The Effect of an Extremely Low-Frequency Electromagnetic Field on the Drought Sensitivity of Wheat Plants
Abstract
1. Introduction
- Focus on studying the effect of a low-intensity alternating MF on plants under stress (drought);
- Focus on the Schumann range (from several Hz to several tens of Hz), particularly the second ScR harmonic (chosen on the basis of the results of our previous studies);
- The MF acting on plants throughout the entire growing period, particularly during the development of a response to the stressor;
- The treatment mode chosen based on the assumption that plant signaling systems play an important role in the responses to the MF and stress;
- The use of a wide range of diagnostic tools that enable the non-invasive monitoring of the activities of key physiological processes in plants during the entire period of observation.
2. Results
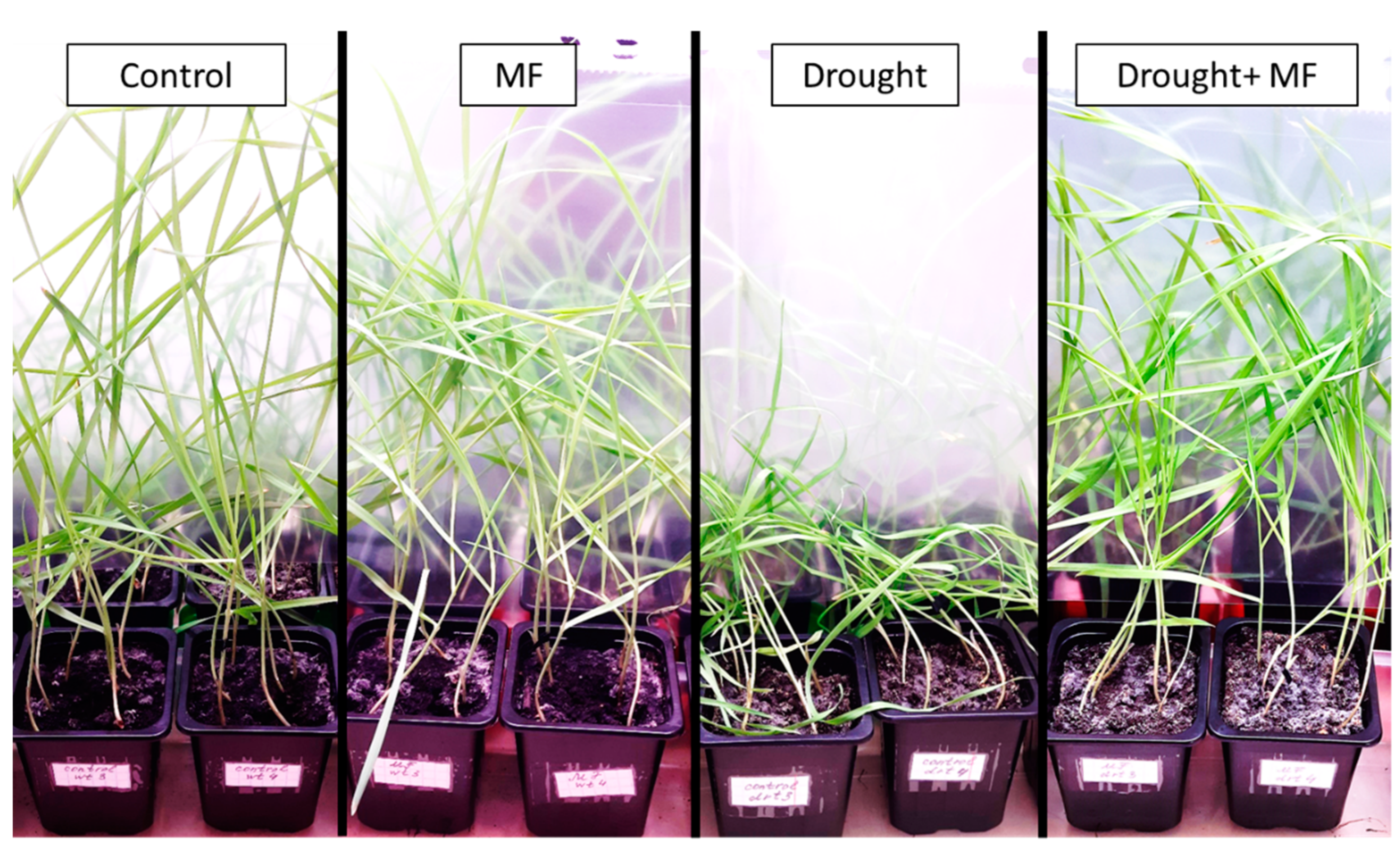
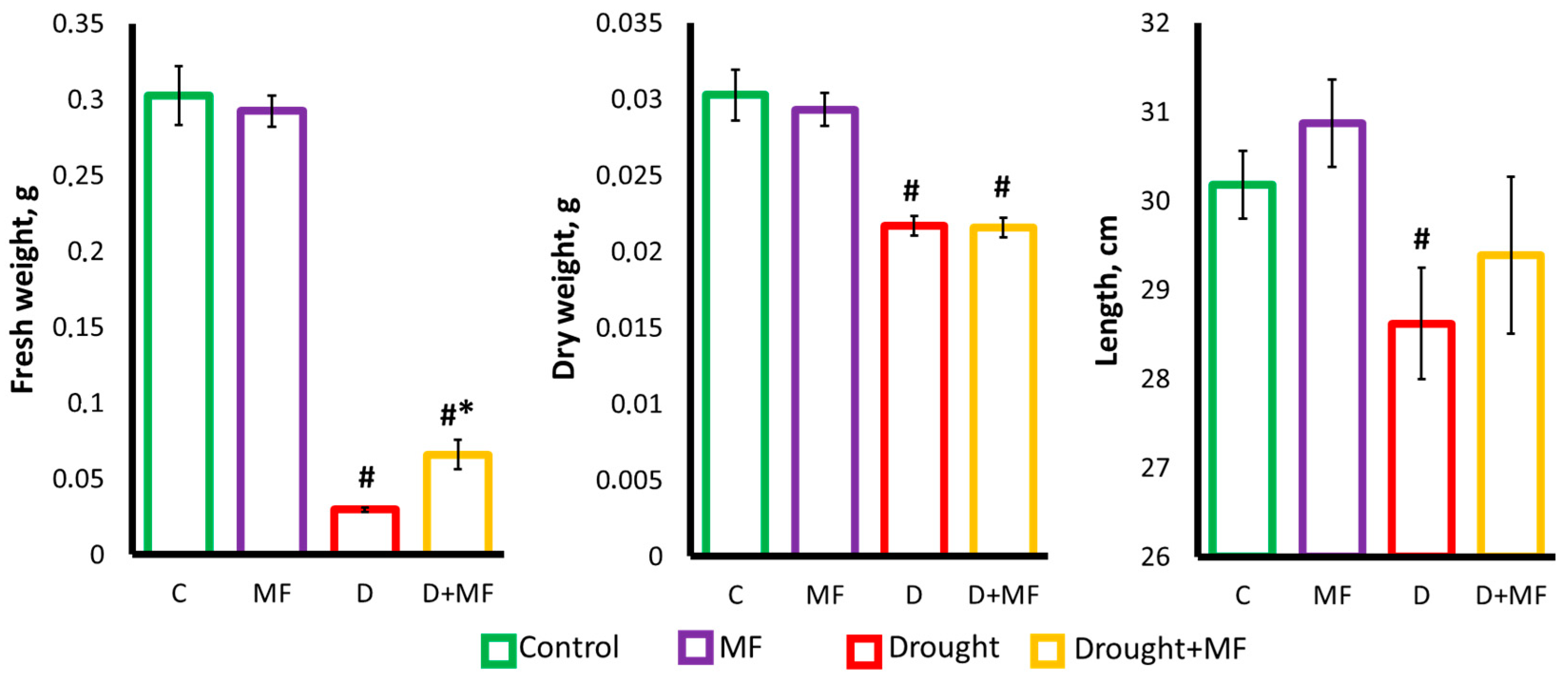
2.1. Electromagnetic Field Effects on Growth Parameters under Control and Drought Conditions
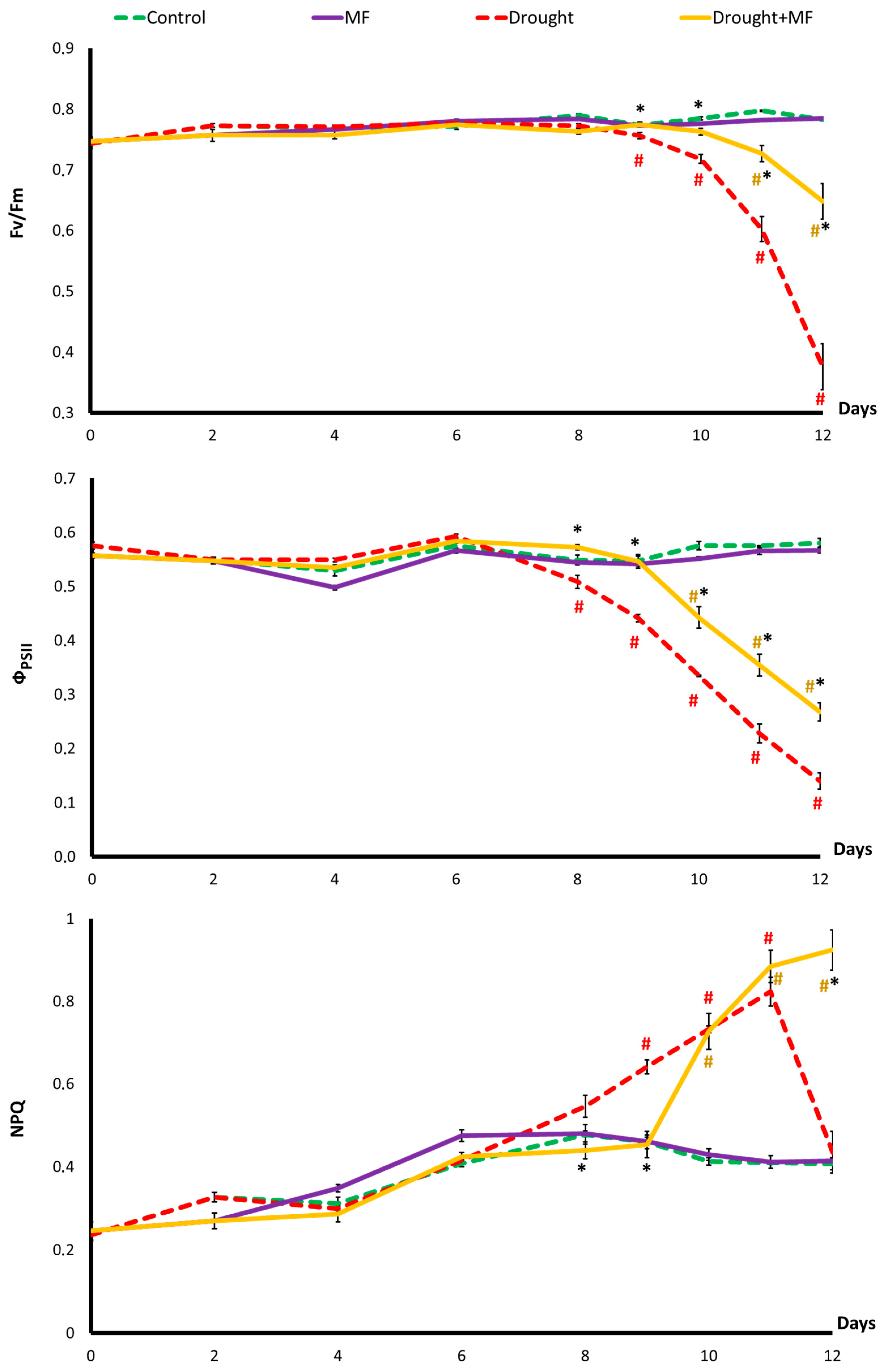
2.2. Electromagnetic Field Effect on Photosynthesis under Control and Drought Conditions
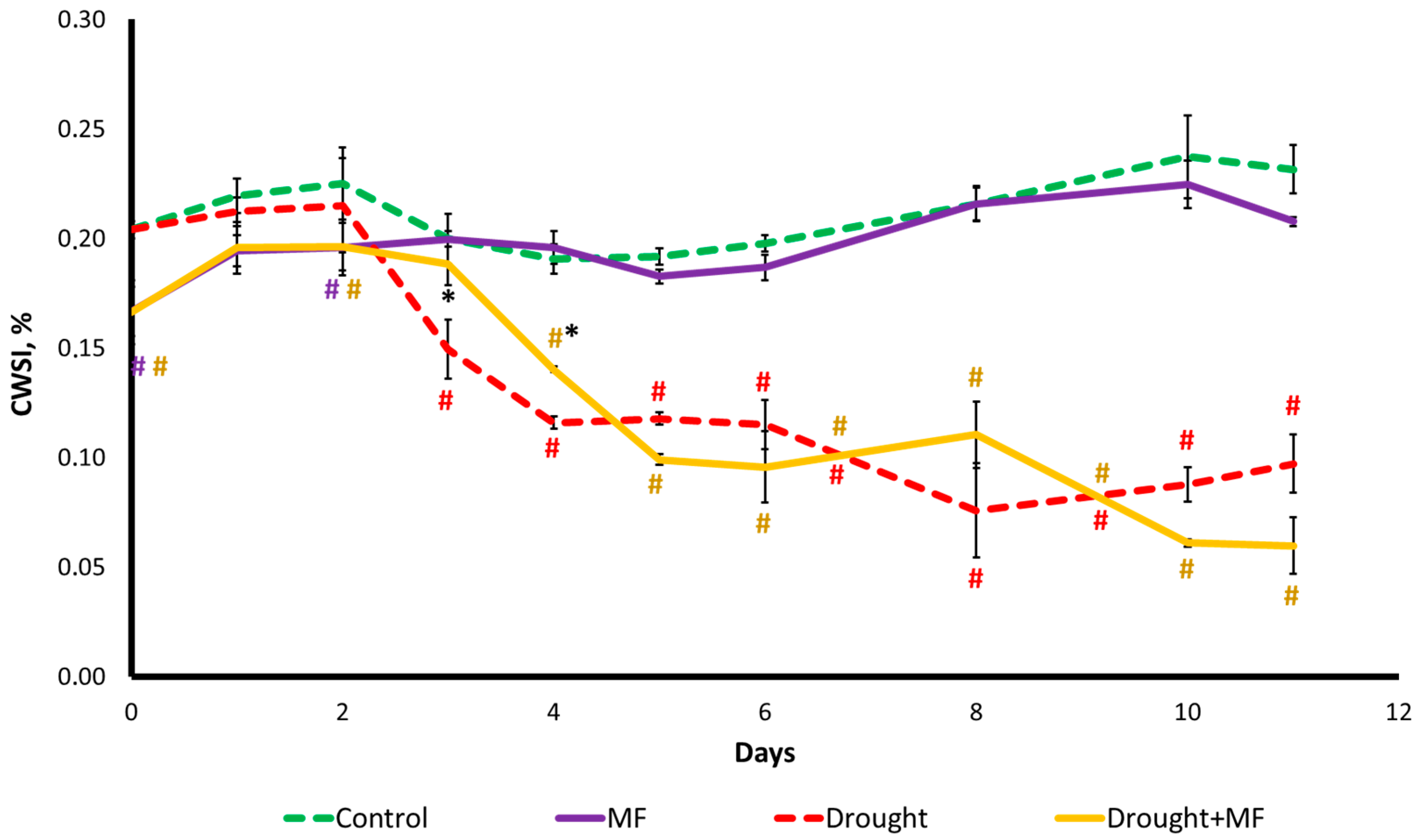
2.3. Magnetic Field Effect on Water Status under Control and Drought Conditions
3. Discussion
4. Materials and Methods
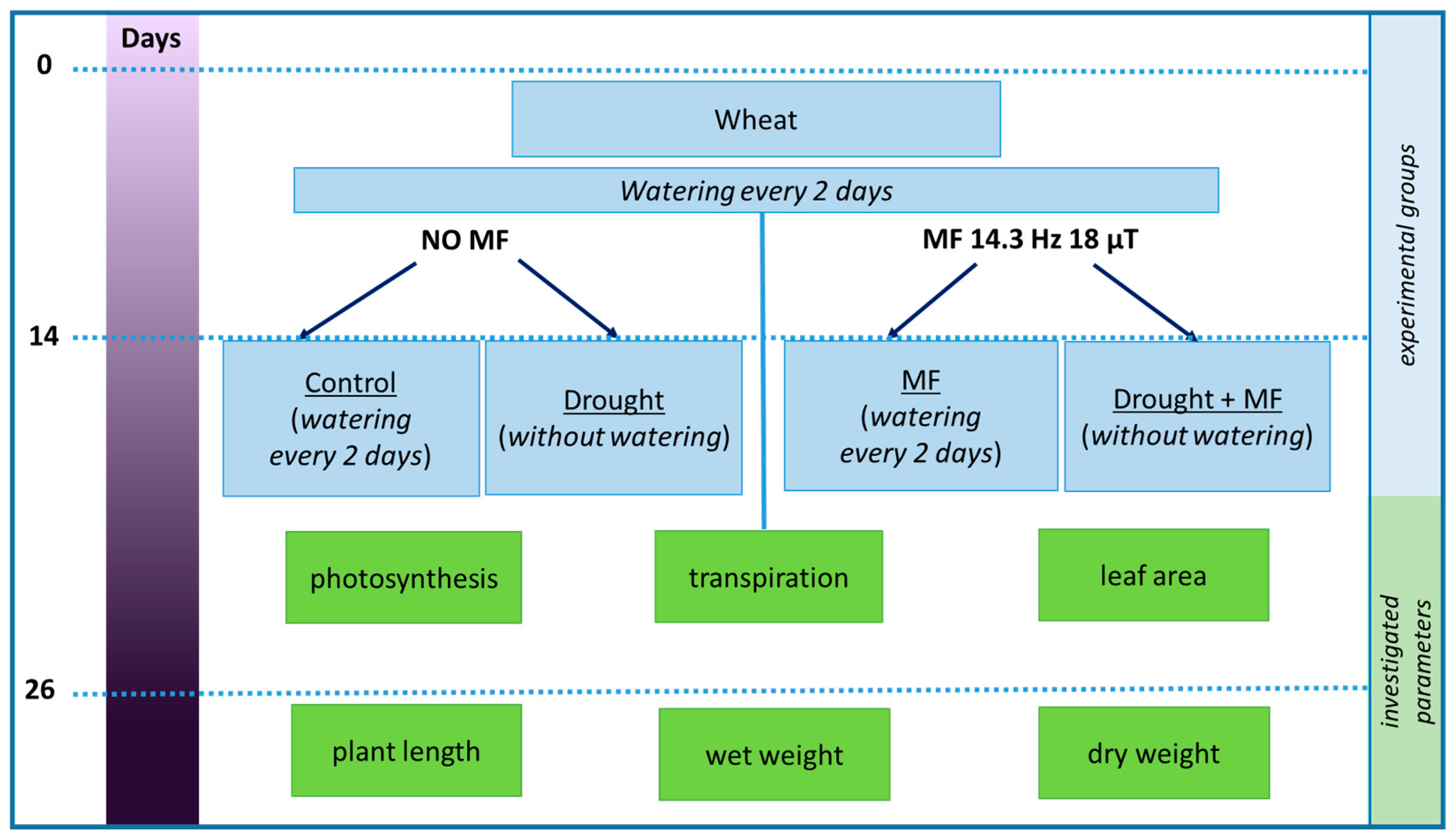
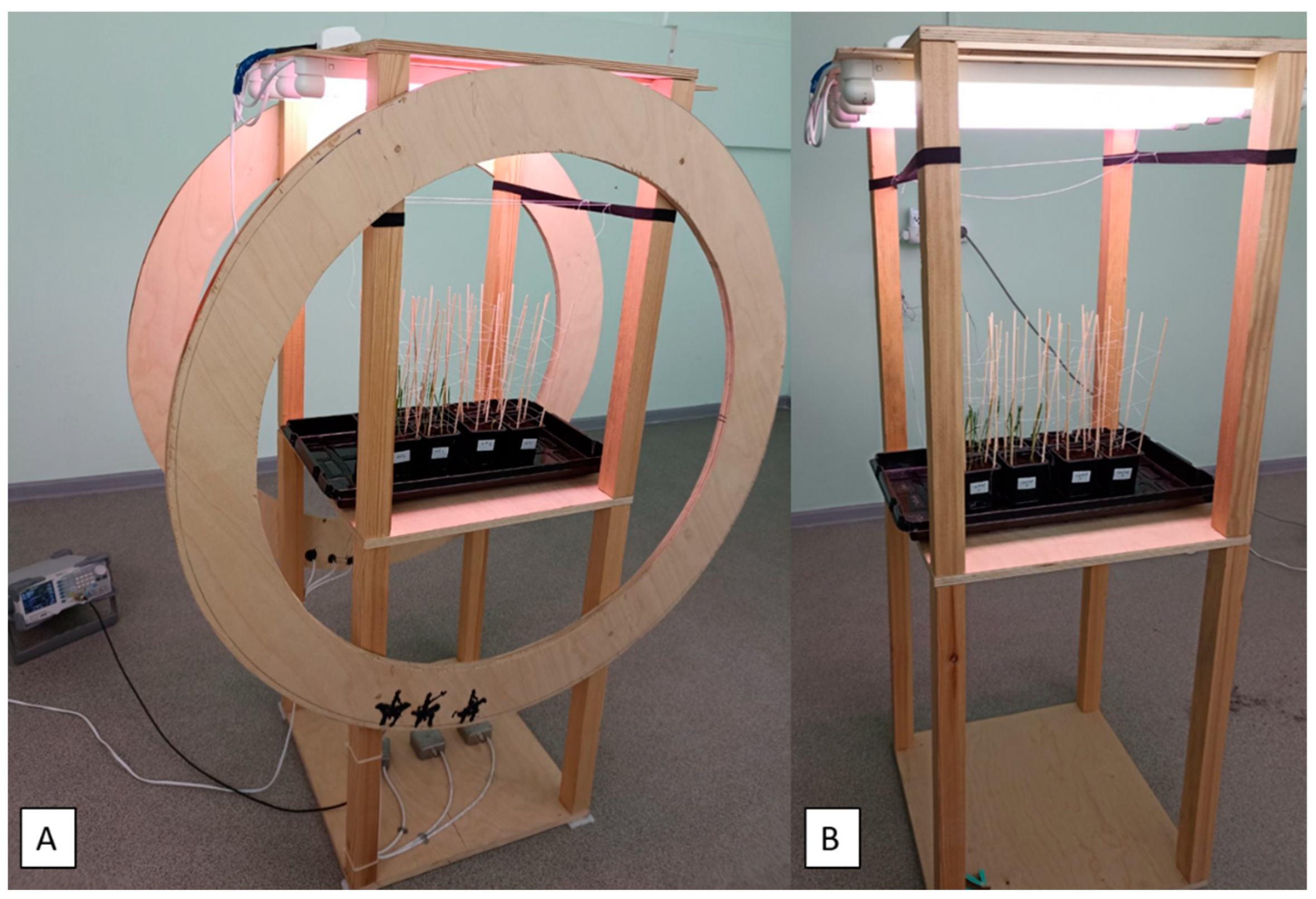
4.1. Experiment Design
4.2. Measurement of Growth Parameters
4.3. Measurement of Photosynthesis and Leaf Area
4.4. Determining Transpiration and the Water Content
4.5. Statistics
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tarduno, J.A.; Cottrell, R.D.; Davis, W.J.; Nimmo, F.; Bono, R.K. A Hadean to Paleoarchean geodynamo recorded by single zircon crystals. Science 2015, 349, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. Magnetic field effects on plant growth, development, and evolution. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.B.; Zahra, N.; Ahmad, N.; Shi, Z.; Raza, A.; Wang, X.; Li, J. Growth, physiological, biochemical and molecular changes in plants induced by magnetic fields: A review. Plant Biol. 2022, 1, 23. [Google Scholar] [CrossRef] [PubMed]
- Recommendation ITU-R V.431-7*. Nomenclature of the Frequency and Wavelength Bands Used in Telecommunications. Available online: https://www.itu.int/dms_pubrec/itu-r/rec/v/R-REC-V.431-7-200005-S!!PDF-E.pdf (accessed on 29 January 2022).
- Lai, H. Exposure to Static and Extremely-Low Frequency Electromagnetic Fields and Cellular Free Radicals. Electromagn. Biol. Med. 2019, 38, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Elhalel, G.; Price, C.; Fixler, D.; Shainberg, A. Cardioprotection from stress conditions by weak magnetic fields in the Schumann Resonance band. Sci. Rep. 2019, 9, 1645. [Google Scholar] [CrossRef]
- Price, C.; Williams, E.; Elhalel, G.; Sentman, D. Natural ELF fields in the atmosphere and in living organisms. Int. J. Biometeorol. 2021, 65, 85–92. [Google Scholar] [CrossRef]
- De Souza-Torres, A.; Sueiro-Pelegrín, L.; Zambrano-Reyes, M.; Macías-Socarras, I.; González-Posada, M.; García-Fernández, D. Extremely low frequency non-uniform magnetic fields induce changes in water relations, photosynthesis and tomato plant growth. Int. J. Radiat. Biol. 2020, 96, 951–957. [Google Scholar] [CrossRef]
- Fatima, A.; Kataria, S.; Prajapati, R.; Jain, M.; Agrawal, A.K.; Singh, B.; Kashyap, Y.; Tripathi, D.K.; Singh, V.P.; Gadre, R. Magnetopriming effects on arsenic stress-induced morphological and physiological variations in soybean involving synchrotron imaging. Physiol. Plant. 2021, 173, 88–99. [Google Scholar] [CrossRef]
- Yang, P.; Gan, T.; Pi, W.; Cao, M.; Chen, D.; Luo, J. Effect of using Celosia argentea grown from seeds treated with a magnetic field to conduct Cd phytoremediation in drought stress conditions. Chemosphere 2021, 280, 130724. [Google Scholar] [CrossRef]
- Soja, G.; Kunsch, B.; Gerzabek, M.; Reichenauer, T.; Soja, A.-M.; Rippar, G.; Bolhàr-Nordenkampf, H.R. Growth and yield of winter wheat (Triticum aestivum L.) and corn (Zea mays L.) near a high voltage transmission line. Bioelectromagnetics 2003, 24, 91–102. [Google Scholar] [CrossRef]
- Fischer, G.; Tausz, M.; Köck, M.; Grill, D. Effects of weak 16⅔ Hz magnetic fields on growth parameters of young sunflower and wheat seedlings. Bioelectromagnetics 2004, 25, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Sukhov, V.; Sukhova, E.; Sinitsyna, Y.; Gromova, E.; Mshenskaya, N.; Ryabkova, A.; Ilin, N.; Vodeneev, V.; Mareev, E.; Price, C. Influence of Magnetic Field with Schumann Resonance Frequencies on Photosynthetic Light Reactions in Wheat and Pea. Cells 2021, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Sukhova, E.; Gromova, E.; Yudina, L.; Kior, A.; Vetrova, Y.; Ilin, N.; Mareev, E.; Vodeneev, V.; Sukhov, V. Change in H+ Transport across Thylakoid Membrane as Potential Mechanism of 14.3 Hz Magnetic Field Impact on Photosynthetic Light Reactions in Seedlings of Wheat (Triticum aestivum L.). Plants 2021, 10, 2207. [Google Scholar] [CrossRef]
- Grinberg, M.; Mudrilov, M.; Kozlova, E.; Sukhov, V.; Sarafanov, F.; Evtushenko, A.; Ilin, N.; Vodeneev, V.; Price, C.; Mareev, E. Effect of extremely low-frequency magnetic fields on light-induced electric reactions in wheat. Plant Signal. Behav. 2022, 17, 2021664. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Sukhov, V.; Sukhova, E.; Vodeneev, V. Long-distance electrical signals as a link between the local action of stressors and the systemic physiological responses in higher plants. Prog. Biophys. Mol. Biol. 2019, 146, 63–84. [Google Scholar] [CrossRef]
- Javed, N.; Ashraf, M.; Akram, N.A.; Al-Qurainy, F. Alleviation of Adverse Effects of Drought Stress on Growth and Some Potential Physiological Attributes in Maize (Zea mays L.) by Seed Electromagnetic Treatment. Photochem. Photobiol. 2011, 87, 1354–1362. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Li, R.; He, J.-M. Magnetic field can alleviate toxicological effect induced by cadmium in mungbean seedlings. Ecotoxicology 2011, 20, 760–769. [Google Scholar] [CrossRef]
- Anand, A.; Nagarajan, S.S.; Verma, A.; Joshi, D.K.; Pathak, P.C.; Bhardwaj, J. Pre-treatment of seeds with static magnetic field ameliorates soil water stress in seedlings of maize (Zea mays L.). Indian J. Biochem. Biophys. 2012, 49, 63–70. [Google Scholar]
- Radhakrishnan, R. Magnetic field regulates plant functions, growth and enhances tolerance against environmental stresses. Physiol. Mol. Biol. Plants 2019, 25, 1107–1119. [Google Scholar] [CrossRef]
- Selim, A.-F.H.; El-Nady, M.F. Physio-anatomical responses of drought stressed tomato plants to magnetic field. Acta Astronaut. 2011, 69, 387–396. [Google Scholar] [CrossRef]
- Karimi, S.; Hojati, S.; Eshghi, S.; Nazary Moghaddam, R.; Jandoust, S. Magnetic exposure improves tolerance of fig ‘Sabz’ explants to drought stress induced in vitro. Sci. Hortic. 2012, 137, 95–99. [Google Scholar] [CrossRef]
- Yano, A.; Ohashi, Y.; Hirasaki, T.; Fujiwara, K. Effects of a 60 Hz magnetic field on photosynthetic CO2 uptake and early growth of radish seedlings. Bioelectromagnetics 2004, 25, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Novitskii, Y.I.; Novitskaya, G.V.; Serdyukov, Y.A. Lipid utilization in radish seedlings as affected by weak horizontal extremely low frequency magnetic field. Bioelectromagnetics 2014, 35, 91–99. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Grinberg, M.A.; Sukhov, V.; Vodeneev, V. Effect of ionizing radiation on physiological and molecular processes in plants. J. Environ. Radioact. 2019, 202, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Isaac, A.E.; Oliveira, M.R.; Almeida, L.A.; Chaves, S.S.; Lorenzo, G.-O.J.; Antonio, C.-J. Effects of 60 Hz sinusoidal magnetic field on in vitro establishment, multiplication, and acclimatization phases of Coffea arabica seedlings. Bioelectromagnetics 2014, 35, 414–425. [Google Scholar] [CrossRef]
- Mildažienė, V.; Aleknavičiūtė, V.; Žūkienė, R.; Paužaitė, G.; Naučienė, Z.; Filatova, I.; Lyushkevich, V.; Haimi, P.; Tamošiūnė, I.; Baniulis, D. Treatment of Common Sunflower (Helianthus annus L.) Seeds with Radio-frequency Electromagnetic Field and Cold Plasma Induces Changes in Seed Phytohormone Balance, Seedling Development and Leaf Protein Expression. Sci. Rep. 2019, 9, 6437. [Google Scholar] [CrossRef]
- Serdyukov, Y.A.; Novitskii, Y.I. Impact of weak permanent magnetic field on antioxidant enzyme activities in radish seedlings. Russ. J. Plant Physiol. 2013, 60, 69–76. [Google Scholar] [CrossRef]
- Podleśny, J.; Podleśna, A.; Gładyszewska, B.; Bojarszczuk, J. Effect of Pre-Sowing Magnetic Field Treatment on Enzymes and Phytohormones in Pea (Pisum sativum L.) Seeds and Seedlings. Agronomy 2021, 11, 494. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Y.; Feng, S.; Hu, Y.; Cao, M.; Luo, J. Reactive effects of pre-sowing magnetic field exposure on morphological characteristics and antioxidant ability of Brassica juncea in phytoextraction. Chemosphere 2022, 303, 135046. [Google Scholar] [CrossRef]
- Mshenskaya, N.; Sinitsyna, Y.; Kalyasova, E.; Valeria, K.; Zhirova, A.; Karpeeva, I.; Ilin, N. Influence of Schumann Range Electromagnetic Fields on Components of Plant Redox Metabolism in Wheat and Peas. Plants 2022, 11, 1955. [Google Scholar] [CrossRef] [PubMed]
- Kornarzyński, K.; Dziwulska-Hunek, A.; Kornarzyńska-Gregorowicz, A.; Sujak, A. Effect of Electromagnetic Stimulation of Amaranth Seeds of Different Initial Moisture on the Germination Parameters and Photosynthetic Pigments Content. Sci. Rep. 2018, 8, 14023. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Deamici, K.M.; Taimourya, H.; Islam, M.; Kataria, S.; Raipuria, R.K.; Abdi, G.; Brestic, M. Effect of Magnetopriming on Photosynthetic Performance of Plants. Int. J. Mol. Sci. 2021, 22, 9353. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Kataria, S.; Taimourya, H.; Santos, L.O.; Menegatti, R.D.; Jain, M.; Ihtisham, M.; Liu, S. Magnetic Field (MF) Applications in Plants: An Overview. Plants 2020, 9, 1139. [Google Scholar] [CrossRef]
- Fatima, A.; Kataria, S.; Baghel, L.; Guruprasad, K.N.; Agrawal, A.K.; Singh, B.; Sarkar, P.S.; Shripathi, T.; Kashyap, Y. Synchrotron-based phase-sensitive imaging of leaves grown from magneto-primed seeds of soybean. J. Synchrotron Radiat. 2017, 24, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Postiglione, A.E.; Muday, G.K. The Role of ROS Homeostasis in ABA-Induced Guard Cell Signaling. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Leelapriya, T.; Kumari, B.D.R. Effects of pulsed magnetic field treatment of soybean seeds on calli growth, cell damage, and biochemical changes under salt stress. Bioelectromagnetics 2012, 33, 670–681. [Google Scholar] [CrossRef]
- Nogués, S.; Baker, N.R. Effects of drought on photosynthesis in Mediterranean plants grown under enhanced UV-B radiation. J. Exp. Bot. 2000, 51, 1309–1317. [Google Scholar] [CrossRef]
- Singh, S.K.; Raja Reddy, K. Regulation of photosynthesis, fluorescence, stomatal conductance and water-use efficiency of cowpea (Vigna unguiculata [L.] Walp.) under drought. J. Photochem. Photobiol. B Biol. 2011, 105, 40–50. [Google Scholar] [CrossRef]
- Yoshida, T.; Fernie, A.R. Remote Control of Transpiration via ABA. Trends Plant Sci. 2018, 23, 755–758. [Google Scholar] [CrossRef]
- Ehonen, S.; Yarmolinsky, D.; Kollist, H.; Kangasjärvi, J. Reactive Oxygen Species, Photosynthesis, and Environment in the Regulation of Stomata. Antioxid. Redox Signal. 2019, 30, 1220–1237. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Suzuki, T.; Osakabe, Y.; Betsuyaku, S.; Kondo, Y.; Dohmae, N.; Fukuda, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 2018, 556, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.B.; Reginato, R.J.; Pinter, P.J. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Vialet-Chabrand, S.; Lawson, T. Thermography methods to assess stomatal behaviour in a dynamic environment. J. Exp. Bot. 2020, 71, 2329–2338. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mshenskaya, N.S.; Grinberg, M.A.; Kalyasova, E.A.; Vodeneev, V.A.; Ilin, N.V.; Slyunyaev, N.N.; Mareev, E.A.; Sinitsyna, Y.V. The Effect of an Extremely Low-Frequency Electromagnetic Field on the Drought Sensitivity of Wheat Plants. Plants 2023, 12, 826. https://doi.org/10.3390/plants12040826
Mshenskaya NS, Grinberg MA, Kalyasova EA, Vodeneev VA, Ilin NV, Slyunyaev NN, Mareev EA, Sinitsyna YV. The Effect of an Extremely Low-Frequency Electromagnetic Field on the Drought Sensitivity of Wheat Plants. Plants. 2023; 12(4):826. https://doi.org/10.3390/plants12040826
Chicago/Turabian StyleMshenskaya, N. S., M. A. Grinberg, E. A. Kalyasova, V. A. Vodeneev, N. V. Ilin, N. N. Slyunyaev, E. A. Mareev, and Y. V. Sinitsyna. 2023. "The Effect of an Extremely Low-Frequency Electromagnetic Field on the Drought Sensitivity of Wheat Plants" Plants 12, no. 4: 826. https://doi.org/10.3390/plants12040826
APA StyleMshenskaya, N. S., Grinberg, M. A., Kalyasova, E. A., Vodeneev, V. A., Ilin, N. V., Slyunyaev, N. N., Mareev, E. A., & Sinitsyna, Y. V. (2023). The Effect of an Extremely Low-Frequency Electromagnetic Field on the Drought Sensitivity of Wheat Plants. Plants, 12(4), 826. https://doi.org/10.3390/plants12040826





