characteristics (3D) of DOM
3.3. Identification of WSOC Components and Their Correlations with Each Other
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, J.N.; Gross, C.D.; Dwivedi, P.; Myers, T.; Santos, F.; Bernardi, R.; de Faria, M.F.; Guerrini, I.A.; Harrison, R.; Butman, D. Land use change alters the radiocarbon age and composition of soil and water-soluble organic matter in the Brazilian Cerrado. Geoderma 2019, 345, 38–50. [Google Scholar] [CrossRef]
- Wilson, H.F.; Xenopoulos, M.A. Effects of agricultural land use on the composition of fluvial dissolved organic matter. Nat. Geosci. 2009, 2, 37–41. [Google Scholar] [CrossRef]
- Gabor, R.S.; Burns, M.A.; Lee, R.H.; Elg, J.B.; Kemper, C.J.; Barnard, H.R.; McKnight, D.M. Influence of leaching solution and catchment location on the fluorescence of water-soluble organic matter. Environ. Sci. Technol. 2015, 49, 4425–4432. [Google Scholar] [CrossRef]
- Caricasole, P.; Hatcher, P.G.; Ohno, T. Biodegradation of crop residue-derived organic matter is influenced by its heteroatomic stoichiometry and molecular composition. Appl. Soil Ecol. 2018, 130, 21–25. [Google Scholar] [CrossRef]
- Duarte, R.M.; Piñeiro-Iglesias, M.; López-Mahía, P.; Muniategui-Lorenzo, S.; Moreda-Piñeiro, J.; Silva, A.M.; Duarte, A.C. Comparative study of atmospheric water-soluble organic aerosols composition in contrasting suburban environments in the Iberian Peninsula Coast. Sci. Total Environ. 2019, 648, 430–441. [Google Scholar] [CrossRef]
- Herencia, J.F.; Ruiz, J.C.; Melero, S.; Galavís, P.G.; Maqueda, C. A short-term comparison of organic v. conventional agriculture in a silty loam soil using two organic amendments. J. Agric. Sci. 2008, 146, 677–687. [Google Scholar] [CrossRef]
- Jiang, S.; Dai, G.; Liu, Z.; He, T.; Zhong, J.; Ma, Y.; Shu, Y. Field-scale fluorescence fingerprints of biochar-derived dissolved organic matter (DOM) provide an effective way to trace biochar migration and the downward co-migration of Pb, Cu and As in soil. Chemosphere 2022, 301, 134738. [Google Scholar] [CrossRef]
- Fan, Q.; Sun, J.; Quan, G.; Yan, J.; Gao, J.; Zou, X.; Cui, L. Insights into the effects of long-term biochar loading on water-soluble organic matter in soil: Implications for the vertical co-migration of heavy metals. Environ. Int. 2020, 136, 105439. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Ok, Y.S.; El-Naggar, A.; Kim, H.; Song, F.; Kang, S.; Tsang, Y.F. Dissolved organic matter characterization of biochars produced from different feedstock materials. J. Environ. Manag. 2019, 233, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Bian, R.; Joseph, S.; Shi WLi, L.; Taherymoosavi, S.; Pan, G. Biochar DOM for plant promotion but not residual biochar for metal immobilization depended on pyrolysis temperature. Sci. Total Environ. 2019, 662, 571–580. [Google Scholar] [CrossRef]
- Lee, M.H.; Ok, Y.S.; Hur, J. Dynamic variations in dissolved organic matter and the precursors of disinfection by-products leached from biochars: Leaching experiments simulating intermittent rain events. Environ. Pollut. 2018, 242, 1912–1920. [Google Scholar] [CrossRef]
- Rombolà, A.G.; Torri, C.; Vassura, I.; Venturini, E.; Reggiani, R.; Fabbri, D. Effect of biochar amendment on organic matter and dissolved organic matter composition of agricultural soils from a two-year field experiment. Science of The Total Environment 2022, 812, 151422. [Google Scholar] [CrossRef]
- Kasozi, G.N.; Zimmerman, A.R.; Nkedi-Kizza, P.; Gao, B. Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars). Environ. Sci. Technol. 2010, 44, 6189–6195. [Google Scholar] [CrossRef]
- Kraska, P.; Oleszczuk, P.; Andruszczak, S.; Kwiecińska-Poppe, E.; Różyło, K.; Pałys, E. Effect of various biochar rates on winter rye yield and the concentration of available nutrients in the soil. Plant Soil Environ. 2016, 62, 483–489. [Google Scholar] [CrossRef]
- Zhang, A.; Zhou, X.; Li, M.; Wu, H. Impacts of biochar addition on soil dissolved organic matter characteristics in a wheat-maize rotation system in Loess Plateau of China. Chemosphere 2017, 186, 986–993. [Google Scholar] [CrossRef]
- Novotny, E.H.; Maia, C.M.B.D.F.; Carvalho, M.T.D.M.; Madari, B.E. Biochar: Pyrogenic carbon for agricultural use-a critical review. Rev. Bras. De Ciência Do Solo 2015, 39, 321–344. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, P.; Huang, L.; Min, H.; Sun, H. Sorption and molecular fractionation of biochar-derived dissolved organic matter on ferrihydrite. J. Hazard. Mater. 2020, 392, 122260. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Niu, C.; Jiang, J.; Sun, H. Positive Effects of Biochar on the Degraded Forest Soil and Tree Growth in China: A Systematic Review. Phyton-Int. J. Exp. Bot. 2022, 91, 1601–1616. [Google Scholar] [CrossRef]
- Sun, H.; Chen, Y.; Yi, Z. After-Effects of Hydrochar Amendment on Water Spinach Production, N Leaching, and N2O Emission from a Vegetable Soil under Varying N-Inputs. Plants 2022, 11, 3444. [Google Scholar] [CrossRef]
- Zhang, Y.; Jeyakumar, P.; Xia, C.; Lam, S.S.; Jiang, J.; Sun, H.; Shi, W. Being applied at rice or wheat season impacts biochar’s effect on gaseous nitrogen pollutants from the wheat growth cycle. Environ. Pollut. 2022, 306, 119409. [Google Scholar] [CrossRef]
- Gámiz, B.; Velarde, P.; Spokas, K.A.; Cox, L. Dynamic effect of fresh and aged biochar on the behavior of the herbicide mesotrione in soils. J. Agric. Food Chem. 2019, 67, 9450–9459. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Nie, X.; Huang, M.; Wang, D.; Xiao, H.; Liu, C.; Peng, H.; Jiang, J.; Zeng, G. The role of dissolved organic matter in soil organic carbon stability under water erosion. Ecol. Indic. 2019, 102, 724–733. [Google Scholar] [CrossRef]
- Gao, J.; Shi, Z.; Wu, H.; Lv, J. Fluorescent characteristics of dissolved organic matter released from biochar and paddy soil incorporated with biochar. RSC Adv. 2020, 10, 5785–5793. [Google Scholar] [CrossRef]
- Boguta, P.; Cybulak, M.; Sokołowska, Z.; Zarzycki, R.; Kacprzak, A.; Kobyłecki, R. Quality and quantity of humic-like and fulvic-like acids entrapped in biochars–The effect of various forestry feedstock and pyrolysis temperature of biochars. Fuel 2023, 333, 126405. [Google Scholar] [CrossRef]
- Bis, Z.; Kobyłecki, R.; Ścisłowska, M.; Zarzycki, R. Biochar–Potential tool to combat climate change and drought. Ecohydrol. Hydrobiol. 2018, 18, 441–453. [Google Scholar] [CrossRef]
- Krzyszczak, A.; Dybowski, M.P.; Zarzycki, R.; Kobyłecki, R.; Oleszczuk, P.; Czech, B. Long-term physical and chemical aging of biochar affected the amount and bioavailability of PAHs and their derivatives. J. Hazard. Mater. 2022, 440, 129795. [Google Scholar] [CrossRef]
- Kuboń, M.; Niemiec, M.; Klimek-Kopyra, A.; Gliniak, M.; Sikora, J.; Sadowska, U.; Latawiec, A.E.; Kobyłecki, R.; Zarzycki, R.; Kacprzak, A.; et al. Assessment of Greenhouse Gas Emissions in Soybean Cultivation Fertilized with Biochar from Various Utility Plants. Agronomy 2021, 11, 2224. [Google Scholar] [CrossRef]
- Gluba, Ł.; Rafalska-Przysucha, A.; Szewczak, K.; Łukowski, M.; Szlązak, R.; Vitková, J.; Kobyłecki, R.; Bis, Z.; Wichliński, M.; Zarzycki, R.; et al. Effect of fine size-fractionated sunflower husk biochar on water retention properties of arable sandy soil. Materials 2021, 14, 1335. [Google Scholar] [CrossRef]
- Kulczycki, G.; Magnucka, E.G.; Oksińska, M.P.; Kucińska, J.; Kobyłecki, R.; Pawęska, K.; Zarzycki, R.; Kacprzak, A.; Pietr, S.J. The Effect of Various Types of Biochar Mixed with Mineral Fertilization on the Development and Ionome of Winter Wheat (Triticum aestivum L.) Seedlings and Soil Properties in a Pot Experiment. Agronomy 2020, 10, 1903. [Google Scholar] [CrossRef]
- Sun, H.; Yi, Z.; Jeyakumar, P.; Xia, C.; Feng, Y.; Lam, S.S.; Sonne, C.; Wang, H.; Shi, W. Citric acid modified biochar application at a low dosage can synchronically mitigate the nitrogenous gas pollutants emission from rice paddy soils. Environ. Pollut. 2022, 312, 120068. [Google Scholar] [CrossRef]
- Zimmerman, A.R. Abiotic and microbial oxidation of laboratory-produced black carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lee, M.H.; Hur, J. A new molecular weight (MW) descriptor of dissolved organic matter to represent the MW-dependent distribution of aromatic condensation: Insights from biodegradation and pyrene binding experiments. Sci. Total Environ. 2019, 660, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Bausenwein, U.; Gattinger, A.; Langer, U.; Embacher, A.; Hartmann, H.P.; Sommer, M. Exploring soil microbial communities and soil organic matter: Variability and interactions in arable soils under minimum tillage practice. Appl. Soil Ecol. 2008, 40, 67–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Guo, H.; Meng, D.; Wang, Y.; Wong, P.K. Interaction between microbes DNA and atrazine in black soil analyzed by spectroscopy. CLEAN–Soil Air Water 2015, 43, 867–871. [Google Scholar] [CrossRef]
- Ledesma, J.L.; Grabs, T.; Bishop, K.H.; Schiff, S.L.; Köhler, S.J. Potential for long-term transfer of dissolved organic carbon from riparian zones to streams in boreal catchments. Glob. Chang. Biol. 2015, 21, 2963–2979. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; Spencer, R.G. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Ohno, T. Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods 2013, 5, 6557–6566. [Google Scholar] [CrossRef]
- Chen, M.; Huang, Y.; Wang, C.; Gao, H. The conversion of organic nitrogen by functional bacteria determines the end-result of ammonia in compost. Bioresour. Technol. 2020, 299, 122599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, Z.; Li, Z.; Wu, H. Impacts of aeration and biochar addition on extracellular polymeric substances and microbial communities in constructed wetlands for low C/N wastewater treatment: Implications for clogging. Chem. Eng. J. 2020, 396, 125349. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Chen, J.; LeBoeuf, E.J.; Dai, S.; Gu, B. Fluorescence spectroscopic studies of natural organic matter fractions. Chemosphere 2003, 50, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Thurman, E.M. Functional Groups of Dissolved Organic Carbon. In Organic Geochemistry of Natural Waters; Springer: Dordrecht, The Netherlands, 1985; pp. 87–101. [Google Scholar]
- Li, W.; Jia, X.; Li, M.; Wu, H. Insight into the vertical characteristics of dissolved organic matter in 5-m soil profiles under different land-use types on the Loess Plateau. Sci. Total Environ. 2019, 692, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Mohinuzzaman, M.; Yuan, J.; Yang, X.; Senesi, N.; Li, S.-L.; Ellam, R.; Mostofa, K.; Liu, C.-Q. Insights into solubility of soil humic substances and their fluorescence character-isation in three characteristic soils. Sci. Total Environ. 2020, 720, 137395. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Du, Y.; Ma, T.; Deng, Y.; Tao, Y.; Xu, Y.; Leng, Z. Dissolved organic matter characterization in high and low ammonium groundwater of Dongting Plain, central China. Ecotoxicol. Environ. Saf. 2020, 208, 111779. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, M.; Jiang, X.; Guan, D.; Wei, D.; Zhao, B. Impact of 36 years of nitrogen fertilization on microbial community composition and soil carbon cycling-related enzyme activities in rhizospheres and bulk soils in northeast China. Appl. Soil Ecol. 2019, 136, 148–157. [Google Scholar] [CrossRef]
- Catalá, T.S.; Reche, I.; Fuentes-Lema, A.; Romera-Castillo, C.; Nieto-Cid, M.; Ortega-Retuerta, E.; Calvo, E.; Alvarez, M.; Marrasé, C.; Stedmon, C.A.; et al. Turnover time of fluorescent dissolved organic matter in the dark global ocean. Nat. Commun. 2015, 6, 5986. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, R.; Verrecchia, E.; Pfeifer, H.R. The use of solid-phase fluorescence spectroscopy in the characterisation of organic matter transformations. Talanta 2015, 134, 453–459. [Google Scholar] [CrossRef]
- Lee, B.M.; Seo, Y.S.; Hur, J. Investigation of adsorptive fractionation of humic acid on graphene oxide using fluorescence EEM-PARAFAC. Water Res. 2015, 73, 242–251. [Google Scholar] [CrossRef]
- Mangalassery, S.; Mooney, S.J.; Sparkes, D.L.; Fraser, W.T.; Sjögersten, S. Impacts of zero tillage on soil enzyme activities, microbial characteristics and organic matter functional chemistry in temperate soils. Eur. J. Soil Biol. 2015, 68, 9–17. [Google Scholar] [CrossRef]
- Oginni, O.; Singh, K. Pyrolysis characteristics of Arundo donax harvested from a reclaimed mine land. Ind. Crops Prod. 2019, 133, 44–53. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, M.H.; Hur, J.; Lee, Y.H.; Igalavithana, A.D.; Shaheen, S.M. Biochar-induced metal immobilization and soil biogeochemical process: An integrated mechanistic approach. Sci. Total Environ. 2020, 698, 134112. [Google Scholar] [CrossRef]
- Fu, P.; Kawamura, K.; Chen, J.; Qin, M.; Ren, L.; Sun, Y.; Wang, Z.; Barrie, L.A.; Tachibana, E.; Ding, A.; et al. Fluorescent water-soluble organic aerosols in the High Arctic atmosphere. Sci. Rep. 2015, 5, 9845. [Google Scholar] [CrossRef]
- Sanchez-Monedero, M.A.; Cayuela, M.L.; Roig, A.; Jindo, K.; Mondini, C.; Bolan, N.J.B.T. Role of biochar as an additive in organic waste composting. Bioresour. Technol. 2018, 247, 1155–1164. [Google Scholar] [CrossRef]
- Nielsen, S.; Joseph, S.; Ye, J.; Chia, C.; Munroe, P.; van Zwieten, L.; Thomas, T. Crop-season and residual effects of sequentially applied mineral enhanced biochar and N fertiliser on crop yield, soil chemistry and microbial communities. Agric. Ecosyst. Environ. 2018, 255, 52–61. [Google Scholar] [CrossRef]
- Jindo, K.; Sánchez-Monedero, M.A.; Matsumoto, K.; Sonoki, T. The efficiency of a low dose of biochar in enhancing the aromaticity of humic-like substance extracted from poultry manure compost. Agronomy 2019, 9, 248. [Google Scholar] [CrossRef]
- Tye, A.M.; Lapworth, D.J. Characterising changes in fluorescence properties of dissolved organic matter and links to N cycling in agricultural floodplains. Agric. Ecosyst. Environ. 2016, 221, 245–257. [Google Scholar] [CrossRef]
- Speratti, A.B.; Johnson, M.S.; Sousa, H.M.; Dalmagro, H.J.; Couto, E.G. Biochar feedstock and pyrolysis temperature effects on leachate: DOC characteristics and nitrate losses from a Brazilian Cerrado Arenosol mixed with agricultural waste biochars. J. Environ. Manag. 2018, 211, 256–268. [Google Scholar] [CrossRef]

| Depth | Total N | Total P | Total K | Avail. * N | Avail. * P | Avail. * K | SOM ** | pH | BD *** |
|---|---|---|---|---|---|---|---|---|---|
| cm | g/kg | g/kg | g/kg | mg/kg | mg/kg | mg/kg | g/kg | g/cm3 | |
| 0–30 | 1.45 | 1.05 | 25.52 | 163.3 | 20.61 | 187.92 | 29.87 | 6.74 | 1.31 |
| 30–60 | 0.61 | 0.66 | 24.06 | 86.80 | 14.50 | 179.00 | 13.85 | 7.45 | 1.41 |
| Particle Components (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SOC * g/kg | Ca g/kg | K g/kg | Mg g/kg | N g/kg | O g/kg | P g/kg | Si g/kg | pH | <0.1 mm | 0.1–2 mm | >2 mm |
| 598 | 3 | 17.0 | 2 | 7.85 | 166 | 1.327 | 60 | 8.69 | 15.0 | 60.2 | 24.8 |
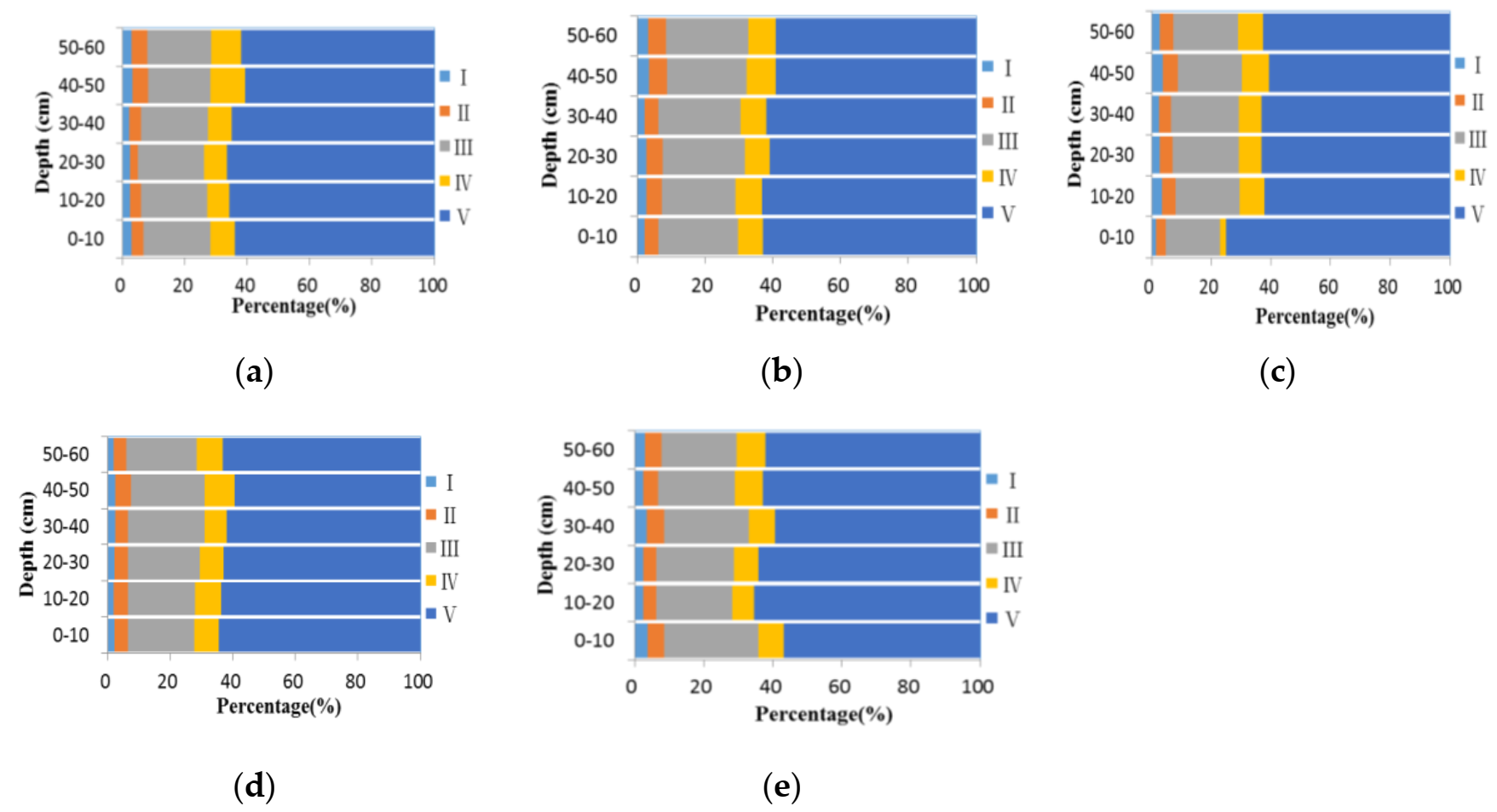
| EX/EM | 0–10 cm | 10–20 cm | 20–30 cm | 30–40 cm | 40–50 cm | 50–60 cm | |
|---|---|---|---|---|---|---|---|
| CK | Ⅰ | 2.9 | 2.4 | 2.4 | 2.3 | 3.2 | 3.1 |
| Ⅱ | 3.8 | 3.7 | 2.7 | 3.7 | 5.1 | 4.9 | |
| Ⅲ | 21.6 | 21.1 | 21.1 | 21.5 | 20.1 | 20.5 | |
| Ⅳ | 7.8 | 7.3 | 7.3 | 7.6 | 11.0 | 9.5 | |
| Ⅴ | 64.0 | 65.6 | 66.5 | 64.9 | 60.6 | 62.0 | |
| NPK | Ⅰ | 2.21 | 2.77 | 2.72 | 2.23 | 3.55 | 3.34 |
| Ⅱ | 4.05 | 4.58 | 4.83 | 4.19 | 5.34 | 5.19 | |
| Ⅲ | 23.45 | 21.60 | 24.28 | 24.17 | 23.42 | 24.18 | |
| Ⅳ | 7.27 | 7.79 | 7.36 | 7.44 | 8.54 | 8.21 | |
| Ⅴ | 63.02 | 63.26 | 60.82 | 61.97 | 59.15 | 59.08 | |
| BC | Ⅰ | 1.9 | 3.0 | 1.0 | 4.5 | 4.8 | 4.0 |
| Ⅱ | 3.6 | 4.6 | 4.5 | 5.8 | 5.6 | 6.3 | |
| Ⅲ | 22.2 | 23.4 | 23.2 | 23.1 | 22.9 | 22.9 | |
| Ⅳ | 7.0 | 7.5 | 6.4 | 7.6 | 7.8 | 7.5 | |
| Ⅴ | 65.3 | 61.6 | 64.9 | 59.0 | 59.0 | 59.2 | |
| NPK + BC | Ⅰ | 1.53 | 3.11 | 3.63 | 2.18 | 2.75 | 2.39 |
| Ⅱ | 5.62 | 5.61 | 5.36 | 3.95 | 4.76 | 4.21 | |
| Ⅲ | 21.09 | 22.83 | 23.39 | 23.56 | 22.92 | 21.08 | |
| Ⅳ | 6.51 | 6.68 | 6.96 | 6.31 | 7.89 | 7.46 | |
| Ⅴ | 65.25 | 61.77 | 60.66 | 64.00 | 61.68 | 64.87 | |
| N +BC | Ⅰ | 3.01 | 2.40 | 0.74 | 2.14 | 3.19 | 3.21 |
| Ⅱ | 4.42 | 3.67 | 3.43 | 3.74 | 4.55 | 4.77 | |
| Ⅲ | 25.81 | 22.80 | 21.75 | 21.88 | 21.77 | 21.66 | |
| Ⅳ | 9.17 | 6.93 | 6.58 | 7.46 | 8.33 | 7.16 | |
| Ⅴ | 57.59 | 64.21 | 67.50 | 64.78 | 62.15 | 63.19 | |
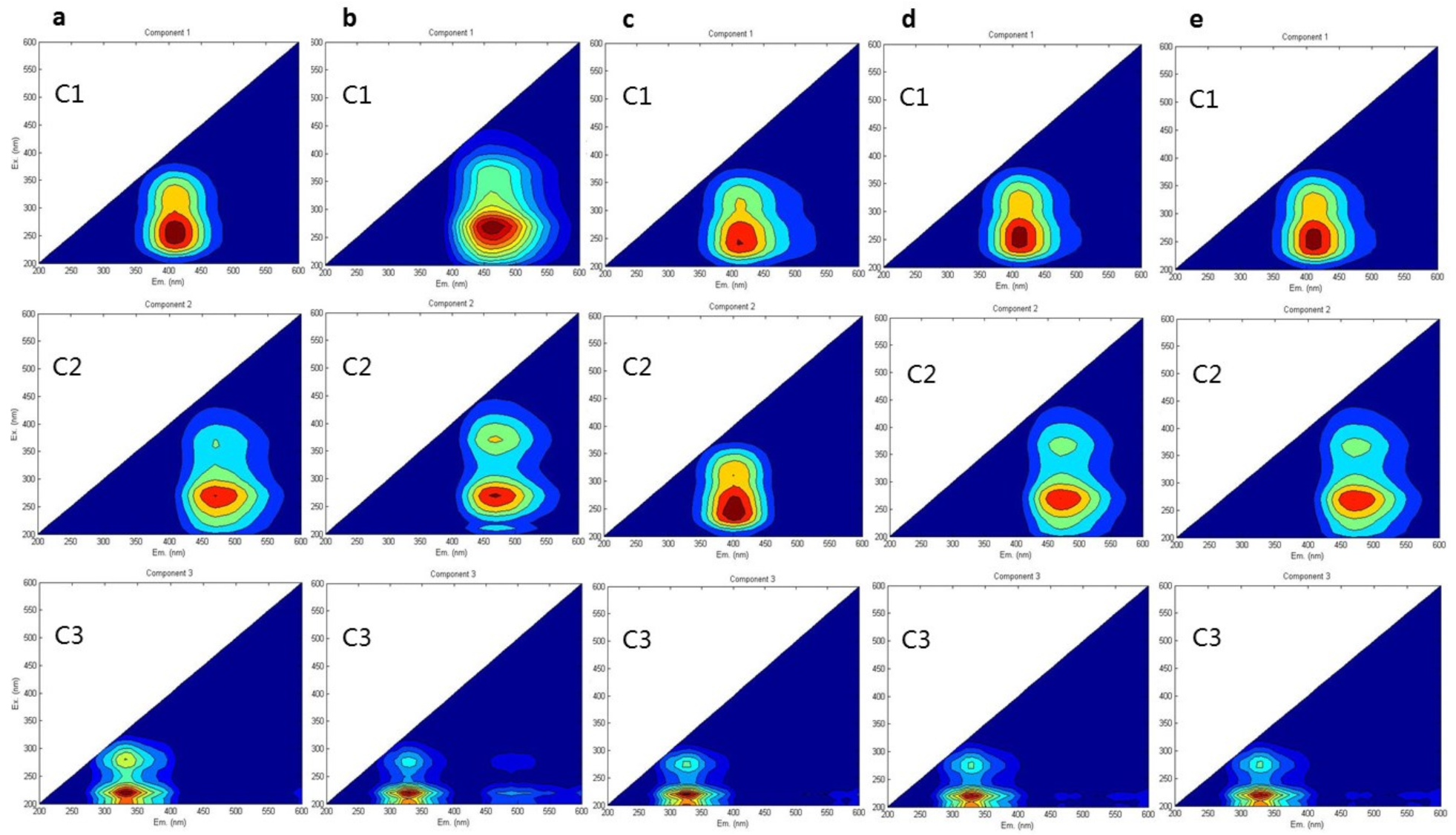
| Group | Approximate EEM Location | Fluorescence Compounds | ||||
|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C1 | C2 | C3 | |
| CK | Em: 407 nm Ex: 256 nm | Em: 474 nm Ex: 263 nm | Em: 332 nm Ex: 221 nm | Ultraviolet A (UVA) fulvic acid-like (terrestrial humic substance) | Ultraviolet C (UVC) humic acid-like (terrestrial humic substance) | Tryptophan-like substance (protein components) |
| NPK | Em: 412 nm Ex: 243 nm | Em: 468 nm Ex: 270 nm | Em: 325 nm Ex: 220 nm | UVA fulvic-like acid (terrestrial humic substance) | UVC humic acid-like (terrestrial humic substance) | Tryptophan-like substance (protein components) |
| BC | Em: 461 nm Ex: 272 nm | Em: 399 nm Ex: 243 nm | Em: 324 nm Ex: 219 nm | UVC humic acid-like (terrestrial humic substance) | UVA fulvic acid-like (aquatic humic substance) | Tryptophan-like substance (protein components) |
| NPK + BC | Em: 410 nm Ex: 249 nm | Em: 467 nm Ex: 270 nm | Em: 326 nm Ex: 221 nm | UVA fulvic-like acid (terrestrial humic substance) | UVC humic acid-like (terrestrial humic substance) | Tryptophan-like substance (protein components) |
| N + BC | Em: 409 nm Ex: 245 nm | Em: 468.5 nm Ex: 269 nm | Em: 325.5 nm Ex: 220 nm | UVA fulvic-like acid (terrestrial humic substance) | UVC humic acid-like (terrestrial humic substance) | Tryptophan-like substance (protein components) |
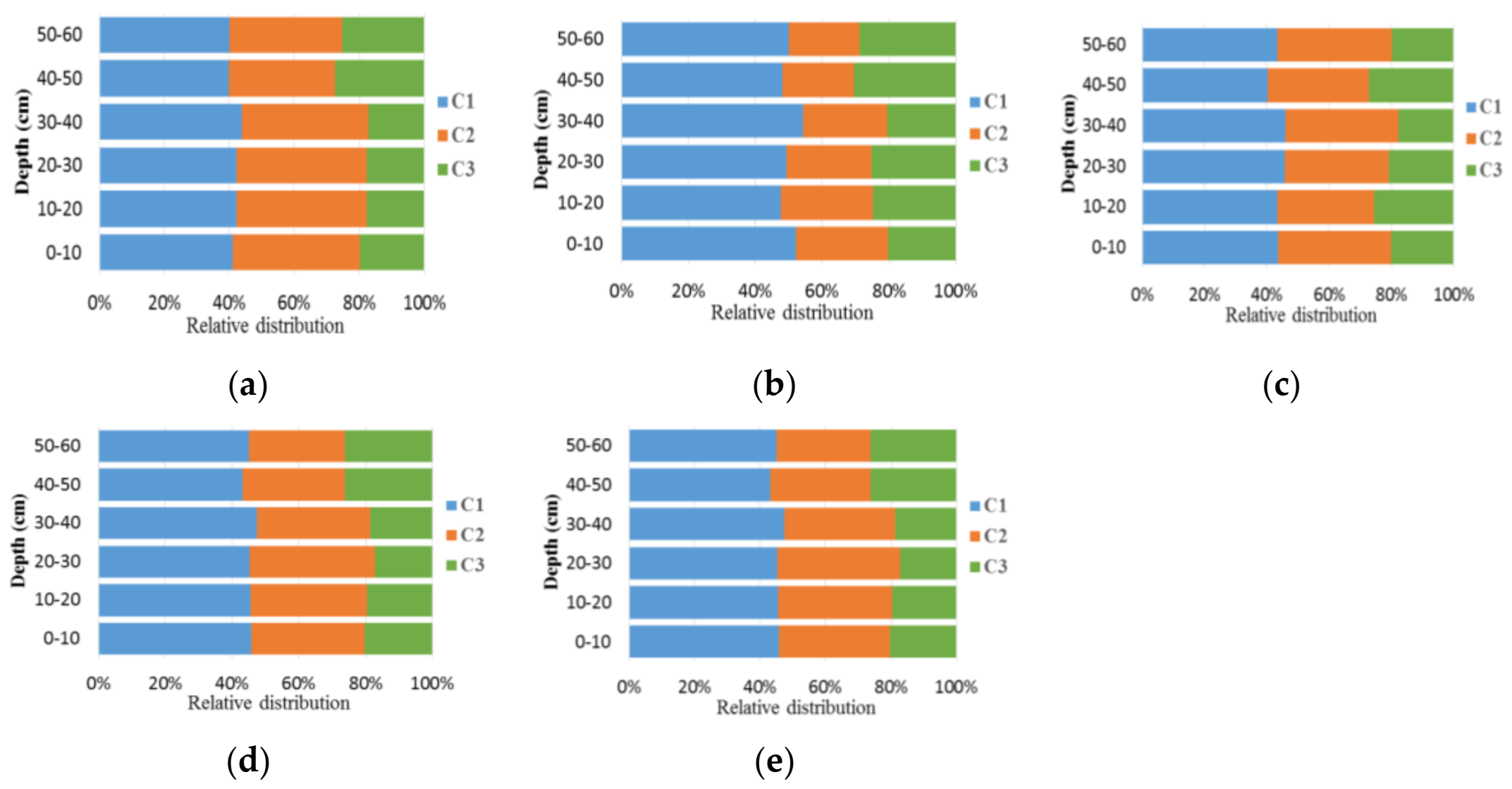
| Depth (cm) | 0–10 | 10–20 | 20–30 | 30–40 | 40–50 | 50–60 | |
|---|---|---|---|---|---|---|---|
| CK | C1 | 41.10 | 42.28 | 42.28 | 43.93 | 39.81 | 40.11 |
| C2 | 39.10 | 40.10 | 40.10 | 38.92 | 32.73 | 34.53 | |
| C3 | 19.80 | 17.62 | 17.62 | 17.15 | 27.46 | 25.36 | |
| NPK | C1 | 52.16 | 47.60 | 49.31 | 54.22 | 48.20 | 49.92 |
| C2 | 27.51 | 27.71 | 25.31 | 25.35 | 21.24 | 21.15 | |
| C3 | 20.32 | 24.69 | 25.38 | 20.43 | 30.56 | 28.93 | |
| BC | C1 | 45.79 | 45.60 | 45.41 | 47.38 | 43.27 | 45.02 |
| C2 | 33.85 | 34.77 | 37.32 | 34.10 | 30.58 | 28.86 | |
| C3 | 20.36 | 19.62 | 17.27 | 18.52 | 26.15 | 26.12 | |
| NPK +BC | C1 | 45.70 | 45.50 | 45.32 | 47.29 | 43.16 | 44.91 |
| C2 | 33.96 | 34.88 | 37.41 | 34.20 | 30.70 | 28.98 | |
| C3 | 20.34 | 19.62 | 17.27 | 18.51 | 26.14 | 26.11 | |
| N +BC | C1 | 45.70 | 45.51 | 45.33 | 47.29 | 43.18 | 44.93 |
| C2 | 33.96 | 34.88 | 37.42 | 34.20 | 30.70 | 28.98 | |
| C3 | 20.34 | 19.61 | 17.26 | 18.50 | 26.13 | 26.10 | |
| WSOC (mg/kg) | C1 | C2 | C3 | FIX | HIX | BIX | |
|---|---|---|---|---|---|---|---|
| WSOC(mg/kg) | 1 | ||||||
| C1 | −0.089 | 1 | |||||
| C2 | −0.376 ** | 0.510 ** | 1 | ||||
| C3 | 0.319 ** | 0.409 ** | 0.020 | 1 | |||
| FIX | −0.180 | −0.023 | −0.366 ** | 0.475 ** | 1 | ||
| HIX | −0.324 ** | −0.236 | 0.432 ** | −0.200 | −0.359 ** | 1 | |
| BIX | −0.50 * | 0.058 | −0.205 | 0.718 ** | 0.777 ** | −0.118 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Wei, D.; Li, Y.; Zou, G.; Wang, L.; Ding, J.; Zhang, Y.; Sun, L.; Wang, W.; Ma, X.; et al. Effects of Biochar on the Fluorescence Spectra of Water-Soluble Organic Matter in Black Soil Profile after Application for Six Years. Plants 2023, 12, 831. https://doi.org/10.3390/plants12040831
Jin L, Wei D, Li Y, Zou G, Wang L, Ding J, Zhang Y, Sun L, Wang W, Ma X, et al. Effects of Biochar on the Fluorescence Spectra of Water-Soluble Organic Matter in Black Soil Profile after Application for Six Years. Plants. 2023; 12(4):831. https://doi.org/10.3390/plants12040831
Chicago/Turabian StyleJin, Liang, Dan Wei, Yan Li, Guoyuan Zou, Lei Wang, Jianli Ding, Yitao Zhang, Lei Sun, Wei Wang, Xingzhu Ma, and et al. 2023. "Effects of Biochar on the Fluorescence Spectra of Water-Soluble Organic Matter in Black Soil Profile after Application for Six Years" Plants 12, no. 4: 831. https://doi.org/10.3390/plants12040831
APA StyleJin, L., Wei, D., Li, Y., Zou, G., Wang, L., Ding, J., Zhang, Y., Sun, L., Wang, W., Ma, X., Shen, H., Wang, Y., Wang, J., Lu, X., Sun, Y., Ding, X., Li, D., & Yin, D. (2023). Effects of Biochar on the Fluorescence Spectra of Water-Soluble Organic Matter in Black Soil Profile after Application for Six Years. Plants, 12(4), 831. https://doi.org/10.3390/plants12040831




