ADH Gene Cloning and Identification of Flooding-Responsive Genes in Taxodium distichum (L.) Rich
Abstract
1. Introduction
2. Results
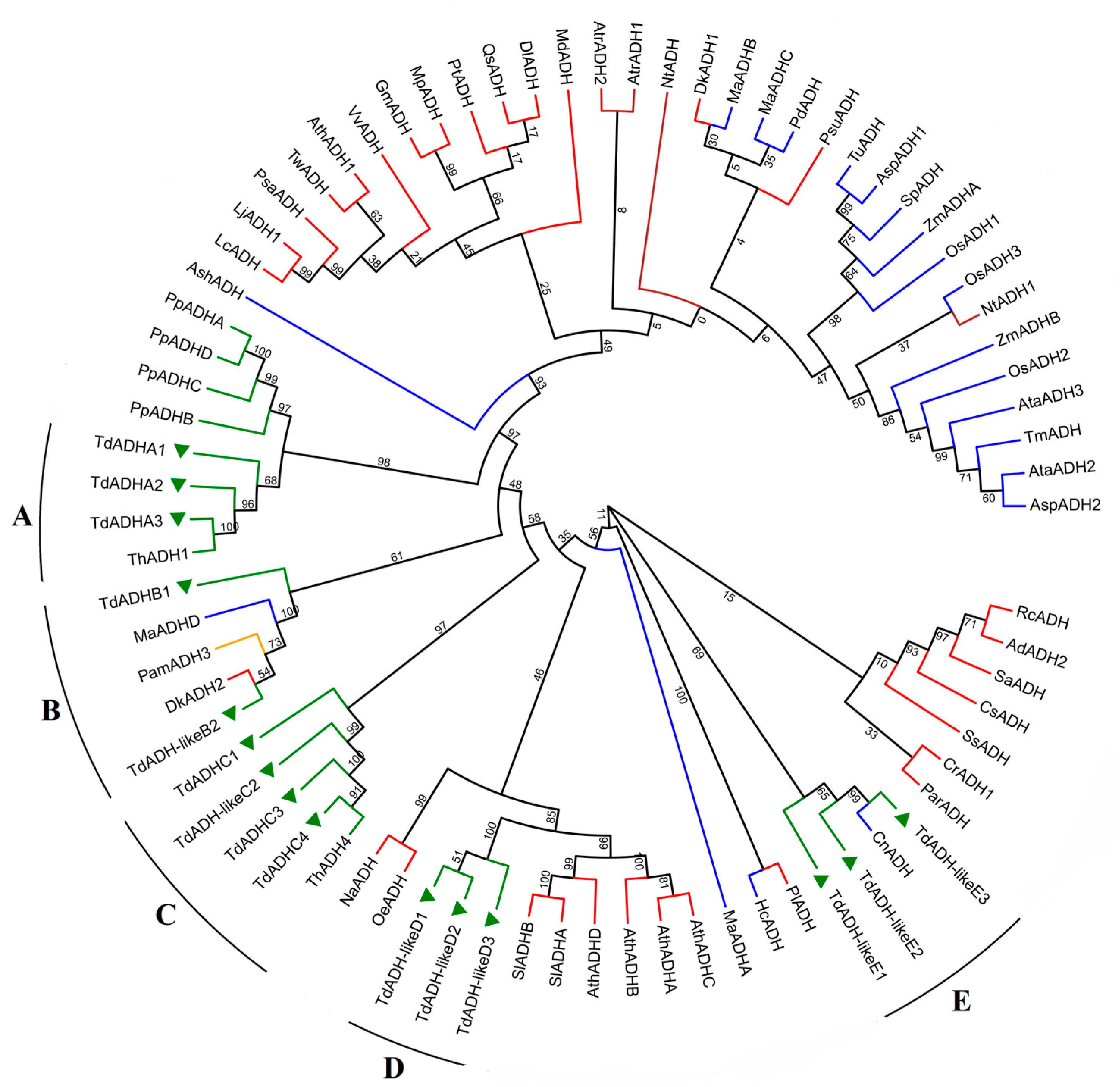
2.1. TdADH Identification and Phylogeny Analysis
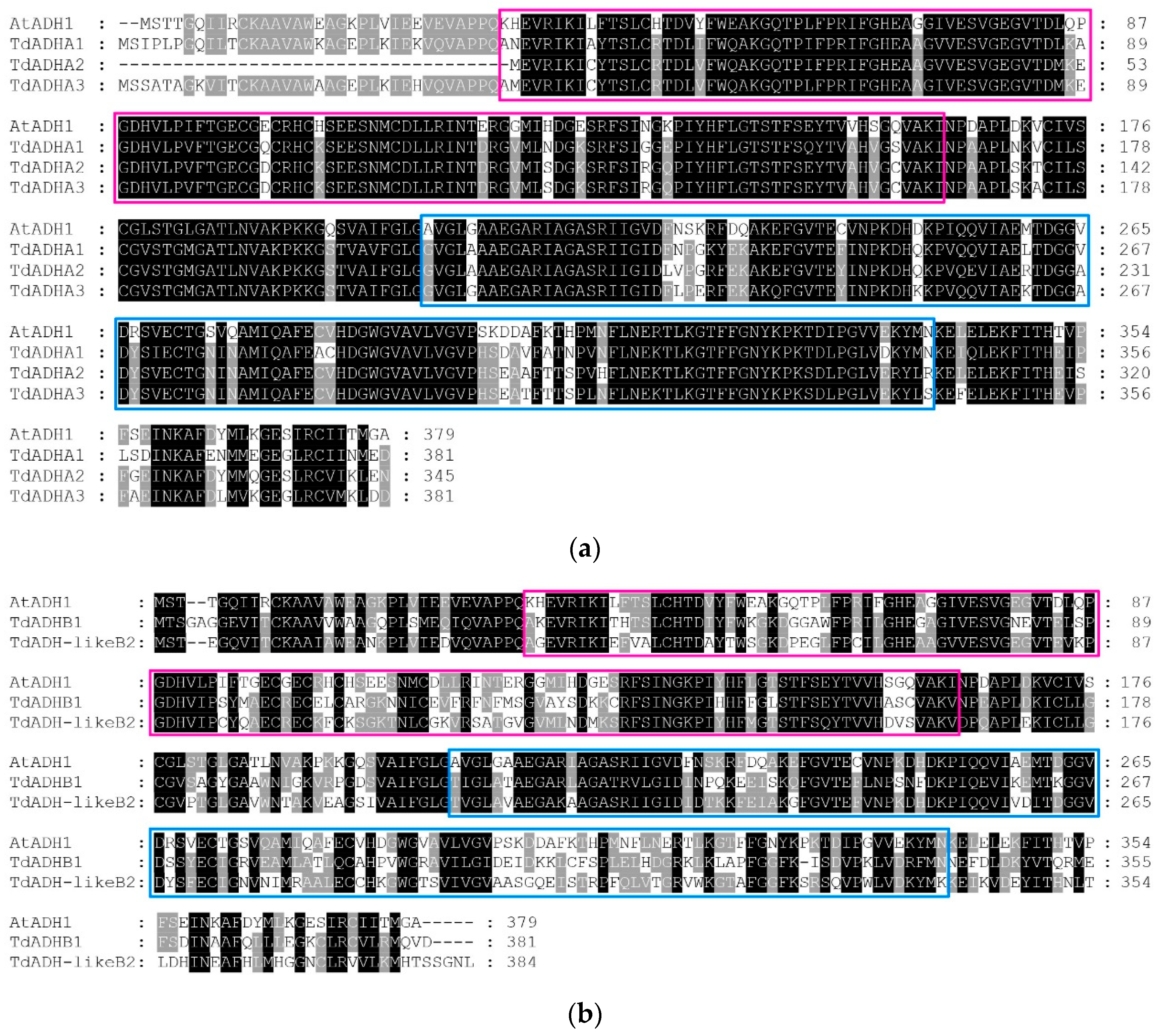
2.2. Multisequence Alignment
2.3. Motif Composition Analysis and Bioinformatics Analysis
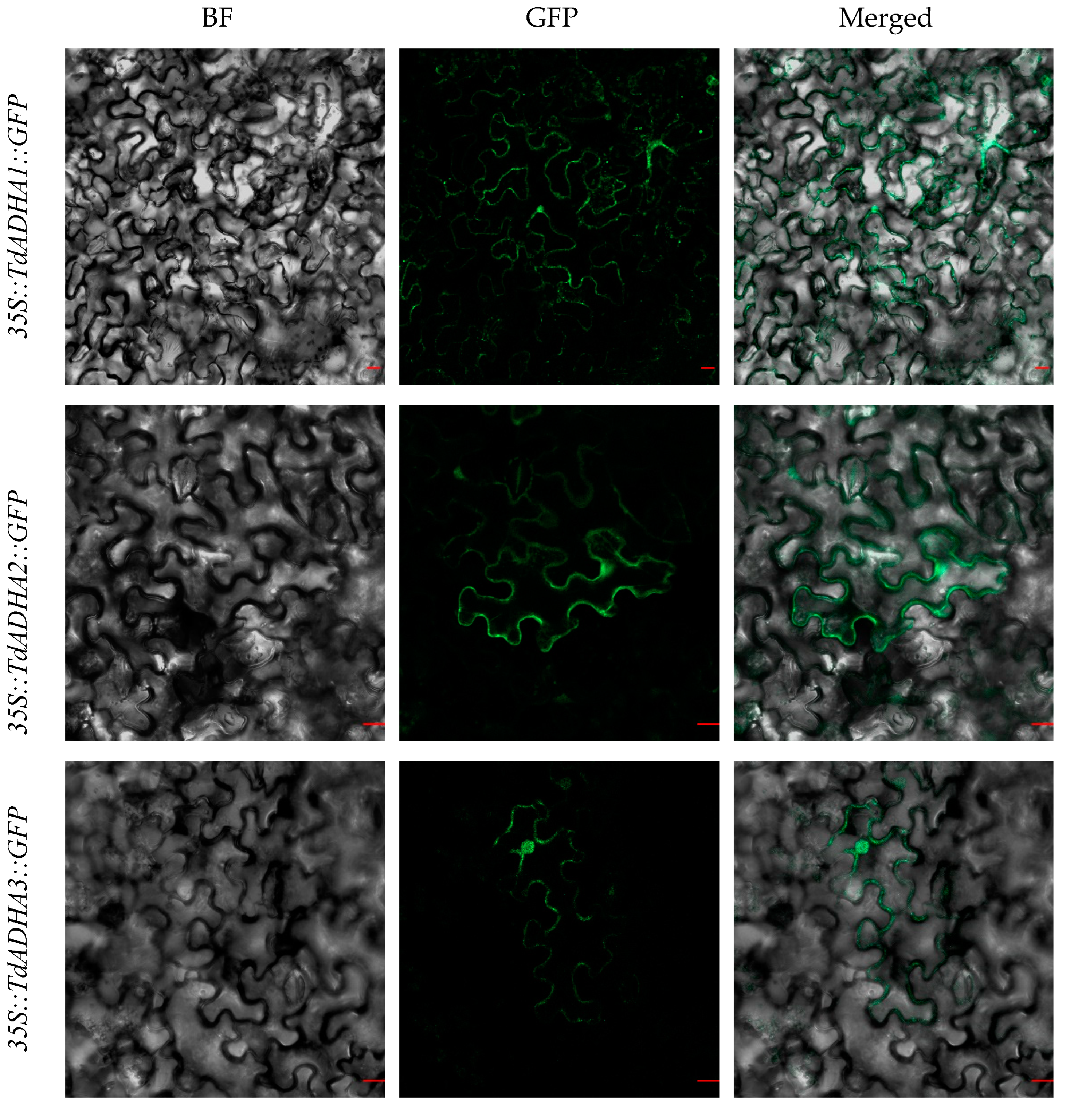
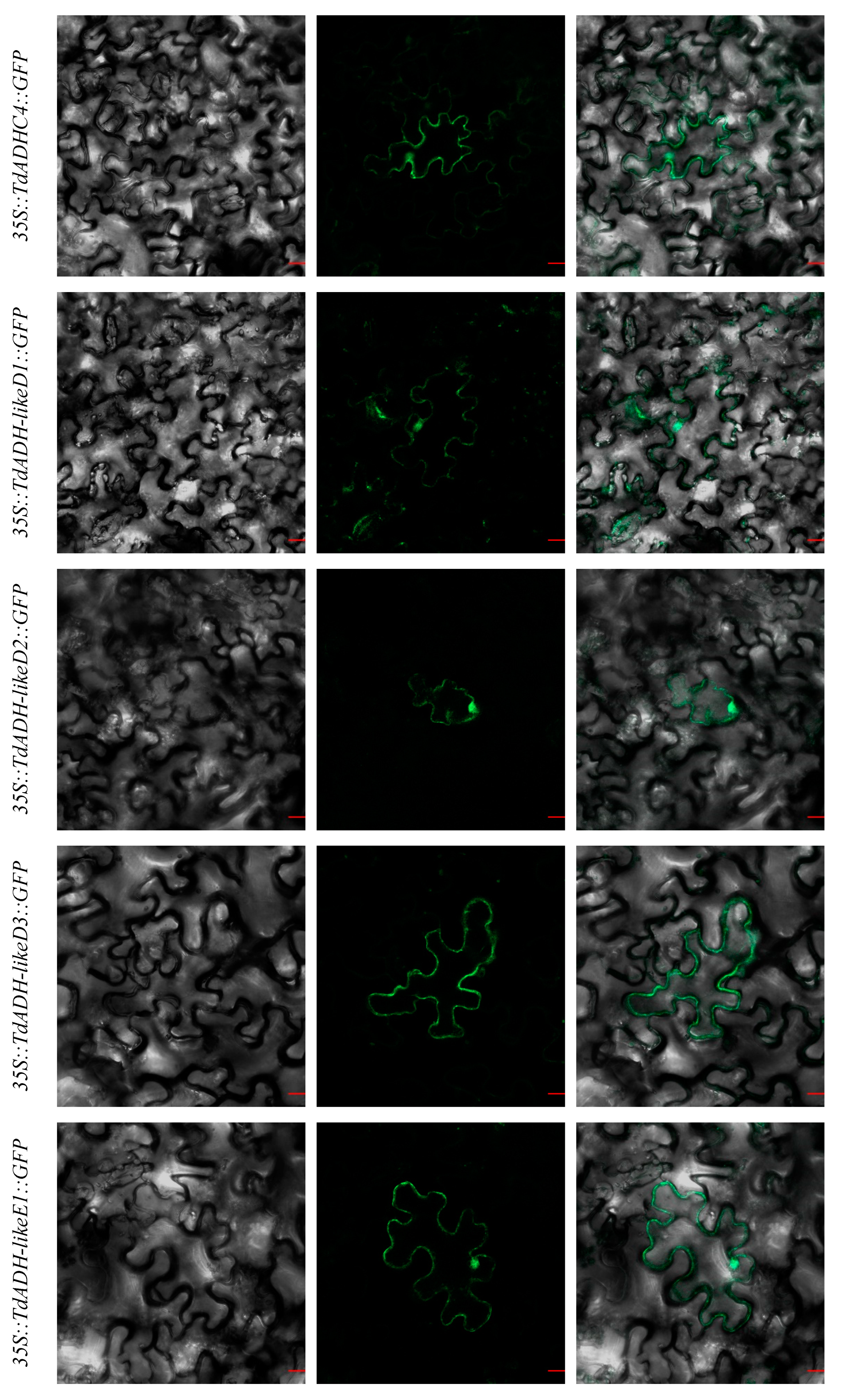
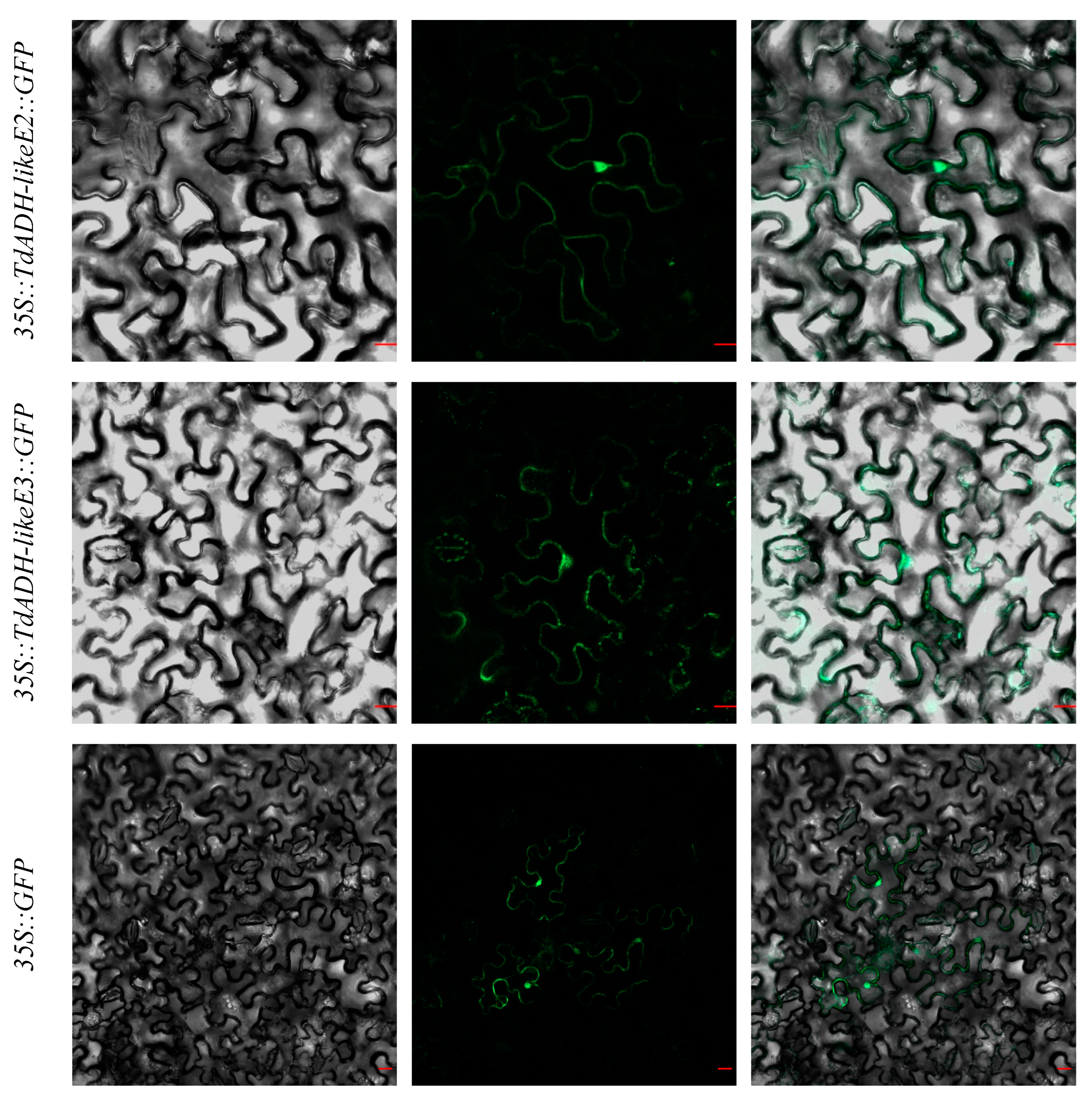
2.4. Subcellular Localization
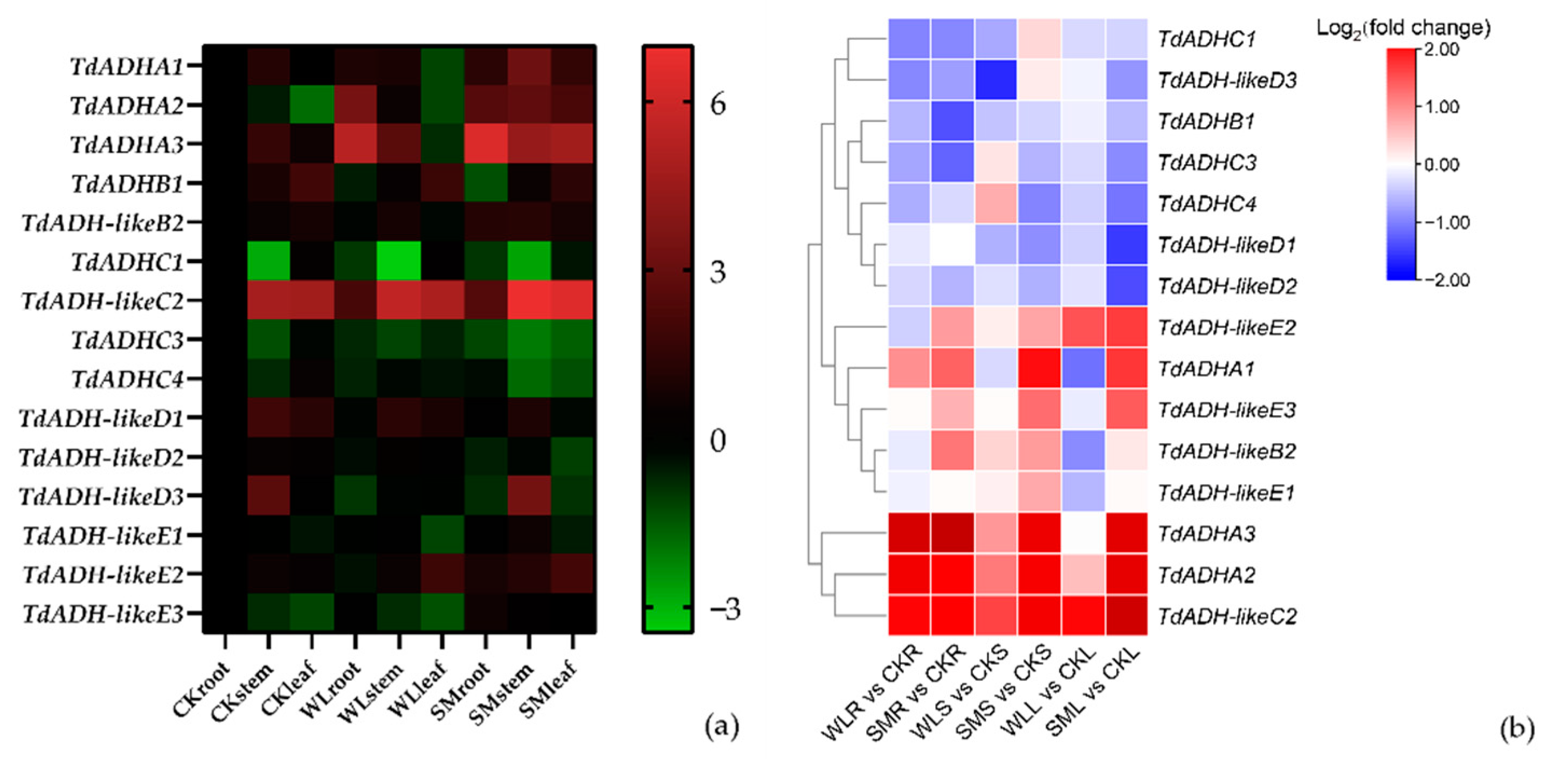
2.5. Expression Pattern under Flooding Stress
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Materials
5.2. Identification of TdADH Sequences
5.3. RNA Extraction and Cloning
5.4. Sequence Alignment and Phylogenetic Analysis
5.5. Motif Composition Analysis and Bioinformatic Analysis
5.6. Subcellular Localization
5.7. Quantitative Real-Time PCR Analysis
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. Contribution Of Working Group I To The Sixth Assessment Report Of The Intergovernmental Panel On Climate Change. In Climate Change 2021: The Physical Science Basis; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, O.; Perata, P.; Voesenek, L.A.C.J. Flooding and low oxygen responses in plants. Funct. Plant Biol. 2017, 44, iii–vi. [Google Scholar] [CrossRef]
- Yamauchi, T.; Colmer, T.D.; Pedersen, O.; Nakazono, M. Regulation of Root Traits for Internal Aeration and Tolerance to Soil Waterlogging-Flooding Stress. Plant Physiol. 2018, 176, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.A.C.J.; Colmer, T.D.; Pierik, R.; Millenaar, F.F.; Peeters, A.J.M. How plants cope with complete submergence. New Phytol. 2006, 170, 213–226. [Google Scholar] [CrossRef]
- Vriezen, W.H. Regulation of Submergence-induced Enhanced Shoot Elongation in Oryza sativa L. Ann. Bot. 2003, 91, 263–270. [Google Scholar] [CrossRef]
- Geigenberger, P. Response of plant metabolism to too little oxygen. Curr. Opin. Plant Biol. 2003, 6, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Bailey-Serres, J. Plant responses to hypoxia—Is survival a balancing act? Trends Plant Sci. 2004, 9, 449–456. [Google Scholar] [CrossRef]
- Perata, P.; Alpi, A. Plant responses to anaerobiosis. Plant Sci. 1993, 93, 1–17. [Google Scholar] [CrossRef]
- Bui, L.T.; Novi, G.; Lombardi, L.; Iannuzzi, C.; Rossi, J.; Santaniello, A.; Mensuali, A.; Corbineau, F.; Giuntoli, B.; Perata, P.; et al. Conservation of ethanol fermentation and its regulation in land plants. J. Exp. Bot. 2019, 70, 1815–1827. [Google Scholar] [CrossRef]
- Strommer, J. The plant ADH gene family. Plant J. 2011, 66, 128–142. [Google Scholar] [CrossRef]
- Ismond, K.P.K.P.; Dolferus, R.R.; De Pauw, M.M.; Dennis, E.S.E.S.; Good, A.G.A.G. Enhanced low oxygen survival in Arabidopsis through increased metabolic flux in the fermentative pathway. Plant Physiol. 2003, 132, 1292–1302. [Google Scholar] [CrossRef]
- Tougou, M.; Hashiguchi, A.; Yukawa, K.; Nanjo, Y.; Hiraga, S.; Nakamura, T.; Nishizawa, K.; Komatsu, S. Responses to flooding stress in soybean seedlings with the alcohol dehydrogenase transgene. Plant Biotechnol. 2012, 29, 301–305. [Google Scholar] [CrossRef]
- Ellis, M.H.; Millar, A.A.; Llewellyn, D.J.; Peacock, W.J.; Dennis, E.S. Transgenic cotton (Gossypium hirsutum) over-expressing alcohol dehydrogenase shows increased ethanol fermentation but no increase in tolerance to oxygen deficiency. Funct. Plant Biol. 2000, 27, 1041–1050. [Google Scholar] [CrossRef]
- Zeng, W.; Qiao, X.; Li, Q.; Liu, C.; Wu, J.; Yin, H.; Zhang, S. Genome-wide identification and comparative analysis of the ADH gene family in Chinese white pear (Pyrus bretschneideri) and other Rosaceae species. Genomics 2020, 112, 3484–3496. [Google Scholar] [CrossRef]
- Garabagi, F.; Strommer, J. Distinct genes produce the alcohol dehydrogenases of pollen and maternal tissues in Petunia hybrida. Biochem. Genet. 2004, 42, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Käsbauer, C.L.; Pathuri, I.P.; Hensel, G.; Kumlehn, J.; Hückelhoven, R.; Proels, R.K. Barley ADH-1 modulates susceptibility to Bgh and is involved in chitin-induced systemic resistance. Plant Physiol. Biochem. 2018, 123, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Ren, Y.; Wang, D.; Su, Y.; Feng, J.; Zhang, C.; Tang, H.; Xu, L.; Muhammad, K.; Que, Y. The alcohol dehydrogenase gene family in sugarcane and its involvement in cold stress regulation. Bmc Genom. 2020, 21, 521. [Google Scholar] [CrossRef]
- Senthil-Kumar, M.; Hema, R.; Suryachandra, T.R.; Ramegowda, H.V.; Gopalakrishna, R.; Rama, N.; Udayakumar, M.; Mysore, K.S. Functional characterization of three water deficit stress-induced genes in tobacco and Arabidopsis: An approach based on gene down regulation. Plant Physiol. Biochem. 2010, 48, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.Y.; Ku, S.S.; Sim, H.; Kim, S.; Park, J.H.; Lyu, J.I.; So, E.J.; Choi, S.Y.; Kim, J.; Ahn, M.S.; et al. An Alcohol Dehydrogenase Gene from Synechocystis sp. Confers Salt Tolerance in Transgenic Tobacco. Front. Plant Sci. 2017, 8, 1965. [Google Scholar] [CrossRef]
- Ventura, I.; Brunello, L.; Iacopino, S.; Valeri, M.C.; Novi, G.; Dornbusch, T.; Perata, P.; Loreti, E. Arabidopsis phenotyping reveals the importance of alcohol dehydrogenase and pyruvate decarboxylase for aerobic plant growth. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, C.; Liu, W.; Tang, Y.; Qi, H.; Chen, H.; Cao, S. The Alcohol Dehydrogenase Gene Family in Melon (Cucumis melo L.): Bioinformatic Analysis and Expression Patterns. Front. Plant Sci. 2016, 7, 670. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Hua, J.; Zhang, F.; Wang, Z.; Pei, X.; Yang, Y.; Yin, Y.; Creech, D.L. Identification and Functional Analysis of ThADH1 and ThADH4 Genes Involved in Tolerance to Waterlogging Stress in Taxodium hybrid ‘Zhongshanshan 406’. Genes 2021, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Yuan, J.; Ou, X.; Ren, X.; Li, X. Genome-wide identification of alcohol dehydrogenase (ADH) gene family under waterlogging stress in wheat (Triticum aestivum). Peerj 2021, 9, e11861. [Google Scholar] [CrossRef] [PubMed]
- Bowe, L.M.; Coat, G.; Depamphilis, C.W. Phylogeny of seed plants based on all three genomic compartments: Extant gymnosperms are monophyletic and Gnetales’ closest relatives are conifers. Proc. Natl. Acad. Sci. USA 2000, 97, 4092–4097. [Google Scholar] [CrossRef]
- Eckardt, N.A. A sense of self: The role of DNA sequence elimination in allopolyploidization. Plant Cell 2001, 13, 1699–1704. [Google Scholar] [CrossRef]
- De La Torre, A.R.; Li, Z.; Van de Peer, Y.; Ingvarsson, P.K. Contrasting Rates of Molecular Evolution and Patterns of Selection among Gymnosperms and Flowering Plants. Mol. Biol. Evol. 2017, 34, 1363–1377. [Google Scholar] [CrossRef]
- Hahn, M.W. Distinguishing Among Evolutionary Models for the Maintenance of Gene Duplicates. J. Hered. 2009, 100, 605–617. [Google Scholar] [CrossRef]
- Nordling, E.; Jörnvall, H.; Persson, B. Medium-chain dehydrogenases/reductases (MDR). Eur. J. Biochem. 2002, 269, 4267–4276. [Google Scholar] [CrossRef]
- Chase, T.J. Alcohol dehydrogenases: Identification and names for gene families. Plant Mol. Biol. Rep. 1999, 17, 333–350. [Google Scholar] [CrossRef]
- Shi, H.; Liu, W.; Yao, Y.; Wei, Y.; Chan, Z. Alcohol dehydrogenase 1 (ADH1) confers both abiotic and biotic stress resistance in Arabidopsis. Plant Sci. 2017, 262, 24–31. [Google Scholar] [CrossRef]
- Liu, S.; Guo, L.; Zhou, Q.; Jiang, Z.; Jin, L.; Zhu, J.; Xie, H.; Wei, C. Identification and Functional Analysis of Two Alcohol Dehydrogenase Genes Involved in Catalyzing the Reduction of (Z)-3-Hexenal into (Z)-3-Hexenol in Tea Plants (Camellia sinensis). J. Agric. Food Chem. 2022, 70, 1830–1839. [Google Scholar] [CrossRef] [PubMed]
- Eprintsev, A.T.; Komarova, N.R.; Falaleeva, M.I.; Larchenkov, V.M. Distinctive Features of the Functioning of Lactic Acid and the Alcohol Fermentation Enzyme in Sorghum and Pea Leaves under the Conditions of Oxygen Deficiency. Biol. Bull 2018, 45, 426–431. [Google Scholar] [CrossRef]
- Tadege, M.; Dupuis, I.; Kuhlemeier, C. Ethanolic fermentation: New functions for an old pathway. Trends Plant Sci. 1999, 4, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Kreuzwieser, J.; Papadopoulou, E.; Rennenberg, H. Interaction of Flooding with Carbon Metabolism of Forest Trees. Plant Biol. 2008, 6, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kreuzwieser, J.; Hauberg, J.; Howell, K.A.; Carroll, A.; Rennenberg, H.; Millar, A.H.; Whelan, J. Differential Response of Gray Poplar Leaves and Roots Underpins Stress Adaptation during Hypoxia. Plant Physiol. 2009, 149, 461–473. [Google Scholar] [CrossRef]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H. Development of Nucleotide Sequence Analysis Software Based on Windows. Chin. J. Bioinform. 2003, 1, 13–17. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Nicholas, K.B.; Nicholas, H.B.J. GeneDoc: A tool for editing and annotating multiple sequence alignments. Embnet. News 1997, 4, 1–4. [Google Scholar]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Yu, C.; Chen, Y.; Lu, C.; Hwang, J. Prediction of protein subcellular localization. Proteins Struct. Funct. Bioinform. 2006, 64, 643–651. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, C.; Xuan, L.; Hua, J.; Shi, Q.; Fan, W.; Yin, Y.; Yu, F. Identification of suitable reference genes in Taxodium ‘Zhongshanshan’ under abiotic stresses. Trees 2017, 31, 1519–1530. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]



| Number of Amino Acids | Molecular Weight | Theoretical Isoelectric Point | Instability Index | Aliphatic Index | Grand Average of Hydropathicity | |
|---|---|---|---|---|---|---|
| TdADHA1 | 381 | 40,959.12 | 6.17 | 28.87 | 86.51 | −0.036 |
| TdADHA2 | 345 | 37,495.02 | 6.22 | 27.00 | 82.49 | −0.065 |
| TdADHA3 | 381 | 41,030.28 | 6.56 | 28.57 | 82.41 | −0.025 |
| TdADHB1 | 381 | 41,587.03 | 6.33 | 26.34 | 87.27 | 0.000 |
| TdADH-likeB2 | 384 | 41,198.52 | 6.63 | 30.97 | 88.54 | 0.066 |
| TdADHC1 | 392 | 42,462.73 | 6.23 | 25.15 | 85.54 | −0.034 |
| TdADH-likeC2 | 377 | 40,877.36 | 6.16 | 13.01 | 91.75 | 0.018 |
| TdADHC3 | 403 | 43,639.58 | 6.03 | 24.28 | 92.61 | 0.081 |
| TdADHC4 | 403 | 43,703.58 | 5.91 | 24.59 | 90.92 | 0.064 |
| TdADH-likeD1 | 384 | 41,800.44 | 6.03 | 33.42 | 87.99 | 0.048 |
| TdADH-likeD2 | 277 | 30,129.37 | 8.64 | 30.48 | 74.15 | −0.268 |
| TdADH-likeD3 | 394 | 43,075.93 | 5.97 | 30.43 | 86.24 | 0.003 |
| TdADH-likeE1 | 388 | 42,450.25 | 6.56 | 30.59 | 102.91 | 0.103 |
| TdADH-likeE2 | 348 | 37,321.25 | 5.95 | 31.07 | 96.75 | 0.093 |
| TdADH-likeE3 | 314 | 34,159.43 | 8.68 | 38.27 | 82.32 | −0.052 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Xuan, L.; Ni, L.; Yang, Y.; Zhang, Y.; Wang, Z.; Yin, Y.; Hua, J. ADH Gene Cloning and Identification of Flooding-Responsive Genes in Taxodium distichum (L.) Rich. Plants 2023, 12, 678. https://doi.org/10.3390/plants12030678
Zhang R, Xuan L, Ni L, Yang Y, Zhang Y, Wang Z, Yin Y, Hua J. ADH Gene Cloning and Identification of Flooding-Responsive Genes in Taxodium distichum (L.) Rich. Plants. 2023; 12(3):678. https://doi.org/10.3390/plants12030678
Chicago/Turabian StyleZhang, Rui, Lei Xuan, Longjie Ni, Ying Yang, Ya Zhang, Zhiquan Wang, Yunlong Yin, and Jianfeng Hua. 2023. "ADH Gene Cloning and Identification of Flooding-Responsive Genes in Taxodium distichum (L.) Rich" Plants 12, no. 3: 678. https://doi.org/10.3390/plants12030678
APA StyleZhang, R., Xuan, L., Ni, L., Yang, Y., Zhang, Y., Wang, Z., Yin, Y., & Hua, J. (2023). ADH Gene Cloning and Identification of Flooding-Responsive Genes in Taxodium distichum (L.) Rich. Plants, 12(3), 678. https://doi.org/10.3390/plants12030678







