Foliar Application of Potassium Salts to Olive, with Focus on Accompanying Anions
Abstract
:1. Introduction
2. Results
2.1. Physico-Chemical Properties of K Compounds
2.2. Olive Leaf K and Anion Absorption
3. Discussion
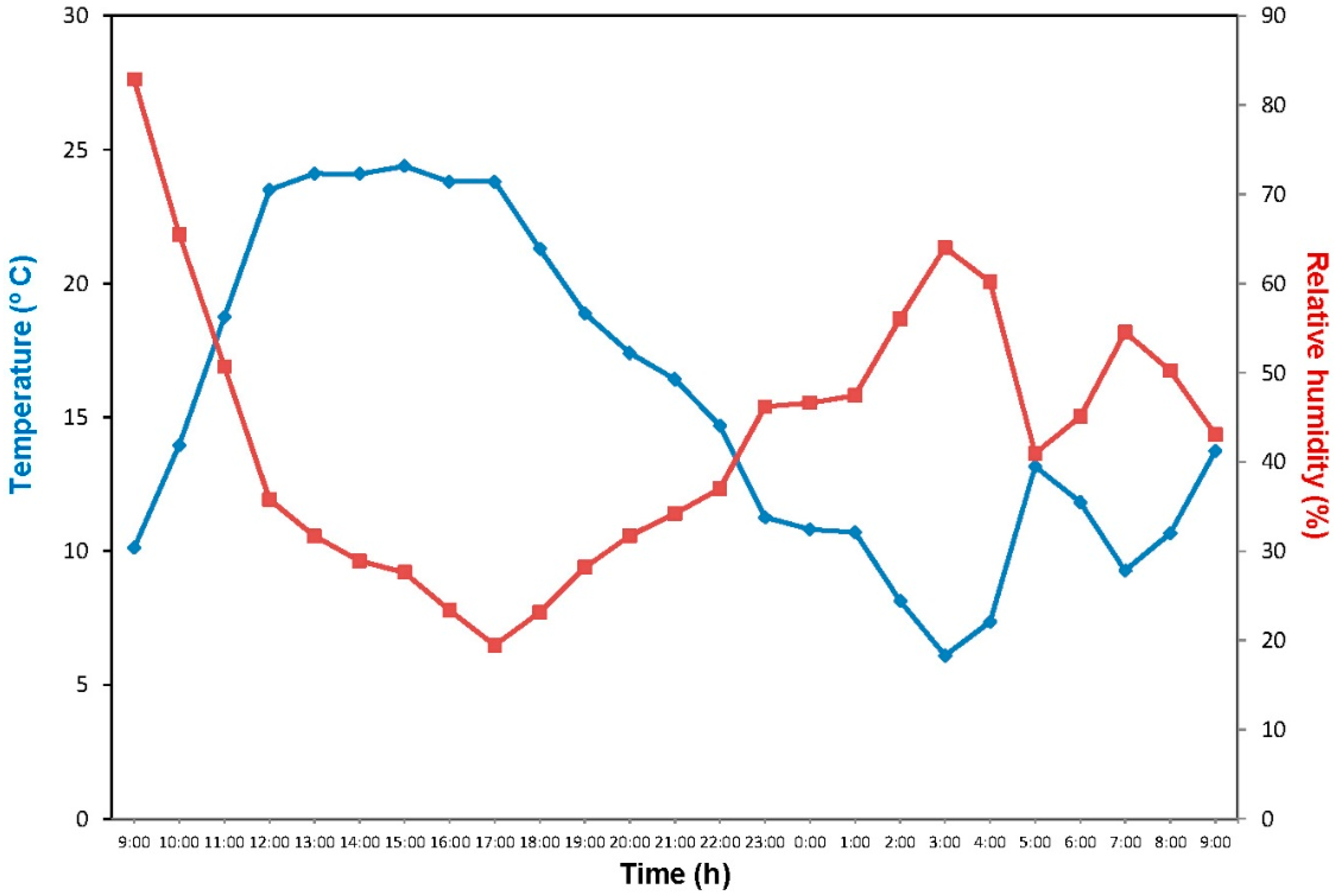
4. Materials and Methods
4.1. Chemicals
4.2. Point of Deliquescence (POD) and Efflorescence (POE) Estimation
4.3. Plant Material
4.4. Tissue Elemental Analysis
4.5. Data Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- White, P.J. Potassium in Crop Physiology. In Achieving Sustainable Crop Nutrition; Rengel, Z., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2020; pp. 213–236. ISBN 978-0-429-27584-5. [Google Scholar]
- Shabala, S.; Pottosin, I. Regulation of Potassium Transport in Plants under Hostile Conditions: Implications for Abiotic and Biotic Stress Tolerance. Physiol. Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Benlloch-González, M.; Sánchez-Lucas, R.; Bejaoui, M.A.; Benlloch, M.; Fernández-Escobar, R. Global Warming Effects on Yield and Fruit Maturation of Olive Trees Growing under Field Conditions. Sci. Hortic. 2019, 249, 162–167. [Google Scholar] [CrossRef]
- Besnard, G.; Terral, J.-F.; Cornille, A. On the Origins and Domestication of the Olive: A Review and Perspectives. Ann. Bot. 2018, 121, 385–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MAFF, (Ministry of Agriculture, Fisheries and Food of Spain) Aceite de oliva. Available online: https://www.mapa.gob.es/es/agricultura/temas/producciones-agricolas/aceite-oliva-y-aceituna-mesa/aceite.aspx (accessed on 14 December 2022).
- Fernández, V.; Sotiropoulos, T.; Brown, P. Foliar Fertilization: Scientific Principles and Field Pratices; International Fertilizer Industry Association (IFA): Paris, France, 2013; ISBN 979-10-92366-00-6. [Google Scholar]
- Miserere, A.; Rousseaux, M.C.; Ploschuk, E.L.; Brizuela, M.M.; Curcio, M.H.; Zabaleta, R.; Searles, P.S. Effects of Prolonged Elevated Temperature on Leaf Gas Exchange and Other Leaf Traits in Young Olive Trees. Tree Physiol. 2021, 41, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Benlloch-González, M.; Romera, J.; Cristescu, S.; Harren, F.; Fournier, J.M.; Benlloch, M. K+ Starvation Inhibits Water-Stress-Induced Stomatal Closure via Ethylene Synthesis in Sunflower Plants. J. Exp. Bot. 2010, 61, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.; Eichert, T. Uptake of Hydrophilic Solutes Through Plant Leaves: Current State of Knowledge and Perspectives of Foliar Fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef] [Green Version]
- Fernández, V.; Brown, P.H. From Plant Surface to Plant Metabolism: The Uncertain Fate of Foliar-Applied Nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef] [Green Version]
- Fernández, V.; Gil-Pelegrín, E.; Eichert, T. Foliar Water and Solute Absorption: An Update. Plant J. 2021, 105, 870–883. [Google Scholar] [CrossRef]
- Restrepo-Diaz, H.; Benlloch, M.; Fernández-Escobar, R. Plant Water Stress and K+ Starvation Reduce Absorption of Foliar Applied K+ by Olive Leaves. Sci. Hortic. 2008, 116, 409–413. [Google Scholar] [CrossRef]
- Elloumi, O.; Ghrab, M.; Ben Mimoun, M. Responses of Olive Trees (Cv. Chemlali) after Five Years of Experiment to Potassium Mineral Nutrition under Rainfed Condition. The Proceedings of the International Plant Nutrition Colloquium XVI 2009. Available online: https://escholarship.org/uc/item/2zb9p060 (accessed on 1 December 2022).
- Restrepo-Díaz, H.; Benlloch, M.; Fernández-Escobar, R. Leaf Potassium Accumulation in Olive Plants Related to Nutritional K Status, Leaf Age, and Foliar Application of Potassium Salts. J. Plant Nutr. 2009, 32, 1108–1121. [Google Scholar] [CrossRef]
- Sarrwy, S.; Mohamed, E.; Hassan, H. Effect of Foliar Sprays with Potassium Nitrate and Mono-Potassium Phosphate on Leaf Mineral Contents, Fruit Set, Yield and Fruit Quality of Picual Olive Trees Grown under Sandy Soil Conditions. Am. -Eurasian J. Agric. Environ. Sci. 2010, 8, 420–430. [Google Scholar]
- Zivdar, S.; Arzani, K.; Souri, M.K.; Moallemi, N.; Seyyednejad, S.M. Physiological and Biochemical Response of Olive (Olea Europaea L.) Cultivars to Foliar Potassium Application. J. Agric. Sci. Technol. 2016, 18, 1897–1908. [Google Scholar]
- Saykhul, A.; Chatzissawidis, C.; Therios, I.; Dimassi, K.; Chatzistathis, T. Growth and Nutrient Status of Olive Plants as Influenced by Foliar Potassium Applications. J. Soil Sci. Plant Nutr. 2014, 14, 602–615. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, I.Q.; Arrobas, M.; Moutinho-Pereira, J.; Correia, C.; Rodrigues, M.Â. Olive Response to Potassium Applications under Different Water Regimes and Cultivars. Nutr. Cycl. Agroecosyst 2018, 112, 387–401. [Google Scholar] [CrossRef] [Green Version]
- Schönherr, J.; Luber, M. Cuticular Penetration of Potassium Salts: Effects of Humidity, Anions, and Temperature. Plant Soil 2001, 236, 117–122. [Google Scholar] [CrossRef]
- Shafer, W.E.; Reed, D.W. The Foliar Absorption of Potassium from Organic and Inorganic Potassium Carriers. J. Plant Nutr. 1986, 9, 143–157. [Google Scholar] [CrossRef]
- Corrêa, C.G.; Rebouças, M.T.; Diniz, M.; Pereira de Carvalho, H.W. Effect of Counterions on the Foliar Absorption and Transport of Potassium in Soybeans [Glycine Max (L.) Merrill]. ACS Agric. Sci. Technol. 2021, 1, 728–734. [Google Scholar] [CrossRef]
- Schönherr, J. Cuticular Penetration of Calcium Salts: Effects of Humidity, Anions, and Adjuvants. J. Plant Nutr. Soil Sci. 2001, 164, 225–231. [Google Scholar] [CrossRef]
- Allan, M.; Mauer, L.J. Comparison of Methods for Determining the Deliquescence Points of Single Crystalline Ingredients and Blends. Food Chem. 2016, 195, 29–38. [Google Scholar] [CrossRef]
- Fernández, V.; Pimentel, C.; Bahamonde, H.A. Salt Hydration and Drop Drying of Two Model Calcium Salts: Implications for Foliar Nutrient Absorption and Deposition. J. Plant Nutr. Soil Sci. 2020, 183, 592–601. [Google Scholar] [CrossRef]
- Khvorostyanov, V.I.; Curry, J.A. Deliquescence and Efflorescence in Atmospheric Aerosols. In Thermodynamics, Kinetics, and Microphysics of Clouds; Curry, J.A., Khvorostyanov, V.I., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 547–576. ISBN 978-1-107-01603-3. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. U.S. Geological Survey Techniques and Methods 6, Chap. A43.; U.S. Geological Survey Techniques and Methods (USGS): Reston, VA, USA, 2013; Volume A43. [Google Scholar]
- Landorfa-Svalbe, Z.; Andersone-Ozola, U.; Ievinsh, G. Type of Anion Largely Determines Salinity Tolerance in Four Rumex Species. Plants 2023, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Aluko, O.O.; Li, C.; Yuan, G.; Nong, T.; Xiang, H.; Wang, Q.; Li, X.; Liu, H. Differential Effects of Ammonium (NH4+) and Potassium (K+) Nutrition on Photoassimilate Partitioning and Growth of Tobacco Seedlings. Plants 2022, 11, 3295. [Google Scholar] [CrossRef] [PubMed]
- Bulawa, B.; Sogoni, A.; Jimoh, M.O.; Laubscher, C.P. Potassium Application Enhanced Plant Growth, Mineral Composition, Proximate and Phytochemical Content in Trachyandra divaricata Kunth (Sandkool). Plants 2022, 11, 3183. [Google Scholar] [CrossRef]
- Leigh, R.A.; Wyn Jones, R.G. A Hypothesis Relating Critical Potassium Concentrations for Growth to the Distribution and Functions of This Ion in the Plant Cell. New Phytol. 1984, 97, 1–13. [Google Scholar] [CrossRef]
- MacRobbie, E.A.C.; Lettau, J. Potassium Content and Aperture in “Intact” Stomatal and Epidermal Cells of Commelina Communis L. J. Membrain Biol. 1980, 56, 249–256. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-0-429-17019-5. [Google Scholar]
| Compound | Solubility (M) | [K] (mM) | [anion] (mM) | POD (%) | POE (%) |
|---|---|---|---|---|---|
| K3PO4 | 7.87 | 390 | 130 | 25 | <10 |
| KNO3 | 4.47 | 130 | 130 | 95 | 85 |
| K2SO4 | 0.75 | 260 | 130 | >95 | 75 |
| KCl | 5.59 | 130 | 130 | 85 | 70 |
| K2CO3 | 10.94 | 260 | 130 | 45 | 35 |
| KHCO3 | 4.25 | 130 | 130 | 85 | 65 |
| Treatment | [K] (%) | [C] (%) | ||
|---|---|---|---|---|
| 3 h | 24 h | 3 h | 24 h | |
| No K | 0.88 ± 0.11 a | 0.92 ± 0.08 a | 47.66 ± 0.50 a | 47.19 ± 0.35 a |
| K3PO4 | 1.00 ± 0.22 a | 1.11 ± 0.10 a | 47.90 ± 0.34 a | 48.11 ± 0.51 a |
| KNO3 | 1.10 ± 0.26 a | 0.98 ± 0.08 a | 47.54 ± 0.30 a | 47.25 ± 0.14 a |
| K2SO4 | 0.96 ± 0.16 a | 1.03 ± 0.15 a | 47.42 ± 0.53 a | 47.54 ± 0.27 a |
| KCl | 1.05 ± 0.19 a | 1.13 ± 0.01 a | 47.35 ± 0.52 a | 47.54 ± 0.14 a |
| K2CO3 | 1.01 ± 0.13 a | 1.04 ± 0.19 a | 47.45 ± 0.51 a | 47.80 ± 0.35 a |
| KHCO3 | 1.03 ± 0.65 a | 1.12 ± 0.26 a | 47.35 ± 0.52 a | 47.81 ± 0.63 a |
| Treatment (T) | 0.96 (0.468) | 1.64 (0.172) | ||
| Hours (H) | 0.77 (0.388) | 0.44 (0.514) | ||
| T × H | 0.32 (0.921) | 1.05 (0.417) | ||
| Treatment | [Cl−] (mg g−1) | [NO3−] (mg g−1) | [SO42−] (mg g−1) | [PO43−] (mg g−1) | ||||
|---|---|---|---|---|---|---|---|---|
| 3 h | 24 h | 3 h | 24 h | 3 h | 24 h | 3 h | 24 h | |
| No K | 0.75 ± 0.12 a | 0.65 ± 0.07 a | 0.15 ± 0.03 b | 0.13 ± 0.01 b,c | 1.85 ± 0.24 a | 1.64 ± 0.49 a | 3.46 ± 0.64 a | 5.44 ± 0.71 a |
| K3PO4 | 0.92 ± 0.05 a | 0.84 ± 0.13 a | <0.02 | <0.02 | 1.22 ± 0.25 a | 1.01 ± 0.03 a | 3.77 ± 0.16 a | 5.12 ± 2.47 a |
| KNO3 | 0.87 ± 0.18 a | 0.69 ± 0.05 a | 0.13 ± 0.02 b | 0.10 ± 0.05 b | 1.43 ± 0.70 a | 1.22 ± 0.44 a | 2.83 ± 0.15 a | 4.67 ± 1.71 a |
| K2SO4 | 0.92 ± 0.27 a | 0.68 ± 0.03 a | 0.07 ± 0.02 a | 0.10 ± 0.02 b | 1.37 ± 0.43 a | 1.12 ± 0.45 a | 3.47 ± 0.90 a | 5.64 ± 1.57 a |
| KCl | 1.35 ± 0.17 b | 0.83 ± 0.13 a | 0.14 ± 0.03 b | 0.16 ± 0.03 c | 1.63 ± 0.24 a | 1.28 ± 0.12 a | 3.64 ± 0.67 a | 4.96 ± 0.50 a |
| K2CO3 | 0.64 ± 0.07 a | 0.77 ± 0.10 a | 0.06 ± 0.03 a | 0.04 ± 0.01 a | 1.36 ± 0.19 a | 1.28 ± 0.24 a | 2.56 ± 1.06 a | 4.88 ± 0.96 a |
| KHCO3 | 0.75 ± 0.03 a | 0.78 ± 0.12 a | 0.10 ± 0.01 a | 0.17 ± 0.01 c | 1.95 ± 0.36 a | 1.36 ± 0.20 a | 3.65 ± 0.76 a | 5.48 ± 1.53 a |
| Treatment (T) | 6.79 (<0.001) | 11 (<0.001) | 3.3 (0.014) | 0.59 (0.734) | ||||
| Hours (H) | 12.17 (0.002) | 0.92 (0.349) | 4.42 (0.045) | 25.99 (<0.001) | ||||
| T × H | 3.99 (0.005) | 3.1 (0.029) | 0.45 (0.839) | 0.16 (0.985) | ||||
| Treatment | [K] (mg L−1 m−2) | |
|---|---|---|
| 3 h | 24 h | |
| No K | 6.44 ± 2.58 a | 12.86 ± 4.01 a |
| K3PO4 | 287.75 ± 28.76 e | 324.69 ± 35.76 d |
| KNO3 | 91.72 ± 10.68 b | 100.23 ± 8.03 b |
| K2SO4 | 204.24 ± 12.25 d | 197.63 ± 24.62 c |
| KCl | 66.39 ± 7.3 b | 74.61 ±123.41 b |
| K2CO3 | 138.61 ± 9.7 c | 194.03 ± 19.68 c |
| KHCO3 | 80.97 ± 15.09 b | 94.18 ± 16.01 b |
| Treatment (T) | 170 (<0.001) | |
| Hours (H) | 9.14 (0.005) | |
| T × H | 1.96 (0.105) | |
| Treatment | [Cl−] (mg L−1 m−2) | [NO3−] (mg L−1 m−2) | [SO42−] (mg L−1 m−2) | [PO4−3] (mg−1 m−2) | ||||
|---|---|---|---|---|---|---|---|---|
| 3 h | 24 h | 3 h | 24 h | 3 h | 24 h | 3 h | 24 h | |
| No K | 91.9 ± 42.2 a | 300.7 ± 107.9 a | 10.4 ± 4.4 a | 18.6 ± 6.4 a | 142.1 ± 37.4 a | 266.2 ± 96.7 a.b | 5.3 ± 2.4 a | 7.1 ± 3.2 a |
| K3PO4 | 198.6 ± 13.9 a,b | 179.5 ± 73.9 a | 8.7 ± 2.2 a | 6.2 ± 5.6 a | 157.8 ± 11.5 a | 143.8± 60.6 a | 268.7 ± 41.2 b | 280.0 ± 38.0 b |
| KNO3 | 283.1 ± 19.8 a,b | 229.5 ± 80.4 a | 148.5 ± 3.8 b | 166.3 ± 21.9 b | 269.2 ± 17.0 a,b | 218.1 ± 44.9 a,b | 3.04 ± 3.5 a | 1.0 ± 0.0 a |
| K2SO4 | 165.5 ± 11.8 a,b | 197.8 ± 39.7 a | 12.0 ± 1.9 a | 11.7 ± 1.5 a | 390.6 ± 46.7 b | 387,2± 28.3 b | 1.0 ± 0.0 a | 1.0 ± 0.0 a |
| KCl | 346.5 ± 88.1 b | 337.4 ± 33.5 a | 13.2 ± 2.9 a | 16.6 ± 2.4 a | 240.8 ± 66.3 a,b | 250.9 ± 32.0 a,b | 1.0 ± 0.0 a | 5.2 ± 3.7 a |
| K2CO3 | 153.2 ± 13.7 a,b | 295.7 ± 82.2 a | 8.7 ± 1.7 a | 15.1 ± 3.2 a | 136.2 ± 12.8 a | 258.8 ± 4.2 a,b | 7.0 ± 1.7 a | 7.6 ± 1.5 a |
| KHCO3 | 180.9 ± 36.7 a,b | 130.0 ± 36.1 a | 10.7 ± 2.5 a | 10.3 ± 2.8 a | 153.3 ± 30.3 a | 110.8 ± 28.2 a | 1.0 ± 0.0 a | 1.0 ± 0.0 a |
| Treatment (T) | 3.35 (0.013) | 399 (<0.001) | 8.25 (<0.001) | 277 (<0.001) | ||||
| Hours (H) | 2.38 (0.134) | 5.02 (0.033) | 2.3 (0.138) | 0.28 (0.599) | ||||
| T × H | 1.86 (0.124) | 1.6 (0.184) | 2 (0.099) | 0.13 (0.992) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahamonde, H.A.; Pimentel, C.; Lara, L.A.; Bahamonde-Fernández, V.; Fernández, V. Foliar Application of Potassium Salts to Olive, with Focus on Accompanying Anions. Plants 2023, 12, 472. https://doi.org/10.3390/plants12030472
Bahamonde HA, Pimentel C, Lara LA, Bahamonde-Fernández V, Fernández V. Foliar Application of Potassium Salts to Olive, with Focus on Accompanying Anions. Plants. 2023; 12(3):472. https://doi.org/10.3390/plants12030472
Chicago/Turabian StyleBahamonde, Héctor A., Carlos Pimentel, Luis Adrián Lara, Vikingur Bahamonde-Fernández, and Victoria Fernández. 2023. "Foliar Application of Potassium Salts to Olive, with Focus on Accompanying Anions" Plants 12, no. 3: 472. https://doi.org/10.3390/plants12030472
APA StyleBahamonde, H. A., Pimentel, C., Lara, L. A., Bahamonde-Fernández, V., & Fernández, V. (2023). Foliar Application of Potassium Salts to Olive, with Focus on Accompanying Anions. Plants, 12(3), 472. https://doi.org/10.3390/plants12030472







