Evaluation of Selenium Nanoparticles in Inducing Disease Resistance against Spot Blotch Disease and Promoting Growth in Wheat under Biotic Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Plant Extract
2.2. Synthesis of Selenium Nanoparticles
2.3. Characterization of Selenium Nanoparticles
2.4. In Vitro Antifungal Assay
2.4.1. Sample Collection and Isolation and Identification of Fungus
2.4.2. Evaluation of Antifungal Activity of SeNP-Well Diffusion Assay
2.5. In Vivo Activity
2.5.1. Greenhouse Experiment
2.5.2. Fungal Inoculum’s Preparation and Application
2.5.3. Evaluation of the Disease Incidence
2.5.4. Collection of Samples
2.6. Plant-Growth Promotion Study
2.7. Analysis of Plant Physiological Parameters
2.7.1. Chlorophyll and Carotenoid Content
2.7.2. Membrane Stability Index (%)
2.8. Assessment of Plant Biochemical Parameters
2.8.1. Proline Content
2.8.2. Soluble Sugar
2.8.3. Total Phenolic Content
2.8.4. Total Flavonoid Content
3. Results
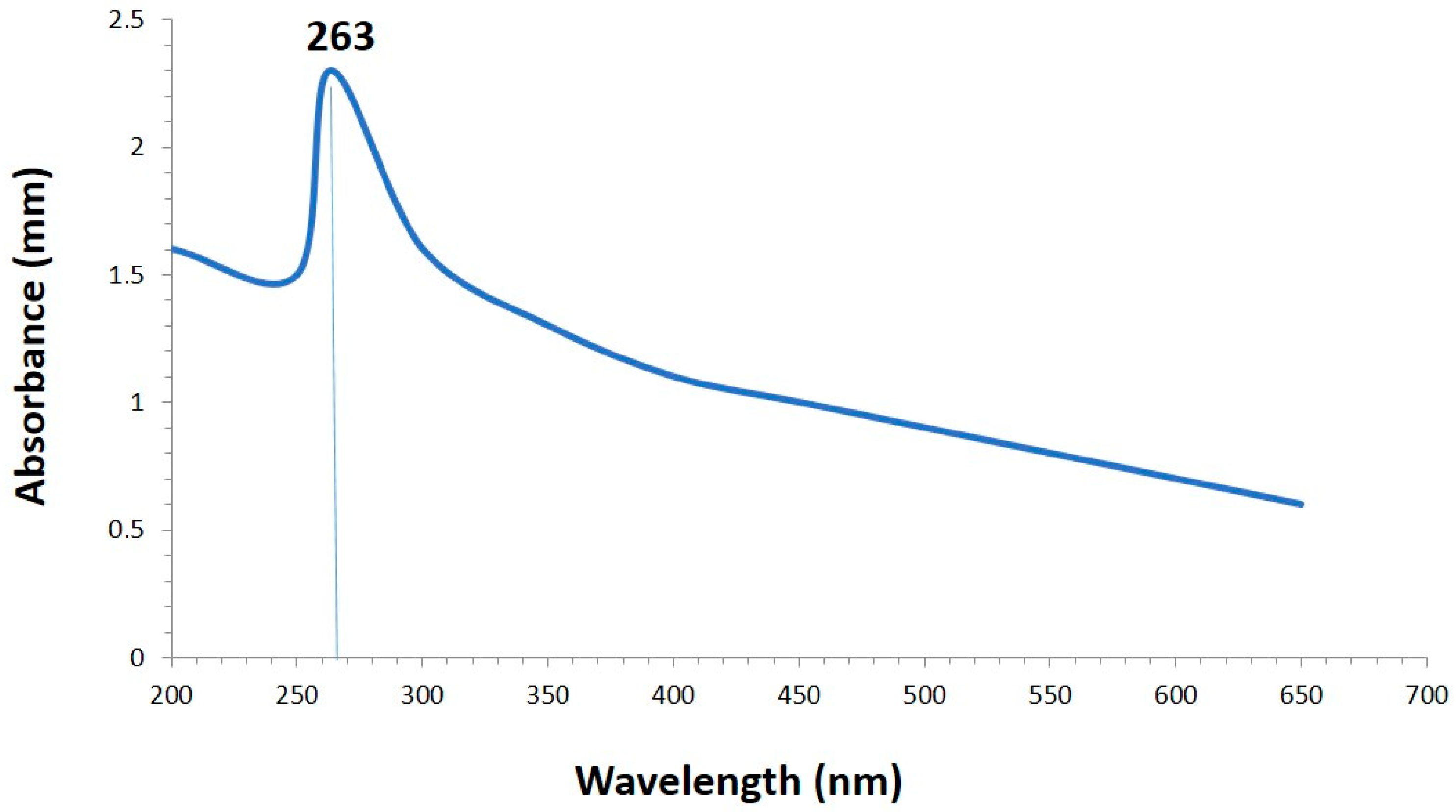
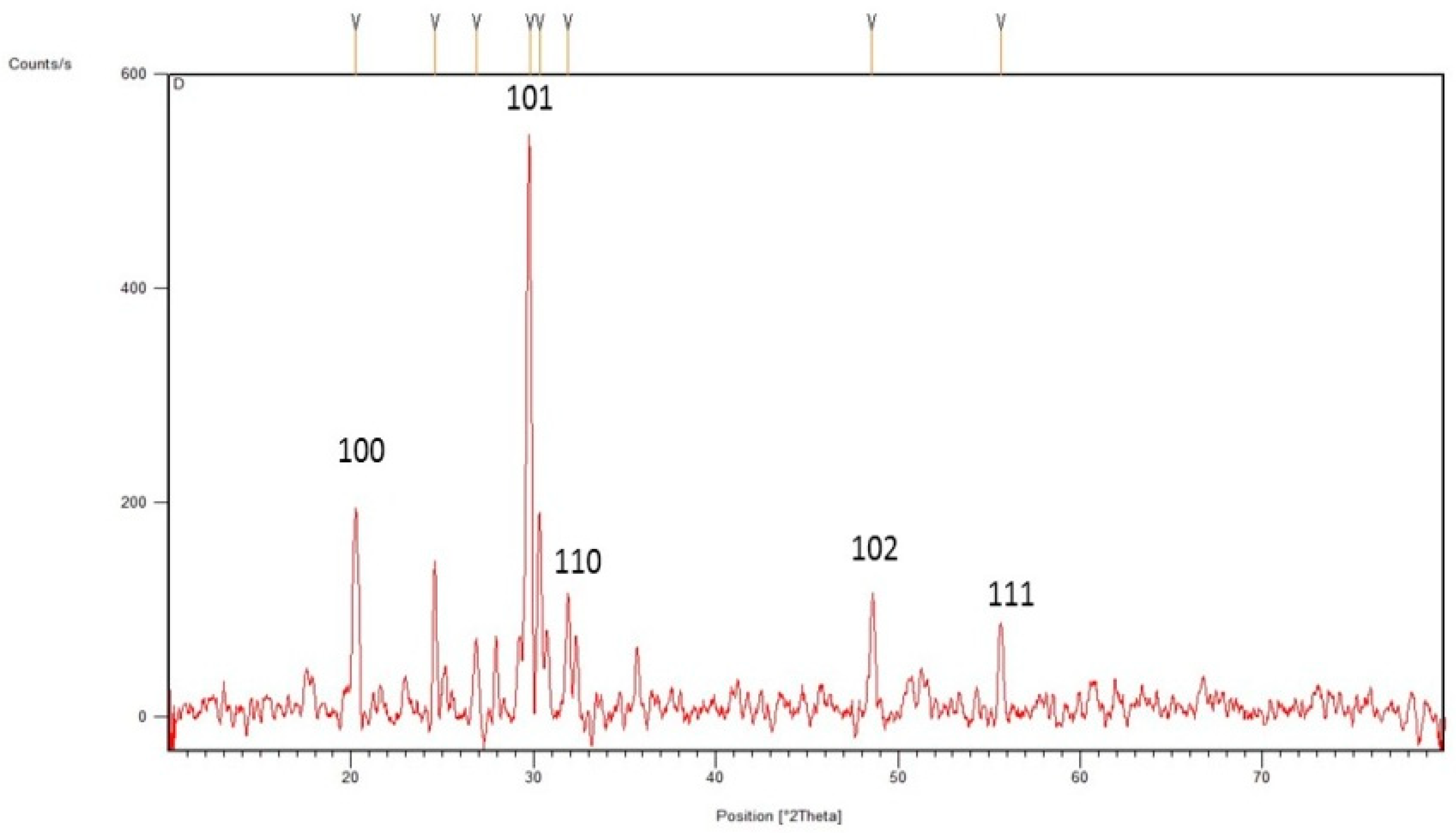
3.1. Green Synthesis and Characterization of Selenium Nanoparticles
3.2. Morphological and Microscopic Identifications
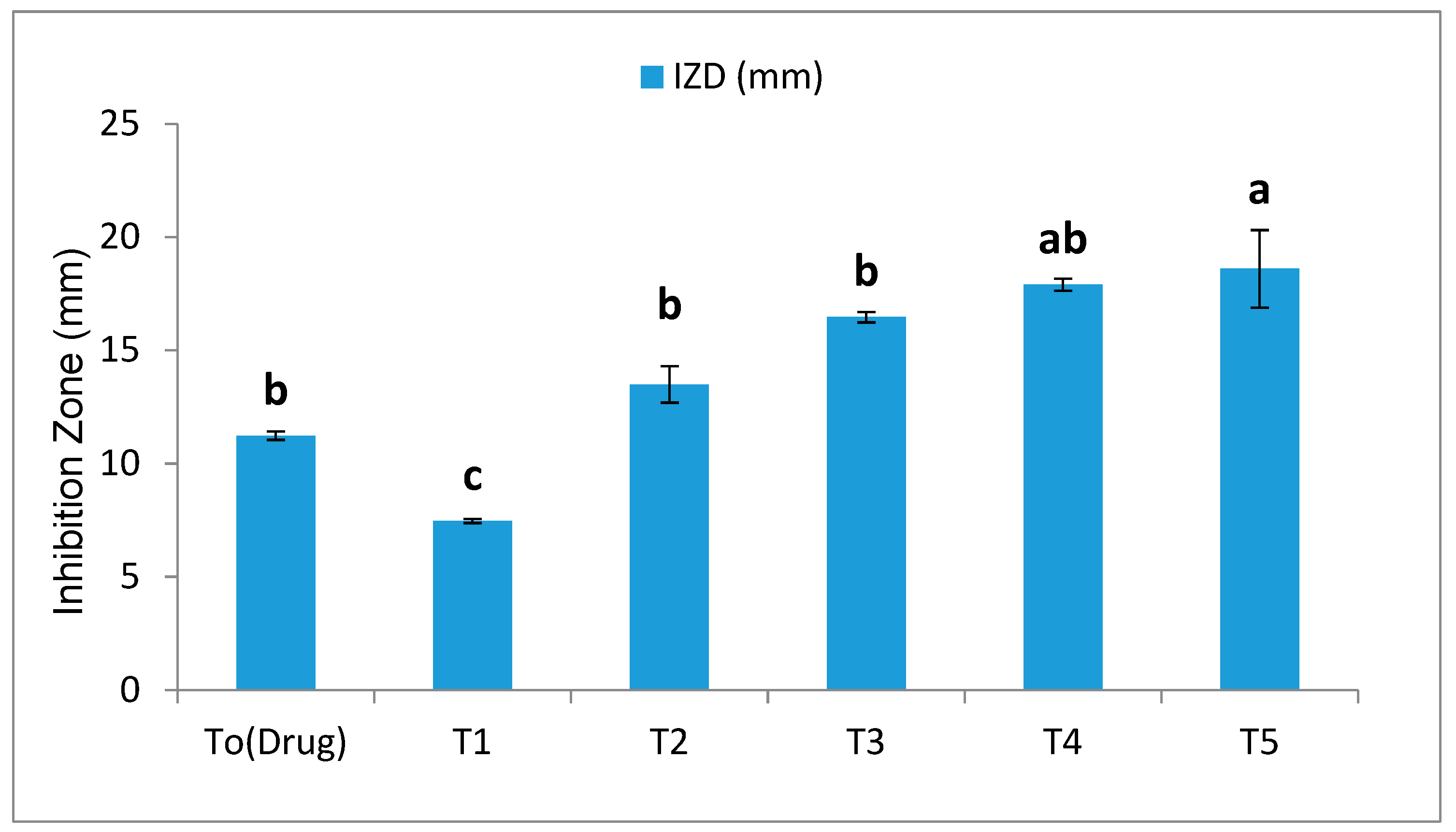
3.2.1. Antifungal Assay (Well Diffusion Method)
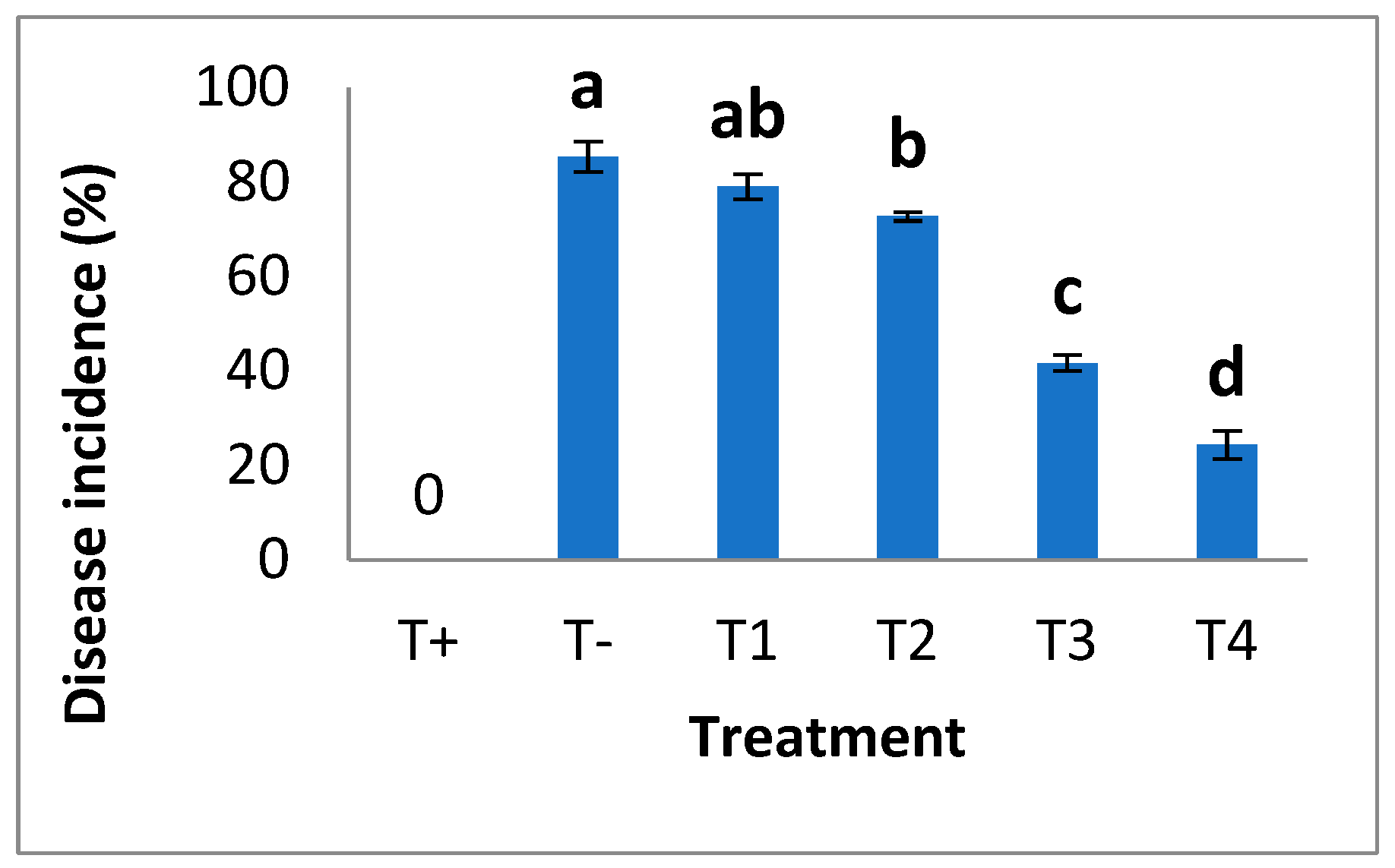
3.2.2. Assessment of Disease Incidence (%)
3.2.3. Effect of Biosynthesized Selenium Nanoparticles on Plant Morphological Aspects
3.3. Evaluation of Plant Physiological Parameters
3.4. Assessment of Plant Biochemical Parameters
4. Discussion
4.1. Synthesis and Characterization of SeNPs
4.2. Effect of SeNPs on Fungal Growth Inhibition
4.3. Effect of SeNPs on Plant Morphological Parameters
4.4. Effect of SeNPs on Plant Physiological Parameters
4.5. Effect of SeNPs on Plant Biochemical Parameters
4.6. Advantages of Using Green Nanotechnology over PGPR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fones, H.N.; Bebber, D.P.; Chaloner, T.M.; Kay, W.T.; Steinberg, G.; Gurr, S.J. Author Correction: Threats to global food security from emerging fungal and oomycete crop pathogens. Nat. Food 2020, 1, 455–456. [Google Scholar] [CrossRef]
- El-Lethy, S.R.; Abdelhamid, M.T.; Fatma, R. Effect of Potassium Application on Wheat (Triticum aestivum L.) Cultivars grown under salinity stress. World Appl. Sci. 2013, 26, 840–850. [Google Scholar]
- Usman, M. Contribution of agriculture sector in the GDP growth rate of Pakistan. J. Glob. Econ. 2016, 4, 184. [Google Scholar] [CrossRef]
- Al-Sadi, A.M. Bipolaris sorokiniana-induced black point, common root rot, and spot blotch diseases of wheat: A review. Front. Cell. Infect. Microbiol. 2021, 11, 584899. [Google Scholar] [CrossRef] [PubMed]
- Navathe, S.; Yadav, P.S.; Chand, R.; Mishra, V.K.; Vasistha, N.K.; Meher, P.K.; Joshi, A.K.; Gupta, P.K. ToxA-Tsn1 interaction for spot blotch susceptibility in Indian wheat: An example of inverse gene-for-gene relationship. Plant Dis. 2020, 104, 71–81. [Google Scholar] [PubMed]
- Gupta, P.K.; Chand, R.; Vasistha, N.; Pandey, S.P.; Kumar, U.; Mishra, V.K.; Joshi, A.K. Spot blotch disease of wheat: The current status of research on genetics and breeding. Plant Pathol. 2017, 67, 508–531. [Google Scholar]
- Devi, H.M.; Mahapatra, S.; Das, S. Assessment of yield loss of wheat caused by spot blotch using regression model. Indian Phytopathol. 2018, 71, 291–294. [Google Scholar] [CrossRef]
- Hovmøller, M.S.; Walter, S.; Bayles, R.A.; Hubbard, A.; Flath, K.; Sommerfeldt, N.; Leconte, M.; Czembor, P.; Rodriguez-Algaba, J.; Thach, T.; et al. Replacement of the European wheat yellow rust population by new races from the centre of diversity in the near-Himalayan region. Plant Pathol. 2016, 65, 402–411. [Google Scholar] [CrossRef]
- Welbaum, G.E.; Sturz, A.V.; Dong, Z.; Nowak, J. Managing soil microorganisms to improve productivity of agro-ecosystems. Crit. Rev. Plant Sci. 2004, 23, 175–193. [Google Scholar] [CrossRef]
- Singh, A.; Singh, K.P.; Singh, M.; Bhareti, P.; Singh, U.P. Antifungal activity of some strains of plant growthpromoting rhizobacteria. J. Pharmacogn. Phytochem. 2017, 6, 577–582. [Google Scholar]
- Paulitz, T.C.; Bélanger, R.R. Biological control in greenhouse systems. Annu. Rev. Phytopathol. 2001, 39, 103–133. [Google Scholar] [PubMed]
- Nargund, V.B.; Vinay, J.U.; Basavesha, K.N.; Chikkanna, S.; Jahagirdar, S.; Patil, R.R. Green Nanotechnology and Its Application in Plant Disease Management. In Emerging Trends in Plant Pathology; Springer: Singapore, 2020; pp. 591–609. [Google Scholar] [CrossRef]
- Elmer, W.; White, J.C. The future of nanotechnology in plant pathology. Annu. Rev. Phytopathol. 2018, 56, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Zonaro, E.; Lampis, S.; Turner, R.J.; Qazi, S.J.S.; Vallini, G. Biogenic selenium and tellurium nanoparticles synthesized by environmental microbial isolates efficaciously inhibit bacterial planktonic cultures and biofilms. Front. Microbiol. 2015, 6, 584. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.S.; Baker, D.H.A.; Ahmed, E.A. Cytotoxicity and antimicrobial efficiency of selenium nanoparticles biosynthesized by Spirulina platensis. Arch. Microbiol. 2021, 203, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Mukhopadhyay, M. Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioprocess Biosyst. Eng. 2015, 38, 1723–1730. [Google Scholar] [CrossRef]
- Hashem, A.H.; Abdelaziz, A.M.; Askar, A.A.; Fouda, H.M.; Khalil, A.M.; Abd-Elsalam, K.A.; Khaleil, M.M. Bacillus megaterium-mediated synthesis of selenium nanoparticles and their antifungal activity against Rhizoctonia solani in Faba Bean Plants. J. Fungi 2021, 7, 195. [Google Scholar] [CrossRef]
- El-Gazzar, N.; Ismail, A.M. The potential use of Titanium, Silver and Selenium nanoparticles in controlling leaf blight of tomato caused by Alternaria alternata. Biocatal. Agric. Biotechnol. 2020, 27, 101708. [Google Scholar]
- Liang, T.; Qiu, X.; Ye, X.; Liu, Y.; Li, Z.; Tian, B.; Yan, D. Biosynthesis of selenium nanoparticles and their effect on changes in urinary nanocrystallites in calcium oxalate stone formation. 3 Biotech 2020, 10, 23. [Google Scholar] [CrossRef]
- Alagesan, V.; Venugopal, S. Green synthesis of selenium nanoparticle using leaves extract of withania somnifera and its biological applications and photocatalytic activities. Bionanoscience 2019, 9, 105–116. [Google Scholar] [CrossRef]
- Fardsadegh, B.; Jafarizadeh-Malmiri, H. Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their in vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains. Green Process. Synth. 2019, 8, 399–407. [Google Scholar] [CrossRef]
- Satgurunathan, T.; Bhavan, P.S.; Komachi, S. Green synthesis of selenium nanoparticles from sodium selenite using garlic extract and its enrichment on Artemia nauplii to feed the freshwater prawn Macrobrachium rosenbergii post–larvae. Res. J. Chem. Environ. 2017, 21, 1–12. [Google Scholar]
- Ullah, A.; Yin, X.; Wang, F.; Xu, B.; Mirani, Z.A.; Xu, B.; Chan, M.W.H.; Ali, A.; Usman, M.; Ali, N.; et al. Biosynthesis of Selenium Nanoparticles (via Bacillus subtilis BSN313), and Their Isolation, Characterization, and Bioactivities. Molecules 2021, 26, 5559. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, N.H.; Davalos, A.F.; Puente, P.A.; Rodríguez, A.Y.; Caicedo, P.A. Antioxidant activity of exo-metabolites produced by Fusarium oxysporum: An endophytic fungus isolated from leaves of Otoba gracilipes. Microbiologyopen 2019, 8, e903. [Google Scholar] [CrossRef]
- Usmani, S.M.H.; Ghaffar, A. Polyethylene mulching of soil to reduce viability of sclerotia of Sclerotiumoryzae. Soil Biol. Biochem. 1982, 14, 203–206. [Google Scholar] [CrossRef]
- Raza, M.; Hussain, M.; Ghazanfer, M.U. Characterization and pathogenicity of Biplolarissorokiniana caused spot blotch of wheat in Pakistan. FUUAST J. Biol. 2014, 4, 97–100. [Google Scholar]
- Abdel-Moneim, A.-M.E.; El-Saadony, M.T.; Shehata, A.M.; Saad, A.M.; Aldhumri, S.A.; Ouda, S.M.; Mesalam, N.M. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2022, 29, 1197–1209. [Google Scholar] [CrossRef]
- Menon, S.; Agarwal, H.; Rajeshkumar, S.; Rosy, P.J.; Shanmugam, V.K. Investigating the antimicrobial activities of the biosynthesized selenium nanoparticles and its statistical analysis. Bionanoscience 2020, 10, 122–135. [Google Scholar] [CrossRef]
- Satti, S.H.; Raja, N.I.; Javed, B.; Akram, A.; Mashwani, Z.-U.-R.; Ahmad, M.S.; Ikram, M. Titanium dioxide nanoparticles elicited agro-morphological and physicochemical modifications in wheat plants to control Bipolaris sorokiniana. PLoS ONE 2021, 16, e0246880. [Google Scholar] [CrossRef]
- Iftikhar, S.; Asad, S.; Munir, A.N.J.U.M.; Ahmad, I.; Sultan, A. Prevalence and distribution of foliar blight pathogens of wheat in different agro ecological zones of Pakistan with special reference to Bipolaris sorokiniana. Pak. J. Bot. 2006, 38, 205–210. [Google Scholar]
- Iqbal, M.; Raja, N.I.; Mashwani, Z.-U.-R.; Hussain, M.; Ejaz, M.; Yasmeen, F. Effect of Silver Nanoparticles on Growth of Wheat Under Heat Stress. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 387–395. [Google Scholar] [CrossRef]
- Bruinsma, J. Investigation of the chlorophyll contents in the leaves extracts. Photochem. Photobiol. 1963, 2, 241–244. [Google Scholar]
- Mullan, D.; Pietragalla, J. Leaf relative water content. In Physiological Breeding II: A Field Guide to Wheat Phenotyping; Cimmyt: Veracruz, Mexico, 2012; pp. 25–27. [Google Scholar]
- Sairam, R.K.; Deshmukh, P.S.; Shukla, D.S. Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J. Agron. Crop. Sci. 1997, 178, 171–178. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Qayyum, A.; Razzaq, A.; Ahmad, M.; Jenks, M.A. Water stress causes differential effects on germination indices, total soluble sugar and proline content in wheat (Triticum aestivum L.) genotypes. Afr. J. Biotechnol. 2011, 10, 14038–14045. [Google Scholar] [CrossRef]
- Hussein, H.-A.A.; Darwesh, O.M.; Mekki, B.B. Environmentally friendly nano-selenium to improve antioxidant system and growth of groundnut cultivars under sandy soil conditions. Biocatal. Agric. Biotechnol. 2019, 18, 101080. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef] [PubMed]
- Gunti, L.; Dass, R.S.; Kalagatur, N.K. Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: Antioxidant, antimicrobial, and biocompatibility. Front. Microbiol. 2019, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.; Ghazal, H.; Othman, H.A.; Hassabo, A.G. Synthesis of different nanometals using Citrus sinensis peel (orange peel) waste extraction for valuable functionalization of cotton fabric. Chem. Pap. 2022, 76, 639–660. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Elbaz, A.F.; Badawy, S.E.; Moussa, S.H. Anticancer and antibacterial activity of cadmium sulfide nanoparticles by Aspergillus niger. Adv. Polym. Technol. 2020, 2020, 13. [Google Scholar] [CrossRef]
- Gonzalo, J.; Serna, R.; Solís, J.; Babonneau, D.; Afonso, C.N. Morphological and interaction effects on the surface plasmon resonance of metal nanoparticles. J. Phys. Condens. Matter 2003, 15, S3001. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Rajagopal, M.; Krishnan, A.; Sreerama, S.K. A review on recent trends in nanomaterials and nanocomposites for environmental applications. Curr. Anal. Chem. 2021, 17, 202–243. [Google Scholar]
- Anu, K.; Singaravelu, G.; Murugan, K.; Benelli, G. Green-synthesis of selenium nanoparticles using garlic cloves (Allium sativum): Biophysical characterization and cytotoxicity on vero cells. J. Clust. Sci. 2017, 28, 551–563. [Google Scholar]
- Joshi, S.M.; De Britto, S.; Jogaiah, S.; Ito, S.I. Mycogenic selenium nanoparticles as potential new generation broad spectrum antifungal molecules. Biomolecules 2019, 9, 419. [Google Scholar] [PubMed]
- Sharma, G.; Sharma, A.R.; Bhavesh, R.; Park, J.; Ganbold, B.; Nam, J.-S.; Lee, S.-S. Biomolecule-mediated synthesis of selenium nanoparticles using dried Vitis vinifera (raisin) extract. Molecules 2014, 19, 2761–2770. [Google Scholar] [PubMed]
- Malhotra, S.; Jha, N.; Desai, K.A. Superficial synthesis of selenium nanospheres using wet chemical approach. Int. J. Nanotechnol. Appl. 2014, 3, 2277–4777. [Google Scholar]
- Verma, P.; Maheshwari, S.K. Preparation of sliver and selenium nanoparticles and its characterization by dynamic light scattering and scanning electron microscopy. J Microsc. Ultrastruct. 2018, 6, 182–187. [Google Scholar] [CrossRef]
- Ikram, M.; Raja, N.I.; Javed, B.; Mashwani, Z.-U.; Hussain, M.; Hussain, M.; Ehsan, M.; Rafique, N.; Malik, K.; Sultana, T.; et al. Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress. Green Process. Synth. 2020, 9, 706–714. [Google Scholar] [CrossRef]
- Shahbaz, M.; Fatima, N.; Mashwani, Z.U.R.; Akram, A.; Haq, E.U.; Mehak, A.; Abasi, F.; Ajmal, M.; Yousaf, T.; Raja, N.I.; et al. Effect of Phytosynthesized Selenium and Cerium Oxide Nanoparticles on Wheat (Triticum aestivum L.) against Stripe Rust Disease. Molecules 2022, 27, 8149. [Google Scholar]
- Ezhuthupurakkal, P.B.; Polaki, L.R.; Suyavaran, A.; Subastri, A.; Sujatha, V.; Thirunavukkarasu, C. Selenium nanoparticles synthesized in aqueous extract of Allium sativum perturbs the structural integrity of Calf thymus DNA through intercalation and groove binding. Mater. Sci. Eng. C 2017, 74, 597–608. [Google Scholar] [CrossRef]
- Fritea, L.; Laslo, V.; Cavalu, S.; Costea, T.; Vicas, S.I. Green biosynthesis of selenium nanoparticles using parsley (Petroselinum crispum) leaves extract. Stud. Univ. Vasile Goldis Arad. Seria Stiintele Vietii Life Sci. Ser. 2017, 27, 203–208. [Google Scholar]
- Ramamurthy, C.H.; Sampath, K.S.; Arunkumar, P.; Kumar, M.S.; Sujatha, V.; Premkumar, K.; Thirunavukkarasu, C. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst. Eng. 2013, 36, 1131–1139. [Google Scholar] [PubMed]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green silver and gold nanoparticles: Biological synthesis approaches and potentials for biomedical applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shen, Y.; Xie, A.; Yu, X.; Zhang, X.; Yang, L.; Li, C. Rapid, room-temperature synthesis of amorphous selenium/protein composites using Capsicum annuum L. extract. Nanotechnology 2007, 18, 405101. [Google Scholar] [CrossRef]
- Joseph, M.M.; George, S.K.; Sreelekha, T.T. Bridging ‘green’with nanoparticles: Biosynthesis approaches for cancer management and targeting of cancer stem cells. Curr. Nanosci. 2016, 12, 47–62. [Google Scholar] [CrossRef]
- Matai, I.; Pandey, S.K.; Garg, D.; Rani, K.; Sachdev, A. Phytogreen synthesis of multifunctional nano selenium with antibacterial and antioxidant implications. Nano Express 2020, 1, 010031. [Google Scholar] [CrossRef]
- Menon, S.; Shrudhi Devi, K.S.; Agarwal, H.; Shanmugam, V.K. Efficacy of biogenic selenium nanoparticles from an extract of ginger towards the Evaluation of anti-microbial and antioxidant activities. Colloid Interface Sci. Commun. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Ghaderi, R.S.; Adibian, F.; Sabouri, Z.; Davoodi, J.; Kazemi, M.; Jamehdar, S.A.; Meshkat, Z.; Soleimanpour, S.; Daroudi, M. Green synthesis of selenium nanoparticle by Abelmoschus esculentus extract and assessment of its antibacterial activity. Mater. Technol. 2021, 37, 1289–1297. [Google Scholar] [CrossRef]
- Hussain, M.; Raja, N.I.; Mashwani, Z.-U.-R.; Naz, F.; Iqbal, M.; Aslam, S. Green synthesis and characterisation of silver nanoparticles and their effects on antimicrobial efficacy and biochemical profiling in Citrus reticulata. IET Nanobiotechnol. 2018, 12, 514–519. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8. [Google Scholar]
- Alvi, G.B.; Iqbal, M.S.; Ghaith, M.M.S.; Haseeb, A.; Ahmed, B.; Qadir, M.I. Biogenic selenium nanoparticles (SeNPs) from citrus fruit have antibacterial activities. Sci. Rep. 2021, 11, 4811. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Fakhimi, A.; Mosavat, G.; Jafari-Fesharaki, P.; Rezaie, S.; Rezayat, S.M. Antifungal activity of biogenic selenium nanoparticles. World Appl. Sci. J. 2010, 10, 918–922. [Google Scholar]
- Kazempour, Z.B.; Yazdi, M.H.; Rafii, F.; Shahverdi, A.R. Sub-inhibitoryconcentration of biogenic selenium nanoparticles lacks post antifungal effectfor Aspergillus niger and Candida albicans and stimulates the growth of Aspergillusniger. Iran. J. Microbiol. 2013, 5, 81. [Google Scholar]
- Anyasi, T.A.; Jideani, A.I.O.; Mchau, G.R.A. Effects of organic acid pretreatmenton microstructure, functional and thermal properties of unripe banana flour. J. Food Meas. Charact. 2017, 11, 99–110. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis ofantimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; El-Wafai, N.A.; El-Fattah, H.I.A.; Mahgoub, S.A. Biosynthesis, optimization and characterization of silver nanoparticles using a soil isolate ofBacilluspseudomycoides MT32 and their antifungal activity against some pathogenic fungi. Adv. Anim. Vet. Sci. 2019, 7, 238–249. [Google Scholar]
- Khshan, K.T.; Alkafaje, H.A. Biosynthesis of Silver Nanoparticles Using Calendula officinalis (L.) Extract and Evaluating their Antioxidant Activity. IOP Conf. Ser. Earth Environ. Sci. 2021, 735, 012073. [Google Scholar]
- Shenashen, M.; Derbalah, A.; Hamza, A.; Mohamed, A.; Safty, S.E. Antifungalactivity of fabricated mesoporous alumina nanoparticles against root rotdisease of tomato caused by Fusarium oxysporium. Pest Manag. Sci. 2017, 73, 1121–1126. [Google Scholar] [PubMed]
- Yilmaz, M.T.; Ispirli, H.; Taylan, O.; Dertli, E. A green nano-biosynthesis of selenium nanoparticles with Tarragon extract: Structural, thermal, and antimicrobial characterization. LWT 2021, 141, 110969. [Google Scholar] [CrossRef]
- Germ, M.; Kreft, I.; Osvald, J. Influence of UV-B exclusion and selenium treatment on photochemical efficiency of photosystem II, yield and respiratory potential in pumpkins (Cucurbita pepo L.). Plant Physiol. Biochem. 2005, 43, 445–448. [Google Scholar] [CrossRef]
- Bao-shan, L.; Shao-qi, D.; Chun-hui, L.; Li-jun, F.; Shu-chun, Q.; Min, Y. Effect of TMS (nanostructured silicon dioxide) on growth of Changbai larch seedlings. J. For. Res. 2004, 15, 138–140. [Google Scholar] [CrossRef]
- Boldrin, P.F.; de Figueiredo, M.A.; Yang, Y.; Luo, H.; Giri, S.; Hart, J.J.; Faquin, V.; Guilherme, L.R.; Thannhauser, T.W.; Li, L. Selenium promotes sulfur accumulation and plant growth in wheat (Triticum aestivum). Physiol. Plant. 2016, 158, 80–91. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Blinov, A.V.; Serov, A.V.; Gvozdenko, A.A.; Kravtsov, A.A.; Nagdalian, A.A.; Raffa, V.; Maglakelidze, D.; Blinova, A.; Kobina, A.; et al. Effect of selenium nanoparticles on germination of Hordéum vulgáre barley seeds. Coatings 2021, 11, 862. [Google Scholar] [CrossRef]
- Chernikova, O.V.; Ampleeva, L.E.; Mazhaisky, Y.A. Effect of selenium nanoparticles on the formation of corn yield. Russ. Agric. Sci. 2019, 45, 256–259. [Google Scholar] [CrossRef]
- Silva, V.M.; Tavanti, R.F.R.; Gratão, P.L.; Alcock, T.D.; dos Reis, A.R. Selenate and selenite affect photosynthetic pigments and ROS scavenging through distinct mechanisms in cowpea (Vigna unguiculata (L.) walp) plants. Ecotoxicol. Environ. Saf. 2020, 201, 110777. [Google Scholar] [CrossRef] [PubMed]
- Quiterio-Gutiérrez, T.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Hernández-Fuentes, A.D.; Sandoval-Rangel, A.; Benavides-Mendoza, A.; la Fuente, M.C.-d.; Juarez-Maldonado, A. The application of selenium and copper nanoparticles modifies the biochemical responses of tomato plants under stress by Alternaria solani. Int. J. Mol. Sci. 2019, 20, 1950. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Hoveizeh, N.F.; Tran, L.-S.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ. Pollut. 2019, 253, 246–258. [Google Scholar] [CrossRef]
- Dong, J.Z.; Wang, Y.; Wang, S.H.; Yin, L.P.; Xu, G.J.; Zheng, C.; Lei, C.; Zhang, M.Z. Selenium increases chlorogenic acid, chlorophyll and carotenoids of Lyciumchinense leaves. J. Sci. Food Agric. 2013, 93, 310–315. [Google Scholar] [CrossRef]
- Dai, H.; Wei, S.; Skuza, L.; Jia, G. Selenium spiked in soil promoted zinc accumulation of Chinese cabbage and improved its antioxidant system and lipid peroxidation. Ecotoxicol. Environ. Saf. 2019, 180, 179–184. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, M.; Li, Y.; Che, Y.; Xiao, Y. Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci. Total Environ. 2019, 655, 1150–1158. [Google Scholar]
- Zhang, H.; Zhao, Z.; Zhang, X.; Zhang, W.; Huang, L.; Zhang, Z.; Yuan, L.; Liu, X. Effects of foliar application of selenate and selenite at different growth stages on Selenium accumulation and speciation in potato (Solanum tuberosum L.). Food Chem. 2019, 286, 550–556. [Google Scholar] [CrossRef]
- Rady, M.M.; Desoky, E.-S.M.; Ahmed, S.M.; Majrashi, A.; Ali, E.F.; Arnaout, S.M.A.I.; Selem, E. Foliar nourishment with nano-selenium dioxide promotes physiology, biochemistry, antioxidant defenses, and salt tolerance in phaseolus vulgaris. Plants 2021, 10, 1189. [Google Scholar] [CrossRef]
- Nasibi, F.; Aminian, F.; Mohammadinejad, G.; Hassanshahian, M. Seed priming with selenium nanoparticle and plant growth promoting rhizobacteria improve seedling development of foxtail millet (Setariaitalica) under salinity stress. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Mahakham, W.; Theerakulpisut, P.; Maensiri, S.; Phumying, S.; Sarmah, A.K. Environmentally benign synthesis of phytochemicals-capped gold nanoparticles as nanopriming agent for promoting maize seed germination. Sci. Total Environ. 2016, 573, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Adrees, M.; Qayyum, M.F.; Khalid, S.; Rehman, M.Z.U.; Sarwar, M.A. Effects of silicon nanoparticles on growth and physiology of wheat in cadmium contaminated soil under different soil moisture levels. Environ. Sci. Pollut. Res. 2020, 27, 4958–4968. [Google Scholar] [CrossRef] [PubMed]
- Asgari, F.; Majd, A.; Jonoubi, P.; Najafi, F. Effects of silicon nanoparticles on molecular, chemical, structural and ultrastructural characteristics of oat (Avena sativa L.). Plant Physiol. Biochem. 2018, 127, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Adrees, M.; Khan, Z.S.; Ali, S.; Hafeez, M.; Khalid, S.; Rehman, M.Z.U.; Hussain, A.; Hussain, K.; Chatha, S.A.S.; Rizwan, M. Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere 2020, 238, 124681. [Google Scholar] [CrossRef] [PubMed]
- Nasirzadeh, L.; Kvarnheden, A.; Sorkhilaleloo, B.; Hervan, E.M.; Fatehi, F. Foliar-Applied Selenium Nanoparticles Can Alleviate Soil-Cadmium Stress Through Physio-chemical and Stomatal Changes to Optimize Yield, Antioxidant Capacity, and Fatty Acid Profile of Wheat (Triticum aestivum L.). J. Soil Sci. Plant Nutr. 2022, 22, 2469–2480. [Google Scholar] [CrossRef]
- Senthil-Kumar, M.; Mysore, K.S. Ornithine-delta-aminotransferase and proline dehydrogenase genes play a role in non-host disease resistance by regulating pyrroline-5-carboxylate metabolism-induced hypersensitive response. Plant Cell Environ. 2012, 35, 1329–1343. [Google Scholar] [CrossRef]
- Morkunas, I.; Ratajczak, L. The role of sugar signaling in plant defense responses against fungal pathogens. Acta Physiol. Plant. 2014, 36, 1607–1619. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd-Allah, E.F.; Gucel, S.; Tran, L.S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef]
- Tripathi, D.; Singh, M.; Pandey-Rai, S. Crosstalk of nanoparticles and phytohormones regulate plant growth and metabolism under abiotic and biotic stress. Plant Stress 2022, 6, 100107. [Google Scholar]
- Sardar, R.; Ahmed, S.; Shah, A.A.; Yasin, N.A. Selenium nanoparticles reduced cadmium uptake, regulated nutritional homeostasis and antioxidative system in Coriandrum sativum grown in cadmium toxic conditions. Chemosphere 2022, 287, 132332. [Google Scholar]
- El-Hoseiny, H.M.; Helaly, M.N.; Elsheery, N.I.; Alam-Eldein, S.M. Humic acid and boron to minimize the incidence of alternate bearing and improve the productivity and fruit quality of mango trees. Hortscience 2020, 55, 1026–1037. [Google Scholar] [CrossRef]
- Subramanyam, K.; Du Laing, G.; Van Damme, E.J.M. Sodium selenate treatment using a combination of seed priming and foliar spray alleviates salinity stress in rice. Front. Plant Sci. 2019, 10, 116. [Google Scholar] [PubMed]
- Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef]
- Chandra, S.; Chakraborty, N.; Dasgupta, A.; Sarkar, J.; Panda, K.; Acharya, K. Chitosan nanoparticles: A positive modulator of innate immune responses in plants. Sci. Rep. 2015, 5, 15195. [Google Scholar]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef]
- Mellersh, D.G.; Foulds, I.V.; Higgins, V.J.; Heath, M.C. H2O2 plays different roles in determining penetration failure in three diverse plant–fungal interactions. Plant J. 2002, 29, 257–268. [Google Scholar] [CrossRef]
- Weintraub, P.G.; Jones, P. (Eds.) Phytoplasmas: Genomes, Plant Hosts and Vectors; CABI: Wallingford, UK, 2009. [Google Scholar]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-Ur-Rehman, M.; Farid, M; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2016, 322, 2–16. [Google Scholar] [CrossRef]
- Sharma, K.; Ko, E.Y.; Assefa, A.D.; Ha, S.; Nile, S.H.; Lee, E.T.; Park, S.W. Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J. Food Drug Anal. 2015, 23, 243–252. [Google Scholar]
- López-Vargas, E.R.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; de Alba Romenus, K.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Foliar application of copper nanoparticles increases the fruit quality and the content of bioactive compounds in tomatoes. Appl. Sci. 2018, 8, 1020. [Google Scholar]
- Shahraki, B.; Bayat, H.; Aminifard, M.H.; Atajan, F.A. Effects of Foliar Application of Selenium and Nano-Selenium on Growth, Flowering, and Antioxidant Activity of Pot Marigold (Calendula officinalis L.) Under Salinity Stress Conditions. Commun. Soil Sci. Plant Anal. 2022, 53, 2749–2765. [Google Scholar] [CrossRef]
- Guleria, A.; Neogy, S.; Raorane, B.S.; Adhikari, S. Room temperature ionic liquid assisted rapid synthesis of amorphous Se nanoparticles: Their prolonged stabilization and antioxidant studies. Mater. Chem. Phys. 2020, 253, 123369. [Google Scholar]
- Kondaparthi, P.; Flora, S.J.S.; Naqvi, S. Selenium nanoparticles: An insight on its pro-oxidant and antioxidant properties. Front. Nanosci. Nanotechnol. 2019, 6, 1–5. [Google Scholar]
- Mellinas, C.; Jiménez, A.; Garrigós, M.D.C. Microwave-assisted green synthesis and antioxidant activity of selenium nanoparticles using Theobroma cacao L. bean shell extract. Molecules 2019, 24, 4048. [Google Scholar] [PubMed]
- Boroumand, S.; Safari, M.; Shaabani, E.; Shirzad, M.; Faridi-Majidi, R. Selenium nanoparticles: Synthesis, characterization and study of their cytotoxicity, antioxidant and antibacterial activity. Mater. Res. Express 2019, 6, 0850d8. [Google Scholar]
- Dumore, N.D.; Mukhopadhyay, M. Antioxidant of aqueous selenium nanoparticles (ASeNPs) and its catalysts activity for 1,1′-diphenyl-2-picrylhydrazyl (DPPH) reduction. J. Mol. Struct. 2020, 1205, 127637. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Mendis, H.C.; Thomas, V.P.; Schwientek, P.; Salamzade, R.; Chien, J.T.; Waidyarathne, P.; Kloepper, J.; De La Fuente, L. Strain-specific quantification of root colonization by plant growth promoting rhizobacteria Bacillus firmus I-1582 and Bacillus amyloliquefaciens QST713 in non-sterile soil and field conditions. PLoS ONE 2018, 13, e0193119. [Google Scholar] [CrossRef]
- Pandey, P.; Bisht, S.; Sood, A.; Aeron, A.; Sharma, G.D.; Maheshwari, D.K. Consortium of plantgrowth-promoting bacteria: Future perspective in agriculture. In Bacteria in Agrobiology: Plant Probiotics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 185–200. [Google Scholar]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.-P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2013, 378, 1–33. [Google Scholar]
- Maiyappan, S.; Amalraj, E.L.D.; Santhosh, A.; Peter, A.J. 2011 Isolation, Evaluation and formulation of selected microbial consortia for sustainable agriculture. J. Biofertil. Biopestic. 2020, 2, 2–7. [Google Scholar] [CrossRef]
- Shalaby, T.A.; Bayoumi, Y.; Abdalla, N.; Taha, H.; Alshaal, T.; Shehata, S.; Amer, M.; Domokos-Szabolcsy, É.; El-Ramady, H. Nanoparticles, soils, plants and sustainable agriculture. In Nanoscience in Food and Agriculture 1; Springer: Berlin/Heidelberg, Germany, 2016; pp. 283–312. [Google Scholar]



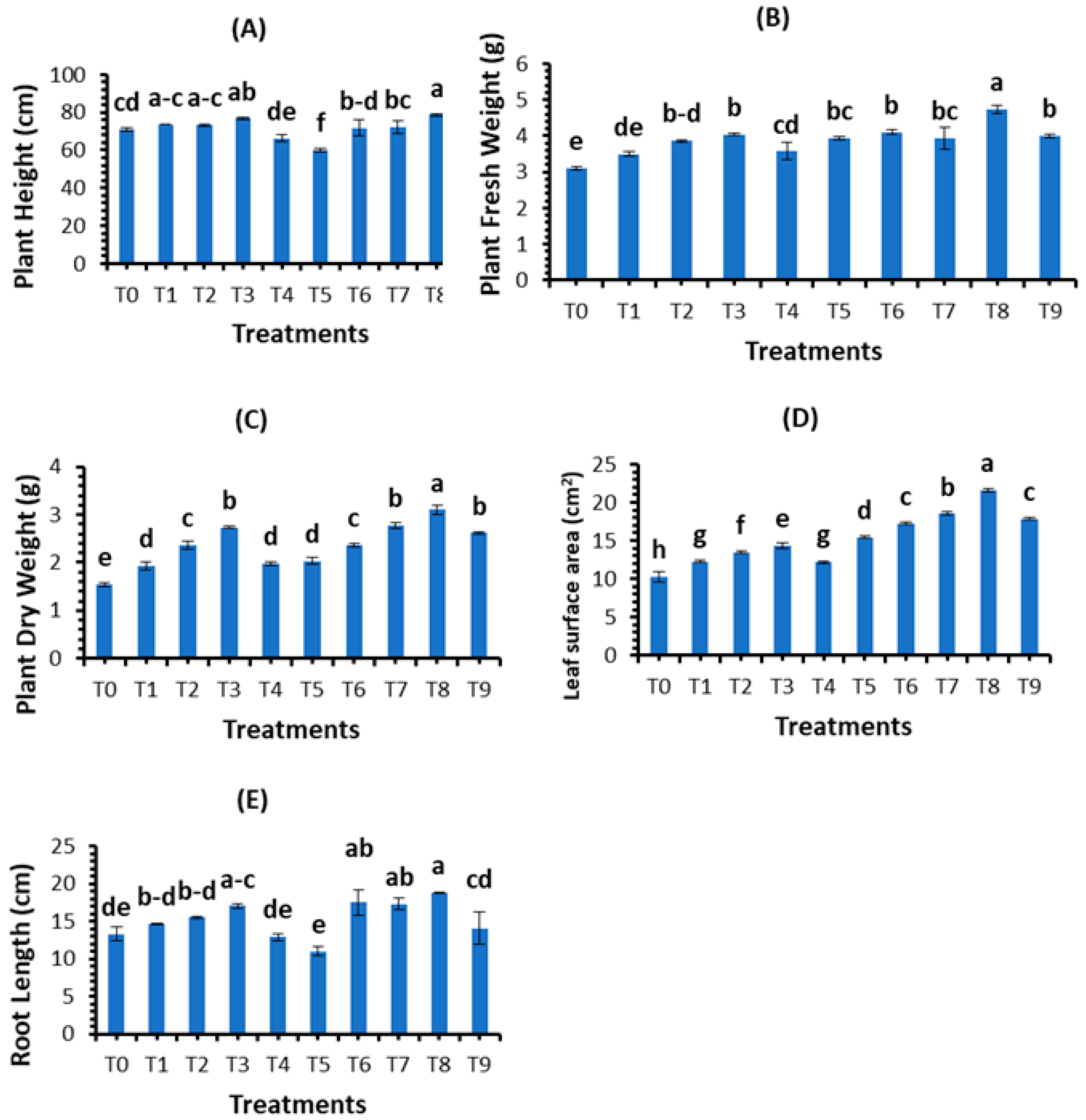
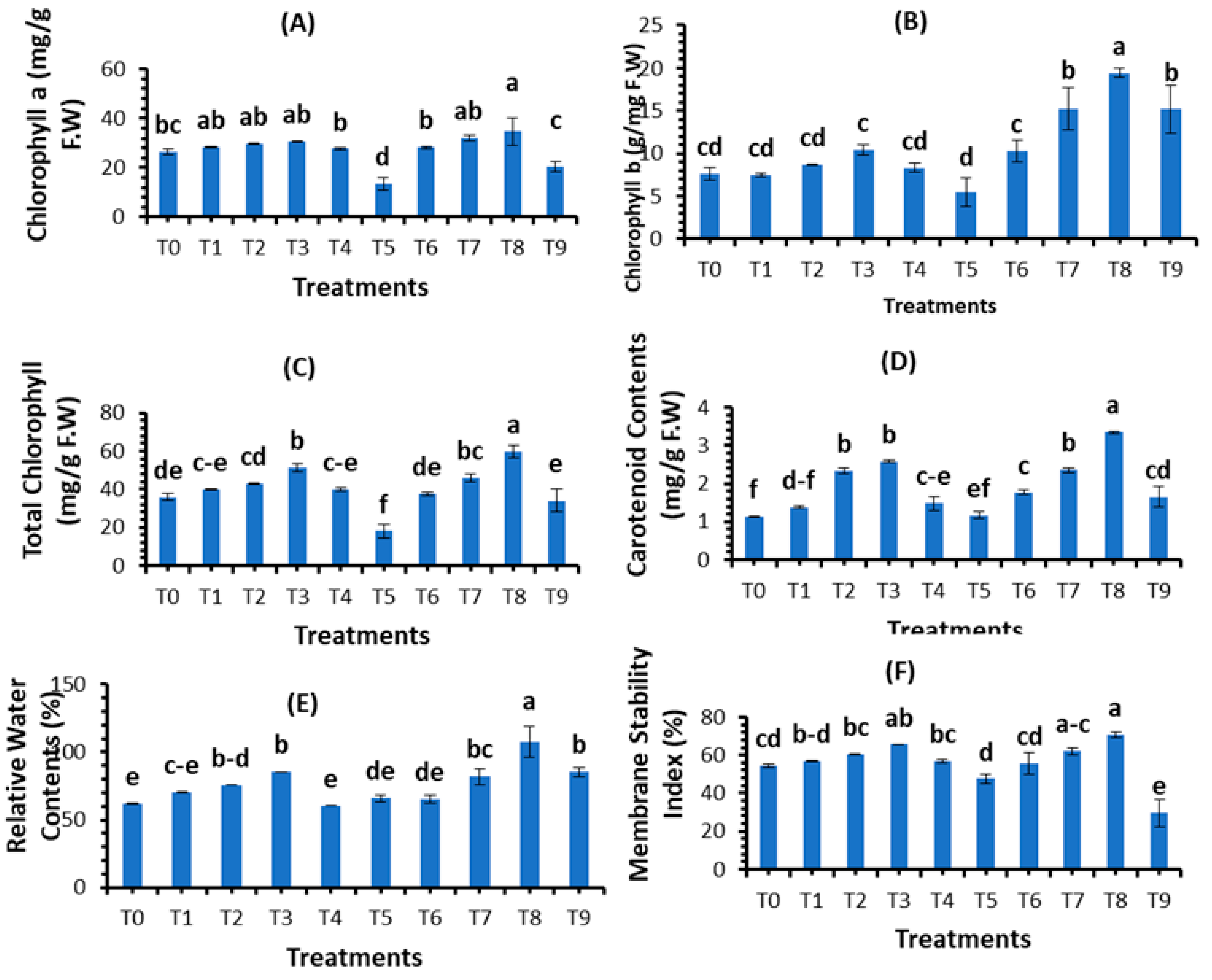
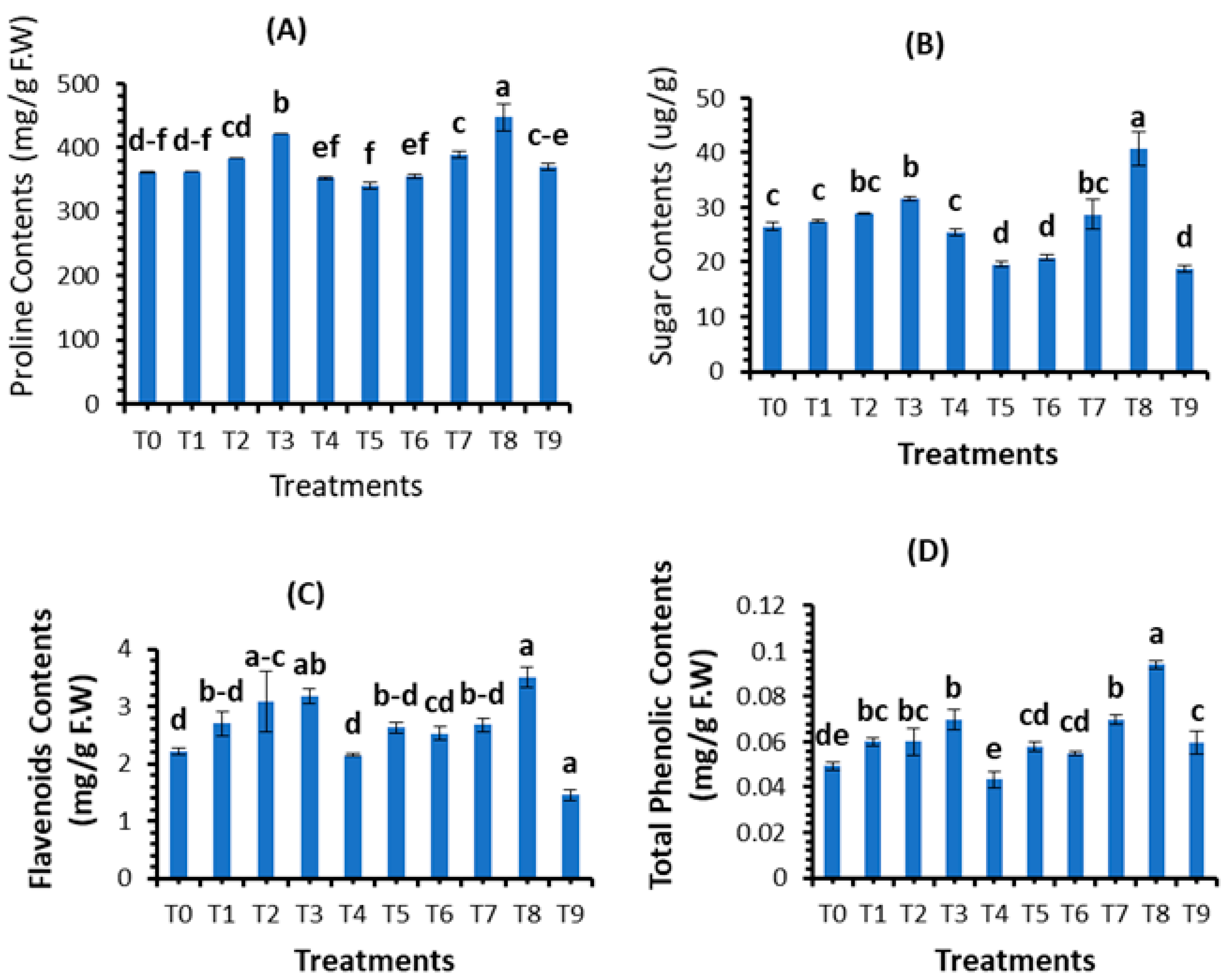
| Treatments | Concentrations (mg/L) |
|---|---|
| To | Control |
| T1 | Control+ 10 mg/L SeNPs |
| T2 | Control + 20 mg/L SeNPs |
| T3 | Control + 30 mg/L SeNPs |
| T4 | Control + 40 mg/L SeNPs |
| T5 | Fungus Inoculated Wheat |
| T6 | Pathogen + 10 mg/L SeNPs |
| T7 | Pathogen + 20 mg/L SeNPs |
| T8 | Pathogen + 30 mg/L SeNPs |
| T9 | Pathogen + 40 mg/L SeNPs |
| Number | Symptoms Level | Resistant Level |
|---|---|---|
| 0 | No symptoms | Resistant |
| 1 | 1–5% of spot on the leaves | Moderately Resistant |
| 2 | 6–20% of spot on the leaves | Moderately Resistant |
| 3 | 21–40% of spot on the leaves | Moderately susceptible |
| 4 | 41–60% of spot on the leaves | Moderately susceptible |
| 5 | Above 61% spot on leaves | Susceptible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahbaz, M.; Akram, A.; Mehak, A.; Haq, E.u.; Fatima, N.; Wareen, G.; Fitriatin, B.N.; Sayyed, R.Z.; Ilyas, N.; Sabullah, M.K. Evaluation of Selenium Nanoparticles in Inducing Disease Resistance against Spot Blotch Disease and Promoting Growth in Wheat under Biotic Stress. Plants 2023, 12, 761. https://doi.org/10.3390/plants12040761
Shahbaz M, Akram A, Mehak A, Haq Eu, Fatima N, Wareen G, Fitriatin BN, Sayyed RZ, Ilyas N, Sabullah MK. Evaluation of Selenium Nanoparticles in Inducing Disease Resistance against Spot Blotch Disease and Promoting Growth in Wheat under Biotic Stress. Plants. 2023; 12(4):761. https://doi.org/10.3390/plants12040761
Chicago/Turabian StyleShahbaz, Muhammad, Abida Akram, Asma Mehak, Ehsan ul Haq, Noor Fatima, Gull Wareen, Betty Natalie Fitriatin, R. Z. Sayyed, Noshin Ilyas, and Mohd Khalizan Sabullah. 2023. "Evaluation of Selenium Nanoparticles in Inducing Disease Resistance against Spot Blotch Disease and Promoting Growth in Wheat under Biotic Stress" Plants 12, no. 4: 761. https://doi.org/10.3390/plants12040761
APA StyleShahbaz, M., Akram, A., Mehak, A., Haq, E. u., Fatima, N., Wareen, G., Fitriatin, B. N., Sayyed, R. Z., Ilyas, N., & Sabullah, M. K. (2023). Evaluation of Selenium Nanoparticles in Inducing Disease Resistance against Spot Blotch Disease and Promoting Growth in Wheat under Biotic Stress. Plants, 12(4), 761. https://doi.org/10.3390/plants12040761






