Precision Horticulture: Application of Optical Sensor Technology for Nitrogen Monitoring Status in Cocoplum, a Native Landscaping Plant
Abstract
1. Introduction
2. Results
2.1. Growth Characteristics Relative Chlorophyll Content (atLEAF), and Normalized Difference Vegetation Index (NDVI)
2.2. Relative Chlorophyll Content (SPAD)
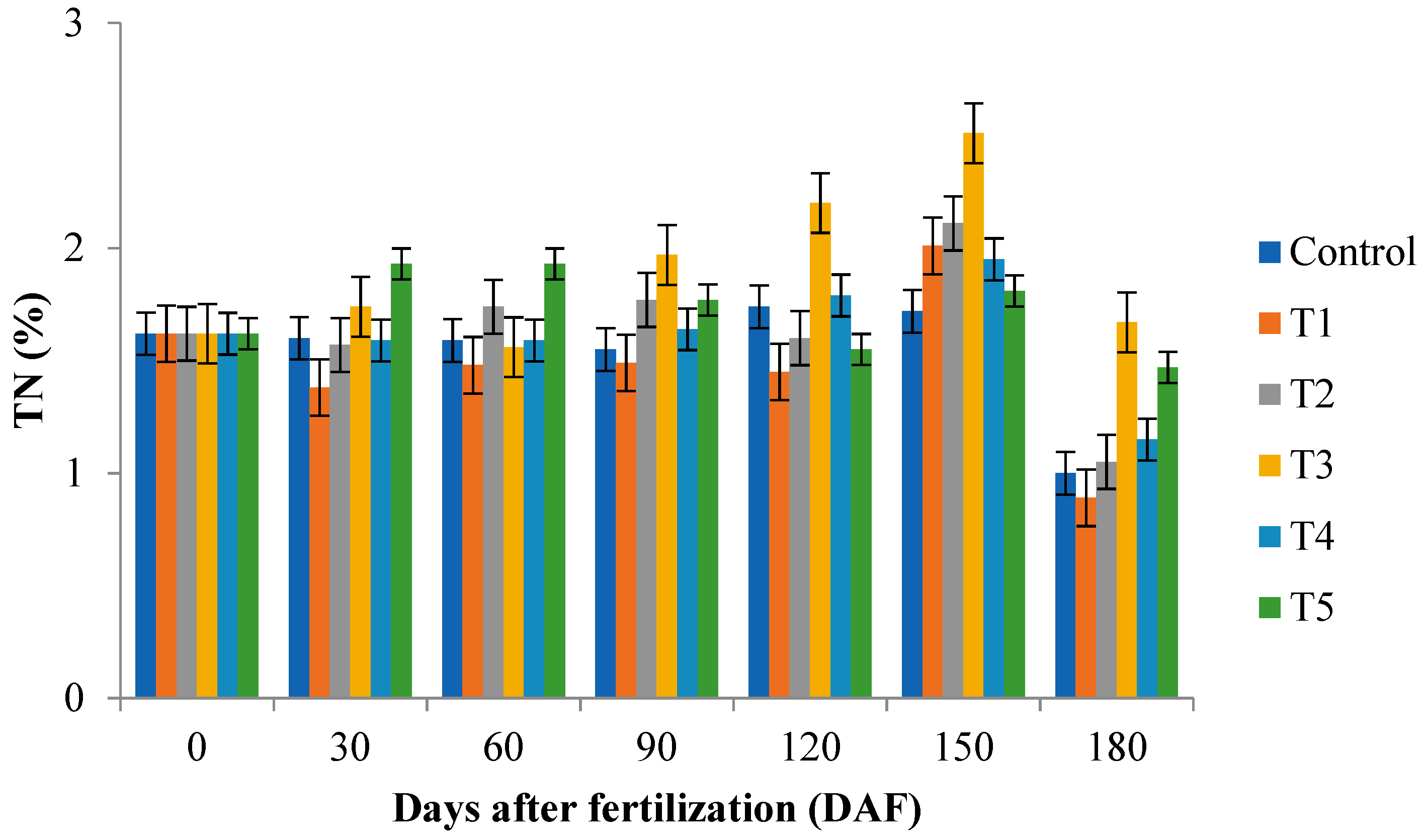
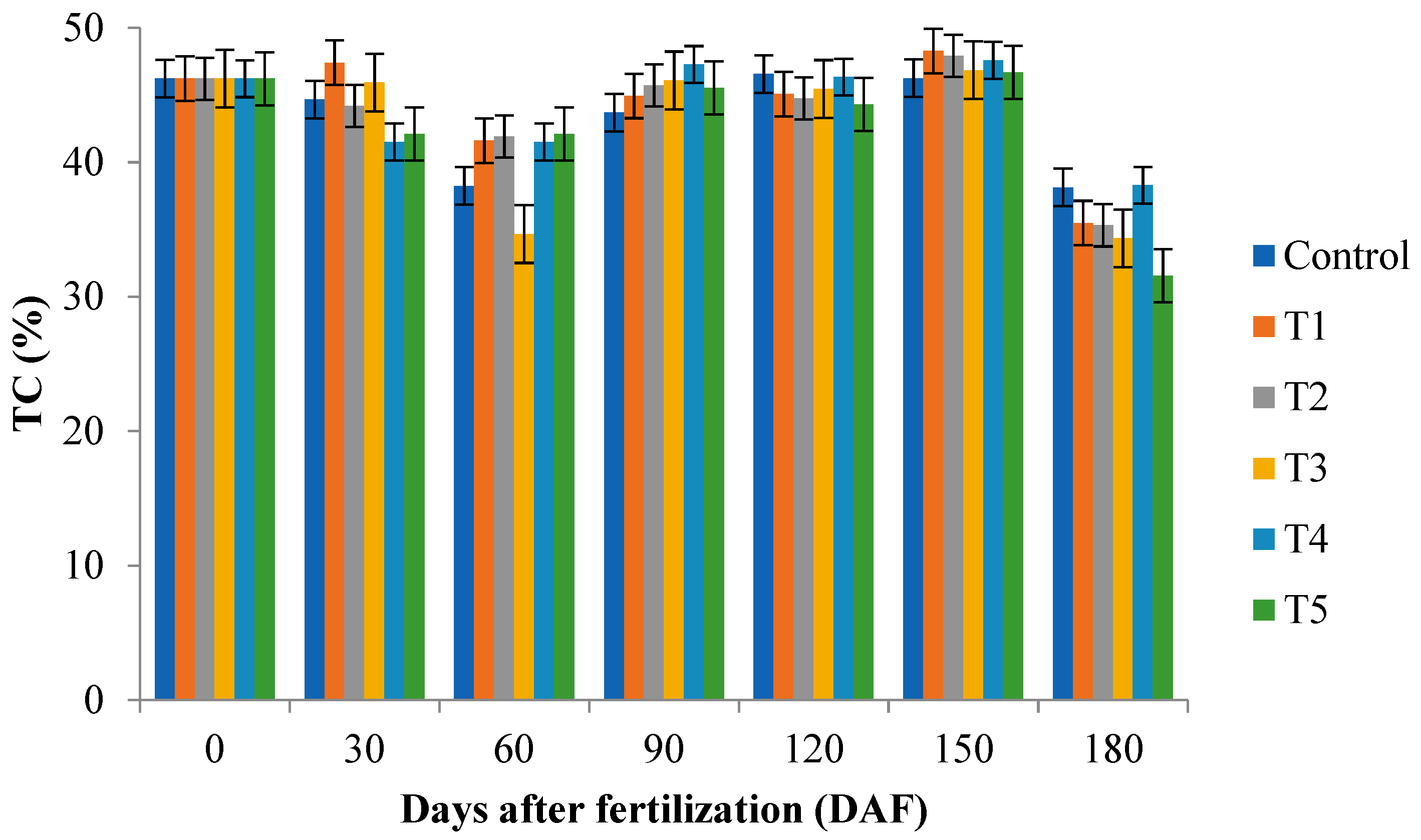
2.3. Total Nitrogen (TN) and Total Carbon (TC) of Leaf and Substrate Samples
2.4. Salt, Electric Conductivity (EC), and Total Nitrogen (TN) of Leachate Samples
2.5. Correlation Coefficient between Sensor Parameters, Number of Leaves (NL), and Total Nitrogen (TN) and Total Carbon (TC) of Leaf Samples
3. Discussion
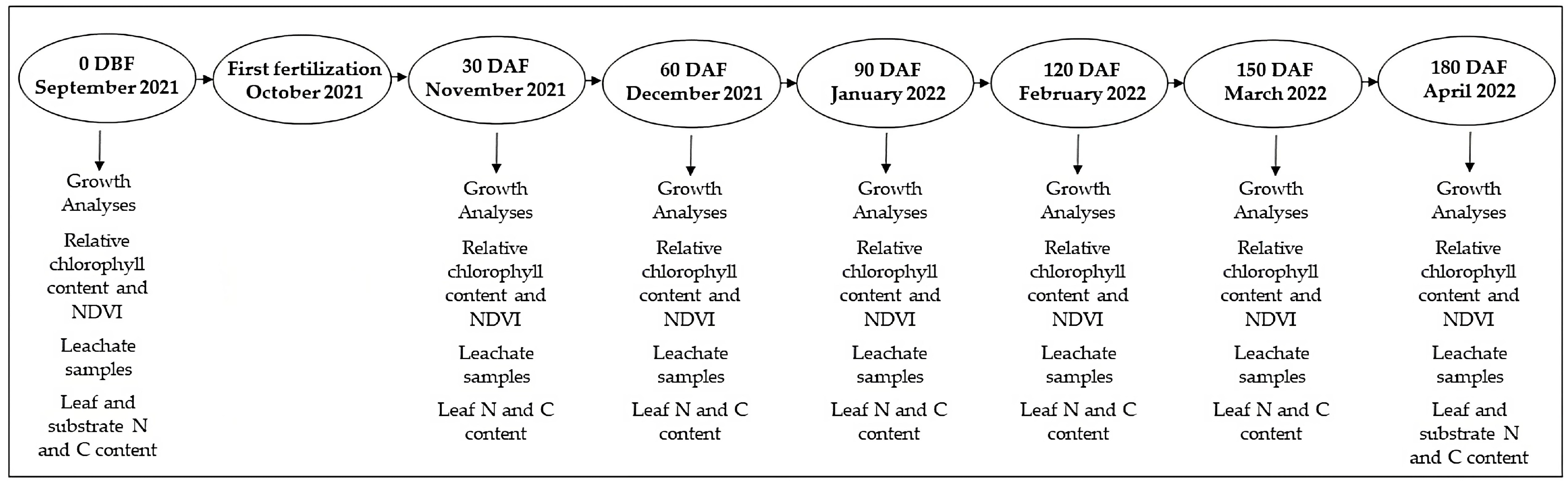
4. Materials and Methods
4.1. Growth Analyses
4.2. Relative Chlorophyll Content and NDVI
4.3. Leachate Samples
4.4. Leaf and Substrate N and C Content
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture. Southern Region News Release Floriculture Production & Sales; USDA: Washington, DC, USA, 2021.
- Brown, S.H.; Frank, M.S. Cocoplum (Chrysobalanusicaco L.) Identification and Uses: ENH1289/EP553, 3/2018. EDIS 2018. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Wang, H.; Huang, S.; Cheng, S.; Khosla, R.; Jiang, R. Non-destructive estimation of rice plant nitrogen status with Crop Circle multispectral active canopy sensor. Field Crop. Res. 2013, 154, 133–144. [Google Scholar] [CrossRef]
- Ju, X.; Kou, C.; Christie, P.; Dou, Z.; Zhang, F. Changes in the soil environment from excessive application of fertilizers and manures to two contrasting intensive cropping systems on the North China Plain. Environ. Pollut. 2007, 145, 497–506. [Google Scholar] [CrossRef]
- Cameron, K.; Di, H.; Moir, J. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Randall, G.W.; Goss, M.J. Nitrate losses to surface water through subsurface, tile drainage. In Nitrogen in the Environment: Sources, Problems and Management; Follett, R.F., Hatfield, J.L., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2001; pp. 95–122. [Google Scholar]
- Department of Regulatory & Economic Resources Division of Environmental Resources Management (DERM). Fertilizer Regu-lations. 2021. Available online: https://www.miamidade.gov/global/service.page?Mduid_service=ser1620843942468395 (accessed on 11 September 2022).
- Lemaire, G.; Jeuffroy, M.-H.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage: Theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Thompson, R.B.; Tremblay, N.; Fink, M.; Gallardo, M.; Padilla, F.M. Tools and strategies for sustainable nitrogen fertilization of vegetable crops. In Advances in Research on Fertilization Management in Vegetable Crops; Tei, F., Nicola, S., Benincasa, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 11–63. [Google Scholar]
- Schnitkey, G.; Swanson, K.; Paulson, N.; Zulauf, C.; Coppess, J.; Baltz, J. Nitrogen Fertilizer Outlook for 2023 Decisions. Department of Agricultural and Consumer Economics, University of Illinois at Urbana-Champaign, 19 July 2022. Farmdoc Dly. 2022, 12, 106. [Google Scholar]
- Kountios, G. The role of agricultural consultants and precision agriculture in the adoption of good agricultural practices and sustainable water management. Int. J. Sustain. Agric. Manag. Inform. 2022, 8, 144. [Google Scholar] [CrossRef]
- Solano-Alvarez, N.; Valencia-Hernández, J.A.; Vergara-Pineda, S.; Millán-Almaraz, J.R.; Torres-Pacheco, I.; Guevara-González, R.G. Comparative Analysis of the NDVI and NGBVI as Indicators of the Protective Effect of Beneficial Bacteria in Conditions of Biotic Stress. Plants 2022, 11, 932. [Google Scholar] [CrossRef]
- Forero, M.G.; Mambuscay, C.L.; Monroy, M.F.; Miranda, S.L.; Méndez, D.; Valencia, M.O.; Selvaraj, M.G. Comparative Analysis of Detectors and Feature Descriptors for Multispectral Image Matching in Rice Crops. Plants 2021, 10, 1791. [Google Scholar] [CrossRef]
- Swaminathan, B.; Palani, S.; Vairavasundaram, S. Fertility level prediction in precision agriculture based on an ensemble classifier model. Int. J. Sustain. Agric. Manag. Inform. 2021, 7, 270. [Google Scholar] [CrossRef]
- Argento, F.; Anken, T.; Abt, F.; Vogelsanger, E.; Walter, A.; Liebisch, F. Site-specific nitrogen management in winter wheat supported by low-altitude remote sensing and soil data. Precis. Agric. 2020, 22, 364–386. [Google Scholar] [CrossRef]
- Russello, H. Convolutional Neural Networks for Crop Yield Prediction. Ph.D. Thesis, University of Amsterdam, Amsterdam, The Netherlands, 2018. [Google Scholar]
- Habib, A.; Han, Y.; Xiong, W.; He, F.; Zhang, Z.; Crawford, M. Automated Ortho-Rectification of UAV-Based Hyperspectral Data over an Agricultural Field Using Frame RGB Imagery. Remote Sens. 2016, 8, 796. [Google Scholar] [CrossRef]
- Basyouni, R.; Dunn, B. Use of Optical Sensors to Monitor Plant Nitrogen Status in Horticultural Plants (HLA-6719-4); Oklahoma Cooperative Extension Service, Stillwater: Oklahoma City, OK, USA, 2013. [Google Scholar]
- Hatfield, J.L.; Gitelson, A.A.; Schepers, J.S.; Walthall, C.L. Application of Spectral Remote Sensing for Agronomic Decisions. Agron. J. 2008, 100, S-117–S-131. [Google Scholar] [CrossRef]
- Knipling, E.B. Physical and physiological basis for the reflectance of visible and near-infrared radiation from vegetation. Remote Sens. Environ. 1970, 1, 155–159. [Google Scholar] [CrossRef]
- Peñuelas, J.; Gamon, J.; Fredeen, A.; Merino, J.; Field, C. Reflectance indices associated with physiological changes in nitrogen- and water-limited sunflower leaves. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Ollinger, S.V. Sources of variability in canopy reflectance and the convergent properties of plants. New Phytol. 2010, 189, 375–394. [Google Scholar] [CrossRef] [PubMed]
- Bannari, A.; Morin, D.; Bonn, F.; Huete, A.R. A review of vegetation indices. Remote Sens. Rev. 1995, 13, 95–120. [Google Scholar] [CrossRef]
- Scotford, I.; Miller, P. Applications of Spectral Reflectance Techniques in Northern European Cereal Production: A Review. Biosyst. Eng. 2005, 90, 235–250. [Google Scholar] [CrossRef]
- Sellers, P.J. Canopy reflectance, photosynthesis and transpiration. Int. J. Remote Sens. 1985, 6, 1335–1372. [Google Scholar] [CrossRef]
- Freidenreich, A.; Barraza, G.; Jayachandran, K.; Khoddamzadeh, A.A. Precision Agriculture Application for Sustainable Nitrogen Management of Justicia brandegeana Using Optical Sensor Technology. Agriculture 2019, 9, 98. [Google Scholar] [CrossRef]
- Ali, A.M.; Ibrahim, S.; Singh, B. Wheat grain yield and nitrogen uptake prediction using atLeaf and GreenSeeker portable optical sensors at jointing growth stage. Inf. Process. Agric. 2019, 7, 375–383. [Google Scholar] [CrossRef]
- Khoddamzadeh, A.A.; Dunn, B.L. Application of Optical Sensors for Nitrogen Management in Chrysanthemum. Hortscience 2016, 51, 915–920. [Google Scholar] [CrossRef]
- Dunn, B.L.; Singh, H.; Payton, M.; Kincheloe, S. Effects of nitrogen, phosphorus, and potassium on SPAD-502 and atLEAF sensor readings of Salvia. J. Plant Nutr. 2018, 41, 1674–1683. [Google Scholar]
- Swearengin, L.; Dunn, B.L.; Singh, H.; Goad, C. Evaluation of a mobile phone plant nitrogen recommendation application in the greenhouse. J. Plant Nutr. 2018, 41, 2615–2625. [Google Scholar] [CrossRef]
- Vesali, F.; Omid, M.; Kaleita, A.; Mobli, H. Development of an android app to estimate chlorophyll content of corn leaves based on contact imaging. Comput. Electron. Agric. 2015, 116, 211–220. [Google Scholar] [CrossRef]
- Dunn, B.L.; Shrestha, A.; Goad, C.; Khoddamzadeh, A.A. Use of optical sensors to monitor Gaillardia Foug. nitrogen status. J. Appl. Hortic. 2015, 17, 181–185. [Google Scholar] [CrossRef]
- Geraldson, C.M.; Tyler, K.B. Plant analysis as an aid in fertilizing vegetable crops. In Soil Testing and Plant Analysis; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1990; pp. 549–5621. [Google Scholar]
- Hartz, T.; Hochmuth, G. Fertility Management of Drip-irrigated Vegetables. Horttechnology 1996, 6, 168–172. [Google Scholar] [CrossRef]
- Padilla, F.M.; Farneselli, M.; Gianquinto, G.; Tei, F.; Thompson, R.B. Monitoring nitrogen status of vegetable crops and soils for optimal nitrogen management. Agric. Water Manag. 2020, 241, 106356. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Pan, K.; Jin, Y.; Liang, J.; Li, W.; Zhang, L. Photosynthetic carbon and nitrogen metabolism and the rela-tionship between their metabolites and lipid peroxidation in dwarf bamboo (Fargesiarufa Yi) during drought and subsequent recovery. Trees 2015, 29, 1633–1647. [Google Scholar]
- Ren, C.; Liu, J.; Gong, Q. Functions of autophagy in plant carbon and nitrogen metabolism. Front. Plant Sci. 2014, 5, 301. [Google Scholar] [CrossRef]
- Sun, W.; Huang, A.; Sang, Y.; Fu, Y.; Yang, Z. Carbon–Nitrogen Interaction Modulates Plant Growth and Expression of Metabolic Genes in Rice. J. Plant Growth Regul. 2013, 32, 575–584. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciênc. Agrotec. 2011, 35, 1039–1042. [Google Scholar] [CrossRef]



| Acronyms | Sensors | Measures |
|---|---|---|
| DBF—Day before fertilization | SPAD | Relative chlorophyll content |
| DAF—Days after fertilization NDVI—Normalized Difference Vegetation Index | ||
| TN—Total nitrogen TC—Total carbon | atLEAF | Relative chlorophyll content |
| EC—Electric conductivity | ||
| NL—Number of leaves | ||
| SPAD—Soil Plant Analytical Development | GreenSeeker | NDVI |
| Treatments | NL | Plant Height (cm) | atLEAF | NDVI |
|---|---|---|---|---|
| Control | 182.91 b | 47.44 a | 61.97 c | 0.83 a |
| T1 | 189.34 b | 45.21 a | 64.06 b | 0.82 a |
| T2 | 195.74 ab | 45.42 a | 63.55 bc | 0.83 a |
| T3 | 205.03 ab | 47.39 a | 63.69 bc | 0.82 a |
| T4 | 193.83 ab | 45.80 a | 64.99 ab | 0.82 a |
| T5 | 215.09 a | 45.81 a | 66.22 a | 0.83 a |
| Days after Fertilization (DAF) | NL | Plant Height (cm) | atLEAF | NDVI |
|---|---|---|---|---|
| 0 | 107.20 c | 33.43 e | 59.43 d | 0.79 e |
| 30 | 171.53 b | 34.28 e | 61.85 c | 0.81 de |
| 60 | 187.10 b | 46.90 d | 64.60 b | 0.83 bc |
| 90 | 227.07 a | 48.97 cd | 66.64 a | 0.83 bcd |
| 120 | 238.70 a | 51.13 bc | 64.90 b | 0.87 a |
| 150 | 223.67 a | 52.82 ab | 65.68 ab | 0.84 b |
| 180 | 223.67 a | 55.73 a | 65.45 ab | 0.81 cde |
| Treatments | Days after Fertilization (DAF) | |
|---|---|---|
| 0 | 180 | |
| TN (%) | ||
| Control | 0.85 aB | 1.00 eA |
| T1 | 0.85 aB | 0.89 fA |
| T2 | 0.85 aB | 1.05 dA |
| T3 | 0.85 aB | 1.67 aA |
| T4 | 0.85 aB | 1.15 cA |
| T5 | 0.85 aB | 1.47 bA |
| 0 | 180 | |
| TC (%) | ||
| Control | 32.94 aB | 38.13 bA |
| T1 | 32.94 aB | 35.48 cA |
| T2 | 32.94 aB | 35.31 dA |
| T3 | 32.94 aB | 34.34 eA |
| T4 | 32.94 aB | 38.29 aA |
| T5 | 32.94 aB | 31.57 fA |
| Treatments | Days after Fertilization (DAF) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | 150 | 180 | |
| Salt (ppm) | |||||||
| Control | 277. 60 aA | 846.20 bA | 488.60 cA | 679.60 cA | 373.80 aA | 448.00 bA | 386.00 cA |
| T1 | 277. 60 aE | 2830.00 aA | 897.60 bcCDE | 1510.00 abBC | 735.60 aDE | 2156.00 aAB | 1084.80 abCD |
| T2 | 277. 60 aC | 2538.00 aA | 1547.60 abB | 1483.80 bB | 575.60 aC | 619.40 bC | 527.40 bcC |
| T3 | 277. 60 aC | 2576.00 aA | 1235.80 abcB | 1213.60 bcB | 580.00 aAB | 2140.00 aA | 805.00 abcAB |
| T4 | 277. 60 aB | 2968.00 aA | 1163.80 abcAB | 1488.20 bB | 942.80 aABC | 766.80 bAB | 582.00 bcAB |
| T5 | 277. 60 aE | 2990.00 aA | 1622.60 aBC | 2194.00 aB | 760.40 aDE | 1966.00 aBC | 1343.60 aCD |
| 0 | 30 | 60 | 90 | 120 | 150 | 180 | |
| EC (µs) | |||||||
| Control | 581.00 a | 1654.60 bA | 998.60 bA | 1370.00 bA | 775.60 aA | 925.40 bA | 800.60 bA |
| T1 | 581.00 aE | 5206.00 aA | 3789.00 aABC | 2868.80 abBCD | 1486.20 aDE | 4038.00 aAB | 2118.20 abCDE |
| T2 | 581.00 aD | 4680.00 aA | 2612.00 aAB | 2932.00 abBC | 1166.80 aBCD | 1137.20 bBCD | 1081.00 abCD |
| T3 | 581.00 aD | 4742.00 aA | 2395.20 abBC | 2315.00 bBCD | 1181.60 aCD | 4040.00 aAB | 1607.80 abCD |
| T4 | 581.00 aC | 5470.00 aA | 2284.40 abBC | 2804.60 abB | 2018.40 aBC | 1551.80 bBC | 1179. 00 abBC |
| T5 | 581.00 aD | 5460.00 aA | 3047.60 aBC | 4116.00 aAB | 1518.80 aCD | 3658.00 aB | 2626.80 aBC |
| 0 | 30 | 60 | 90 | 120 | 150 | 180 | |
| TN (ppm) | |||||||
| Control | 2.903 aB | 229.000 aA | 8.367 dB | 4.200 aB | 1.300 aB | 3.093 bB | 1.484 bB |
| T1 | 2.903 aD | 217.667 abA | 53.000 cC | 4.300 aD | 7.667 aD | 124.470 aB | 29.764 abCD |
| T2 | 2.903 aB | 102.74 cA | 115.250 abA | 5.333 aB | 4.633 aB | 3.450 bB | 2.362 bB |
| T3 | 2.903 aB | 8.244 dB | 82.750 bcA | 17.167 aB | 3.333 aB | 15.350 bB | 10.838 abB |
| T4 | 2.903 aC | 175.72 bA | 80.750 bcB | 5.933 aC | 14.867 aC | 5.933 bC | 2.845 bC |
| T5 | 2.903 aD | 250.667 aA | 147.500 aB | 10.167 aCD | 6.900 aCD | 106.500 aB | 46.106 aC |
| atLEAF | NDVI | TN (%) | TC (%) | NL | |
|---|---|---|---|---|---|
| 30 DAF | |||||
| SPAD | 0.461 | −0.048 | 0.336 | −0.613 | 0.310 |
| atLEAF | −0.394 | 0.573 | −0.120 | 0.636 | |
| NDVI | −0.540 | −0.360 | 0.039 | ||
| TN (%) | −0.499 | 0.472 | |||
| TC (%) | −0.409 | ||||
| 60 DAF | |||||
| SPAD | 0.719 | −0.317 | 0.175 | 0.066 | 0.698 |
| atLEAF | −0.055 | 0.637 | 0.174 | 0.490 | |
| NDVI | 0.693 | 0.578 | −0.857 * | ||
| TN (%) | 0.409 | −0.310 | |||
| TC (%) | −0.500 | ||||
| 90 DAF | |||||
| SPAD | 0.883 * | 0.177 | 90 DAF | 0.694 | 0.741 |
| atLEAF | 0.123 | −0.183 | 0.783 | 0.769 | |
| NDVI | 0.073 | 0.035 | 0.717 | ||
| TN (%) | 0.432 | 0.441 | 0.365 | ||
| TC (%) | 0.624 | ||||
| 120 DAF | |||||
| SPAD | 0.533 | −0.637 | −0.565 | 0.123 | −0.099 |
| atLEAF | −0.849 * | −0.251 | −0.448 | 0.357 | |
| NDVI | −0.023 | −0.006 | 0.180 | ||
| TN (%) | 0.396 | −0.454 | |||
| TC (%) | −0.811 * | ||||
| 150 DAF | |||||
| SPAD | 0.616 | −0.246 | 0.252 | −0.084 | −0.132 |
| atLEAF | 0.083 | −0.134 | −0.007 | −0.028 | |
| NDVI | 0.247 | −0.399 | 0.812 * | ||
| TN (%) | 0.226 | 0.724 | |||
| TC (%) | −0.128 | ||||
| 180 DAF | |||||
| SPAD | 0.558 | 0.666 | 0.311 | −0.316 | −0.149 |
| atLEAF | −0.029 | 0.288 | 0.295 | 0.042 | |
| NDVI | 0.567 | −0.369 | −0.038 | ||
| TN (%) | 0.426 | 0.663 | |||
| TC (%) | 0.499 | ||||
| Treatments | FT | SFT | Number and Month of Application (SFT) |
|---|---|---|---|
| Control | 15 g | ---- | ---- |
| T1 | 15 g | 15 g | 2—November and March |
| T2 | 15 g | 15 g | 1—November |
| T3 | 30 g | 15 g | 2—November and March |
| T4 | 30 g | 15 g | 1—November |
| T5 | 45 g | 15 g | 2—November and March |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, B.N.S.; Tucker, D.A.; Khoddamzadeh, A.A. Precision Horticulture: Application of Optical Sensor Technology for Nitrogen Monitoring Status in Cocoplum, a Native Landscaping Plant. Plants 2023, 12, 760. https://doi.org/10.3390/plants12040760
Costa BNS, Tucker DA, Khoddamzadeh AA. Precision Horticulture: Application of Optical Sensor Technology for Nitrogen Monitoring Status in Cocoplum, a Native Landscaping Plant. Plants. 2023; 12(4):760. https://doi.org/10.3390/plants12040760
Chicago/Turabian StyleCosta, Bárbara Nogueira Souza, Daniel A. Tucker, and Amir Ali Khoddamzadeh. 2023. "Precision Horticulture: Application of Optical Sensor Technology for Nitrogen Monitoring Status in Cocoplum, a Native Landscaping Plant" Plants 12, no. 4: 760. https://doi.org/10.3390/plants12040760
APA StyleCosta, B. N. S., Tucker, D. A., & Khoddamzadeh, A. A. (2023). Precision Horticulture: Application of Optical Sensor Technology for Nitrogen Monitoring Status in Cocoplum, a Native Landscaping Plant. Plants, 12(4), 760. https://doi.org/10.3390/plants12040760









