Modulation of Morpho-Physiological and Metabolic Profiles of Lettuce Subjected to Salt Stress and Treated with Two Vegetal-Derived Biostimulants
Abstract
1. Introduction
2. Results
2.1. Lettuce Growth and Morphometric Parameters
2.2. Leaf Photosynthetic and Biochemical Parameters
2.3. Leaf Mineral Contents
2.4. Leaf Pigment Content and Antioxidant Activity
2.5. Leaf Polyphenolic Contents
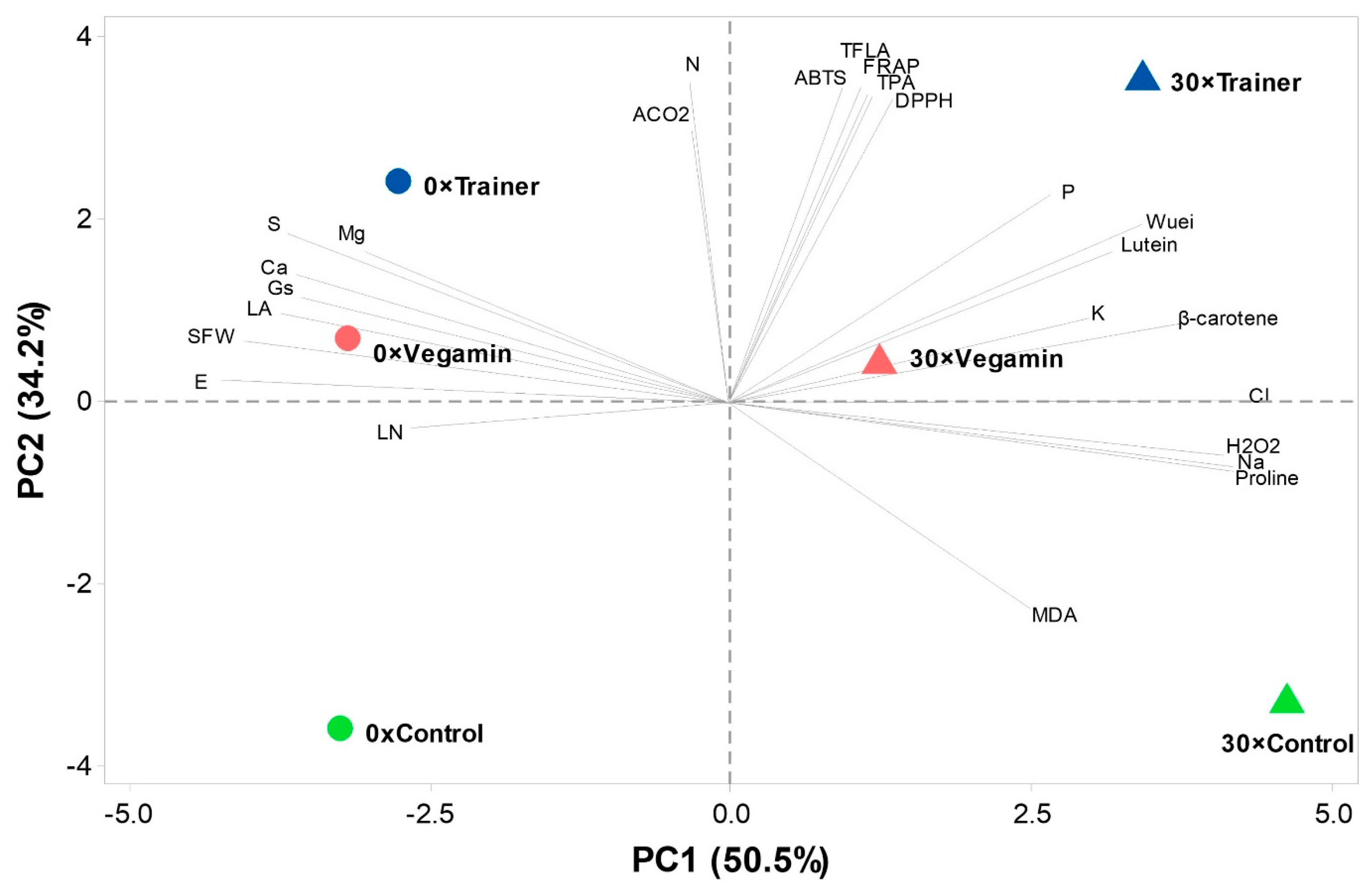
2.6. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Growth Conditions, Experimental Design and Plant Material
4.2. Biostimulant Treatments
4.3. Yield, Growth Assessment, Leaf Area Measurement and Sampling
4.4. Leaf gas Exchange and Biochemistry Parameters
4.5. Leaf Total Nitrogen and Mineral Analysis
4.6. Leaf Carotenoid Contents, Antioxidant Activity
4.7. Leaf Polyphenolic Contents
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GSASmap|Global Soil Partnership|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/global-soil-partnership/gsasmap/en (accessed on 13 December 2022).
- Jacobsen, S.-E.; Jensen, C.R.; Liu, F. Improving Crop Production in the Arid Mediterranean Climate. Field Crops Res. 2012, 128, 34–47. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS Regulation During Abiotic Stress Responses in Crop Plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [PubMed]
- Keisham, M.; Mukherjee, S.; Bhatla, S.C. Mechanisms of Sodium Transport in Plants—Progresses and Challenges. Int. J. Mol. Sci. 2018, 19, 647. [Google Scholar] [CrossRef] [PubMed]
- Majdoubi, M.; El Malahi, S.; Choukr-Allah, R.; Mokhtari, M. Setting Up of a New Local and Ecological Substrate for Tomato Soil-Less Cultivation to Cope with Saline Soils. Environ. Sci. Proc. 2022, 16, 6. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and Salinity: A Comparison of Their Effects on Mineral Nutrition of Plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Li, J.; Van Gerrewey, T.; Geelen, D. A Meta-Analysis of Biostimulant Yield Effectiveness in Field Trials. Front. Plant Sci. 2022, 13, 836702. [Google Scholar] [CrossRef]
- Schaafsma, G. Safety of Protein Hydrolysates, Fractions Thereof and Bioactive Peptides in Human Nutrition. Eur. J. Clin. Nutr. 2009, 63, 1161–1168. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Rouphael, Y. Biostimulant Substances for Sustainable Agriculture: Origin, Operating Mechanisms and Effects on Cucurbits, Leafy Greens, and Nightshade Vegetables Species. Biomolecules 2021, 11, 1103. [Google Scholar] [CrossRef]
- Colantoni, A.; Recchia, L.; Bernabei, G.; Cardarelli, M.; Rouphael, Y.; Colla, G. Analyzing the Environmental Impact of Chemically-Produced Protein Hydrolysate from Leather Waste vs. Enzymatically-Produced Protein Hydrolysate from Legume Grains. Agriculture 2017, 7, 62. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M.; et al. A Combined Phenotypic and Metabolomic Approach for Elucidating the Biostimulant Action of a Plant-Derived Protein Hydrolysate on Tomato Grown Under Limited Water Availability. Front. Plant Sci. 2019, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The Effect of a Plant-Derived Biostimulant on Metabolic Profiling and Crop Performance of Lettuce Grown under Saline Conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, W.; Wang, W.; Lv, H.; Liang, B.; Li, J. Exogenous Pig Blood-Derived Protein Hydrolysates as a Promising Method for Alleviation of Salt Stress in Tomato (Solanum Lycopersicum L.). Sci. Hortic. 2022, 294, 110779. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Cozzolino, E.; Ottaiano, L.; Petropoulos, S.A.; Nocerino, S.; Pelosi, M.E.; Rouphael, Y.; Mori, M.; Di Mola, I. Effect of Biostimulant Application on Plant Growth, Chlorophylls and Hydrophilic Antioxidant Activity of Spinach (Spinacia Oleracea L.) Grown under Saline Stress. Horticulturae 2022, 8, 971. [Google Scholar] [CrossRef]
- Ceccarelli, A.V.; Miras-Moreno, B.; Buffagni, V.; Senizza, B.; Pii, Y.; Cardarelli, M.; Rouphael, Y.; Colla, G.; Lucini, L. Foliar Application of Different Vegetal-Derived Protein Hydrolysates Distinctively Modulates Tomato Root Development and Metabolism. Plants 2021, 10, 326. [Google Scholar] [CrossRef]
- Carillo, P.; De Micco, V.; Ciriello, M.; Formisano, L.; El-Nakhel, C.; Giordano, M.; Colla, G.; Rouphael, Y. Morpho-Anatomical, Physiological, and Mineral Composition Responses Induced by a Vegetal-Based Biostimulant at Three Rates of Foliar Application in Greenhouse Lettuce. Plants 2022, 11, 2030. [Google Scholar] [CrossRef] [PubMed]
- Cristofano, F.; El-Nakhel, C.; Pannico, A.; Giordano, M.; Colla, G.; Rouphael, Y. Foliar and Root Applications of Vegetal-Derived Protein Hydrolysates Differentially Enhance the Yield and Qualitative Attributes of Two Lettuce Cultivars Grown in Floating System. Agronomy 2021, 11, 1194. [Google Scholar] [CrossRef]
- Ünlükara, A.; Cemek, B.; Karaman, S.; Erşahin, S. Response of Lettuce (Lactuca Sativa Var. Crispa) to Salinity of Irrigation Water. N. Z. J. Crops Hortic. Sci. 2008, 36, 265–273. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Garcia-Perez, P.; Cardarelli, M.; Senizza, B.; Miras-Moreno, B.; Colla, G.; Lucini, L. Plant Biostimulants from Seaweeds or Vegetal Proteins Enhance the Salinity Tolerance in Greenhouse Lettuce by Modulating Plant Metabolism in a Distinctive Manner. Sci. Hortic. 2022, 305, 111368. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ and K+ Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Breś, W.; Kleiber, T.; Markiewicz, B.; Mieloszyk, E.; Mieloch, M. The Effect of NaCl Stress on the Response of Lettuce (Lactuca Sativa L.). Agronomy 2022, 12, 244. [Google Scholar] [CrossRef]
- Gall, H.L.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell Wall Metabolism in Response to Abiotic Stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Gedeon, S.; Ioannou, A.; Balestrini, R.; Fotopoulos, V.; Antoniou, C. Application of Biostimulants in Tomato Plants (Solanum Lycopersicum) to Enhance Plant Growth and Salt Stress Tolerance. Plants 2022, 11, 3082. [Google Scholar] [CrossRef]
- Souza, A.C.; Zandonadi, D.B.; Santos, M.P.; Canellas, N.O.A.; de Paula Soares, C.; da Silva Irineu, L.E.S.; de Rezende, C.E.; Spaccini, R.; Piccolo, A.; Olivares, F.L.; et al. Acclimation with Humic Acids Enhances Maize and Tomato Tolerance to Salinity. Chem. Biol. Technol. Agric. 2021, 8, 40. [Google Scholar] [CrossRef]
- Formisano, L.; Ciriello, M.; Cirillo, V.; Pannico, A.; El-Nakhel, C.; Cristofano, F.; Duri, L.G.; Giordano, M.; Rouphael, Y.; De Pascale, S. Divergent Leaf Morpho-Physiological and Anatomical Adaptations of Four Lettuce Cultivars in Response to Different Greenhouse Irradiance Levels in Early Summer Season. Plants 2021, 10, 1179. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt Tolerance and Salinity Effects on Plants: A Review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A Global Perspective on Carotenoids: Metabolism, Biotechnology, and Benefits for Nutrition and Health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed]
- Jahns, P.; Holzwarth, A.R. The Role of the Xanthophyll Cycle and of Lutein in Photoprotection of Photosystem II. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant Growth under Water/Salt Stress: ROS Production; Antioxidants and Significance of Added Potassium under Such Conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; El-Nakhel, C.; Carillo, P.; Colla, G.; Graziani, G.; Di Mola, I.; Mori, M.; Kyriacou, M.C.; Rouphael, Y.; Soteriou, G.A.; et al. Plant-Derived Biostimulants Differentially Modulate Primary and Secondary Metabolites and Improve the Yield Potential of Red and Green Lettuce Cultivars. Agronomy 2022, 12, 1361. [Google Scholar] [CrossRef]
- Yang, X.; Gil, M.I.; Yang, Q.; Tomás-Barberán, F.A. Bioactive Compounds in Lettuce: Highlighting the Benefits to Human Health and Impacts of Preharvest and Postharvest Practices. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4–45. [Google Scholar] [CrossRef] [PubMed]
- Benmeziane, F.; Cadot, Y.; Djamai, R.; Djermoun, L. Determination of Major Anthocyanin Pigments and Flavonols in Red Grape Skin of Some Table Grape Varieties (Vitis Vinifera Sp.) by High-Performance Liquid Chromatography–Photodiode Array Detection (HPLC-DAD). OENO One 2016, 50, 125–135. [Google Scholar] [CrossRef]
- Mannino, G.; Gentile, C.; Ertani, A.; Serio, G.; Bertea, C.M. Anthocyanins: Biosynthesis, Distribution, Ecological Role, and Use of Biostimulants to Increase Their Content in Plant Foods—A Review. Agriculture 2021, 11, 212. [Google Scholar] [CrossRef]
- Carillo, P.; Pannico, A.; Cirillo, C.; Ciriello, M.; Colla, G.; Cardarelli, M.; De Pascale, S.; Rouphael, Y. Protein Hydrolysates from Animal or Vegetal Sources Affect Morpho-Physiological Traits, Ornamental Quality, Mineral Composition, and Shelf-Life of Chrysanthemum in a Distinctive Manner. Plants 2022, 11, 2321. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Colla, G.; Cardarelli, M.; Pii, Y.; Lucini, L.; Rouphael, Y. Tracking the Biostimulatory Effect of Fractions from a Commercial Plant Protein Hydrolysate in Greenhouse-Grown Lettuce. Antioxidants 2023, 12, 107. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Mannino, G.; Beekwilder, J.; Contartese, V.; Karlova, R.; Bertea, C.M. The Application of a Biostimulant Based on Tannins Affects Root Architecture and Improves Tolerance to Salinity in Tomato Plants. Sci. Rep. 2021, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, M.; De Diego, N.; Ugena, L.; Spíchal, L.; Lucini, L.; Miras-Moreno, B.; Zhang, L.; Rouphael, Y.; Colla, G.; Panzarová, K. Seed Priming With Protein Hydrolysates Improves Arabidopsis Growth and Stress Tolerance to Abiotic Stresses. Front. Plant Sci. 2021, 12, 837. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.; Bonini, P.; Colla, G. Plant- and Seaweed-Based Extracts Increase Yield but Differentially Modulate Nutritional Quality of Greenhouse Spinach through Biostimulant Action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Kumar, P.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Kalunke, R.M.; Colla, G. Insight into the Role of Grafting and Arbuscular Mycorrhiza on Cadmium Stress Tolerance in Tomato. Front. Plant Sci. 2015, 6, 477. [Google Scholar] [CrossRef]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1965; pp. 1149–1178. ISBN 978-0-89118-204-7. [Google Scholar]
- Ciriello, M.; Formisano, L.; Pannico, A.; El-Nakhel, C.; Fascella, G.; Duri, L.G.; Cristofano, F.; Gentile, B.R.; Giordano, M.; Rouphael, Y.; et al. Nutrient Solution Deprivation as a Tool to Improve Hydroponics Sustainability: Yield, Physiological, and Qualitative Response of Lettuce. Agronomy 2021, 11, 1469. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Functional Quality in Novel Food Sources: Genotypic Variation in the Nutritive and Phytochemical Composition of Thirteen Microgreens Species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Palladino, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Phenolic Constitution, Phytochemical and Macronutrient Content in Three Species of Microgreens as Modulated by Natural Fiber and Synthetic Substrates. Antioxidants 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Formisano, L.; Ciriello, M.; El-Nakhel, C.; Poledica, M.; Starace, G.; Graziani, G.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Pearl Grey Shading Net Boosts the Accumulation of Total Carotenoids and Phenolic Compounds That Accentuate the Antioxidant Activity of Processing Tomato. Antioxidants 2021, 10, 1999. [Google Scholar] [CrossRef]
| Source of Variance | Leaf Number | Leaf Area | Shoot Fresh Weight | Leaf Dry Matter |
|---|---|---|---|---|
| (no. Plant−1) | (cm2) | (g Plant−1) | (%) | |
| Salinity (S; mM NaCl) | ||||
| 0 | 34.7 ± 0.3 | 2357 ± 28 a | 313.5 ± 3.3 a | 5.29 ± 0.05 b |
| 30 | 34.2 ± 0.4 | 2026 ± 37 b | 240.5 ± 5.0 b | 6.28 ± 0.03 a |
| t-test | ns | *** | *** | *** |
| Biostimulant (B) | ||||
| Control | 34.3 ± 0.5 | 2116 ± 72 b | 265.1 ± 17.5 c | 5.82 ± 0.22 |
| Trainer | 34.2 ± 0.1 | 2251 ± 60 a | 288.7 ± 14.4 a | 5.81 ± 0.24 |
| Vegamin | 34.8 ± 0.5 | 2207 ± 103 ab | 277.2 ± 17.8 b | 5.73 ± 0.22 |
| ns | * | *** | ns | |
| S × B | ||||
| 0 × Control | 35.3 ± 0.6 | 2276 ± 12 ab | 303.1 ± 4.7 | 5.34 ± 0.13 |
| 0 × Trainer | 34.0 ± 0.2 | 2356 ± 50 a | 320.7 ± 3.1 | 5.28 ± 0.11 |
| 0 × Vegamin | 34.8 ± 0.6 | 2438 ± 10 a | 316.7 ± 3.9 | 5.24 ± 0.02 |
| 30 × Control | 33.4 ± 0.2 | 1957 ± 27 e | 227.1 ± 8.1 | 6.29 ± 0.04 |
| 30 × Trainer | 34.3 ± 0.2 | 2147 ± 69 bc | 256.7 ± 1.1 | 6.33 ± 0.02 |
| 30 × Vegamin | 34.9 ± 0.9 | 1976 ± 7.8 cd | 237.7 ± 3.8 | 6.23 ± 0.08 |
| ns | * | ns | ns |
| Source of Variance | ACO2 | gs | E | WUEi | Proline | MDA | H2O2 |
|---|---|---|---|---|---|---|---|
| (μmol CO2 m−2 s−1) | (mol H2O m−2 s−1) | (mol H2O m−2 s−1) | (μmol CO2 mol H2O−1) | (mM 100 g−1 FW) | (μM 100 g−1 FW) | (mM 100 g−1 FW) | |
| Salinity (S; mM NaCl) | |||||||
| 0 | 17.73 ± 0.28 | 0.19 ± 0.00 a | 3.96 ± 0.07 a | 4.49 ± 0.12 b | 19.4 ± 0.78 b | 0.95 ± 0.04 b | 4.66 ± 0.16 b |
| 30 | 17.94 ± 0.74 | 0.16 ± 0.01 b | 3.41 ± 0.14 b | 5.33 ± 0.26 a | 45.5 ± 2.28 a | 1.05 ± 0.06 a | 7.09 ± 0.22 a |
| t-test | ns | ** | ** | ** | *** | ** | *** |
| Biostimulant (B) | |||||||
| Control | 16.33 ± 0.44 b | 0.16 ± 0.01 b | 3.56 ± 0.23 | 4.66 ± 0.27 b | 38.09 ± 7.26 a | 1.17 ± 0.05 a | 6.29 ± 0.66 |
| Trainer | 19.04 ± 0.38 a | 0.17 ± 0.01 ab | 3.65 ± 0.17 | 5.29 ± 0.33 a | 29.29 ± 5.02 b | 0.96 ± 0.04 b | 5.66 ± 0.44 |
| Vegamin | 18.18 ± 0.43 a | 0.19 ± 0.01 a | 3.85 ± 0.13 | 4.66 ± 0.20 b | 29.97 ± 5.35 b | 0.87 ± 0.03 b | 5.67 ± 0.62 |
| *** | * | ns | * | *** | *** | ns | |
| S × B | |||||||
| 0 × Control | 17.13 ± 0.27 cd | 0.18 ± 0.00 | 3.98 ± 0.06 | 4.31 ± 0.13 | 21.90 ± 0.94 c | 1.08 ± 0.04 b | 4.94 ± 0.28 |
| 0 × Trainer | 18.57 ± 0.52 abc | 0.19 ± 0.00 | 3.96 ± 0.12 | 4.71 ± 0.27 | 18.06 ± 0.38 c | 0.88 ± 0.02 c | 4.72 ± 0.21 |
| 0 × Vegamin | 17.50 ± 0.18 bcd | 0.19 ± 0.01 | 3.95 ± 0.19 | 4.45 ± 0.19 | 18.24 ± 1.22 c | 0.89 ± 0.03 c | 4.31 ± 0.27 |
| 30 × Control | 15.53 ± 0.49 d | 0.14 ± 0.00 | 3.15 ± 0.29 | 5.01 ± 0.47 | 54.29 ± 0.72 a | 1.26 ± 0.03 a | 7.64 ± 0.52 |
| 30 × Trainer | 19.51 ± 0.49 a | 0.16 ± 0.02 | 3.34 ± 0.17 | 5.87 ± 0.35 | 40.52 ± 0.11 b | 1.04 ± 0.02 b | 6.6 ± 0.19 |
| 30 × Vegamin | 19.2 ± 0.01 ab | 0.19 ± 0.02 | 3.74 ± 0.20 | 4.98 ± 0.33 | 41.70 ± 1.96 b | 0.86 ± 0.05 c | 7.04 ± 0.03 |
| ** | Ns | ns | ns | ** | * | ns |
| Source of Variance | Total N | P | K | S | Ca | Mg | Na | Cl | Na/K Ratio |
|---|---|---|---|---|---|---|---|---|---|
| (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | ||
| Salinity (S; mM NaCl) | |||||||||
| 0 | 32.85 ± 0.32 | 4.52 ± 0.12 | 49.12 ± 1.72 | 0.76 ± 0.01 a | 2.86 ± 0.09 a | 1.89 ± 0.05 a | 2.67 ± 0.25 b | 8.74 ± 0.22 b | 0.06 ± 0.01 b |
| 30 | 32.84 ± 0.34 | 4.81 ± 0.13 | 53.59 ± 1.53 | 0.70 ± 0.02 b | 2.20 ± 0.12 b | 1.63 ± 0.04 b | 9.92 ± 0.90 a | 19.73 ± 0.87 a | 0.18 ± 0.01 a |
| t-test | ns | ns | ns | * | ** | *** | *** | *** | *** |
| Biostimulant (B) | |||||||||
| Control | 32.2 ± 0.45 | 4.52 ± 0.15 | 50.72 ± 2.81 | 0.70 ± 0.02 | 2.33 ± 0.21 b | 1.67 ± 0.04 b | 7.91 ± 2.37 a | 15.13 ± 2.72 | 0.15 ± 0.04 a |
| Trainer | 33.3 ± 0.36 | 4.89 ± 0.17 | 52.80 ± 1.68 | 0.75 ± 0.02 | 2.79 ± 0.16 a | 1.90 ± 0.07 a | 5.47 ± 1.43 b | 14.41 ± 2.89 | 0.10 ± 0.03 b |
| Vegamin | 33.04 ± 0.25 | 4.59 ± 0.12 | 50.84 ± 2.02 | 0.74 ± 0.03 | 2.38 ± 0.17 b | 1.68 ± 0.09 b | 6.08 ± 1.31 b | 14.06 ± 2.14 | 0.12 ± 0.03 ab |
| ns | ns | ns | ns | * | ** | ** | ns | ** | |
| S × B | |||||||||
| 0 × Control | 32.13 ± 0.85 | 4.25 ± 0.16 | 44.50 ± 0.65 b | 0.73 ± 0.02 | 2.79 ± 0.02 | 1.76 ± 0.03 | 2.62 ± 0.27 c | 9.15 ± 0.34 | 0.06 ± 0.01 c |
| 0 × Trainer | 33.27 ± 0.23 | 4.67 ± 0.21 | 51.57 ± 2.60 ab | 0.78 ± 0.02 | 3.04 ± 0.11 | 2.03 ± 0.05 | 2.40 ± 0.63 c | 8.26 ± 0.28 | 0.05 ± 0.01 c |
| 0 × Vegamin | 33.13 ± 0.26 | 4.70 ± 0.12 | 52.38 ± 3.15 ab | 0.78 ± 0.03 | 2.68 ± 0.34 | 1.89 ± 0.07 | 3.14 ± 0.24 c | 8.84 ± 0.39 | 0.06 ± 0.01 c |
| 30 × Control | 32.27 ± 0.52 | 4.78 ± 0.10 | 56.94 ± 0.47 a | 0.66 ± 0.03 | 1.87 ± 0.11 | 1.58 ± 0.03 | 13.19 ± 0.46 a | 21.11 ± 1.05 | 0.23 ± 0.01 a |
| 30 × Trainer | 33.32 ± 0.77 | 5.11 ± 0.24 | 54.03 ± 2.43 ab | 0.72 ± 0.03 | 2.53 ± 0.23 | 1.76 ± 0.07 | 8.54 ± 0.66 b | 20.55 ± 2.01 | 0.16 ± 0.01 b |
| 30 × Vegamin | 32.95 ± 0.47 | 4.53 ± 0.19 | 49.81 ± 3.01 ab | 0.71 ± 0.03 | 2.19 ± 0.06 | 1.54 ± 0.03 | 8.04 ± 0.96 b | 17.53 ± 0.37 | 0.16 ± 0.01 b |
| ns | ns | * | ns | ns | ns | ** | ns | ** |
| Source of Variance | Lutein | β-carotene | DPPH | ABTS | FRAP |
|---|---|---|---|---|---|
| (mg kg−1 DW) | (mg kg−1 DW) | (mmol Trolox kg−1 DW) | (mmol Trolox kg−1 DW) | (mmol Trolox kg−1 DW) | |
| Salinity (S; mM NaCl) | |||||
| 0 | 427.8 ± 11.2 b | 219.5 ± 8.1 b | 33.71 ± 1.58 b | 43.60 ± 2.33 b | 41.05 ± 2.80 b |
| 30 | 595.6 ± 39.3 a | 286.0 ± 11.3 a | 36.85 ± 1.22 a | 48.62 ± 3.68 a | 46.05 ± 2.95 a |
| t-test | *** | *** | *** | * | *** |
| Biostimulant (B) | |||||
| Control | 474.4 ± 19.7 b | 254.3 ± 12.7 ab | 30.23 ± 1.18 c | 35.91 ± 1.31 c | 33.70 ± 1.27 c |
| Trainer | 585.9 ± 72.1 a | 278.4 ± 20.5 a | 39.49 ± 0.63 a | 54.84 ± 2.70 a | 53.31 ± 1.65 a |
| Vegamin | 474.7 ± 31.4 b | 225.6 ± 16.3 b | 36.13 ± 0.71 b | 47.57 ± 1.94 b | 43.64 ± 0.87 b |
| ** | ** | *** | *** | *** | |
| S × B | |||||
| 0 × Control | 436.5 ± 17.3 b | 227.6 ± 7.7 | 27.83 ± 0.57 | 34.69 ± 0.69 | 30.89 ± 0.18 |
| 0 × Trainer | 428.6 ± 27.0 b | 234.9 ± 14.6 | 38.30 ± 0.36 | 49.77 ± 0.75 | 50.12 ± 0.54 |
| 0 × Vegamin | 418.3 ± 20.1 b | 196.1 ± 9.8 | 35.00 ± 0.99 | 46.32 ± 1.24 | 42.14 ± 0.91 |
| 30 × Control | 512.3 ± 14.1 b | 281.0 ± 6.2 | 32.63 ± 0.94 | 37.13 ± 2.58 | 36.50 ± 0.42 |
| 30 × Trainer | 743.2 ± 22.5 a | 321.8 ± 2.4 | 40.68 ± 0.67 | 59.91 ± 3.17 | 56.50 ± 1.78 |
| 30 × Vegamin | 531.1 ± 36.8 b | 255.2 ± 19.2 | 37.25 ± 0.56 | 48.82 ± 3.97 | 45.14 ± 0.83 |
| ** | ns | ns | ns | ns |
| Source of Variance | Chlorogenic Acid | Coumaroyl-diglucoside | Disinapoylgentobiose | Ferulic Acid | Synapoyl-hexose | Total Phenolic Acids |
|---|---|---|---|---|---|---|
| (mg kg−1 DW) | (mg kg−1 DW) | (mg kg−1 DW) | (mg kg−1 DW) | (mg kg−1 DW) | (mg kg−1 DW) | |
| Salinity (S; mM NaCl) | ||||||
| 0 | 1278 ± 86 b | 1.51 ± 0.1 b | 0.39 ± 0.02 b | 37.67 ± 4.18 | 46.54 ± 1.79 | 1364 ± 89 b |
| 30 | 1439 ± 77 a | 1.88 ± 0.16 a | 0.44 ± 0.03 a | 39.87 ± 2.87 | 48.98 ± 3.82 | 1530 ± 80 a |
| t-test | * | *** | ** | ns | ns | * |
| Biostimulant (B) | ||||||
| Control | 1078 ± 57 b | 1.25 ± 0.05 c | 0.32 ± 0.02 c | 33.59 ± 2.23 | 39.63 ± 2.63 c | 1153 ± 57 b |
| Trainer | 1551 ± 65 a | 2.12 ± 0.15 a | 0.48 ± 0.02 a | 43.7 ± 4.87 | 56.17 ± 2.6 a | 1654 ± 68 a |
| Vegamin | 1447 ± 61 a | 1.72 ± 0.09 b | 0.43 ± 0.00 b | 39.02 ± 4.84 | 47.48 ± 1.56 b | 1536 ± 58 a |
| *** | *** | *** | ns | *** | *** | |
| S × B | ||||||
| 0 × Control | 953 ± 5.9 | 1.16 ± 0.05 | 0.30 ± 0.00 | 28.69 ± 0.65 | 43.35 ± 4.32 bc | 1026 ± 3.6 |
| 0 × Trainer | 1472 ± 53 | 1.81 ± 0.07 | 0.43 ± 0.00 | 45.53 ± 9.98 | 50.77 ± 1.98 ab | 1571 ± 63.2 |
| 0 × Vegamin | 1410 ± 72 | 1.57 ± 0.11 | 0.42 ± 0.00 | 38.78 ± 6.11 | 45.5 ± 1.04 bc | 1497 ± 69.4 |
| 30 × Control | 1203 ± 19 | 1.34 ± 0.05 | 0.34 ± 0.03 | 38.5 ± 0.63 | 35.91 ± 1.4 c | 1280 ± 20.7 |
| 30 × Trainer | 1631 ± 110 | 2.43 ± 0.06 | 0.53 ± 0.01 | 41.86 ± 3.95 | 61.56 ± 0.89 a | 1737 ± 111 |
| 30 × Vegamin | 1483 ± 111 | 1.86 ± 0.10 | 0.44 ± 0.00 | 39.26 ± 8.93 | 49.46 ± 2.69 b | 1574 ± 102 |
| ns | ns | ns | ns | * | ns |
| Source of Variance | Isorhamnetin 3-rutinoside | Kaempferol 3,7-diglucoside | Kaempferol 3-glucoside | Quercetin 3-glucoside | Rutin | Total Flavonoids |
|---|---|---|---|---|---|---|
| (mg kg−1 DW) | (mg kg−1 DW) | (mg kg−1 DW) | (mg kg−1 DW) | (mg kg−1 DW) | (mg kg−1 DW) | |
| Salt (S; mM NaCl) | ||||||
| 0 | 0.37 ± 0.05 b | 0.33 ± 0.02 b | 1.21 ± 0.10 b | 10.31 ± 1.27 b | 0.55 ± 0.1 b | 12.77 ± 1.49 b |
| 30 | 0.55 ± 0.04 a | 0.48 ± 0.04 a | 1.39 ± 0.09 a | 12.10 ± 0.93 a | 0.72 ± 0.12 a | 15.25 ± 1.12 a |
| t-test | *** | *** | *** | *** | *** | *** |
| Biostimulant (B) | ||||||
| Control | 0.29 ± 0.06 c | 0.52 ± 0.05 a | 0.95 ± 0.06 c | 7.44 ± 0.72 c | 0.20 ± 0.03 b | 9.41 ± 0.9 c |
| Trainer | 0.58 ± 0.04 a | 0.38 ± 0.03 b | 1.56 ± 0.05 a | 14.88 ± 0.35 a | 0.90 ± 0.05 a | 18.3 ± 0.42 a |
| Vegamin | 0.50 ± 0.03 b | 0.32 ± 0.04 b | 1.40 ± 0.03 b | 11.3 ± 0.50 b | 0.81 ± 0.06 a | 14.33 ± 0.58 b |
| *** | *** | *** | *** | *** | *** | |
| S × B | ||||||
| 0 × Control | 0.17 ± 0.04 d | 0.41 ± 0.02 | 0.81 ± 0.03 d | 5.91 ± 0.47 d | 0.15 ± 0.01 | 7.46 ± 0.48 d |
| 0 × Trainer | 0.49 ± 0.01 bc | 0.32 ± 0.01 | 1.45 ± 0.02 b | 14.57 ± 0.39 a | 0.79 ± 0.04 | 17.62 ± 0.37 a |
| 0 × Vegamin | 0.45 ± 0.02 c | 0.25 ± 0.01 | 1.38 ± 0.04 b | 10.46 ± 0.51 bc | 0.70 ± 0.02 | 13.24 ± 0.44 c |
| 30 × Control | 0.42 ± 0.01 c | 0.63 ± 0.02 | 1.09 ± 0.02 c | 8.97 ± 0.09 c | 0.25 ± 0.04 | 11.36 ± 0.14 c |
| 30 × Trainer | 0.67 ± 0.02 a | 0.44 ± 0.01 | 1.67 ± 0.02 a | 15.18 ± 0.6 a | 1.01 ± 0.01 | 18.97 ± 0.56 a |
| 30 × Vegamin | 0.56 ± 0.02 b | 0.39 ± 0.04 | 1.42 ± 0.04 b | 12.14 ± 0.55 b | 0.91 ± 0.09 | 15.42 ± 0.56 b |
| * | ns | ** | * | ns | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristofano, F.; El-Nakhel, C.; Colla, G.; Cardarelli, M.; Pii, Y.; Lucini, L.; Rouphael, Y. Modulation of Morpho-Physiological and Metabolic Profiles of Lettuce Subjected to Salt Stress and Treated with Two Vegetal-Derived Biostimulants. Plants 2023, 12, 709. https://doi.org/10.3390/plants12040709
Cristofano F, El-Nakhel C, Colla G, Cardarelli M, Pii Y, Lucini L, Rouphael Y. Modulation of Morpho-Physiological and Metabolic Profiles of Lettuce Subjected to Salt Stress and Treated with Two Vegetal-Derived Biostimulants. Plants. 2023; 12(4):709. https://doi.org/10.3390/plants12040709
Chicago/Turabian StyleCristofano, Francesco, Christophe El-Nakhel, Giuseppe Colla, Mariateresa Cardarelli, Youry Pii, Luigi Lucini, and Youssef Rouphael. 2023. "Modulation of Morpho-Physiological and Metabolic Profiles of Lettuce Subjected to Salt Stress and Treated with Two Vegetal-Derived Biostimulants" Plants 12, no. 4: 709. https://doi.org/10.3390/plants12040709
APA StyleCristofano, F., El-Nakhel, C., Colla, G., Cardarelli, M., Pii, Y., Lucini, L., & Rouphael, Y. (2023). Modulation of Morpho-Physiological and Metabolic Profiles of Lettuce Subjected to Salt Stress and Treated with Two Vegetal-Derived Biostimulants. Plants, 12(4), 709. https://doi.org/10.3390/plants12040709











