A Review of Ampelometry: Morphometric Characterization of the Grape (Vitis spp.) Leaf
Abstract
1. Introduction
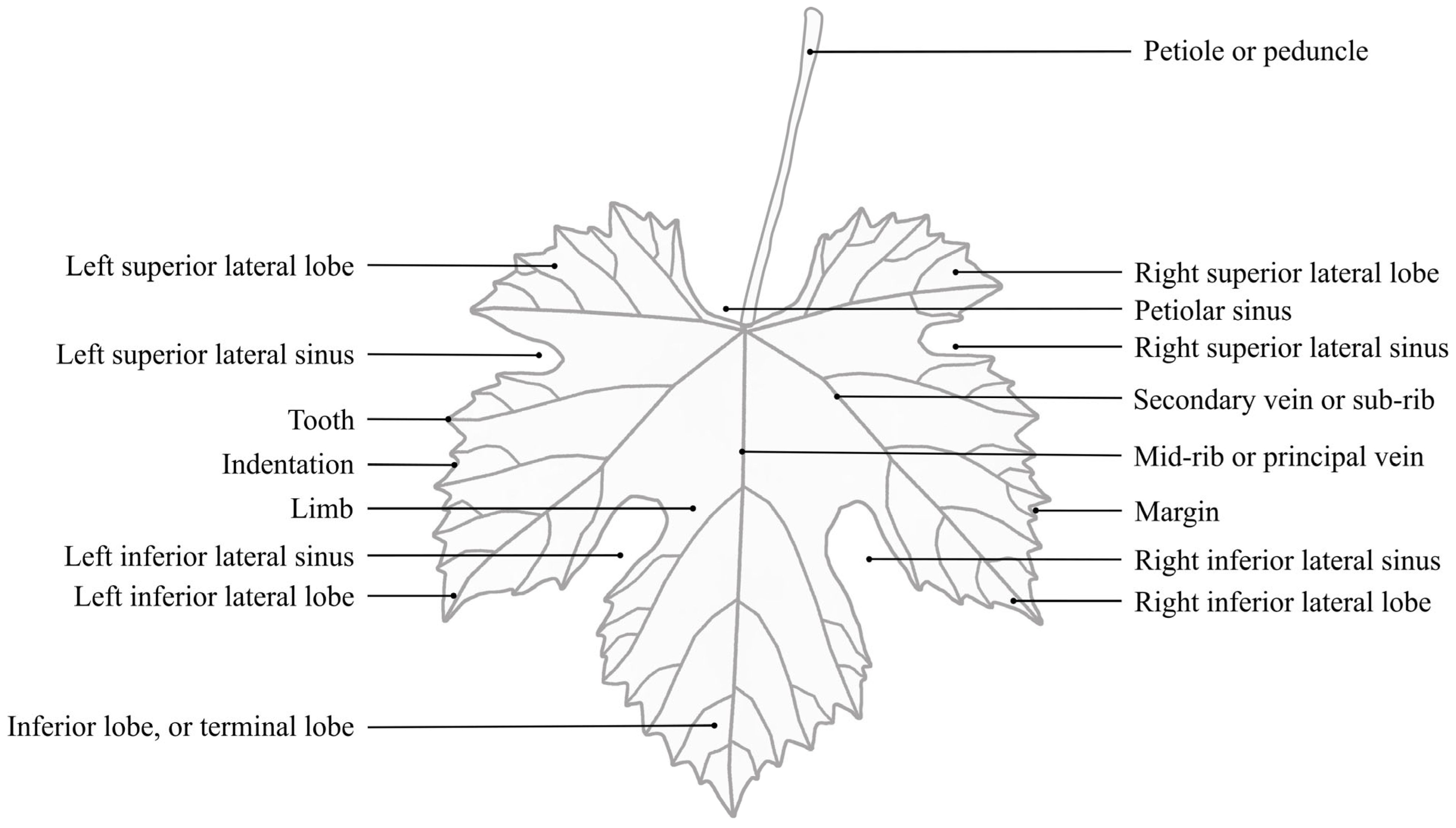
Grapevine Leaf Morphological Traits
2. History and Development of Ampelometry
2.1. Prelude of Ampelometry
2.2. Ampelometry by Ravaz
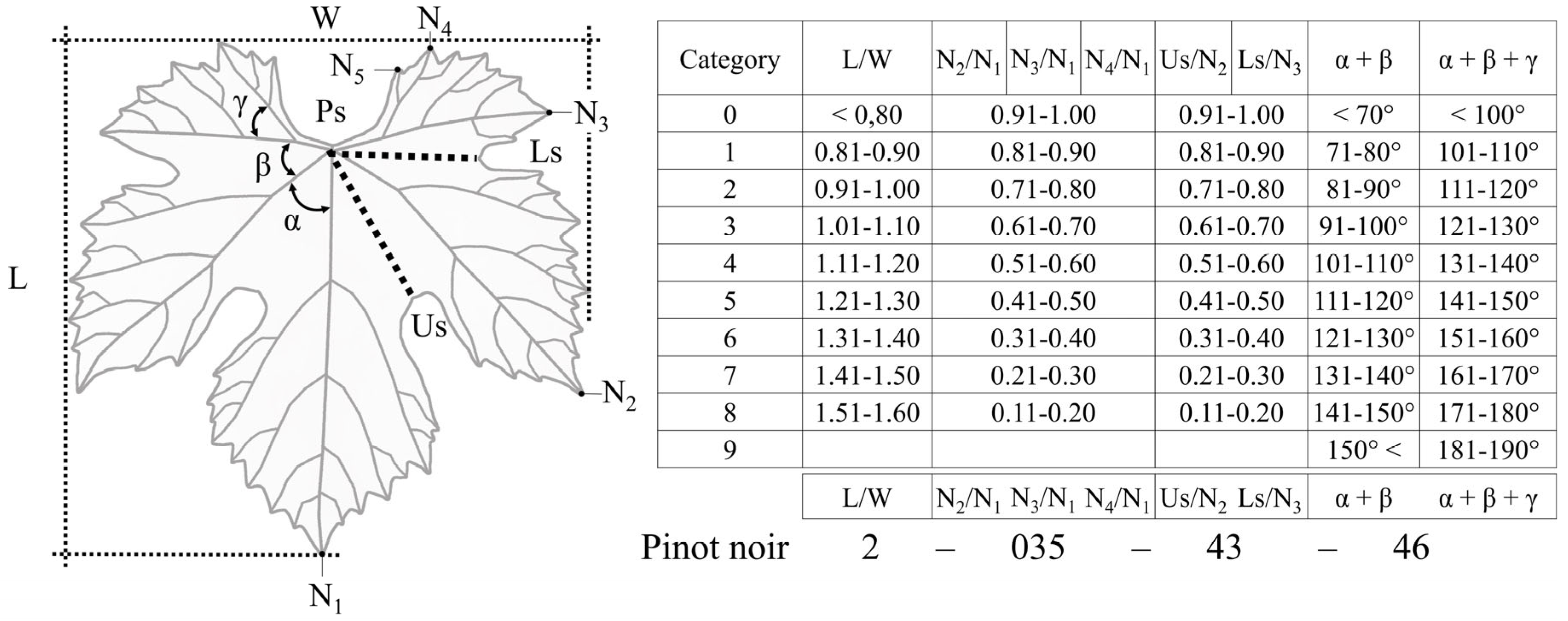
2.3. Galet’s Ampelometric Index
- L/W—length of the leaf/width of the leaf;
- N2/N1—length of the distal vein/length of the mid vein;
- N3/N1—length of the proximal vein/length of the mid vein;
- N4/N1—length of the petiolar vein/length of the main vein;
- Us/N3—depth of the upper sinus/length of the proximal vein;
- Ls/N2—depth of the lower sinus/length of the distal vein.
- α + β;
- α + β + γ;
2.4. OIV Descriptor List
| Code N° | Characteristic on the Mature Leaf | Notations | ||||
|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 9 | ||
| OIV 601 | Length of vein N1 | very short up to about 75 mm | short about 105 mm | medium about 135 mm | long about 165 mm | very long about 195 mm and more |
| OIV 602 | Length of vein N2 | very short up to about 65 mm | short about 85 mm | medium about 105 mm | long about 125 mm | very long about 145 mm and more |
| OIV 603 | Length of vein N3 | very short up to about 35 mm | short about 55 mm | medium about 75 mm | long about 95 mm | very long about 115 mm and more |
| OIV 604 | Length of vein N4 | very short up to about 15 mm | short about 25 mm | medium about 35 mm | long about 45 mm | very long about 55 mm and more |
| OIV 605 | Length petiole sinus to upper lateral leaf sinus | very short up to about 30 mm | short about 50 mm | medium about 70 mm | long about 90 mm | very long about 110 mm and more |
| OIV 606 | Length petiole sinus to lower lateral leaf sinus | very short up to about 30 mm | short about 45 mm | medium about 60 mm | long about 75 mm | very long about 90 mm and more |
| OIV 607 | Angle between N1 and N2 measured at the first ramification | very small up to about 30° | small about 30°–45° | medium about 46°–55° | large about 56°–70° | very large about 70° and more |
| OIV 608 | Angle between N2 and N3 measured at the first ramification | very small up to about 30° | small about 30°–45° | medium about 46°–55° | large about 56°–70° | very large about 70° and more |
| OIV 609 | Angle between N3 and N4 measured at the first ramification | very small up to about 30° | small about 30°–45° | medium about 46°–55° | large about 56°–70° | very large about 70° and more |
| OIV 610 | Angle between N3 and the tangent between petiole point and the tooth tip of N5 | very small up to about 30° | small about 30°–45° | medium about 46°–55° | large about 56°–70° | very large about 70° and more |
| OIV 611 | Length of vein N5 | very short up to about 15 mm | short about 25 mm | medium about 35 mm | long about 45 mm | very long about 55 mm and more |
| OIV 612 | Length of tooth of N2 | very short up to about 6 mm | short about 10 mm | medium about 14 mm | long about 18 mm | very long about 22 mm and more |
| OIV 613 | Width of tooth of N2 | very short up to about 6 mm | short about 10 mm | medium about 14 mm | long about 18 mm | very long about 22 mm and more |
| OIV 614 | Length of tooth of N4 | very short up to about 6 mm | short about 10 mm | medium about 14 mm | long about 18 mm | very long about 22 mm and more |
| OIV 615 | Width of tooth of N4 | very short up to about 6 mm | short about 10 mm | medium about 14 mm | long about 18 mm | very long about 22 mm and more |
| OIV 616 | Number of teeth between the tooth tip of N2 and the tooth tip of the first secondary vein of N2 including the limits | very small up to about 3 | small about 4 | medium about 5–6 | large about 7–8 | very large about 9 and more |
| OIV 617 | Length between the tooth tip of N2 and the tooth tip of the first secondary vein of N2 | very small up to about 30 mm | small about 30–45 mm | medium about 46–55 mm | large about 56–70 mm | very large about 70 mm and more |
| OIV 618 | Opening/overlapping of the petiole sinus | wide open up to about −35 mm | open about −15 mm | closed about −5 mm | overlapping about 25 mm | very overlapping about 45 mm and more |
2.5. Ampelometric Data as Continuos Variables
2.6. Geometric Morphometry—Odontometry
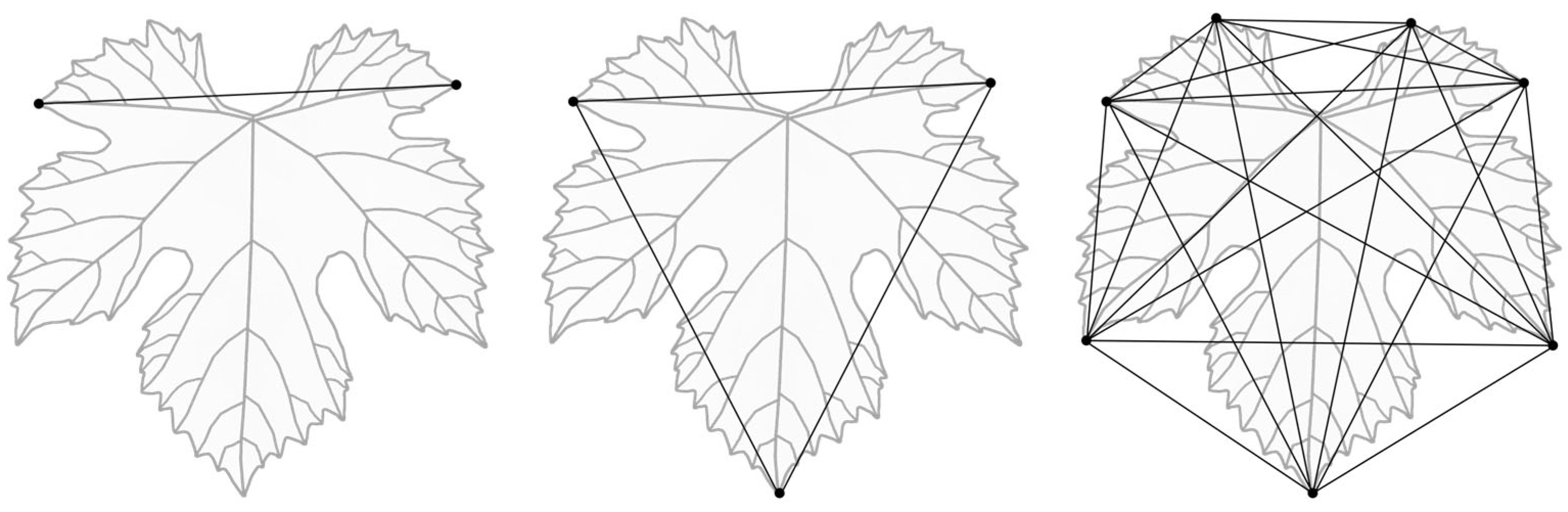
2.7. Landmark-Based Geometric Morphometry
2.8. Elliptic Fourier Descriptors
3. Tools for Ampelometric Evaluations
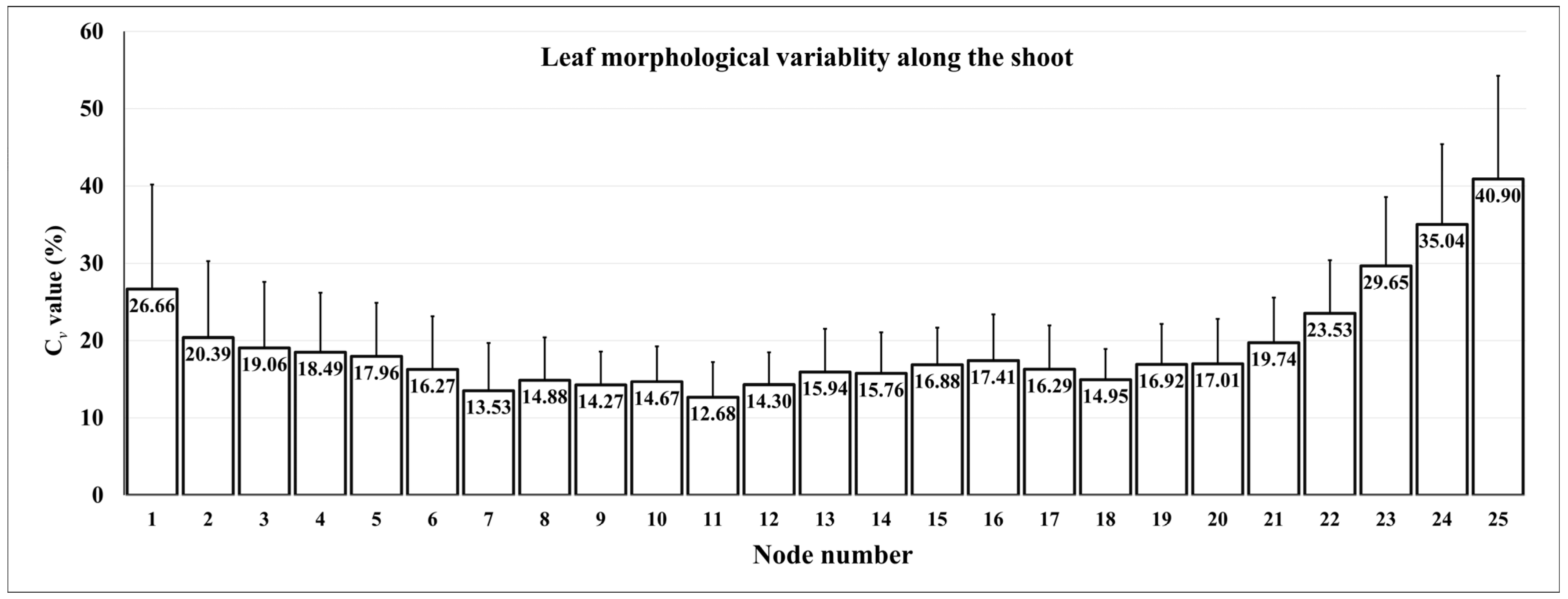
4. Morphological Variability along the Shoot: Which Leaf to Compare?

5. Leaf Morphological Diversity among Species, Cultivars and Clones
5.1. Ampelometric Evaluation of Vitis Species and Rootstock Cultivars
5.2. Morphometric Diversity of the Wild Grape (Vitis Sylvestris C.C. Gmel. Hegi)
5.3. Comparison of Grapevine Cultivars and Clones
6. Factors Influencing Ampelometric Traits
7. Discussion
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mullins, M.G.; Bouquet, A.; Williams, L.E. Biology of the Grapevine; Cambridge University Press: Cambridge, UK, 2003; p. 239. [Google Scholar]
- Goussard, P.G. Grape Cultivars for Wine Production in South Africa; Cheviot Publishing: Greenpoint, South Africa, 2008; p. 166. [Google Scholar]
- Robinson, J.; Harding, J.; Vouillamoz, J. Wine Grapes: A Complete Guide to 1368 Vine Varieties, Including Their Origins and Flavours; HarperCollins Publisher: New York, NY, USA, 2012; p. 1280. [Google Scholar]
- Hungarian Ministry of Agriculture. Geographical Indications and Traditional Terms, Protected Designation of Origin and Protected Geographical Indications. Available online: https://boraszat.kormany.hu/termekleirasok2 (accessed on 28 November 2022).
- Steiner, B.E. Australian wines in the British wine market: A hedonic price analysis. Agribusiness 2004, 20, 287–307. [Google Scholar] [CrossRef]
- Kallas, Z.; Escobar, C.; Gil, J.M. Analysis of consumers’ preferences for a special-occasion red wine: A dual response choice experiment approach. Food Qual. Prefer. 2013, 30, 156–168. [Google Scholar] [CrossRef]
- OIV Descriptor List for Grape Varieties and Vitis Species, 2nd ed.; Office International de la Vigne et du Vin: Paris, France, 2009; p. 177.
- Winkler, A.J.; Cook, J.A.; Kliewer, W.M.; Lider, L.A. General Viticulture; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA; London, UK, 1974; p. 710. [Google Scholar]
- Mazade, M. First Steps in Ampelography: A Guide to Facilitate the Recognition of Vines; Brain, R.S., Ed.; Government Printer: Melbourne, Australia, 1900; p. 95. [Google Scholar]
- Ravaz, L. Les Vignes Americaines: Porte-Greffes et Producteurs Directs (Caracteres Aptitudes); Coulet et Fils, Éditeurs: Montpellier, France, 1902; p. 390. [Google Scholar]
- Seltensperger, C. Dictionnaire D’agriculture et de Viticulture; Librairie, J.-B., Ed.; Bailliére et Fils: Paris, France, 1911; p. 1064. [Google Scholar]
- Kozma, P. Szőlészeti és borászati követelmények a fajtakutatás és nemesítés területén. Agrártudományi Közlemények 1956, 12, 223–257. [Google Scholar]
- Dodoens, R. Stripium Historiae Pemptades Sex. Sive Libri XXX.; Ex Officina Christophori Plantini: Antverpiæ, Belgium, 1583; p. 860. [Google Scholar]
- Bauhin, C. Pinax Theatri Botanici; Sumptibus & Typis Ludovici Regis: Paris, France, 1623; p. 522. [Google Scholar]
- Sachs, P.J. Ampelographia Sive Vitis Viniferae Ejusque Partium Consideratio Physico-Philologico-Historico-Medico-Chymica; Impensis Viti Jacobi Trescheri: Leipzig, Germany, 1661; p. 670. [Google Scholar]
- Rea, J. Flora, Ceres & Pomona; Richard Marriott: London, UK, 1665; p. 211. [Google Scholar]
- Worlidge, J. Vinetum Britannicum. A Treatise of Cider and Other Wines and Drinks Extracted from Fruits; Thomas Dring: London, UK, 1678; p. 278. [Google Scholar]
- Miller, P. The Gardeners Dictionary: Containing the Methods of Cultivating and Improving the Kitchen, Fruit and Flower Garden, as also the Physick Garden, Wilderness, Conservatory, and Vineyard; St. Paul’s Church-Yard: London, UK, 1741; Volume 2, p. 540. [Google Scholar]
- Duhamel, D.M. Traité des Arbres et Arbustes qui se Cultivent en France en Pleine Terre; Desain, Libraire, rue du Foin: Paris, France, 1755; Volume 2, p. 387. [Google Scholar]
- Mills, J. New System of Practical Husbandry; J. Johnson and B. Davenport: London, UK, 1755; Volume IV, p. 465. [Google Scholar]
- Duhamel, D.M. Traité des Arbres Fruitiers: Contenant Leur Figure, Leur Description, Leur Culture; Chez Saillant: Paris, France, 1768; Volume 2, p. 337. [Google Scholar]
- Clement, R.S.R. Ensayo Sobre las Variedades de la vid Comun que Vegetan en Andalucía; En la imprenta de Villalpando: Madrid, Spain, 1807; p. 324. [Google Scholar]
- Vest, L.C. Versuch Einer Systematischen Zusammenstellung der in Steyermark Cultivirten Weinreben: Mit Ihren Diagnosen, mit Beschreibungen, und mit Einem Alphabetischen Index Ihrer Synonymen; Andreas Leykam: Graz, Austria, 1816; p. 103. [Google Scholar]
- Acerbi, G. Delle viti Italiane ossia Materiali per Servire alla Classificazione, Monografia e Sinonimia, Preceduti dal Tentativo di una Classificazione Delle viti; Milano per Giovanni Silvestri: Milano, Italy, 1825; p. 335. [Google Scholar]
- Gok, K.F. Die Wein-Rebe Mit Ihren Arten Und Abarten, Oder, Beiträge Zur Kenntniss Der Eigenschaften Und Zur Classifikation Der Cultivirten Weinreben-Arten; Heinrich Mantler: Stuttgart, Germany, 1829; p. 120. [Google Scholar]
- Babo, L.; Metzger, J. Die Wein- und Tafeltrauben der deutschen Weinberge und Garten; Heimrich Hoff.: Mannheim, Germany, 1836; p. 251. [Google Scholar]
- Burger, J. Systematische Klassifikation und Beschreibung der in Österreichischen Weingärten Vorkommenden Traubenarten, mit den Charakteristischen Merkmalen der Gattungen und Arten, ihren Wissenschaftlichen und Ortsüblichen Benennungen und den Besonderen Eigenschaften der Trauben und der aus Ihnen Gekelterten Weine; Verlag Gerold Wien: Berlin, Germany, 1837; p. 156. [Google Scholar]
- Dierbach. Die Weinrebe (Vitis vinifera L.) und ihre Vorzüglichsten zum Arzneigebrauche Dienenden Varietäten. Lemgo; Meyersche Hof-Buchhandlung: Hof, Germany, 1838; p. 56. [Google Scholar]
- Molon, G. Ampelografia. Descrizione delle Migliori Varietá di Viti; Ulrico Hoepli: Milano, Italy, 1906; p. 1243. [Google Scholar]
- Henderson, A. Traditional morphometrics in plant systematics and its role in palm systematics. Bot. J. Linn. Soc. 2006, 151, 103–111. [Google Scholar] [CrossRef]
- Frege, M.C.A. Versuch einer Classification der Wein-Sorten nach ihre Beeren; Den Karl Friedrich Wilhelm Erbstein: Meissen, Germany, 1804; p. 171. [Google Scholar]
- Metzger, J. Der Rheinische Weinbau in Theoretischer Und Praktischer Beziehung; August Oßwald: Heidelberg, Germany, 1827; p. 344. [Google Scholar]
- Tersánczki, J. Oenologia Azaz: Irányt adó Kalauz; Markbreiter, J., Ed.; Fischel F. Print: Nagy-Kanizsa, Hungary, 1865; p. 108. [Google Scholar]
- Goethe, H. Handbuch der Ampelographie. Beschreibung und Klassifikation der bis jetzt Kultivierten Rebenarten und Trauben-Varietäten mit Angabe Ihrer Synonyme, Kulturverhältnisse und Verwendungsart; Parey, P., Ed.; Berlin 21: Graz, Austria, 1887; p. 219. [Google Scholar]
- Branas, J. Des Méthodes Ampélographiques; Imprimerie Paul Déhan: Montpellier, France, 1943; p. 1177. [Google Scholar]
- Grew, N. The Anatomy of Plants: With an Idea of a Philosophical History of Plants. And Several Other Lectures, Read Before the Royal Society; W. Rawlins for the Author: London, UK, 1682; p. 300. [Google Scholar]
- Seeliger, R. Vererbungs- und Kreuzungsversuche mit der Weinrebe. Zeitschr. F. Indukt. Abstamm.- Und Vererb. 1925, 39, 31–163. [Google Scholar] [CrossRef]
- Moog, H. Beiträge zur Ampelographie; Mitteilungen der Preussischen Rebenveredlungskommission Nr. 6; Buchdruckerei Arthur Jander: Geisenheim, Germany, 1930; p. 103. [Google Scholar]
- Galet, P. Méthode de Description et de Classification des Espécies Variétés et Hybrids de Vignes; Imprimerie Paul Déhan: Montpellier, France, 1951; p. 8. [Google Scholar]
- Galet, P. Précis D’ampélographie Pratique; Imprimerie Paul Déhan: Montpellier, France, 1952; p. 182. [Google Scholar]
- Morton, L. A Practical Ampelography: Grapevine Identification; Cornell University Press: Ithaca, NY, USA, 1979; p. 248. [Google Scholar]
- Galet, P. Les Portes-Greffes de Malégues; Imprimerie Paul Déhan: Montpellier, France, 1953; p. 19. [Google Scholar]
- Galet, P. Cépages et Vignobles de France; Tome I; Déhan: Montpellier, France, 1956; p. 670. [Google Scholar]
- Galet, P. Cépages et Vignobles de France; Tome IV; Déhan: Montpellier, France, 1964; pp. 2902–3500. [Google Scholar]
- Németh, M. Ampelográfiai Album. Termesztett Borszőlőfajták 1.; Mezőgazdasági Kiadó: Budapest, Hungary, 1967; p. 235. [Google Scholar]
- Németh, M. Ampelográfiai Album. Termesztett Borszőlőfajták 2.; Mezőgazdasági Kiadó: Budapest, Hungary, 1970; p. 272. [Google Scholar]
- Németh, M. Ampelográfiai Album 3. Alany-, Direkt Termő és Csemegeszőlő Fajták; Mezőgazdasági Kiadó: Budapest, Hungary, 1975; p. 358. [Google Scholar]
- Hajdu, E. Magyar Szőlőfajták; Mezőgazda Kiadó: Budapest, Hungary, 2003; p. 258. [Google Scholar]
- Martí, C.; Casanova, J.; Montaner, C.; Badia, D. Ampelometric study of mature leaves from two indigenous Vitis cultivars grown in Somontano de Barbastro. J. Wine Res. 2006, 17, 185–194. [Google Scholar] [CrossRef]
- Chitwood, D.H. The shapes of wine and table grape leaves: An ampelometric study inspired by the methods of Pierre Galet. Plants People Planet 2021, 3, 155–170. [Google Scholar] [CrossRef]
- Németh, M. Borszőlőfajták Határozókulcsa; Mezőgazdasági Kiadó: Budapest, Hungary, 1966; p. 239. [Google Scholar]
- Dorsey, M.J. Variation Studies of the Venation Angles and Leaf Dimensions in Vitis. J. Hered. 1912, 1, 227–250. [Google Scholar] [CrossRef]
- Bodor, P.; Hajdu, E.; Baranyai, L.; Deák, T.; Bisztray, G.D.; Bálo, B. Traditional and Landmark-Based Geometric Morphometric Analysis of Table Grape Clone Candidates. Mitt. Klosterneubg. 2017, 67, 20–27. [Google Scholar]
- Alba, V.; Bergamini, C.; Genghi, R.; Gasperro, M.; Perniola, R.; Antoacci, D. Ampelometric leaf trait and SSR loci selection for a multivariate statistical approach in Vitis vinifera L. biodiversity management. Mol. Biotechnol. 2015, 57, 709–719. [Google Scholar] [CrossRef]
- Abiri, K.; Rezaei, M.; Tahanian, H.; Heidari, P.; Khadivi, A. Morphological and pomological variability of a grape (Vitis vinifera L.) germplasm collection. Sci. Hortic. 2020, 266, 109285. [Google Scholar] [CrossRef]
- Martínez Rodríguez, D.C.; Pérez Fernández, J.E. La vid en el Occidente del Principado de Asturias: Descripción Ampelográfica de las Variedades; CSIC: Madrid, Spain, 1999; p. 101. [Google Scholar]
- Díaz-Losada, E.; Cortés-Diéguez, S.; Rodríguez-Torres, I.; Mirás-Avalos, J.M.; Orriols-Fernández, I.; Pereira-Lorenzo, S. Characterization of the nearly extinct ‘Albilla’ cultivar from Galicia and its relationships with other spanish ‘Albillos’. J. Int. Sci. Vigne Vin 2013, 47, 261–268. [Google Scholar] [CrossRef]
- Nieddu, G.; Chessa, I.; Mercenaro, L. Primary and secondary characterization of a Vermentino grape clones collection. In Proceedings of the 2006 First International Symposium on Environment Identities and Mediterranean Area, Corte-Ajaccio, France, 10–13 July 2006; pp. 517–521. [Google Scholar] [CrossRef]
- Ben Slimane Harbi, M.; Snoussi, H.; Bouhlal, R.; Nahdi, H. Ampelometry to test for genetic diversity in Tunisian Vitis sylvestris. Afr. J. Plant Sci. Biotechnol. 2010, 4, 17–22. [Google Scholar]
- Labagnara, T.; Bergamini, C.; Caputo, A.R.; Cirigliano, P. Vitis vinifera L. germplasm diversity: A genetic and ampelometric study in ancient vineyards in the South of Basilicata region (Italy). Vitis 2018, 57, 1–8. [Google Scholar] [CrossRef]
- Preinier, D.; Safner, T.; Karoglan Kontić, J.; Marković, Z.; Šimon, S.; Maletić, E. Analysis of phyllometric parameters efficiency in discrimination of Croatian native V. vinifera cultivars. Vitis 2014, 53, 215–217. [Google Scholar] [CrossRef]
- Beleski, K.; Nedelkovski, D. Identification and classification of grapevine cultivars (Vitis vinifera L.) from the Balkan subgroup by phyllometric descriptors. Vitis 2015, 54, 133–134. [Google Scholar] [CrossRef]
- Rodrigues, M.A. Sôbre o recorte e assimetria da fôlha da videira. Agromonia Lusitana 1942, 4, 137–153. [Google Scholar]
- Rodrigues, M.A. Acêrca do valor taxonomico do numero de dentes da fôlha na separaçao de dois hibrido do genero Vitis L. Agron. Lusit. 1945, 3, 325–340. [Google Scholar]
- Rodrigues, M.A. Um método Filométrico de Caracterização Ampelográfica. Fundamentos, Descrição e Técnica Operatória; Ministério da Economia: Lisboa, Portugal, 1952; p. 42. [Google Scholar]
- Rodrigues, M.A. Sur la variation du nombre de faisceaux foliaires le long du sarment dans differents cepages de Vitis vinifera L. In Proceedings of the Comunicação apresentada à XXXIII Session Plenière de l’Office International du Vin et VII Congrès International de la Vigne et du Vin, Sienne, Rome, 13–20 September 1953; Volume III, pp. 84–101. [Google Scholar]
- Rodrigues, M.A. La determination ampelogrpahique de dix porte-greffes. Agron. Lusit. 1961, 23, 79. [Google Scholar]
- Eynard, I. Sobre a aplicação do método filométrico de Acúrcio Rodrigues na caracterização ampelográfica de dois porta-enxertos, en Itália. Agron. Lusit. 1960, 22, 127–130. [Google Scholar]
- Eynard, I. Studio Ampelografico e Ampelometrico di Alcuni Ancori de vino del Prof. Giovanni Dalmasso; Nota II. Vitigni da uva Bianca; Tipografia Vincenzo Bona: Torino, Italy, 1966; p. 296. [Google Scholar]
- Eiras Dias, J.E.J. Análise odontométrica de uma casta em anos sucessivos. Ciência Téc. Vitiv. 1983, 2, 39–48. [Google Scholar]
- Bookstein, F.L. Morphometric Tools for Landmark Data Geometry and Biology; Cambridge University Press: Cambridge, UK, 1991; p. 435. [Google Scholar]
- Viscosi, V.; Cardini, A. Leaf Morphology, Taxonomy and Geometric Morphometrics: A Simplified Protocol for Beginners. PLoS ONE 2011, 6, e25630. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics software package for education and data analysis. Paleontol. Electron. 2001, 4, 9. [Google Scholar]
- Chitwood, D.H.; Ranjan, A.; Martinez, C.C.; Headland, L.R.; Thiem, T.; Kumar, R.; Convington, M.F.; Hatcher, T.; Naylor, D.T.; Zimmerman, S.; et al. A modern ampelography: A genetic basis for leaf shape and venation patterning in grape. Plant Physiol. 2014, 164, 259–272. [Google Scholar] [CrossRef]
- Chitwood, D.H.; Klein, L.L.; O’hanlon, R.; Chacko, S.; Greg, M.; Kitchen, C.; Miller, A.J.; Londo, J.P. Latent developmental and evolutionary shapes embedded within the grapevine leaf. New Phytol. 2016, 210, 343–355. [Google Scholar] [CrossRef]
- Chitwood, D.H.; Rundell, S.M.; Li, D.Y.; Woodford, Q.L.; Yu, T.T.; Lopez, J.R.; Greenblatt, D.; Kang, J.; Londo, J.P. Climate and developmental plasticity: Interannual variability in grapevine leaf morphology. Plant Physiol. 2016, 170, 1480–1491. [Google Scholar] [CrossRef]
- Bodor, P.; Baranyai, L.; Szekszárdi, A.; Bisztray, G.D.; Bálo, B. Landmark-based morphometry reveals phyllometric diversity along the shoot axis of the grapevine (Vitis vinifera L.). Prog. Agric. Eng. Sci. 2018, 14, 1–9. [Google Scholar]
- Bryson, A.E.; Wilson Brown, M.; Mullins, J.; Dong, W.; Bahmani, K.; Bornowski, N.; Chiu, C.; Engelgau, P.; Gettings, B.; Gomezcano, F.; et al. Composite modeling of leaf shape along shoots discriminates Vitis species better than individual leaves. Appl. Plant Sci. 2020, 8, e11404. [Google Scholar] [CrossRef]
- Diaz, G.; Setzu, M.; Diana, A.; Loi, C.; De Martis, B.; Pala, M.; Boselli, M. Analyse de Fourier de la forme de la feuille de vigne. Premiere application ampelometrique sur un échatillon de 34 cepages implantes en Sardaigne. J. Intern. Sei. Vigne Vin 1991, 25, 37–49. [Google Scholar]
- Mancuso, S. Elliptic Fourier Analysis (EFA) and Artificial Neural Networks (ANNs) for the identification of grapevine (Vitis vinifera L.) genotypes. Vitis 1999, 38, 73–77. [Google Scholar]
- Swanepoel, J.J.; de Villiers, C.E. A Numerical-Taxonomic classification of Vitis spp and Cultivars Based on Leaf Characteristics. South Afr. J. Enol. Vitic. 1987, 8, 31–35. [Google Scholar] [CrossRef]
- Boursiquot, J.-M.; Faber, M.-P.; Blachier, O.; Truel, P. Utilisation par l’informatique et traitement statistique d’un fichier ampelographique. Agron. EDP Sci. 1987, 7, 13–20. [Google Scholar] [CrossRef]
- Schneider, A.; Zeppa, G. Biometria in ampelográfia: L’uso di una tavolessa grafica per effettuare rapidamente misure fillometriche. Vignevini 1988, 9, 37–40. [Google Scholar]
- Martinez, M.C.; Mantilla, J.L.G. Morphological and yield comparison between Vitis vinifera L. cv. Albariňo grown from cuttings and from in vitro propagation. Am. J. Enol. Vitic. 1995, 46, 195–203. [Google Scholar] [CrossRef]
- Alessandri, S.; Vignozzi, N.; Vignini, A. AmpeloCADs (Ampelographic Computer-Aided Digitizing System): An integrated system to digitize and process biometrical data from Vitis spp. Leaves. Am. J. Enol. Vitic. 1996, 47, 257–267. [Google Scholar] [CrossRef]
- Soldavini, C.; Stefanini, M.; Dallaserra, M.; Policarpo, M.; Schneider, A. SuperAmpelo, a software for ampelometric and ampelographic descriptions in Vitis. In Proceedings of the IX International Conference on Grape Genetics and Breeding, Udine, Italy, 31 May 2009; pp. 253–258. [Google Scholar] [CrossRef]
- Strochi, P.; Armanni, A.; Randellini, L.; Giannetto, S.; Meneghetti, S.; Crespan, M. Investigations on the identity of ‘Canaiolo bianco’ and other white grape varietiesof central Italy. Vitis 2011, 50, 59–64. [Google Scholar]
- Zdunić, G.; Šimon, S.; Malenica, N.; Preiner, D.; Maletić, E.; Pejić, I. Morphometric and molecular analysis of a pink-berried mutant within the population of grape cultivar ‘Plavac mali.’. Vitis 2012, 51, 7–13. [Google Scholar]
- Fatehi, S.E.; Ater, M.; Hmimsa, Y. Ampelometric and Ampelographic Characterization of Leaves of Indigenous “Vitis vinifera ssp. Vinifera” in the North of Morocco. Biol. Life Sci. Forum 2021, 2, 8. [Google Scholar]
- Bodor, P.; Baranyai, L.; Bálo, B.; Tóth, E.; Strever, A.; Hunter, J.J.; Bisztray, G.D. GRA.LE.D. (GRApevine LEaf Digitalization) software for the detection and graphic reconstruction of ampelometric differences between Vitis leaves. South Afr. J. Enol. Vitic. 2012, 33, 1–6. [Google Scholar] [CrossRef]
- Bodor, P.; Baranyai, L.; Parrag, V.; Bisztray, G.D. Effect of row orientation and elevation on leaf morphology of grapevine (Vitis vinifera L.) c.v. Furmint. Progr. Agric. Eng. Sci. 2014, 10, 53–69. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image processing with Image. J. Biophotonics Int. 2004, 11, 36–43. [Google Scholar]
- Martinez, M.; Grenan, S. A graphic reconstruction method of an average leaf of vine. Agronomie 1999, 19, 491–507. [Google Scholar] [CrossRef]
- Santiago, J.L.; Boso, S.; Martin, J.P.; Ortiz, J.M.; Martinez, M.C. Characterization and identification of grapevine cultivars (Vitis vinifera L.) from northwestern Spain using microsatellite markers and ampelometric methods. Vitis 2005, 44, 67–72. [Google Scholar]
- Santiago, J.L.; Boso, S.; Martínez, M.C.; Pinto-Carnide, O.; Ortiz, J.M. Ampelographic comparison of grape cultivars (Vitis vinifera L.) grown in Northwestern Spain and Northern Portugal. Am. J. Enol. Vitic. 2005, 56, 287–290. [Google Scholar] [CrossRef]
- Santiago, J.L.; Boso, S.; Gago, P.; Alonso-Villaverde, V.; Martínez, M.C. A contribution to the maintenance of grapevine diversity: The rescue of Tinta Castañal (Vitis vinifera L.), a variety on the edge of extinction. Sci. Hortic. 2008, 116, 199–204. [Google Scholar] [CrossRef]
- Boso, S.; Alonso-Villaverde, V.; Santiago, J.L.; Gago, P.; Durrenberger, M.; Duggelin, M.; Kassemeyer, H.H.; Martinez, M.C. Macro- and microscopic leaf characteristics of six grapevine genotypes (Vitis spp.) with different susceptibilities to grapevine downy mildew. Vitis 2010, 49, 43–50. [Google Scholar] [CrossRef]
- Chadha, K.L.; Randhawa, G.S. Grape varieties in India. Description and classification. ICAR Tech. Bull. 1974, 48, 220. [Google Scholar]
- Demaria, P.P.; Leardi, C. Ampelografia della Provincia di Alessandria: Con Introduzione Sugli Studi Ampelografici, Sulla Viticoltura e sull’Enologia della; Presso Augosto Federico Negro Editore: Torino, Italy, 1875; p. 320. [Google Scholar]
- Cousins, P.; Prins, B. Vitis shoots show reversible change in leaf shape along the shoot axis. In Proceedings of the 2nd Annual National Viticulture Research Conference, Davis, CA, USA, July 9–11 2008; University of California: Davis, CA, USA; pp. 17–18. [Google Scholar]
- Chitwood, D.H.; Mullins, J.; Migicovsky, Z.; Frank, M.; VanBuren, R.; Londo, J.P. Vein-to-blade ratio is an allometric indicator of leaf size and plasticity. Am. J. Bot. 2021, 108, 571–579. [Google Scholar] [CrossRef]
- Gmelin, C.C. Flora Badensis Alsatica et Confinium Regionum Cis et Trans Rhenana; Officina A. Mülleriana: Carlsruhae, Germany, 1806; p. 768. [Google Scholar]
- Anzani, R.; Failla, O.; Scienza, A.; Campostrini, F. Wild Grapevine (Vitis vinifera var. silvestris) in Italy: Distribution, Characteristics and Germplasm Preservation: 1989 Report. Vitis 1990, 29, 97–112. [Google Scholar]
- Söylemezoğlu, G.; Ağaoğlu, Y.S.; Uzun, H.I. Ampelographic Characteristics and Isozymic Analysis of Vitis Vinifera Spp. Sylvestris Gmel. in Southwestern Turkey. Biotechnol. Biotechnol. Equip. 2001, 15, 106–113. [Google Scholar] [CrossRef]
- Susaj, L.; Susaj, E.; Jashari, F. Mature Leaf Features of Wild Grapevine: Populations grown in Three Different River Valleys of North Albania. Albanian J. Agric. Sci. 2014, 135–142. [Google Scholar] [CrossRef]
- Martinez de Toda, F.M.; Sancha, J.C. Characterization of wild vines in La Rioja (Spain). Am. J. Enol. Vitic. 1999, 50, 443–446. [Google Scholar] [CrossRef]
- Cunha, J.; Baleiras-Couto, M.; Cunha, J.P.; Banza, J.; Soveral, A.; Carneiro, L.C.; Eiras-Dias, E. Characterization of Portuguese population of Vitis vinifera L. ssp. Sylvestris (Gmelin) Hegi. Gen. Res. Crop. Evol. 2007, 54, 981–988. [Google Scholar] [CrossRef]
- Ekhvaia, J.; Akhalkatsi, M. Morphological variation and relationships of Georgian populations of Vitis vinifera L. subsp. sylvestris (C.C. Gmel.) Hegi. Flora 2010, 205, 608–617. [Google Scholar] [CrossRef]
- Barth, S.; Forneck, A.; Verzeletti, F.; Blaich, R.; Schumann, F. Genotypes and phenotypes of an ex situ Vitis vinifera ssp. Sylvestris (Gmel.) Beger germplasm collection from the Upper Rhine Valley. Genet. Resour. Crop. Evol. 2009, 56, 1171–1181. [Google Scholar] [CrossRef]
- Bodor, P.; Ladányi, M.; Grzeskowiak, L.; Grando, M.S.; Bisztray, G.D. Ampelometric evaluation of wild grape (Vitis vinifera L. ssp. sylvestris (C.C. Gmel.) Hegi) accessions in the germplasm collection of FEM-IASMA, Italy. Vitis 2015, 54, 213–215. [Google Scholar]
- Asensio, M.L.; Valdés, E.; Cabello, F. Characterization of some Spanish white grapevine cultivars by morphology and amino acid analysis. Sci. Hortic. 2002, 93, 3–4. [Google Scholar] [CrossRef]
- Sabir, A.; Tangolar, S.; Buyukalaca, S.; Kafkas, S. Ampelographic and Molecular Diversity among Grapevine (Vitis spp.) Cultivars. Czech J. Genet. Plant Breed. 2009, 45, 160–168. [Google Scholar] [CrossRef]
- Popescu, C.F.; Dejeu, L.C.; Bejan, C. Ampelographic characterization—Preliminary results of the nine most appreciated autochthonous Vitis vinifera L. varieties from Romania. Vitis 2015, 54, 159–162. [Google Scholar]
- Averna-Sacca, R. Experiment Station Record; 1910; Volume 22. Available online: digital.library.unt.edu/ark:/67531/metadc5032/ (accessed on 29 January 2017).
- Allen, E.W. Experiment Station Record; U.S. Department of Agriculture, Washington Government Printing Office: Washington, DC, USA, 1912; Volume XXVI. [Google Scholar]
- Reiczigel, Z.; Szekszárdi, A.; Bisztray, G.D.; Ladányi, M.; Bálo, B.; Bodor, P. Szőlőfajták Ampelometriai Mutatóinak Statisztikai Elemzése. XXIII. Növénynemesítési Tudományos Nap; MTA Agrártudományok Osztálya Növénynemesítési Tudományos Bizottság Magyar Növénynemesítők Egyesülete: Budapest, Hungary, 2017; p. 137. [Google Scholar]
- Fidelibus, M.W.; Christensen, L.P.; Katayama, D.G.; Verdenal, P.T. Yield components and fruit composition of six Chardonnay grapevine clones in the Central San Joaquin Valley, California. Am. J. Enol. Vitic. 2006, 57, 503–506. [Google Scholar] [CrossRef]
- Miotto, L.C.V.; Mota, R.V.; de Souza, C.R.; França, D.V.C.; Dias, F.A.N.; Pimentel, R.M.A.; Dal’Osto, M.C.; Regina, M.A. Agronomic evaluation of ‘Bordô’ grapevine (Ives) clones. Sci. Agric. 2014, 71, 458–463. [Google Scholar] [CrossRef]
- Petric, I.V.; Košmerl, T.; Pejić, I.; Kubanović, V.; Zlatić, E. Clone candidates differentiation of grapevine Vitis vinifera ‘Škrlet bijeli’ using aroma compounds detected by gas chromatography-mass spectrometry. Acta Agric. Slov. 2016, 107, 483–496. [Google Scholar] [CrossRef]
- Imazio, S.; Labra, M.; Grassi, F.; Winfield, M.; Bardini, M.; Scienza, A. Molecular tools for clone identification: The case of the grapevine cultivar ‘Traminer’. Plant Breed. 2002, 121, 531–535. [Google Scholar] [CrossRef]
- Werner, J.; Tóth-Lencsés, K.; Veres, A.; Kiss, E.; Kozma, P., Jr. Morphological and molecular characterization of varieties and selected clones of Kadarka grape. Mitt. Klosterneubg. 2013, 63, 38–50. [Google Scholar]
- Silvestroni, O.; Intrieri, C.; Credi, R.; Facciolo, F.; Marangoni, B.; Vespignani, G. Clonal variability of several grapevine cultivars (V. vinifera L.) grown in the Emilia-Romagna. Vitis 1990, 29, 500–507. [Google Scholar]
- Santos, M.T.; Rocha, M.L.T.; Martins, J.M.S.; Carneiro, L.C. Effect of grapevine viruses GFLV, GLRV 3 and GFkV on leaf morphology of the Portuguese white variety Arinto by multivariate discriminant analysis. In Proceedings of the Extended Abstracts, 14th ICVG Meeting, Locorotondo/Bari, Italy, 12–17 September 2003; pp. 21–22. [Google Scholar]
- Gribaudo, I.; Mannini, F.; Lisa, A.; Cuozzo, D. Phenotypical modification of micropropagated grapevines. Acta Hortic. 2000, 530, 231–236. [Google Scholar] [CrossRef]
- Martinez, M.C.; Boursiquot, J.M.; Grenan, S.; Boidron, R. Étude ampélométrique de feuilles adultes de somaclones du cv. Grenache N (Vitis vinifera L.). Can. J. Bot. 1997, 75, 333–345. [Google Scholar] [CrossRef]
- Carrión, M.E.; Cutanda, M.C.; Botella, O.; Montero, F.J. Influencia del Sistema de cultivo en la morfología de los órganos de Vitis Vinífera L. In Proceedings of the Actas XXIX Congreso Mundial de la Viña y el Vino, Logroño, Spain, 25–30 June 2006. [Google Scholar]
- Bodor, P.; Baranyai, L.; Ladányi, M.; Bálo, B.; Strever, A.E.; Bisztra, G.D.; Hunter, J.J. Stability of ampelometric characteristics of Vitis vinifera L. cv. ‘Syrah’ and ‘Sauvignon blanc” leaves: Impact of within-vineyard variability and pruning method/bud load. South Afr. J. Enol. Vitic. 2013, 34, 129–137. [Google Scholar] [CrossRef]
- Eynard, I. Ampelografia e Metodi Ampelometrici; Seminario tenutosi il 23 maggio 1969 presso l’Instituto di Coltivazioni Arboree dell’Universitá di Pisa; Universitá di Pisa: Pisa, Italy, 1969; p. 38. [Google Scholar]
- Dettweiler-Münch, E. Ampelography—An international course in San Michele/Italy. Vitis 1991, 30, 265–267. [Google Scholar]
- Ferreira, C.; Cunha, M. Machine Learning predictive model of grapevine yield based on agroclimatic patterns. Eng. Agric. Environ. Food 2019, 12, 443–450. [Google Scholar]
- Bodor-Pesti, P.; Somogyi, E.; Deák, T.; Nyitrainé Sárdy, D.Á.; Ladányi, M. Quantitative image analysis of berry size and berry shape of different grapevine (Vitis vinifera L.) accessions. Mitt. Klosterneubg. 2022, 72, 130–136. [Google Scholar]
- Landa, V.; Shapira, Y.; David, M. Accurate classification of fresh and charred grape seeds to the varietal level, using machine learning based classification method. Sci. Rep. 2021, 11, 13577. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodor-Pesti, P.; Taranyi, D.; Deák, T.; Nyitrainé Sárdy, D.Á.; Varga, Z. A Review of Ampelometry: Morphometric Characterization of the Grape (Vitis spp.) Leaf. Plants 2023, 12, 452. https://doi.org/10.3390/plants12030452
Bodor-Pesti P, Taranyi D, Deák T, Nyitrainé Sárdy DÁ, Varga Z. A Review of Ampelometry: Morphometric Characterization of the Grape (Vitis spp.) Leaf. Plants. 2023; 12(3):452. https://doi.org/10.3390/plants12030452
Chicago/Turabian StyleBodor-Pesti, Péter, Dóra Taranyi, Tamás Deák, Diána Ágnes Nyitrainé Sárdy, and Zsuzsanna Varga. 2023. "A Review of Ampelometry: Morphometric Characterization of the Grape (Vitis spp.) Leaf" Plants 12, no. 3: 452. https://doi.org/10.3390/plants12030452
APA StyleBodor-Pesti, P., Taranyi, D., Deák, T., Nyitrainé Sárdy, D. Á., & Varga, Z. (2023). A Review of Ampelometry: Morphometric Characterization of the Grape (Vitis spp.) Leaf. Plants, 12(3), 452. https://doi.org/10.3390/plants12030452










