Integrating Application Methods and Concentrations of Salicylic Acid as an Avenue to Enhance Growth, Production, and Water Use Efficiency of Wheat under Full and Deficit Irrigation in Arid Countries
Abstract
1. Introduction
2. Results
2.1. Vegetative Growth Parameters
2.2. Physiological Parameters
2.3. Yield Parameters and Irrigation Water Use Efficiency
2.4. Relationship between Different Parameters under the Full and Limited Irrigation Regimes
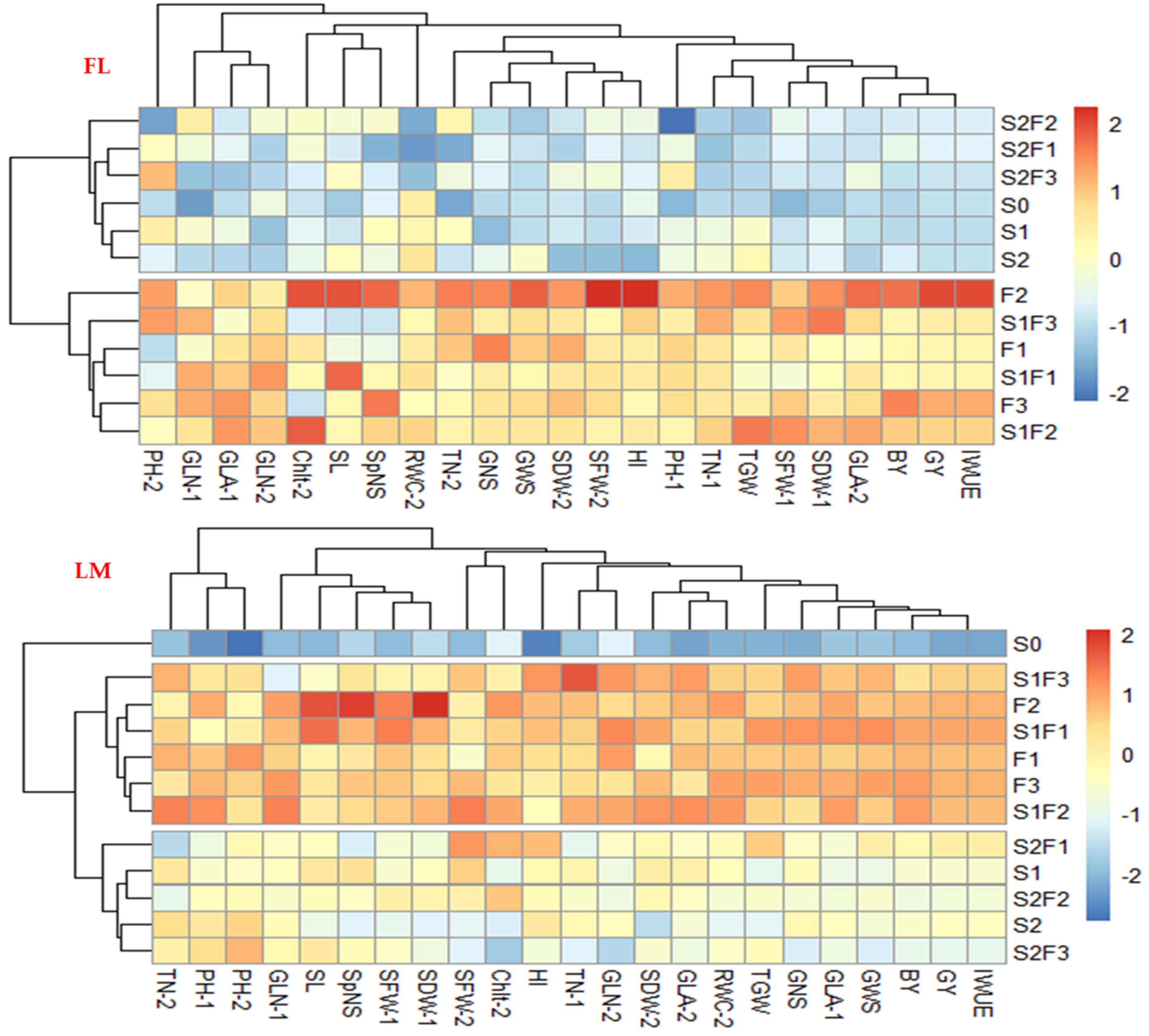
2.5. Multivariate Approach: An Overview of the Responses of Plant Parameters to Different SA Treatments
| Par. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PH-1 (1) | 0.68 | 0.81 | 0.76 | 0.71 | 0.66 | 0.86 | 0.78 | 0.51 | 0.79 | 0.45 | 0.63 | 0.82 | 0.36 | 0.60 | 0.68 | 0.69 | 0.64 | 0.66 | 0.75 | 0.76 | 0.58 | 0.75 | |
| TN-1 (2) | 0.73 | 0.53 | 0.87 | 0.71 | 0.71 | 0.54 | 0.81 | 0.87 | 0.93 | 0.59 | 0.80 | 0.83 | 0.61 | 0.52 | 0.74 | 0.91 | 0.83 | 0.66 | 0.84 | 0.82 | 0.71 | 0.84 | |
| GLN-1 (3) | 0.65 | 0.61 | 0.80 | 0.85 | 0.80 | 0.63 | 0.61 | 0.66 | 0.70 | 0.68 | 0.67 | 0.83 | 0.65 | 0.78 | 0.74 | 0.69 | 0.71 | 0.73 | 0.79 | 0.85 | 0.45 | 0.79 | |
| GLA-1 (4) | 0.64 | 0.79 | 0.72 | 0.90 | 0.84 | 0.60 | 0.74 | 0.90 | 0.90 | 0.66 | 0.83 | 0.93 | 0.65 | 0.70 | 0.79 | 0.94 | 0.96 | 0.86 | 0.96 | 0.97 | 0.68 | 0.96 | |
| SFW-1 (5) | 0.64 | 0.86 | 0.71 | 0.80 | 0.94 | 0.56 | 0.58 | 0.73 | 0.83 | 0.62 | 0.84 | 0.90 | 0.71 | 0.88 | 0.88 | 0.84 | 0.86 | 0.85 | 0.88 | 0.89 | 0.63 | 0.88 | |
| SDW-1 (6) | 0.64 | 0.93 | 0.67 | 0.72 | 0.94 | 0.36 | 0.48 | 0.69 | 0.80 | 0.59 | 0.79 | 0.86 | 0.74 | 0.85 | 0.93 | 0.75 | 0.78 | 0.72 | 0.80 | 0.82 | 0.53 | 0.80 | |
| PH-2 (7) | 0.64 | 0.42 | 0.17 | 0.21 | 0.45 | 0.54 | 0.73 | 0.60 | 0.65 | 0.58 | 0.58 | 0.64 | 0.18 | 0.48 | 0.41 | 0.63 | 0.56 | 0.65 | 0.67 | 0.63 | 0.68 | 0.67 | |
| TN-2 (8) | 0.58 | 0.77 | 0.58 | 0.58 | 0.75 | 0.80 | 0.36 | 0.68 | 0.79 | 0.35 | 0.59 | 0.69 | 0.13 | 0.45 | 0.60 | 0.72 | 0.60 | 0.49 | 0.68 | 0.70 | 0.49 | 0.68 | |
| GLN-2 (9) | 0.60 | 0.76 | 0.70 | 0.83 | 0.74 | 0.70 | 0.01 | 0.59 | 0.84 | 0.59 | 0.71 | 0.78 | 0.66 | 0.46 | 0.61 | 0.89 | 0.91 | 0.73 | 0.87 | 0.88 | 0.63 | 0.87 | |
| GLA-2 (10) | 0.74 | 0.87 | 0.60 | 0.82 | 0.88 | 0.92 | 0.54 | 0.74 | 0.78 | 0.71 | 0.88 | 0.93 | 0.65 | 0.67 | 0.80 | 0.90 | 0.87 | 0.79 | 0.92 | 0.90 | 0.77 | 0.92 | |
| SFW-2 (11) | 0.71 | 0.74 | 0.62 | 0.76 | 0.73 | 0.76 | 0.43 | 0.77 | 0.72 | 0.91 | 0.83 | 0.71 | 0.65 | 0.44 | 0.45 | 0.60 | 0.65 | 0.68 | 0.68 | 0.66 | 0.62 | 0.68 | |
| SDW-2 (12) | 0.78 | 0.84 | 0.67 | 0.84 | 0.83 | 0.80 | 0.39 | 0.80 | 0.85 | 0.90 | 0.91 | 0.91 | 0.66 | 0.73 | 0.80 | 0.81 | 0.84 | 0.84 | 0.84 | 0.84 | 0.65 | 0.84 | |
| RWC-2 (13) | 0.47 | 0.48 | 0.17 | 0.56 | 0.42 | 0.54 | 0.12 | 0.40 | 0.50 | 0.53 | 0.56 | 0.52 | 0.68 | 0.74 | 0.85 | 0.88 | 0.90 | 0.90 | 0.94 | 0.95 | 0.68 | 0.94 | |
| Chlt-2 (14) | 0.39 | 0.54 | 0.23 | 0.50 | 0.55 | 0.57 | 0.06 | 0.53 | 0.45 | 0.55 | 0.54 | 0.53 | 0.42 | 0.58 | 0.58 | 0.60 | 0.75 | 0.71 | 0.71 | 0.71 | 0.63 | 0.71 | |
| SL (15) | 0.56 | 0.51 | 0.35 | 0.56 | 0.36 | 0.44 | 0.18 | 0.47 | 0.48 | 0.64 | 0.74 | 0.54 | 0.38 | 0.61 | 0.87 | 0.67 | 0.63 | 0.69 | 0.71 | 0.69 | 0.60 | 0.71 | |
| SpNS (16) | 0.42 | 0.62 | 0.42 | 0.73 | 0.53 | 0.56 | 0.27 | 0.56 | 0.49 | 0.69 | 0.70 | 0.63 | 0.55 | 0.60 | 0.67 | 0.75 | 0.69 | 0.64 | 0.74 | 0.76 | 0.51 | 0.74 | |
| GNS (17) | 0.78 | 0.83 | 0.47 | 0.78 | 0.81 | 0.77 | 0.26 | 0.71 | 0.79 | 0.83 | 0.86 | 0.92 | 0.53 | 0.63 | 0.58 | 0.51 | 0.95 | 0.83 | 0.96 | 0.93 | 0.83 | 0.96 | |
| GWS (18) | 0.79 | 0.93 | 0.47 | 0.77 | 0.83 | 0.86 | 0.41 | 0.73 | 0.71 | 0.86 | 0.83 | 0.88 | 0.50 | 0.60 | 0.58 | 0.63 | 0.94 | 0.93 | 0.98 | 0.97 | 0.77 | 0.98 | |
| TGW (19) | 0.66 | 0.91 | 0.45 | 0.74 | 0.83 | 0.88 | 0.46 | 0.65 | 0.54 | 0.82 | 0.62 | 0.69 | 0.56 | 0.67 | 0.47 | 0.67 | 0.71 | 0.86 | 0.92 | 0.90 | 0.76 | 0.92 | |
| GY (20) | 0.70 | 0.85 | 0.60 | 0.86 | 0.87 | 0.88 | 0.46 | 0.72 | 0.75 | 0.95 | 0.93 | 0.90 | 0.52 | 0.64 | 0.64 | 0.76 | 0.87 | 0.91 | 0.80 | 0.98 | 0.82 | 1.00 | |
| BY (21) | 0.71 | 0.83 | 0.62 | 0.89 | 0.87 | 0.84 | 0.44 | 0.65 | 0.74 | 0.92 | 0.89 | 0.87 | 0.49 | 0.60 | 0.63 | 0.78 | 0.86 | 0.90 | 0.80 | 0.99 | 0.71 | 0.98 | |
| HI (22) | 0.62 | 0.81 | 0.50 | 0.71 | 0.76 | 0.85 | 0.44 | 0.80 | 0.74 | 0.92 | 0.94 | 0.88 | 0.50 | 0.65 | 0.60 | 0.60 | 0.82 | 0.83 | 0.69 | 0.91 | 0.82 | 0.82 | |
| IWUE (23) | 0.70 | 0.85 | 0.60 | 0.86 | 0.87 | 0.88 | 0.46 | 0.72 | 0.75 | 0.95 | 0.93 | 0.90 | 0.52 | 0.64 | 0.64 | 0.76 | 0.87 | 0.91 | 0.80 | 1.00 | 0.99 | 0.91 |
3. Discussion
4. Materials and Methods
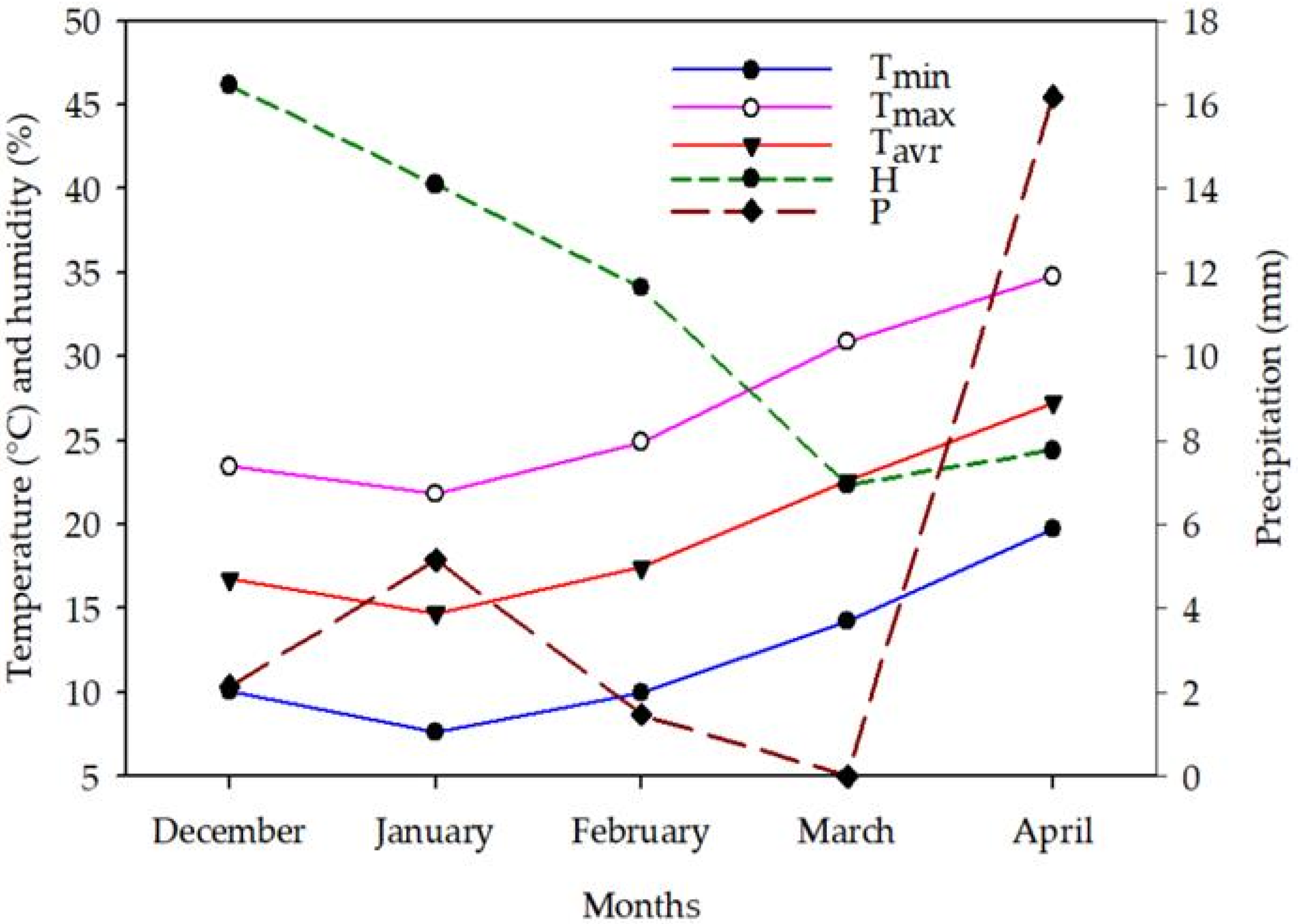
4.1. Experimental Site and Conditions
4.2. Experimental Design and Treatments
4.3. Crop Husbandry
4.4. Data Recorded
4.4.1. Vegetative Growth Parameters
4.4.2. Yield Parameters
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosa, L.; Chiarelli, D.D.; Rulli, M.C.; Dell’Angelo, J.; D’Odorico, P. Global agricultural economic water scarcity. Sci. Adv. 2020, 6, eaaz6031. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Organization of the United Nations Statistics Database. Available online: http://faostat.fao.org/site/567/DesktopDefault.aspx (accessed on 21 June 2022).
- Fereres, F.; Soriano, M.A. Deficit irrigation for reducing agricultural water use. J. Exp. Bot. 2007, 58, 147–159. [Google Scholar] [CrossRef] [PubMed]
- El-Hendawy, S.; Hassan, W.; Al-Suhaibani, N.; Schmidhalter, U. Spectral assessment of drought tolerance indices and grain yield in advanced spring wheat lines grown under full and limited water irrigation. Agric. Water Manag. 2017, 182, 1–12. [Google Scholar] [CrossRef]
- Comas, L.H.; Trout, T.J.; DeJonge, K.C.; Zhang, H.; Gleason, S.M. Water productivity under strategic growth stage- based deficit irrigation in maize. Agric. Water Manag. 2019, 212, 433–440. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Al-Suhaibani, N.; Elsayed, S.; Refay, Y.; Alotaibi, M.; Dewir, Y.H.; Hassan, W.; Schmidhalter, U. Combining biophysical parameters, spectral indices and multivariate hyperspectral models for estimating yield and water productivity of spring wheat across different agronomic practices. PLoS ONE 2019, 14, e0212294. [Google Scholar]
- Azmat, A.; Yasmin, H.; Hassan, M.N.; Nosheen, A.; Naz, R.; Sajjad, M.; Ilyas, N.; Akhtar, M.N. Co-application of bio-fertilizer and salicylic acid improves growth, photosynthetic pigments and stress tolerance in wheat under drought stress. Peer J. 2020, 8, e9960. [Google Scholar] [CrossRef]
- Hafez, E.M.; Osman, H.S.; Gowayed, S.M.; Okasha, S.A.; Omara, A.E.D.; Sami, R.; Abd El-Monem, A.M.; Abd El-Razek, U.A. Minimizing the adversely impacts of water deficit and soil salinity on maize growth and productivity in response to the application of plant growth-promoting rhizobacteria and silica nanoparticles. Agronomy 2021, 11, 676. [Google Scholar] [CrossRef]
- Jesus, C.; Meijón, M.; Monteiro, P.; Correia, B.; Amaral, J.; Escandón, M.; Cañal, M.J.; Pinto, G. Salicylic acid application modulates physiological and hormonal changes in Eucalyptus globulus under water deficit. Environ. Exp. Bot. 2015, 118, 56–66. [Google Scholar] [CrossRef]
- Chai, Q.; Gan, Y.; Zhao, C.; Xu, H.L.; Waskom, R.M.; Niu, Y.; Siddique, K.H.M. Regulated deficit irrigation for crop production under drought stress. A review. Agron. Sustain. Dev. 2016, 36, 3. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Ilyas, N.; Gull, R.; Mazhar, R.; Saeed, M.; Kanwal, S.; Shabir, S.; Bibi, F. Influence of salicylic acid and jasmonic acid on wheat under drought stress. Commun. Soil Sci. Plant Anal. 2017, 48, 2715–2723. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Hashim, A.M.; Alharbi, B.M.; Abdulmajeed, A.M.; Elkelish, A.; Hozzein, W.N.; Hassan, H.M. Oxidative stress responses of some endemic plants to high altitudes by intensifying antioxidants and secondary metabolites content. Plants 2020, 9, 869. [Google Scholar] [CrossRef] [PubMed]
- Tayyab, N.; Naz, R.; Yasmin, H.; Nosheen, A.; Keyani, R.; Sajjad, M.; Hassan, M.N.; Roberts, T.H. Combined seed and foliar pre-treatments with exogenous methyl jasmonate and salicylic acid mitigate drought induced stress in maize. PLoS ONE 2020, 15, e0232269. [Google Scholar] [CrossRef]
- Dutta, T.; Neelapu, N.R.; Wani, S.H.; Challa, S. Compatible solute engineering of crop plants for improved tolerance toward abiotic stresses. In Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress Tolerance in Plants; Elsevier: London, UK, 2018; pp. 221–254. [Google Scholar]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal. Behav. 2021, 16, 8. [Google Scholar] [CrossRef]
- Maruri-López, I.; Aviles-Baltazar, N.Y.; Buchala, A.; Serrano, M. Intra and extracellular journey of the phytohormone salicylic acid. Front. Plant Sci. 2019, 10, 423. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A.; Sareer, O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. South Afr. J. Bot. 2015, 98, 84–94. [Google Scholar] [CrossRef]
- Razmi, N.; Ebadi, A.; Daneshian, J.; Jahanbakhsh, S. Salicylic acid induced changes on antioxidant capacity, pigments and grain yield of soybean genotypes in water deficit condition. J. Plant Interact. 2017, 12, 457–464. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Matin, M.A.; Fardus, J.; Hasanuzzaman, M.; Hossain, M.S.; Parvin, K. Foliar application of salicylic acid improves growth and yield attributes by upregulating the antioxidant defense system in Brassica campestris plants grown in lead-amended soils. Acta Agrobot. 2019, 72, 1765. [Google Scholar] [CrossRef]
- Hafez, E.M.; Kheir, A.; Badawy, S.A.; Rashwan, E.; Farig, M.; Osman, H.S. Differences in physiological and biochemical attributes of wheat in response to single and combined salicylic acid and biochar subjected to limited water irrigation in saline sodic soil. Plants 2020, 9, 1346. [Google Scholar] [CrossRef] [PubMed]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Brestic, M.; Afrin, S.; Sakil, M.A.; Hossain, M.T.; Hossain, M.A.; Hossain, M.A. Exogenous salicylic acid and hydrogen peroxide attenuate drought stress in rice. Plant Soil Environ. 2020, 66, 7–13. [Google Scholar] [CrossRef]
- Kareem, F.; Rihan, H.; Fuller, M. The effect of exogenous applications of salicylic acid and molybdenum on the tolerance of drought in wheat. Agric. Res. Techno. 2017, 9, 97–105. [Google Scholar]
- Korkmaz, A.; Uzunlu, M.; Demirkiran, A.R. Treatment with acetyl salicylic acid protects muskmelon seedlings against drought stress. Acta Physiol. Plant. 2007, 29, 503–508. [Google Scholar] [CrossRef]
- Mevada, K.; Niwas, C.R.; Saiyad, M. Influence of seed soaking and foliar spray of stress mitigating bio-regulators on growth and yield attributes, yield and economics of durum wheat under conserved soil moisture condition. Int. J. Chem. Stud. 2020, 8, 1404–1409. [Google Scholar]
- Farooq, M.; Basra, S.; Wahid, A.; Ahmad, N.; Saleem, B. Improving the drought tolerance in rice (Oryza sativa L.) by exogenous application of salicylic acid. J. Agron. Crop Sci. 2009, 195, 237–246. [Google Scholar] [CrossRef]
- Kang, G.; Li, G.; Xu, W.; Peng, X.; Han, Q.; Zhu, Y.; Guo, T. Proteomics reveals the effects of salicylic acid on growth and tolerance to subsequent drought stress in wheat. J. Proteome Res. 2012, 11, 6066–6079. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011, 168, 807–815. [Google Scholar] [CrossRef]
- Parveen, A.; Ashraf, M.A.; Hussain, I.; Perveen, S.; Rasheed, R.; Mahmood, Q.; Hussain, S.; Ditta, A.; Hashem, A.; Al-Arjani, A.F.; et al. Promotion of growth and physiological characteristics in water-stressed Triticum aestivum in relation to foliar-application of salicylic acid. Water 2021, 13, 1316. [Google Scholar] [CrossRef]
- Senaratna, T.; Merritt, D.; Dixon, K.; Bunn, E.; Touchell, D.; Sivasithamparam, K. Benzoic acid may act as the functional group in salicylic acid and derivatives in the induction of multiple stress tolerance in plants. Plant Growth Regul. 2003, 39, 77–81. [Google Scholar] [CrossRef]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant Physiol. 2003, 160, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef] [PubMed]
- El-Hendawy, S.; Alsamin, B.; Mohammed, N.; Al-Suhaibani, N.; Refay, Y.; Alotaibi, M.; Tola, E.; Mattar, M.A. Combining planting patterns with mulching bolsters the soil water content, growth, yield, and water use efficiency of spring wheat under limited water supply in arid regions. Agronomy 2022, 12, 1298. [Google Scholar] [CrossRef]
- Outoukarte, I.; El Keroumi, A.; Dihazi, A.; Naamani, K. Use of morpho-physiological parameters and biochemical markers to select drought tolerant genotypes of durum wheat. J. Plant Stress Phys. 2019, 5, 1–7. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Meijón, M.; Ferreira, H.; Pinto, G.; Moutinho-Pereira, J.; Correia, C. Salicylic acid modulates olive tree physiological and growth responses to drought and re-watering events in a dose dependent manner. J. Plant Physiol. 2018, 230, 21–32. [Google Scholar] [CrossRef]
- Raza, A.; Mehmood, S.S.; Tabassum, J.; Batool, R. Targeting plant hormones to develop abiotic stress resistance in wheat. In Wheat Production in Changing Environments; Hasanuzzaman, M., Nahar, K., Hossain, M., Eds.; Springer: Singapore, 2019. [Google Scholar]
- González-Villagra, J.; Reyes-Díaz, M.M.; Tighe-Neira, R.; Inostroza-Blancheteau, C.; Escobar, A.L.; Bravo, L.A. Salicylic acid improves antioxidant defense system and photosynthetic performance in Aristotelia chilensis plants subjected to moderate drought stress. Plants 2022, 11, 639. [Google Scholar] [CrossRef]
- Khan, F.S.; Gan, Z.M.; Li, E.Q.; Ren, M.K.; Hu, C.G.; Zhang, J.Z. Transcriptomic and physiological analysis reveals interplay between salicylic acid and drought stress in citrus tree floral initiation. Planta 2022, 255, 24. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Ashraf, M.; Arvin, M.J. Alleviation of field water stress in wheat cultivars by using silicon and salicylic acid applied separately or in combination. Crop. Pasture Sci. 2019, 70, 36–43. [Google Scholar] [CrossRef]
- Khalvandi, M.; Siosemardeh, A.; Roohi, E.; Keramati, S. Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon 2021, 7, e05908. [Google Scholar] [CrossRef]
- Bakry, B.; El-Hariri, D.; Sadak, M.; El-Bassiouny, H. Drought stress mitigation by foliar application of salicylic acid in two linseed varieties grown under newly reclaimed sandy soil. J. Appl. Sci. Res. 2012, 8, 3503–3514. [Google Scholar]
- Munsif, F.; Shah, T.; Arif, M.; Jehangir, M.; Afridi, M.Z.; Ahmad, I.; Jan, B.L.; Alansi, S. Combined effect of salicylic acid and potassium mitigates drought stress through the modulation of physio-biochemical attributes and key antioxidants in wheat. Saudi J. Biolo. Sci. 2022, 29, 103294. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Corpas, F.J.; Ahmad, P. Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J. Hazard. Mater. 2020, 399, 123020. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.S.M.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Aires, E.S.; Ferraz, A.K.L.; Carvalho, B.L.; Teixeira, F.P.; Putti, F.F.; de Souza, E.P.; Rodrigues, J.D.; Ono, E.O. Foliar application of salicylic acid to mitigate water stress in tomato. Plants 2022, 11, 1775. [Google Scholar] [CrossRef]
- Baninasab, B. Induction of drought tolerance by salicylic acid in seedlings of cucumber (Cucumis sativus L.). J. Hortic. Sci. Biotechnol. 2010, 85, 191–196. [Google Scholar] [CrossRef]
- Bandurska, H. An Salicylic Acid: Update on biosynthesis and action in plant response to water deficit and performance under drought. In Salicylic Acid: Plant Growth and Development, 1st ed.; Hayat, S., Ahmad, A., Alyemeni, M.N., Eds.; Springer: Cham, Switzerland, 2013; Volume 1, pp. 1–14. [Google Scholar]
- Chakma, R.; Biswas, A.; Saekong, P.; Ullah, H.; Datta, A. Foliar application and seed priming of salicylic acid affect growth, fruit yield, and quality of grape tomato under drought stress. Sci. Hortic. 2021, 280, 109904. [Google Scholar] [CrossRef]
- Souri, M.K.; Tohidloo, G. Effectiveness of different methods of salicylic acid application on growth characteristics of tomato seedlings under salinity. Chem. Biol. Technol. Agric. 2019, 6, 26. [Google Scholar] [CrossRef]
- Allen, R.; Pereira, L.; Raes, D.; Smith, M. Crop Evapotranspiration. Guidelines for Computing Crop Water Requirements; Irrigation and Drainage Paper no. 56; FAO: Rome, Italy, 1998; p. 300. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach; No. 519.5 S8; McGraw Hill College: New York, NY, USA, 1997. [Google Scholar]
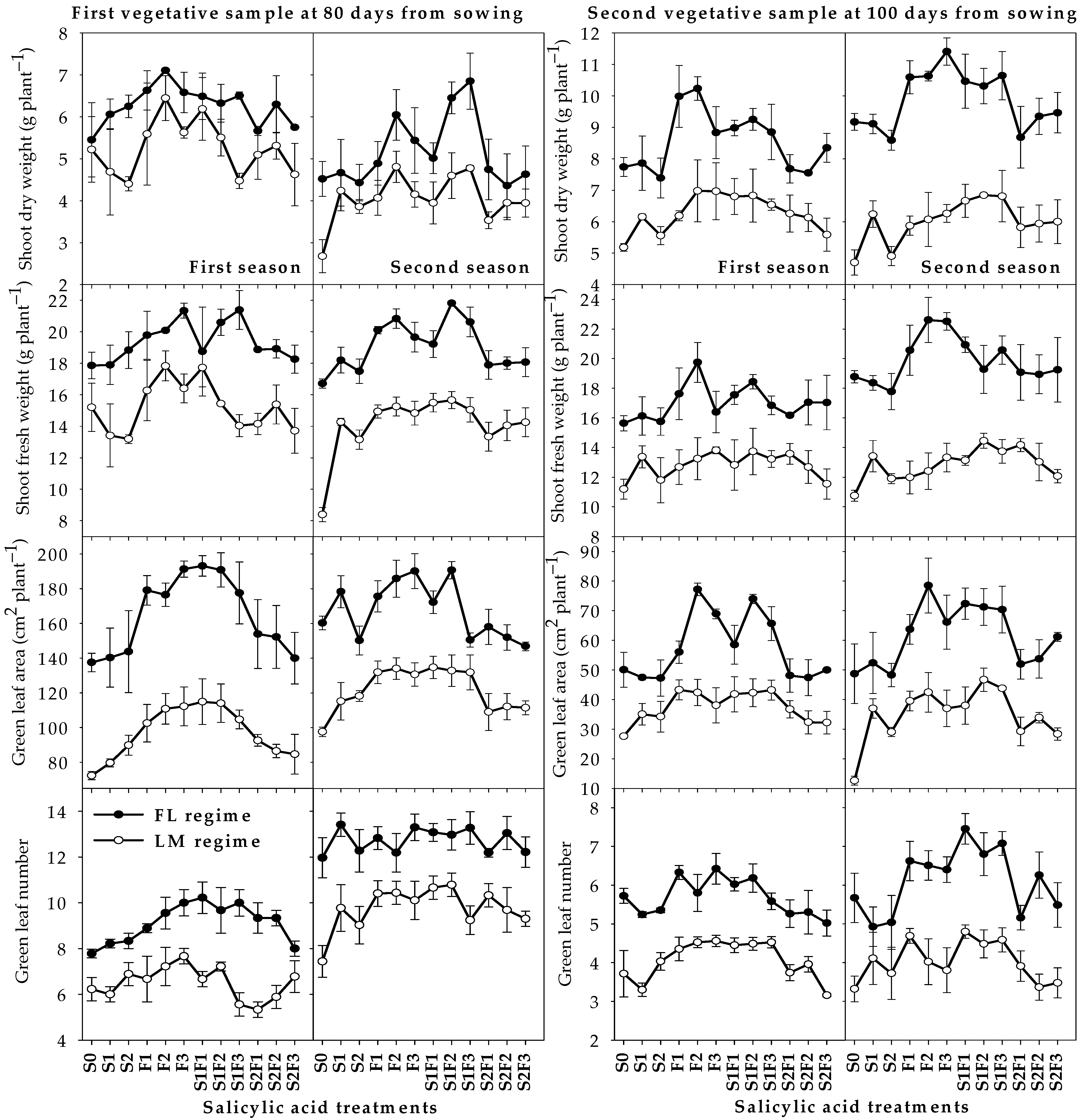
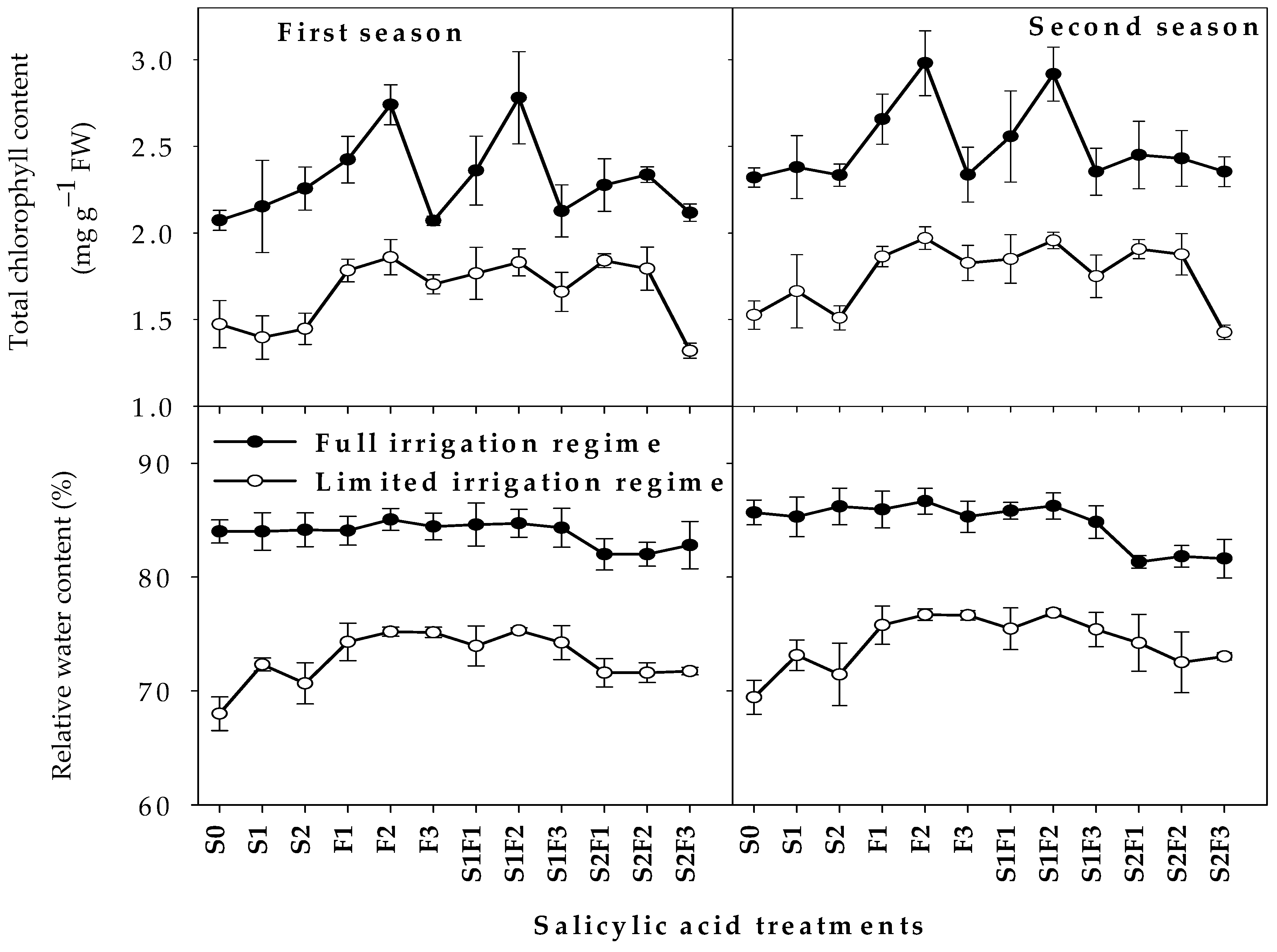
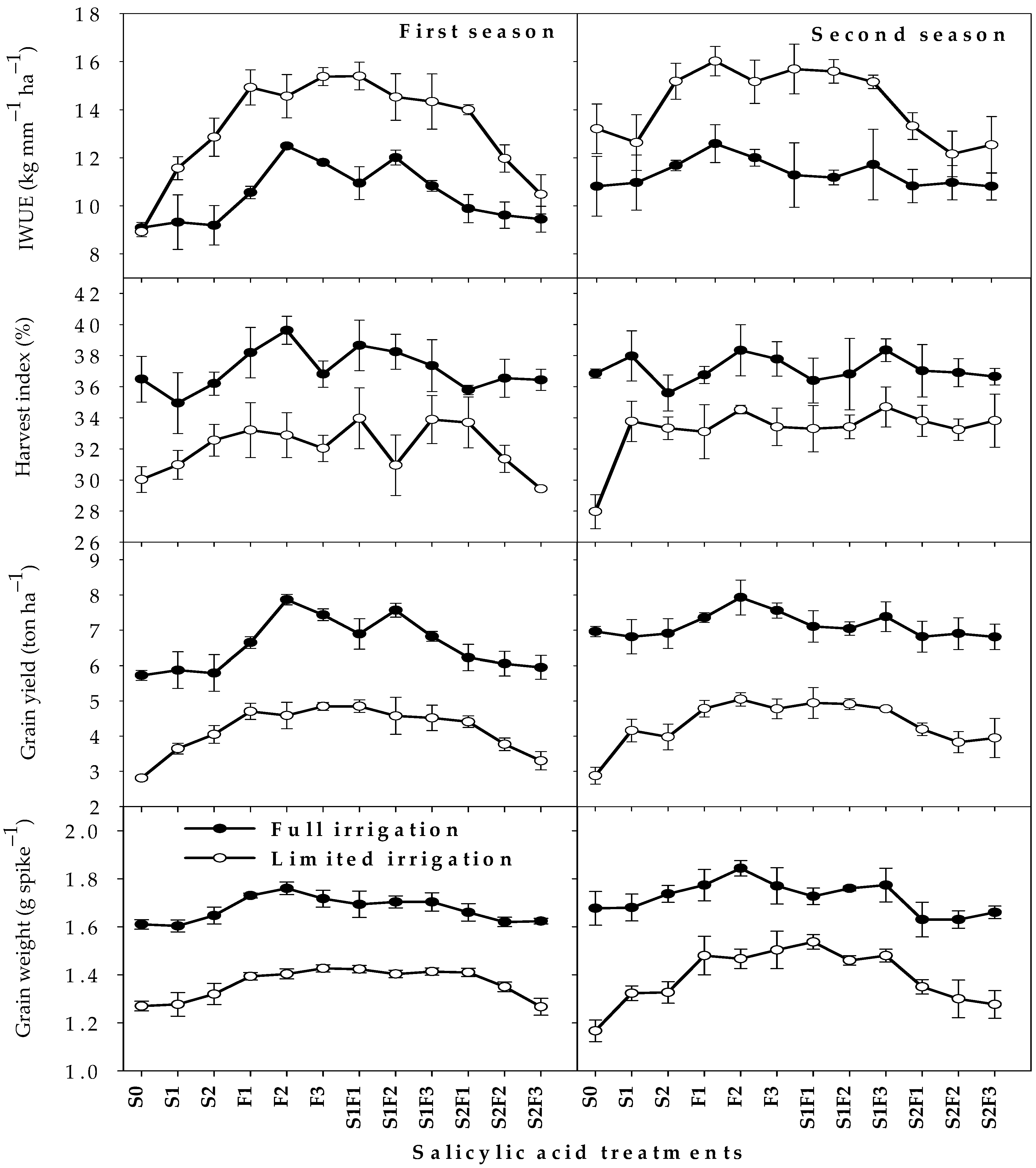
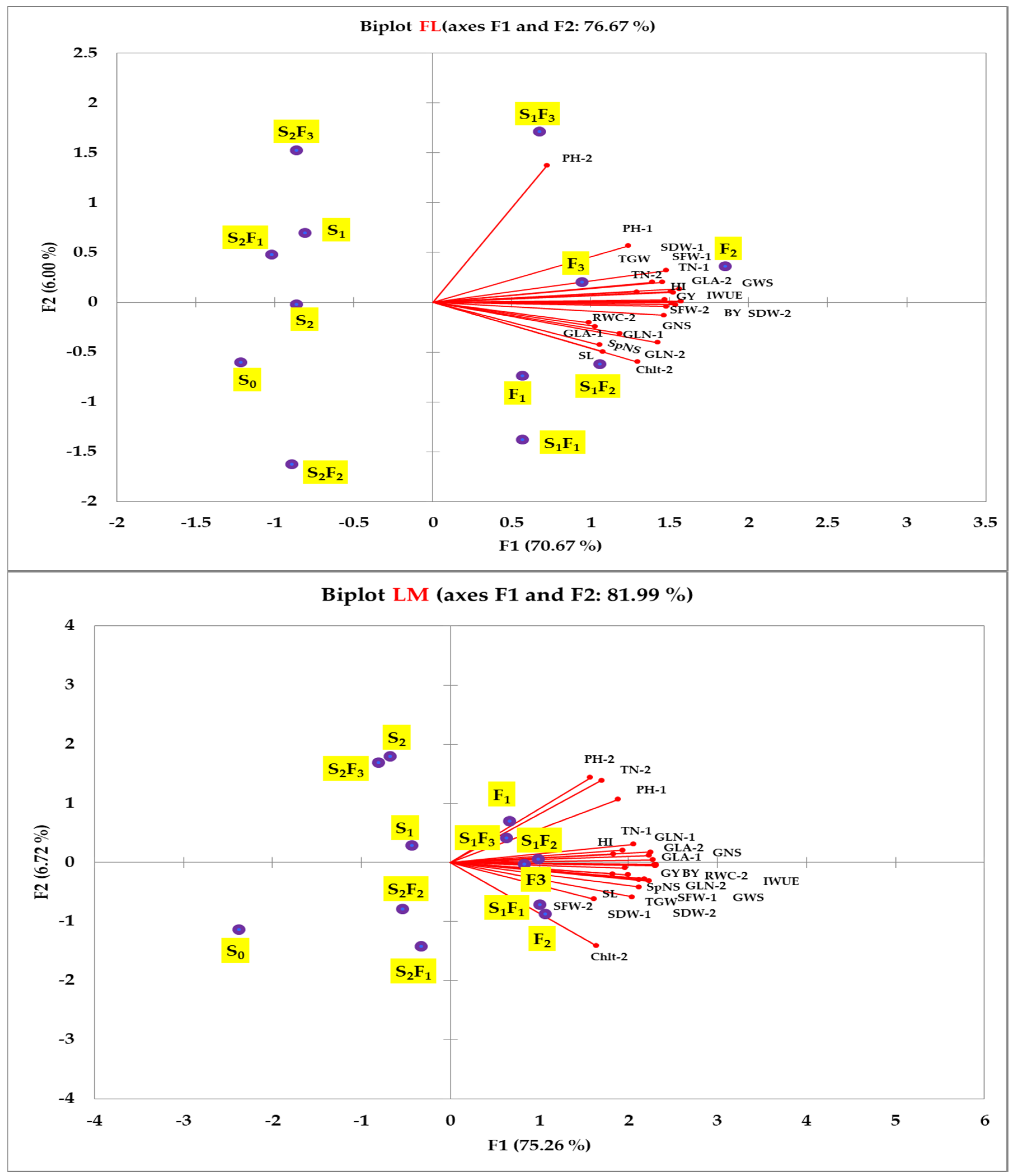
| Par. | First Season (2019/2020) | Second Season (2020/2021) | ||||||
|---|---|---|---|---|---|---|---|---|
| IR | SA | IR × SA | Error | IR | SA | IR × SA | Error | |
| DF | 1 | 11 | 11 | 44 | 1 | 11 | 11 | 44 |
| First vegetative sample at 80 days from sowing | ||||||||
| PH | 3007.78 *** | 4.31 ns | 0.490 ns | 2.40 | 3108.92 * | 16.74 * | 8.76 ns | 8.25 |
| TN | 1.78 * | 0.406 ns | 0.097 ns | 0.225 | 14.81 ** | 1.74 *** | 0.030 ns | 0.168 |
| GLN | 121.91 ** | 2.08 *** | 1.60 *** | 0.314 | 157.95 ** | 2.32 *** | 1.12 * | 0.437 |
| GLA | 82,246.9 *** | 1988.26 *** | 119.48 * | 129.55 | 37,967.1 ** | 999.54 *** | 288.35 *** | 66.23 |
| SFW | 310.13 ** | 7.99 *** | 4.24 * | 1.61 | 449.80 *** | 16.17 *** | 2.60 *** | 0.665 |
| SDW | 17.82 * | 1.47 *** | 0.458 * | 0.240 | 22.75 * | 2.62 *** | 0.489 * | 0.285 |
| Second vegetative sample at 100 days from sowing | ||||||||
| PH | 3631.22 *** | 4.78 ns | 0.571 ns | 2.44 | 3430.13 * | 23.19 ns | 23.55 ns | 12.28 |
| TN | 3.43 * | 0.189 ns | 0.112 ns | 0.114 | 29.79 * | 0.585 ns | 0.285 ns | 0.299 |
| GLN | 47.27 *** | 1.25 *** | 0.153 * | 0.070 | 78.83 ** | 2.30 *** | 0.629 * | 0.254 |
| GLA | 7277.00 ** | 351.56 *** | 103.48 *** | 25.75 | 12,876.92 ** | 499.69 *** | 72.97 * | 33.22 |
| SFW | 320.93 * | 4.38 *** | 2.15 * | 1.00 | 887.54 ** | 5.95 ** | 4.78 ** | 1.74 |
| SDW | 94.65 * | 2.99 *** | 0.784 * | 0.319 | 267.54 *** | 3.04 *** | 0.787 * | 0.369 |
| RWC | 2181.19 *** | 12.45 *** | 5.67 ** | 1.80 | 1987.97 ** | 17.52 *** | 10.44 *** | 2.12 |
| Chlt | 7.68 * | 0.234 *** | 0.052 ** | 0.015 | 9.99 * | 0.219 *** | 0.043 ** | 0.014 |
| Par. | First Season (2019/2020) | Second Season (2020/2021) | ||||
|---|---|---|---|---|---|---|
| FL | LM | Change (%) | FL | LM | Change (%) | |
| First Vegetative Sample at 80 Days from Sowing | ||||||
| PH | 84.66 a | 71.74 b | 15.3 | 85.79 a | 72.65 b | 15.3 |
| TN | 5.23 a | 4.92 b | 5.9 | 4.69 a | 3.79 b | 19.2 |
| GLN | 9.11 a | 6.51 b | 28.6 | 12.73 a | 9.77 b | 23.3 |
| GLA | 164.67 a | 97.08 b | 41.0 | 167.55 a | 121.62 b | 27.4 |
| SFW | 19.38 a | 15.23 b | 21.4 | 19.05 a | 14.06 b | 26.2 |
| SDW | 6.26 a | 5.26 b | 16.0 | 5.17 a | 4.05 b | 21.7 |
| Second vegetative sample at 100 days from sowing | ||||||
| PH | 88.51 a | 74.31 b | 16.0 | 88.90 a | 75.09 b | 15.5 |
| TN | 4.88 a | 4.44 b | 9.0 | 5.18 a | 3.89 b | 24.9 |
| GLN | 5.69 a | 4.07 b | 28.5 | 6.12 a | 4.02 b | 34.3 |
| GLA | 57.65 a | 37.43 b | 35.1 | 61.54 a | 34.80 b | 43.5 |
| SFW | 17.03 a | 12.81 b | 24.8 | 19.89 a | 12.87 b | 35.3 |
| SDW | 8.56 a | 6.26 b | 26.9 | 9.87 a | 6.01 b | 39.1 |
| RWC | 83.85 a | 72.84 b | 13.1 | 84.73 a | 74.22 b | 12.4 |
| Chlt | 2.31 a | 1.66 b | 28.3 | 2.51 a | 1.76 b | 29.7 |
| Par. | S0 | S1 | S2 | F1 | F2 | F3 | S1F1 | S1F2 | S1F3 | S2F1 | S2F2 | S2F3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Vegetative Sample at 80 Days from Sowing | ||||||||||||
| First Season (2019/2020) | ||||||||||||
| PH | 76.67 ns | 77.49 ns | 78.43 ns | 78.25 ns | 79.04 ns | 79.19 ns | 78.95 ns | 78.74 ns | 78.18 ns | 77.92 ns | 76.77 ns | 78.77 ns |
| TN | 5.22 ns | 5.05 ns | 5.17 ns | 5.00 ns | 5.12 ns | 5.17 ns | 5.22 ns | 5.17 ns | 5.61 ns | 4.72 ns | 4.67 ns | 4.78 ns |
| GLN | 7.00 d | 7.11 d | 7.61 cd | 7.78 bc | 8.39 ab | 8.84 a | 8.45 a | 8.45 a | 7.78 bc | 7.33 cd | 7.61 cd | 7.39 cd |
| GLA | 104.9 d | 110.0 cd | 116.8 bcd | 140.8 a | 143.7 a | 151.8 a | 154.0 a | 152.4 a | 141.1 a | 123.3 b | 119.4 bc | 112.3 bcd |
| SFW | 16.53 cde | 15.66 e | 16.02 de | 18.03 ab | 18.96 a | 18.87 a | 18.24 ab | 18.02 ab | 17.72 abc | 16.51 cde | 17.15 bcd | 15.99 de |
| SDW | 5.34 de | 5.37 cde | 5.33 de | 6.11 b | 6.78 a | 6.10 b | 6.34 ab | 5.91 bc | 5.49 cde | 5.38 cde | 5.80 bcd | 5.19 e |
| Second season (2020/2021) | ||||||||||||
| PH | 75.37 b | 78.61 ab | 78.95 a | 81.06 a | 81.00 a | 80.33 a | 78.61 ab | 80.95 a | 80.00 a | 77.84 ab | 78.06 ab | 79.83 a |
| TN | 3.11 d | 4.11 bc | 4.00 c | 4.66 a | 4.94 a | 4.50 ab | 4.50 ab | 4.78 a | 4.72 a | 3.78 c | 4.06 bc | 3.72 c |
| GLN | 9.70 c | 11.59 a | 10.65 b | 11.62 a | 11.31 ab | 11.70 a | 11.87 a | 11.88 a | 11.26 ab | 11.26 ab | 11.37 ab | 10.76 b |
| GLA | 128.9 e | 146.8 bc | 134.2 de | 153.8 ab | 159.9 a | 160.4 a | 153.5 ab | 161.8 a | 141.2 cd | 133.5 de | 132.0 de | 129.1 e |
| SFW | 12.55 d | 16.24 c | 15.32 c | 17.52 b | 18.05 ab | 17.24 b | 17.36 b | 18.74 a | 17.83 ab | 15.62 c | 16.03 c | 16.16 c |
| SDW | 3.60 d | 4.45 bc | 4.15 cd | 4.48 bc | 5.43 a | 4.79 b | 4.48 bc | 5.52 a | 5.81 a | 4.14 cd | 4.16 cd | 4.29 bc |
| Second vegetative sample at 100 days from sowing | ||||||||||||
| First season (2019/2020) | ||||||||||||
| PH | 79.79 ns | 80.72 ns | 81.80 ns | 81.19 ns | 82.48 ns | 82.41 ns | 81.98 ns | 82.04 ns | 81.38 ns | 81.14 ns | 79.94 ns | 82.02 ns |
| TN | 4.57 ns | 4.65 ns | 4.61 ns | 4.87 ns | 4.81 ns | 4.79 ns | 4.83 ns | 4.77 ns | 4.70 ns | 4.28 ns | 4.40 ns | 4.62 ns |
| GLN | 4.72 c | 4.27 de | 4.69 c | 5.34 ab | 5.16 b | 5.49 a | 5.24 ab | 5.34 ab | 5.06 b | 4.50 cd | 4.63 c | 4.09 e |
| GLA | 38.84 d | 41.24 d | 40.73 d | 49.64 c | 59.76 a | 53.50 bc | 50.17 c | 58.12 ab | 54.43 abc | 42.40 d | 39.86 d | 41.08 d |
| SFW | 13.42 e | 14.75 cd | 13.78 de | 15.15 bc | 16.50 a | 15.11 bc | 15.19 bc | 16.09 ab | 15.04 bc | 14.87 cd | 14.87 cd | 14.29 cde |
| SDW | 6.46 c | 7.01 c | 6.48 c | 8.09 ab | 8.60 a | 7.90 b | 7.89 b | 8.04 ab | 7.70 b | 6.97 c | 6.84 c | 6.97 c |
| RWC | 76.00 d | 78.16 bc | 77.40 cd | 79.18 ab | 80.12 a | 79.79 a | 79.28 ab | 80.01 a | 79.29 ab | 76.80 c d | 76.80 cd | 77.27 cd |
| Chlt | 1.77 cd | 1.78 cd | 1.85 cd | 2.10 b | 2.30 a | 1.89 c | 2.06 b | 2.31 a | 1.89 c | 2.06 b | 2.07 b | 1.72 d |
| Second season (2020/2021) | ||||||||||||
| PH | 77.06 ns | 82.44 ns | 81.72 ns | 82.95 ns | 82.39 ns | 83.11 ns | 80.72 ns | 82.00 ns | 84.50 ns | 81.89 ns | 80.72 ns | 84.45 ns |
| TN | 3.78 ns | 4.61 ns | 4.50 ns | 4.77 ns | 4.72 ns | 4.50 ns | 4.50 ns | 4.83 ns | 4.94 ns | 4.16 ns | 4.61 ns | 4.50 ns |
| GLN | 4.50 f | 4.52 ef | 4.38 f | 5.66 abc | 5.27 bcd | 5.10 cde | 6.13 a | 5.64 abc | 5.83 ab | 4.54 ef | 4.81 def | 4.48 f |
| GLA | 30.67 d | 44.66 c | 38.66 c | 51.63 b | 60.45 a | 51.59 b | 55.16 ab | 58.98 a | 57.05 ab | 40.60 c | 43.80 c | 44.77 c |
| SFW | 14.76 d | 15.89cd | 14.84 d | 16.28 bcd | 17.51 ab | 17.92 a | 17.03 abc | 16.87 ab | 17.16 ab | 16.62 abc | 15.98 bcd | 15.66 cd |
| SDW | 6.94 e | 7.67 bcd | 6.75 e | 8.23 abc | 8.35 ab | 8.83 a | 8.56 a | 8.58 a | 8.73 a | 7.25 de | 7.64 cd | 7.73 bcd |
| RWC | 77.56 de | 79.21 bcd | 78.83 cde | 80.86 a | 81.68 a | 80.97 a | 80.64 ab | 81.55 a | 80.11 bc | 77.77 de | 77.17 e | 77.32 e |
| Chlt | 1.92 fg | 2.02 efg | 1.92 fg | 2.26 b | 2.48 a | 2.08 cde | 2.20 bc | 2.44 a | 2.05 def | 2.18 bcd | 2.15 bcde | 1.89 g |
| Par. | First Season (2019/2020) | Second Season (2020/2021) | ||||||
|---|---|---|---|---|---|---|---|---|
| IR | SA | IR × SA | Error | IR | SA | IR × SA | Error | |
| DF | 1 | 11 | 11 | 44 | 1 | 11 | 11 | 44 |
| SL | 13.09 ** | 0.472 ** | 0.052 ns | 0.155 | 2.27 ** | 0.100 * | 0.039 ns | 0.048 |
| SpNS | 8.27 * | 0.148 ns | 0.111 ns | 0.170 | 7.61 ** | 0.379 ** | 0.162 ns | 0.122 |
| GNS | 312.38 ** | 4.30 * | 0.577 ns | 2.07 | 449.60 ** | 11.54 *** | 4.63 ns | 1.29 |
| GWS | 1.72 ** | 0.018 *** | 0.002 * | 7.82 | 1.99 *** | 0.042 *** | 0.010 ** | 0.003 |
| TGW | 262.63 ** | 3.07 ** | 1.66 ns | 0.925 | 230.73 ** | 4.74 *** | 1.87 ns | 1.26 |
| GY | 103.58 *** | 2.63 *** | 0.361 ** | 0.099 | 139.11 ** | 1.23 *** | 0.375 * | 0.169 |
| BY | 400.59 *** | 14.34 *** | 1.35 ns | 0.700 | 680.56 *** | 6.91 *** | 2.10 ns | 1.09 |
| HI | 455.42 ** | 8.59 *** | 3.86 * | 1.81 | 276.75 * | 6.81 ** | 4.16 * | 2.40 |
| IWUE | 142.58 ** | 14.40 *** | 2.98 *** | 0.530 | 112.15 * | 8.81 *** | 4.51 *** | 0.871 |
| Treatments | SL | SpNS | GNS | GWS | TGW | GY | BY | HI | IWUE |
|---|---|---|---|---|---|---|---|---|---|
| First season (2019/2020) | |||||||||
| S0 | 8.17 c | 16.28 ns | 42.83 c | 1.44 d | 32.67 d | 4.27 e | 12.55 f | 33.26 de | 9.00 g |
| S1 | 8.52 bc | 16.40 ns | 43.66 bc | 1.44 d | 33.56 cd | 4.76 d | 14.28 e | 32.96 e | 10.44 ef |
| S2 | 8.48 bc | 16.12 ns | 44.29 abc | 1.48 c | 33.41 cd | 4.92 d | 14.22 e | 34.38 cde | 11.02 e |
| F1 | 8.68 b | 16.58 ns | 45.58 a | 1.56 ab | 34.17 abc | 5.68 bc | 15.81 c | 35.70 abc | 12.74 bcd |
| F2 | 9.20 a | 16.68 ns | 45.20 ab | 1.58 a | 34.91 ab | 6.23 a | 16.91 ab | 36.26 ab | 13.53 ab |
| F3 | 8.67 b | 16.42 ns | 44.94 ab | 1.57 a | 34.73 ab | 6.14 a | 17.67 a | 34.42 cde | 13.59 a |
| S1F1 | 9.15 a | 16.52 ns | 45.56 a | 1.56 ab | 34.18 abc | 5.87 ab | 16.06 bc | 36.31 a | 13.17 abc |
| S1F2 | 8.75 ab | 16.43 ns | 44.85 ab | 1.55 ab | 35.16 a | 6.07 a | 17.29 a | 34.60 cd | 13.27 abc |
| S1F3 | 8.53 bc | 16.43 ns | 45.52 a | 1.56 ab | 34.17 abc | 5.67 bc | 15.80 c | 35.62 abc | 12.59 cd |
| S2F1 | 8.63 b | 16.22 ns | 44.76 ab | 1.54 b | 34.30 abc | 5.32 c | 15.25 cd | 34.74 bcd | 11.94 d |
| S2F2 | 8.55 bc | 16.37 ns | 43.94 abc | 1.49 c | 33.79 bc | 4.91 d | 14.30 de | 33.95 de | 10.79 ef |
| S2F3 | 8.62 bc | 16.28 ns | 44.39 abc | 1.45 d | 33.35 cd | 4.63 de | 13.78 e | 32.93 e | 9.96 f |
| FL | 9.09 a | 16.73 a | 46.71 a | 1.67 a | 35.94 a | 6.57 a | 17.68 a | 37.11 a | 10.43 b |
| LM | 8.24 b | 16.06 b | 42.54 b | 1.36 b | 32.12 b | 4.17 b | 12.97 b | 32.08 b | 13.24 a |
| Change (%) | 9.4 | 4.0 | 8.9 | 18.6 | 10.6 | 36.5 | 26.7 | 13.6 | −27.0 |
| Second season (2020/2021) | |||||||||
| S0 | 8.55 c | 16.65 d | 42.66 d | 1.42 d | 33.07 d | 4.92 e | 14.59 c | 32.41 c | 10.09 c |
| S1 | 8.77 abc | 17.07 abc | 44.06 c | 1.50 bc | 34.01 cd | 5.49 d | 15.14 c | 35.87 ab | 12.01 b |
| S2 | 8.72 abc | 16.97 bcd | 44.41 bc | 1.53 b | 34.36 abc | 5.44 d | 15.65 bc | 34.46 b | 11.80 b |
| F1 | 8.63 bc | 16.77 cd | 46.04 a | 1.63 a | 35.26 abc | 6.07 ab | 17.24 a | 34.93 ab | 13.43 a |
| F2 | 8.92 a | 17.40 a | 46.48 a | 1.66 a | 35.49 ab | 6.49 a | 17.65 a | 36.43 a | 14.31 a |
| F3 | 8.83 ab | 17.38 a | 46.25 a | 1.64 a | 35.34 ab | 6.17 ab | 17.16 a | 35.60 ab | 13.58 a |
| S1F1 | 8.90 a | 17.08 abc | 45.87 a | 1.63 a | 35.55 a | 6.03 ab | 17.24 a | 34.85 ab | 13.49 a |
| S1F2 | 8.72 abc | 17.20 ab | 45.48 ab | 1.61 a | 35.34 ab | 5.98 bc | 16.94 a | 35.11 ab | 13.39 a |
| S1F3 | 8.59 bc | 16.87 bcd | 45.64 ab | 1.63 a | 35.58 a | 6.08 ab | 16.50 ab | 36.52 a | 13.44 a |
| S2F1 | 8.54 c | 16.65 d | 43.46 cd | 1.49 bc | 34.23 bcd | 5.51 cd | 15.45 bc | 35.42 ab | 12.07 b |
| S2F2 | 8.74 abc | 16.88 bcd | 43.76 cd | 1.47 cd | 33.37 d | 5.37 de | 15.12 c | 35.07 ab | 11.56 b |
| S2F3 | 8.82 ab | 16.92 bcd | 42.72 d | 1.47 cd | 34.24 bcd | 5.38 de | 15.14 c | 35.23 ab | 11.68 b |
| FL | 8.90 a | 17.31 a | 47.23 a | 1.72 a | 36.44 a | 7.13 a | 19.23 a | 37.12 a | 11.32 b |
| LM | 8.55 b | 16.66 b | 42.24 b | 1.39 b | 32.86 b | 4.35 b | 13.08 b | 33.20 b | 13.82 a |
| Change (%) | 3.9 | 3.8 | 10.6 | 19.3 | 9.8 | 39.0 | 32.0 | 10.6 | −22.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, N.; El-Hendawy, S.; Alsamin, B.; Mubushar, M.; Dewir, Y.H. Integrating Application Methods and Concentrations of Salicylic Acid as an Avenue to Enhance Growth, Production, and Water Use Efficiency of Wheat under Full and Deficit Irrigation in Arid Countries. Plants 2023, 12, 1019. https://doi.org/10.3390/plants12051019
Mohammed N, El-Hendawy S, Alsamin B, Mubushar M, Dewir YH. Integrating Application Methods and Concentrations of Salicylic Acid as an Avenue to Enhance Growth, Production, and Water Use Efficiency of Wheat under Full and Deficit Irrigation in Arid Countries. Plants. 2023; 12(5):1019. https://doi.org/10.3390/plants12051019
Chicago/Turabian StyleMohammed, Nabil, Salah El-Hendawy, Bazel Alsamin, Muhammad Mubushar, and Yaser Hassan Dewir. 2023. "Integrating Application Methods and Concentrations of Salicylic Acid as an Avenue to Enhance Growth, Production, and Water Use Efficiency of Wheat under Full and Deficit Irrigation in Arid Countries" Plants 12, no. 5: 1019. https://doi.org/10.3390/plants12051019
APA StyleMohammed, N., El-Hendawy, S., Alsamin, B., Mubushar, M., & Dewir, Y. H. (2023). Integrating Application Methods and Concentrations of Salicylic Acid as an Avenue to Enhance Growth, Production, and Water Use Efficiency of Wheat under Full and Deficit Irrigation in Arid Countries. Plants, 12(5), 1019. https://doi.org/10.3390/plants12051019








