Abstract
Pre-harvest sprouting (PHS) of wheat reduces grain yield and quality, and it is strongly affected by seed dormancy. Therefore, identification of quantitative trait loci (QTL) for seed dormancy is essential for PHS resistance breeding. A doubled haploid (DH) population, consisting of 174 lines from the cross between Yangmai16 (YM16) and Zhongmai895 (ZM895) was used to detect QTLs for seed dormancy and grain color. For seed dormancy, a total of seven QTLs were detected on chromosomes 2A, 3A, 3D, 4D, 5B and 5D over four environments, among which Qdor.hzau-3A, Qdor.hzau-3D.1 and Qdor.hzau-3D.2 were stably detected in more than two environments. For grain color, only two QTLs, Qgc.hzau-3A and Qgc.hzau-3D were detected on chromosomes 3A and 3D, which physically overlapped with Qdor.hzau-3A and Qdor.hzau-3D.1, respectively. Qdor.hzau-3D.2 has never been reported elsewhere and is probably a novel locus with allelic effect of seed dormancy contributed by weakly dormant parent ZM895, and a KASP marker was developed and validated in a wheat natural population. This study provides new information on the genetic dissection of seed dormancy, which may aid in further improvement for marker-assisted wheat breeding for PHS resistance.
1. Introduction
Bread wheat (Triticum aestivum L.) is one of the most important cereal crops around the world and contributes nearly 20% of the total calories consumed by humans []. Pre-harvest sprouting (PHS), physiologically mature grains germination in spikes before harvest, causes huge loss in grain yield and quality, particularly in regions with harvest time overlapping with rainy season []. The economic yearly loss caused by PHS in wheat production is more than one billion dollars worldwide []. Thus, it is imperative to identify PHS related QTLs that can be used to breed for PHS-resistant wheat cultivars.
Many factors have been found to be associated with pre-harvest sprouting, with seed dormancy and grain color as two major factors [,]. Seed dormancy can be defined as an intact and viable seed that fails to germinate under favorable conditions []. In wheat, QTLs associated with seed dormancy have been found on all 21 chromosomes, most of which are enriched on chromosomes of the third and fourth chromosome homologous groups []. Several genes related to seed dormancy have been cloned through map-based cloning. TaPHS1 (also called TaMFT-3A), the causal gene for the major QTL Qphs.pseru-3AS, was shown to be a positive regulator of ABA sensitivity and increases seed dormancy [,,,]. TaMKK3 (mitogen activated kinase kinase 3), the causal gene for another major seed dormancy QTL Phs1-4AL, was involved in protein phosphorylation in signal transduction pathways and affected seed dormancy by positively modulating the responsiveness of ABA [,,]. Moreover, several seed dormancy-related genes were cloned in wheat through homology-based cloning. TaVp1, a wheat ortholog of Vp1 (Viviparous-1) gene in maize and ABI3 (ABA Insensitive 3) in Arabidopsis, confers a high level of seed dormancy [,,,,]. TaSdr, an ortholog of OsSdr4 gene in rice, has a significant effect on seed dormancy with a missense mutation SNP643 which resulted in a substitution from Val to Ile [,]. TaQsd1, the ortholog of Qsd1 in barley, which encodes an alanine aminotransferase, has been shown to be necessary in the early seed germination [,,]. TaDOG1, the ortholog of DOG1 (Delay of Germination 1) gene in Arabidopsis, was found to increase seed dormancy via ABA1-dependent inhibition of Hypersensitive Germination 1 [,,,,,].
Grain color is another important factor for seed dormancy. Previous studies showed that red grain wheat is usually more resistant to PHS than white grain wheat [,,]. The R genes (contains R-A1, R-B1 and R-D1) affect grain color by regulating the expression of flavonoid biosynthesis genes, such as CHS, CHI and F3H []. TaMyb10, the casual grain color gene of the R loci, encodes a MYB type of transcription factor and has a large effect on both grain color and seed dormancy [,,,,]. Notably, recent studies confirmed the function of TaMyb10-3B through CRISPR/Cas9 in the wheat variety “Fielder” []. Another grain color related gene, TaDFR, has different expression level between white grain and red grain wheat and has been found to be associated with PHS resistance [,].
In this study, we used a double haploid population derived from a strongly dormant cultivar Yangmai16 (YM16) with red grain color and weakly dormant cultivar Zhongmai895 (ZM895) with white grain color, to detect QTLs for grain color and seed dormancy by evaluating the germination index (GI) and germination percentage (GP). The genetic effect of the identified QTLs for seed dormancy was further confirmed in natural population and DH subpopulation according to grain color. A KASP marker was successfully developed based on the identified QTL Qdor.hzau-3D.2 of seed dormancy and might be useful for PHS-resistance breeding.
2. Results
2.1. Phenotype Characterization and Analysis
GI and GP were assessed in the parental and DH lines over four environments. Positive significant correlation was observed among different environments for both GI and GP (Supplementary Tables S1 and S2). An analysis of variance (ANOVA) results showed significant variance of GI and GP for both genotypes and environments, and the interaction between genotype and environment (Supplementary Tables S3 and S4). The data showed that GI and GP values were distributed continuously and varied widely in DH population across the environments tested (Supplementary Figures S1 and S2). The weakly dormant parent ZM895 showed higher GI and GP values across all environments, while the values for the strongly dormant parent YM16 varied among different environments with much lower GI and GP compared to that of ZM895 (Table 1 and Table 2). The average GI and GP of the DH lines was generally higher in LY21 than in other environments, which could be the result of over-matured seeds when harvesting. Broad-sense heritability was over 0.75 for each of the four environments for GI and GP. Taken together, the heritability for GI and GP across all the four environments was 0.88, indicating that these traits were mainly determined by genetic factors.

Table 1.
GI of the YM16/ZM895 double haploid population and its parents in multiple environments.

Table 2.
GP of the YM16/ZM895 double haploid population and its parents in multiple environments.
Grain color was shown to be a key factor affecting seed dormancy []. Seeds from environment LY21 and XY22 were assessed through the sodium hydroxide immersion method as white grains and red grains exhibited different color after soaking (Supplementary Figure S3). In both environments, the white-grained lines had higher GI and GP than that of the red-grained lines. The Student’s t-test was employed to show that GI and GP was significantly different between red and white DH lines (Supplementary Figure S4). These results confirmed and consisted with previous results that grain color is a major factor for seed dormancy.
2.2. QTL Mapping for Seed Dormancy and Grain Color
For GI and GP, a total of seven QTLs were detected on chromosomes 2A, 3A, 3D, 4D, 5B and 5D. Three stable QTLs on chromosomes 3A and 3D were reproducibly detected in more than two environments for both GI and GP, and they were named Qdor.hzau-3A, Qdor.hzau-3D.1 and Qdor.hzau-3D.2, respectively (Table 3). According to the flanking markers and physical positions using Chinese Spring v1.0 genome as reference, Qdor.hzau-3A (709 Mb) and Qdor.hzau-3D.1 (572 Mb) could be the loci reported previously, TaMyb10-A (703.91 Mb) and TabMyb10-D (570.80 Mb) [], respectively. The locus Qdor.hzau-3D.2 was not found in previous results and could be a novel QTL with the LOD value 3.35–7.36, explained by the phenotypic variation 4.98–10.34%. The QTL was detected at approximately 230 cM on chromosome 3D, with flanking markers AX-110937331 and AX-108883716. Blast search results showed that Qdor.hzau-3D.2 was located in the interval 593.62–600.15 Mb, which contained 96 high-confidence genes. According to tissue expression profiles from Wheat Omics 1.0 website (http://202.194.139.32/, accessed on 25 December 2022), two genes, TraesCS3D02G514000 and TraesCS3D02G514300, were regarded as strong candidate genes for future research as they are mainly expressed in the grain. Interestingly, we found that the additive value of Qdor.hzau-3D.2 was positive among all environments, hinting that the dormant allele was contributed by weakly-dormant parent ZM895. The Student’s t-test results showed a significant difference on GI and GP between lines with YM16 allele and ZM895 allele in this DH population except for WH22 environment (Figure 1).

Table 3.
QTLs for GI and GP detected by ICIM in the YM16/ZM895 DH population.
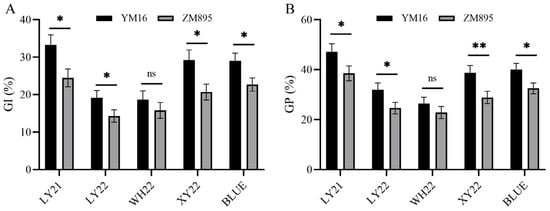
Figure 1.
Effect of Qdor.hzau-3D.2 on GI (A) and GP (B) in the YM16/ZM895 double haploid population. YM16 and ZM895 represented alleles of Qdor.hzau-3D.2 from parent Yangmai16 and Zhongmai895, respectively; * p < 0.05; ** p < 0.01; ns, nonsignificant.
Another four seed dormancy related QTLs were also detected in this study, and they were Qdor.hzau-2A, Qdor.hzau-4D, Qdor.hzau-5B and Qdor.hzau-5D (Table 3). However, each of the four QTLs could only be detected in one environment, indicating that they were not stable QTLs. Further experiments need to be done to confirm these QTLs.
For grain color QTL analysis, two QTLs on chromosomes 3A and 3D were detected in this population, and they were named Qgc.hzau-3A and Qgc.hzau-3D, explained by 19.25–21.11% and 30.25–30.44% phenotypic variation, respectively (Table 4). Based on the genetic position and physical interval of the two QTLs, Qgc.hzau-3A and Qgc.hzau-3D could be the same locus as Qdor.hzau-3A and Qdor.hzau-3D.1, covering the known grain color transcription factor TaMyb10 on chromosomes 3A and 3D, respectively. We did not detect the grain color QTL on chromosome 3B probably because there was no polymorphism between YM16 and ZM895 (Supplementary Figure S5).

Table 4.
QTLs for grain color detected by ICIM in the YM16/ZM895 DH population.
2.3. Effects of Qdor.hzau-3D.2 on Seed Dormancy in DH Population
As the grain color has a major effect on seed dormancy, the effect of Qdor.hzau-3D.2 on seed dormancy was assessed in this DH population when the grain color QTLs were present or absent. To do this, the DH population were divided into four genotypic classes. Class 1 includes the lines without either two grain color QTLs Qdor.hzau-3A/Qgc.hzau-3A, Qdor.hzau-3D.1/Qgc.hzau-3D or Qdor.hzau-3D.2, while class 2 includes the lines without the two grain color QTLs but with Qdor.hzau-3D.2. Class 3 includes the lines with two grain color QTLs but without Qdor.hzau-3D.2, while class 4 includes the lines with both the two grain color QTLs and Qdor.hzau-3D.2 (Table 5).

Table 5.
GI and GP of four genotypic combinations in the YM16/ZM895 DH population.
Based on the comparison results of four different classes, the GI and GP value of class 1 was higher than that of class 2, and the GI and GP value of class 3 was higher than that of class 4 (Table 5 and Figure 2A–D). The Student’s t-test showed a significant difference between class 1 and class 2 except for WH22 (Figure 2A,B), while significant difference was detected between class 3 and class 4 only in WH22 (Figure 2C,D). These results showed that the effect of Qdor.hzau-3D.2 had a minor but stable effect on seed dormancy, and that the effect would be expressed only in the absence of Qdor.hzau-3A/Qgc.hzau-3A and Qdor.hzau-3D.1/Qgc.hzau-3D, while it would be suppressed when the grain color related QTLs were present.
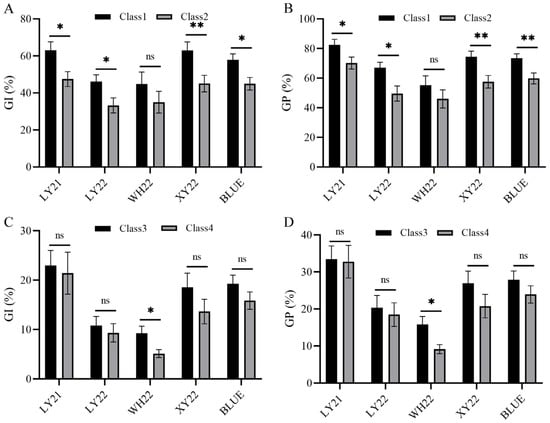
Figure 2.
Effect of Qdor.hzau-3D.2 in the absence or presence of grain color QTLs on GI (A,C) and GP (B,D) for all environments. Class 1 and 2 represented without and with the loci Qdor.hzau-3D.2 in the absence of grain color QTLs, respectively; Class 3 and 4 represented without and with the loci Qdor.hzau-3D.2 in the presence of grain color QTLs, respectively; * p < 0.05; ** p < 0.01; ns, nonsignificant.
In order to further investigate whether the effect of Qdor.hzau-3D.2 is related to grain color, the effect for Qdor.hzau-3D.2 in red and white subpopulations was analyzed based on the grain color assessment results in LY21 and XY22. We found that lines with two different alleles of Qdor.hzau-3D.2 showed significantly different seed dormancy in both red and white grain subpopulations, and lines with allele from ZM895 showed stronger seed dormancy than that with allele from YM16 (Figure 3). These results confirmed that the effect of Qdor.hzau-3D.2 on seed dormancy is independent of grain color, although it could be suppressed when the grain color related QTLs were present as aforementioned.
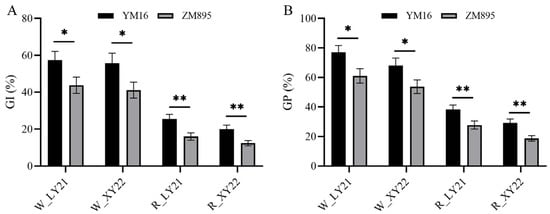
Figure 3.
Effect of Qdor.hzau-3D.2 on GI (A) and GP (B) in red and white-grained DH subpopulations. The uppercase letter W and R on the x-axis represented white- and red-grained DH subpopulations; YM16 and ZM895 represented alleles of Qdor.hzau-3D.2 from Yangmai16 and Zhongmai895, respectively; * p < 0.05; ** p < 0.01.
2.4. QTL Validation of Qdor.hzau-3D.2
The QTL Qdor.hzau-3D.2 was identified as a novel QTL in this study and was stably detected; therefore, the flanking marker AX-108883716 was converted to a KASP marker. This marker was used to validate the effect of Qdor.hzau-3D.2 in a wheat natural population, containing 305 wheat cultivars and 107 wheat landraces, and spike germination percentage (SGP) [] for this population was measured previously. This marker successfully classified the population into two groups, either with T allele from the ZM895, or C allele from the YM16 of Qdor.hzau-3D.2 (Supplementary Figure S6). The frequency of T and C allele in this population were nearly 60.7% and 39.3%, respectively. Overall, the lines with T allele showed lower SGP values than that with the C allele, although no significant difference was detected either in all wheat varieties or in subpopulations of cultivars, the significant difference was detected in subpopulation of landraces (Figure 4).
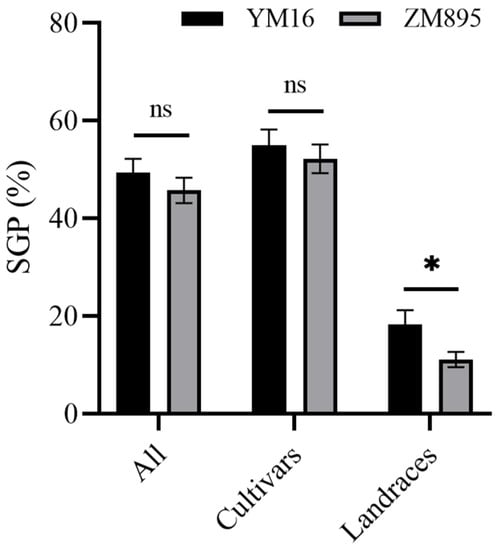
Figure 4.
Effect of Qdor.hzau-3D.2 on spike germination percentage in wheat natural population. YM16, wheat varieties carrying the C allele; ZM895, wheat varieties carrying the T allele; * p < 0.05; ns, nonsignificant.
3. Discussion
Pre-harvest sprouting (PHS) is a complex quantitative trait that is affected by many factors, while seed dormancy and grain color are two important factors. PHS has caused huge loss of yield and quality in wheat production, so it is imperative to explore the QTLs related to seed dormancy for breeding PHS resistant wheat varieties and around 200 QTLs have been identified []. In this study, three stable QTLs were detected on chromosomes 3A and 3D: Qdor.hzau-3A, Qdor.hzau-3D.1 and Qdor.hzau-3D.2. Comparative analysis showed that Qdor.hzau-3A and Qdor.hzau-3D.1 were colocalized with grain color genes TaMyb10-A and TaMyb10-D, respectively. Notably, based on grain color mapping results, the QTLs Qdor.hzau-3A and Qdor.hzau-3D.1 were co-localized with Qgc.hzau-3A and Qgc.hzau-3D, respectively. A significant difference on GI and GP was found between white and red DH lines, indicating grain color has a large effect on seed dormancy. These results confirmed the effect of grain color loci as reported in the previous studies [,,].
The QTL Qdor.hzau-3D.2 was identified as a novel stable seed dormancy related QTL in this study, with a dormant allele from weakly dormant parent ZM895. Qdor.hzau-3D.2 improved seed dormancy only when the grain color-related QTLs Qdor.hzau-3A/Qgc.hzau-3A and Qdor.hzau-3D.1/Qgc.hzau-3D were absent, suggesting that the effect of Qdor.hzau-3D.2 is stable but minor and that there might be an epistatic effect between them. In order to dissect the genetic interaction between Qdor.hzau-3D.2 and Qdor.hzau-3D.1/Qgc.hzau-3D near isogenic lines (NIL) containing either one or both of them, will be developed and further investigated. It will also be used for fine-mapping the causal gene underlying the QTL Qdor.hzau-3D.2. In addition, Qdor.hzau-3D.2 showed a significant difference on GI and GP values in both the red subpopulation and white subpopulation of this DH population, suggesting this QTL might affect seed dormancy independent of grain color. A KASP marker was successfully developed based on the flanking marker AX-108883716 at Qdor.hzau-3D.2, and the marker successfully separated a wheat natural population into two groups. Based on the Student’s t-test results in the wheat natural population, it was shown that this marker was tightly associated with spike germination rate in the wheat landraces while not in cultivars, which further confirmed the minor effect on seed dormancy. Nonetheless, this KASP marker might be useful for marker-assisted breeding PHS resistance in wheat varieties.
In addition, four QTLs, Qdor.hzau-2A, Qdor.hzau-4D, Qdor.hzau-5B and Qdor.hzau-5D, were detected in only one of the four environments, on chromosomes 2A, 4D, 5B and 5D in this study. None of the four unstable QTLs was found in previous studies on seed dormancy or PHS resistance [], indicating they might be novel QTLs for seed dormancy. Interestingly, preliminary results showed that the QTL Qdor.hzau-5D was also detected for heading date, flowering date and tillering angle in this DH population and co-localized with the vernalization-related gene VRN1-5D [], indicating that this QTL might have pleiotropic effects. However, more evidences need to be explored in future research to further validate the authenticity of these QTLs.
4. Materials and Methods
4.1. Plant Materials and Trial Environments
The parents Yangmai16 (YM16) and Zhongmai895 (ZM895) and 174 DH lines were planted at Luoyang (Henan Province) in the 2020–2021 cropping season and at Wuhan (Hubei Province), Xiangyang (Hubei Province) and Luoyang (Henan Province) in the 2021–2022 cropping season, named LY21, WH22, XY22 and LY22 in this study, respectively. Field experiments were arranged in randomized complete blocks with three replications and each plot was 1 m single row in which 20 seeds were sown. The field management followed local agricultural practice. A panel of 412 wheat accessions was utilized for validation of the KASP marker for Qdor.hzau-3D.2, including 305 wheat cultivars and 107 wheat landraces collected from both China and other countries, representing global genetic diversity.
4.2. Phenotype Assessment
In this study, germination index (GI) and germination percentage (GP) were measured for seed dormancy [,]. In physiologically mature stage (hard dough stage), five spikes were harvested from different plants of each of the DH lines. The harvested spikes were air-dried for 2 days at room temperature, hand-threshed to avoid damage to the embryos of seeds, then stored at −20 °C to maintain dormancy until phenotyping []. Seeds were sterilized with 1% (v/v) of sodium hypochlorite for 10 min, followed by three rinses with sterile water. Fifty clean seeds were incubated in a 90 mm Petri dish with a filter paper and 8 mL of distilled water in the dark conditions for 7 days at 22 °C with three replications. Germinated seeds were counted daily and removed. GI and GP were calculated based on the following formula: GI = [(n1 × 7 + n2 × 6 + n3 × 5 + n4 × 4 + n5 × 3 + n6 × 2 + n7 × 1)/(N × 7)] × 100%; GP = [(n1 + n2 + n3 + n4 + n5 + n6 + n7)/N] × 100%. The parameters n1, n2, n3, n4, n5, n6 and n7 represent the number of germinated seed on day 1, day 2, day 3, day 4, day 5, day 6 and day 7, respectively. N represents the total of seeds.
For the wheat natural population, the spike germination percentage was obtained using the whole spike germination method, according to the procedure in the determination of pre-harvest sprouting in wheat (https://www.sdtdata.com/fx/fmrule/tsLibCard.doView, accessed on 15 August 2022). Briefly, five physiologically matured spikes were harvested and soaked in water for 4 h, then spikes were sterilized with 0.1% (v/v) of sodium hypochlorite for 5 min, followed by three rinses with sterile water. Spikes were incubated at 22 °C and RH100% (relative humidity) for 4 days, then dried at 80 °C for 2 days. The processed spikes were hand-threshed and the geminated seeds were counted. SGP was calculated by the formula: SGP = (germinated seeds)/(total seeds) × 100%.
Seeds harvested from the environment LY21 and XY22 were used for grain color assessment. The grain color was assessed according to the procedure provided by Imtiaz et al. []. About 30–40 seeds were soaked with 10 mL of 5% sodium hydroxide solution for 4 h at room temperature. Red grain cultivars showed dark-red color while white grains exhibited straw-yellow color. The number 0 and 1 were used to represent white and red DH lines for data analysis in this study, respectively.
4.3. Genotyping and Linkage Mapping
The parents and 174 DH lines were genotyped with the wheat 660K SNP array, and the genetic linkage map was constructed in the previous study []. There were 14,480 markers on this genetic map covered with 21 wheat chromosomes; the full length of the genetic map was 3681.7 cM and the average marker interval was 0.25 cM.
Markers for grain color loci TaMyb10-A, TaMyb10-B and TaMyb10-D were used for genotyping the parent YM16 and ZM895. In this study, markers for TaMyb10-A contained three pairs of primers, named TaMyb10-A1-F/R (F: 5′-CTACCAGCTCGTTTGGGAAG-3′, R: 5′-CTACCAGCTCGTTTGGGAAG-3), TaMyb10-A2-F/R (F: 5′-TTTCAATCGAGTGGGCATAA-3′, R: 5′-CCTGACGATGAGCTCCTCTT-3′) and TaMyb10-A3-F/R (F: 5′-TGTTATCACATGCTGATCCTGA-3′, R: 5′-TCCCTACATGGGAGACAGAGA-3′), respectively; markers for TaMyb10-B and TaMyb10-D were named TaMyb10-B-F/R (F: 5′-AGGAACCTGCAGTCTCACGG-3′, R: 5′-CTCGTGAACCCCCTCTGCT-3′) and TaMyb10-D-F/R (F: 5′-TAGGCCAACACCTTCTAAACG-3′, R: 5′-AGGCACACCAGCTTATTTGG-3′), respectively; primers information for these markers was obtained from previous studies [,].
4.4. Statistical Analysis
GI and GP correlation coefficients and analysis of variance (ANOVA) were obtained using QTL IciMapping version 4.2 []. The best linear unbiased estimation (BLUE) values were calculated by R package “lme4” using R4.2.2 software []; the mixed linear model was used for BLUE value calculation, the DH lines were used as fixed factors while the year, location and interaction between replication and location were used as random factors. The broad-sense heritability (H2) was estimated by the following formula: H2 = Vg/(Vg + Vge/e + Ve/re), where Vg, Vge and Ve represent genotypic variance, the variance of genotype by environment and error variance, respectively; r and e represent the number of replications and environments, respectively.
4.5. QTL Analysis
GI and GP values in single environment and BLUE values across all environments for the 174 DH lines were used for QTL mapping. For grain color QTL detection, phenotypic data was used from environment LY21 and XY22. Inclusive composite interval mapping (ICIM-ADD) method under the bi-parental populations model was used for QTL analysis. QTLs with LOD score greater than 2.5 were reported and named using standard nomenclature for wheat. The physical positions of QTLs in this study were obtained based on the flanking markers using Chinese Spring v1.0 genome as reference from the Wheat Omics 1.0 website (http://202.194.139.32/, accessed on 20 October 2022). In this study, QTLs with large shared position or peaks of adjacent QTL distanced to less than 1 cM or overlapped with physical interval were regarded as the same QTL [].
4.6. KASP Marker Development and Validation
The flanking marker AX-108883716 linked to the QTL Qdor.hzau-3D.2 was converted to a KASP assay. The KASP primers in this study were designed using the Primer Server tool of Wheat Omics 1.0 website. The forward primers contain FAM-primer and HEX-primer, they were 5′-GAAGGTGACCAAGTTCATGCTgtgagtgtactgaacttggttgT-3′ and 5′-GAAGGTCGGAGTCAACGGATTgtgagtgtactgaacttggttgC-3′, respectively; the reverse primer was 5′-GAATTCGGTGTCCAGGACCT-3′. The PCR reactions were performed in a total volume of 5.25 μL, containing 2.5 μL of KASP master mix (JasonGen, Beijing, China), 0.25 μL of primer mix (0.05 μL FAM primer, 0.05 μL HEX primer and 0.15 μL common reverse primer), 0.5 μL of genomic DNA (concentration of 100 ng/μL) and 2 μL of ddH2O. The PCR was performed on a BioRad CFX96 qPCR machine according to the following steps: 95 °C for 10 min, 10 touchdown cycles for 65–55 °C (decreasing by −1 °C per cycle), followed by 35 additional cycles (95 °C for 15 s and 55 °C for 1 min), and then read fluorescence data at 30 °C for 30 s. The data was downloaded from the inbuilt BioRad CFX96 software and analyzed with Microsoft Excel 2016.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12040759/s1. Figure S1: frequency distributions of GI in the YM16/ZM895 double haploid population for single environment and BLUE value; Figure S2: frequency distributions of GP in the YM16/ZM895 double haploid population for single environment and BLUE value; Figure S3: grain color identification in the YM16/ZM895 double haploid population; Figure S4: effect of grain color on GI (A) and GP (B) in the YM16/ZM895 double haploid population for all environments; Figure S5: genotyping results for DH parents YM16 and ZM895 with the TaMyb10-A/B/D markers; Figure S6: genotyping results in wheat natural population with the KASP marker for Qdor.hzau-3D.2; Table S1: correlation coefficients (r) of GI among all environments in the YM16/ZM895 double haploid population; Table S2: correlation coefficients (r) of GP among all environments in the YM16/ZM895 double haploid population; Table S3: analysis of variance of GI in the YM16/ZM895 double haploid population; Table S4: analysis of variance of GP in the YM16/ZM895 double haploid population.
Author Contributions
G.G. performed most of experiment and wrote the manuscript; S.X. and H.C. performed a partial experiment; H.M. designed the experiment and assisted in writing the manuscript together with Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Fundamental Research Funds for the Central Universities (11041810314).
Data Availability Statement
Not applicable.
Acknowledgments
We thank Y Zhang, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences (CAAS), for critical reading of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gupta, P.K.; Balyan, H.S.; Sharma, S.; Kumar, R. Genetics of yield, abiotic stress tolerance and biofortification in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2020, 133, 1569–1602. [Google Scholar] [CrossRef] [PubMed]
- Vetch, J.M.; Stougaard, R.N.; Martin, J.M.; Giroux, M.J. Review: Revealing the genetic mechanisms of pre-harvest sprouting in hexaploid wheat (Triticum aestivum L.). Plant Sci. 2019, 281, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Bai, G.; Rife, T.W.; Poland, J.; Lin, M.; Liu, S.; Chen, H.; Kumssa, T.; Fritz, A.; Trick, H.; et al. QTL mapping of pre-harvest sprouting resistance in a white wheat cultivar Danby. Theor. Appl. Genet. 2018, 131, 1683–1697. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Groos, C.; Gay, G.; Perretant, M.R.; Gervais, L.; Bernard, M.; Dedryver, F.; Charmet, G. Study of the relationship between pre-harvest sprouting and grain color by quantitative trait loci analysis in a whitexred grain bread-wheat cross. Theor. Appl. Genet. 2002, 104, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.; Wang, H.J.; Xu, X.J.; Sun, W.H.; Ju, L.; Liu, W.T.; Li, W.Q.; Sun, J.; Chen, K.M. Pre-harvest sprouting in cereals: Genetic and biochemical mechanisms. J. Exp. Bot. 2021, 72, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cai, S.; Graybosch, R.; Chen, C.; Bai, G. Quantitative trait loci for resistance to pre-harvest sprouting in US hard white winter wheat Rio Blanco. Theor. Appl. Genet. 2008, 117, 691–699. [Google Scholar] [CrossRef]
- Liu, S.; Sehgal, S.K.; Li, J.; Lin, M.; Trick, H.N.; Yu, J.; Gill, B.S.; Bai, G. Cloning and characterization of a critical regulator for preharvest sprouting in wheat. Genetics 2013, 195, 263–273. [Google Scholar] [CrossRef]
- Nakamura, S.; Abe, F.; Kawahigashi, H.; Nakazono, K.; Tagiri, A.; Matsumoto, T.; Utsugi, S.; Ogawa, T.; Handa, H.; Ishida, H.; et al. A wheat homolog of MOTHER OF FT AND TFL1 acts in the regulation of germination. Plant Cell 2011, 23, 3215–3229. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, L.-X.; Chen, X.-J.; Cao, J.-J.; Wu, Z.-Y.; Liu, K.; Zhang, C.; Wei, W.-X.; Xie, H.-Y.; Li, L.; et al. A novel 33-bp insertion in the promoter of TaMFT-3A is associated with pre-harvest sprouting resistance in common wheat. Mol. Breed. 2018, 38, 69. [Google Scholar] [CrossRef]
- Danquah, A.; de Zelicourt, A.; Boudsocq, M.; Neubauer, J.; Frei Dit Frey, N.; Leonhardt, N.; Pateyron, S.; Gwinner, F.; Tamby, J.P.; Ortiz-Masia, D.; et al. Identification and characterization of an ABA-activated MAP kinase cascade in Arabidopsis thaliana. Plant J. 2015, 82, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Torada, A.; Koike, M.; Ogawa, T.; Takenouchi, Y.; Tadamura, K.; Wu, J.; Matsumoto, T.; Kawaura, K.; Ogihara, Y. A Causal Gene for Seed Dormancy on Wheat Chromosome 4A Encodes a MAP Kinase Kinase. Curr. Biol. 2016, 26, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.A.; Shorinola, O.; Conselman, S.; See, D.; Skinner, D.Z.; Uauy, C.; Steber, C.M. Exome sequencing of bulked segregants identified a novel TaMKK3-A allele linked to the wheat ERA8 ABA-hypersensitive germination phenotype. Theor. Appl. Genet. 2020, 133, 719–736. [Google Scholar] [CrossRef] [PubMed]
- McCarty, D.R.; Hattori, T.; Carson, C.B.; Vasil, V.; Lazar, M.; Vasil, I.K. The Viviparous-1 developmental gene of maize encodes a novel transcriptional activator. Cell 1991, 66, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, Y.Z.; Xu, Z.S.; Chen, X.M.; He, Z.H.; Yu, Z.; Wilkinson, M.; Jones, H.D.; Shewry, P.R.; Xia, L.Q. Isolation and characterization of Viviparous-1 genes in wheat cultivars with distinct ABA sensitivity and pre-harvest sprouting tolerance. J. Exp. Bot. 2007, 58, 2863–2871. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.L.; Liu, S.X.; Sun, Y.Q.; Meng, J.Y.; Xia, L.Q. Characterization of the rich haplotypes of Viviparous-1A in Chinese wheats and development of a novel sequence-tagged site marker for pre-harvest sprouting resistance. Mol. Breed. 2013, 33, 75–88. [Google Scholar] [CrossRef]
- Feng, Y.; Qu, R.; Liu, S.; Yang, Y. Rich haplotypes of Viviparous-1 in Triticum aestivum subsp. spelta with different abscisic acid sensitivities. J. Sci. Food Agric. 2017, 97, 497–504. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, H.; Cheng, M.P.; Dankwa, K.O.; Chen, Z.X.; Li, Z.Y.; Gao, S.; Liu, Y.X.; Jiang, Q.T.; Lan, X.J.; et al. Genome-Wide Association Study for Pre-harvest Sprouting Resistance in a Large Germplasm Collection of Chinese Wheat Landraces. Front. Plant Sci. 2017, 8, 401. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, X.; Xia, X.; He, Z. Cloning of seed dormancy genes (TaSdr) associated with tolerance to pre-harvest sprouting in common wheat and development of a functional marker. Theor. Appl. Genet. 2014, 127, 855–866. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, X.; He, Z. The seed dormancy allele TaSdr-A1a associated with pre-harvest sprouting tolerance is mainly present in Chinese wheat landraces. Theor. Appl. Genet. 2017, 130, 81–89. [Google Scholar] [CrossRef]
- Sato, K.; Yamane, M.; Yamaji, N.; Kanamori, H.; Tagiri, A.; Schwerdt, J.G.; Fincher, G.B.; Matsumoto, T.; Takeda, K.; Komatsuda, T. Alanine aminotransferase controls seed dormancy in barley. Nat. Commun. 2016, 7, 11625. [Google Scholar] [CrossRef] [PubMed]
- Onishi, K.; Yamane, M.; Yamaji, N.; Tokui, M.; Kanamori, H.; Wu, J.; Komatsuda, T.; Sato, K. Sequence differences in the seed dormancy gene Qsd1 among various wheat genomes. BMC Genom. 2017, 18, 497. [Google Scholar] [CrossRef]
- Hisano, H.; Hoffie, R.E.; Abe, F.; Munemori, H.; Matsuura, T.; Endo, M.; Mikami, M.; Nakamura, S.; Kumlehn, J.; Sato, K. Regulation of germination by targeted mutagenesis of grain dormancy genes in barley. Plant Biotechnol. J. 2022, 20, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Bentsink, L.; Jowett, J.; Hanhart, C.J.; Koornneef, M. Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 17042–17047. [Google Scholar] [CrossRef] [PubMed]
- Ashikawa, I.; Abe, F.; Nakamura, S. Ectopic expression of wheat and barley DOG1-like genes promotes seed dormancy in Arabidopsis. Plant Sci. 2010, 179, 536–542. [Google Scholar] [CrossRef]
- Ashikawa, I.; Mori, M.; Nakamura, S.; Abe, F. A transgenic approach to controlling wheat seed dormancy level by using Triticeae DOG1-like genes. Transgenic Res. 2014, 23, 621–629. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Bartsch, M.; Xiang, Y.; Miatton, E.; Pellengahr, S.; Yano, R.; Seo, M.; Soppe, W.J. The time required for dormancy release in Arabidopsis is determined by DELAY OF GERMINATION1 protein levels in freshly harvested seeds. Plant Cell 2012, 24, 2826–2838. [Google Scholar] [CrossRef]
- Rikiishi, K.; Maekawa, M. Seed maturation regulators are related to the control of seed dormancy in wheat (Triticum aestivum L.). PLoS ONE 2014, 9, e107618. [Google Scholar] [CrossRef]
- Nishimura, N.; Tsuchiya, W.; Moresco, J.J.; Hayashi, Y.; Satoh, K.; Kaiwa, N.; Irisa, T.; Kinoshita, T.; Schroeder, J.I.; Yates, J.R., 3rd; et al. Control of seed dormancy and germination by DOG1-AHG1 PP2C phosphatase complex via binding to heme. Nat. Commun. 2018, 9, 2132. [Google Scholar] [CrossRef]
- Flintham, J.E. Different genetic components control coat-imposed and embryo-imposeddormancy in wheat. Seed Sci. Res. 2000, 10, 43–50. [Google Scholar] [CrossRef]
- Warner, R.L.; Kudrna, D.A.; Spaeth, S.C.; Jones, S.S. Dormancy in white-grain mutants of Chinese Spring wheat (Triticum aestivum L.). Seed Sci. Res. 2007, 10, 51–60. [Google Scholar] [CrossRef]
- Himi, E.; Mares, D.J.; Yanagisawa, A.; Noda, K. Effect of grain colour gene (R) on grain dormancy and sensitivity of the embryo to abscisic acid (ABA) in wheat. J. Exp. Bot. 2002, 53, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Himi, E.; Nisar, A.; Noda, K. Colour genes (R and Rc) for grain and coleoptile upregulate flavonoid biosynthesis genes in wheat. Genome 2005, 48, 747–754. [Google Scholar] [CrossRef]
- Himi, E.; Maekawa, M.; Miura, H.; Noda, K. Development of PCR markers for Tamyb10 related to R-1, red grain color gene in wheat. Theor. Appl. Genet. 2011, 122, 1561–1576. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.L.; Meng, J.Y.; Zhang, Y.J.; He, Z.H.; Yang, Y. Characterization of Tamyb10 allelic variants and development of STS marker for pre-harvest sprouting resistance in Chinese bread wheat. Mol. Breed. 2016, 36, 148. [Google Scholar] [CrossRef]
- Mares, D.; Himi, E. The role of TaMYB10-A1 of wheat (Triticum aestivum L.) in determining grain coat colour and dormancy phenotype. Euphytica 2021, 217, 89. [Google Scholar] [CrossRef]
- Lang, J.; Fu, Y.; Zhou, Y.; Cheng, M.; Deng, M.; Li, M.; Zhu, T.; Yang, J.; Guo, X.; Gui, L.; et al. Myb10-D confers PHS-3D resistance to pre-harvest sprouting by regulating NCED in ABA biosynthesis pathway of wheat. New Phytol. 2021, 230, 1940–1952. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, Y.; Fan, Y.; Wang, Y.; Li, P.; Xiong, J.; He, Y.; Cheng, S.; Ye, X.; Wang, F.; et al. CRISPR/Cas9-mediated restoration of Tamyb10 to create pre-harvest sprouting-resistant red wheat. Plant Biotechnol. J. 2022. [Google Scholar] [CrossRef]
- Himi, E.; Noda, K. Isolation and location of three homoeologous dihydroflavonol-4-reductase (DFR) genes of wheat and their tissue-dependent expression. J. Exp. Bot. 2004, 55, 365–375. [Google Scholar] [CrossRef]
- Bi, H.H.; Sun, Y.W.; Xiao, Y.G.; Xia, L.Q. Characterization of DFR allelic variations and their associations with pre-harvest sprouting resistance in a set of red-grained Chinese wheat germplasm. Euphytica 2013, 195, 197–207. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Yan, Y.; Yan, X.; Shi, C.; Zhao, L.; Chen, F. Genome-wide association study of heading and flowering dates and construction of its prediction equation in Chinese common wheat. Theor. Appl. Genet. 2018, 131, 2271–2285. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tan, C.; Lang, J.; Tang, H.; Hao, M.; Tan, Z.; Yu, H.; Zhou, Y.; Liu, Z.; Li, M.; et al. Identification of qPHS.sicau-1B and qPHS.sicau-3D from synthetic wheat for pre-harvest sprouting resistance wheat improvement. Mol. Breed. 2019, 39, 132. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Ou, X.; Wang, M.; Wang, N.; Li, W.; Deng, Y.; Diao, Y.; Sun, Z.; Luo, Q.; et al. Identification of a stable major-effect quantitative trait locus for pre-harvest sprouting in common wheat (Triticum aestivum L.) via high-density SNP-based genotyping. Theor. Appl. Genet. 2022, 135, 4183–4195. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, M.; Ogbonnaya, F.C.; Oman, J.; van Ginkel, M. Characterization of quantitative trait loci controlling genetic variation for preharvest sprouting in synthetic backcross-derived wheat lines. Genetics 2008, 178, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, Z.; Jia, A.; Wang, F.; Wang, J.; Zhang, Y.; Fu, C.; Fu, L.; Bai, G.; Xia, X.; et al. Mapping of QTL for partial resistance to powdery mildew in two Chinese common wheat cultivars. Euphytica 2019, 216, 3. [Google Scholar] [CrossRef]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Usinglme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Wang, H.; Jia, J.; Cai, Z.; Duan, M.; Jiang, Z.; Xia, Q.; Ma, Q.; Lian, T.; Nian, H. Identification of quantitative trait loci (QTLs) and candidate genes of seed Iron and zinc content in soybean [Glycine max (L.) Merr.]. BMC Genom. 2022, 23, 146. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).