Microbes vs. Nematodes: Insights into Biocontrol through Antagonistic Organisms to Control Root-Knot Nematodes
Abstract
1. Introduction
2. Biocontrol Agents against Root-Knot Nematodes
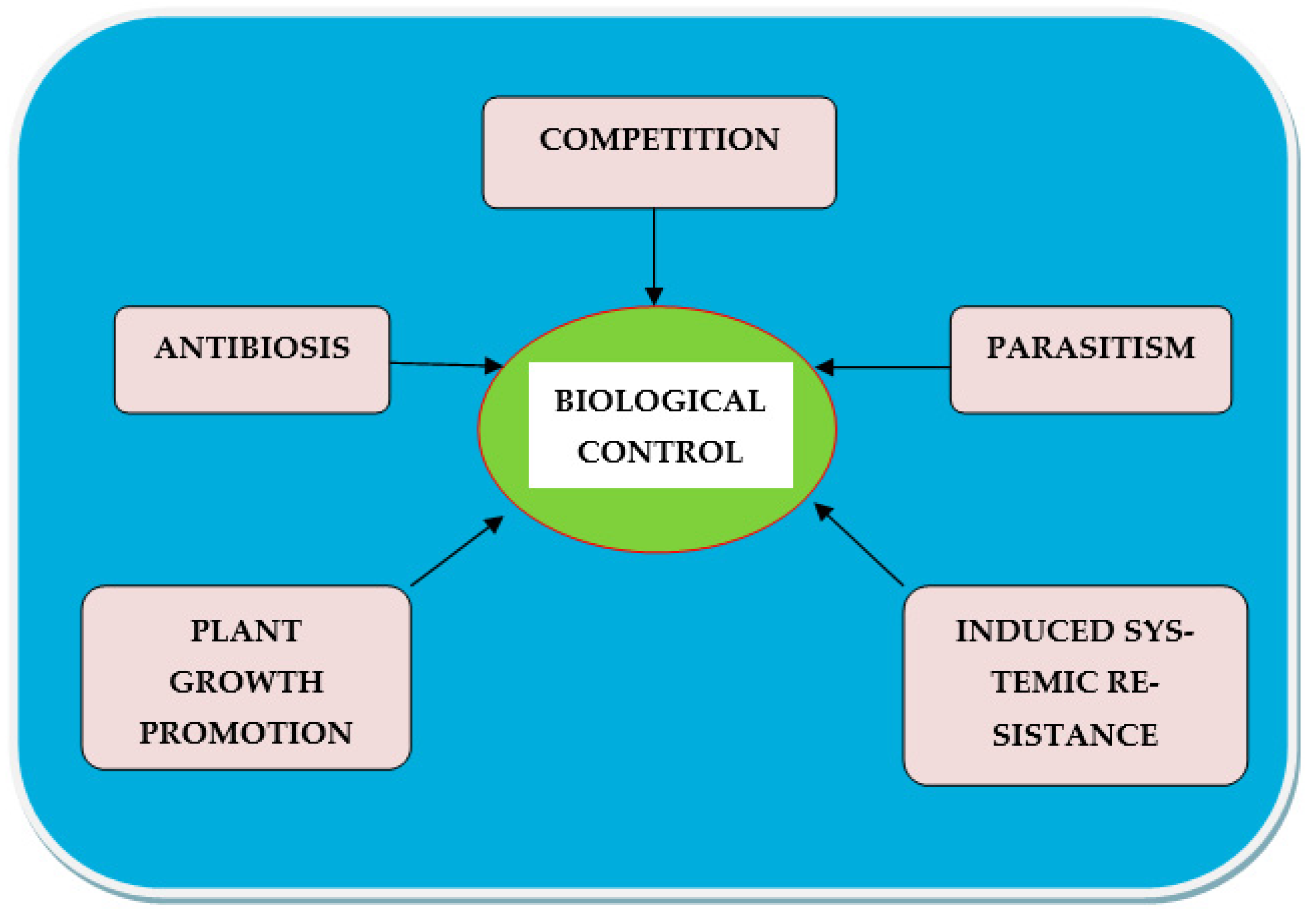
- Competition: Competition (intraspecific and interspecific), mainly for space, nutrients, water, etc. reduces the growth, activity, and reproduction of the organisms involved [22] or affects nematode fitness.
- Antibiosis: This can happen when plant compounds are released from the roots into the soil. Bacteria produce and release certain antibiotics or toxins, which may have an undesirable effect on the infective stage of nematodes [23]. Allelochemicals are known to harm plant-parasitic nematodes as well [24].
- Parasitism: Nematodes are prey for most nematophagous bacteria, which use nematodes as a potential source of nutrients. They can also pierce the cuticle (due to enzymatic action), and kill the nematode host [25].
- Plant growth promotion: Bio-agents aid in the control of plant diseases by increasing plant development through improved nutrient solubilization, higher nutrient uptake, and nutrient sequestration. Plants with higher nutritional status can tolerate more plant-parasitic nematodes in their roots [26].
- Induced systemic resistance: Numerous bacterial products create systemic signaling in plants, which can protect the entire plant from diseases induced by various pathogens or help plants become resistant to various pathogenic organisms [27].
2.1. Bacteria as Biocontrol Agents against Root-Knot Nematodes
2.2. Fungi as Biocontrol Agents against Root-Knot Nematodes
2.2.1. Entomopathogenic Fungi as Biocontrol Agents of Root-Knot Nematodes
2.2.2. Toxin-Producing Fungi as Biocontrol Agents against Root-Knot Nematodes
2.2.3. Nematode-Trapping Fungi as Biocontrol Agents against Root-Knot Nematodes
2.2.4. Endoparasitic Fungi as Biocontrol Agents against Root-Knot Nematodes
2.2.5. Ovicidal Fungi as Biocontrol Agents against Root-Knot Nematodes
2.2.6. Secondary Metabolites and Nematicidal Compounds Obtained from Nematophagous Fungi as Biocontrol Agents against Root-Knot Nematodes
2.3. Plant Extracts as Biocontrol Agents against Root-Knot Nematodes
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shakeel, A.; Khan, A.A.; Bhat, A.H.; Sayed, S. Nitrogen fertilizer alleviates root-knot nematode stress in beet root by suppressing the pathogen while modulating the antioxidant defence system and cell viability of the host. Physiol. Mol. Plant Pathol. 2022, 120, 101838. [Google Scholar] [CrossRef]
- Sikora, R.A.; Coyne, D.; Hallmann, J.; Timper, P. Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; CABI: Wallingford, UK, 2018; pp. 1–19. [Google Scholar]
- Kiewnick, S.; Sikora, R.A. Biological control of the root-knot nematode (Meloidogyne incognita) by Paecilomyces lilacinus strain 251. Biol. Control 2006, 38, 179–187. [Google Scholar] [CrossRef]
- Ashraf, M.S.; Khan, T.A. Integrated approach for the management of Meloidogyne javanica on egg plant using oil cakes and biocontrol agents. Arch. Phytopathol. Plant Prot. 2010, 43, 609–614. [Google Scholar] [CrossRef]
- Weischer, B. Nematode-virus interactions. In Nematode Interactions; Springer: Dordrecht, The Netherlands, 1993; pp. 217–231. [Google Scholar]
- Ismail, W.; Johri, J.K.; Zaidi, A.A.; Singh, B.P. Influence of root-knot nematode, tobaccomosaic virus & complex on the growth & carbohydrates of Solanum khasianum Clarke. Indian J. Exp. Biol. 1979, 17, 1266–1267. [Google Scholar]
- Huang, S.P.; Chu, E.Y. Inhibitory Effect of Watermelon Mosaic Virus on Meloidogyne javanica (Treub) Chitwood infecting Cucurbitapepo L. J. Nematol. 1984, 16, 109. [Google Scholar] [PubMed]
- Alam, M.M.; Samad, A.; Anver, S. Interaction between tomato mosaic virus and Meloidogyne incognita in tomato. Nematol. Mediterr. 1990, 18, 131–133. [Google Scholar]
- Moura, R.M.; Powell, N.T. Estudos sobre o complexo TMV M. incognita em tomate. Soc. Bras. De Nematol. Publicacao 1977, 2, 175–181. [Google Scholar]
- Weischer, B. Further studies on the population development of Ditylenchus dipsaci and Aphelenchoides ritzemabosi in virus-infected and virus-free tobacco. Nematologica 1975, 21, 213–218. [Google Scholar] [CrossRef]
- Showler, A.T.; Reagan, T.E.; Shao, K.P. Nematode interactions with weeds and sugarcane mosaic virus in Louisiana sugarcane. J. Nematol. 1990, 22, 31. [Google Scholar]
- Moens, M.; Perry, R.N.; Starr, J.L. Meloidogyne species–a diverse group of novel and important plant parasites. Root-Knot Nematodes 2009, 1, 483. [Google Scholar]
- Coyne, D.L.; Cortada, L.; Dalzell, J.J.; Claudius-Cole, A.O.; Haukeland, S.; Luambano, N.; Talwana, H. Plant-parasitic nematodes and food security in Sub-Saharan Africa. Annu. Rev. Phytopathol. 2018, 56, 381. [Google Scholar] [CrossRef] [PubMed]
- Phani, V.; Khan, M.R.; Dutta, T.K. Plant-parasitic nematodes as a potential threat to protected agriculture: Current status and management options. Crop Prot. 2021, 144, 105573. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.X.; Song, B. Chemical nematicides: Recent research progress and outlook. J. Agric. Food Chem. 2020, 68, 12175–12188. [Google Scholar] [CrossRef]
- Dong, J.Y.; Li, X.P.; Li, L.; Li, G.H.; Liu, Y.J.; Zhang, K.Q. Preliminary results on nematicidal activity from culture filtrate s of Basidiomycetes against the pinewood nematode, Bursaphelenchus xylophilus (Aphelenchoididae). Ann. Microbiol. 2006, 56, 163–166. [Google Scholar] [CrossRef]
- Sharma, P.; Rakesh, P. Biologicalcontrol of root-knot nematode; Meloidogyne incognita in the medicinal plant; Withania somnifera and the effect of biocontrol agents on plant growth. Afr. J. Agric. Res. 2009, 4, 564–567. [Google Scholar]
- MBTOC. Report of the Methyl Bromide Technical Options Committee. In Non-Chemical Alternatives Adopted as Replacements to Methyl Bromide on a Large Scale; United Nation Environmental Programme, UNON Publishing Section Services: Nairobi, Kenya, 2006; pp. 39–73. [Google Scholar]
- EC. Directive of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council. In Directives 79/117/EEC and 91/414/EEC. Official Journal of the European Union, 24.11. 2009, L 309/1-50; EC: Brussels, Belgium; Luxembourg, 2009. [Google Scholar]
- Zarb, J.; Ghorbani, R.; Koocheki, A.; Leifert, C. The importance of microorganisms in organic agriculture. Outlooks Pest Manag. 2005, 16, 52–55. [Google Scholar] [CrossRef]
- Stirling, G.R. Biological control of plant-parasitic nematodes. In Diseases of Nematodes; CRC Press: Boca Raton, FL, USA, 2018; pp. 103–150. [Google Scholar]
- Pal, K.K.; Gardener, B.M. Biological control of plant pathogens. Plant Health Instr. 2006, 2, 1117–1142. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant growth promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.K.; Koppenhöfer, A.M. Effects of microbial and other antagonistic organism and competitionon entomopathogenic nematodes. Biocontrol. Sci. Technol. 1996, 6, 357–372. [Google Scholar] [CrossRef]
- Tian, B.; Yang, J.; Zhang, K.Q. Bacteria used in the biological control of plant-parasitic nematodes: Populations, mechanisms of action, and future prospects. FEMS Microbiol. Ecol. 2007, 61, 197–213. [Google Scholar] [CrossRef]
- Schouteden, N.; DeWaele, D.; Panis, B.; Vos, C.M. Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: A review of the mechanisms involved. Front. Microbiol. 2015, 6, 1280. [Google Scholar] [CrossRef] [PubMed]
- Junaid, J.M.; Dar, N.A.; Bhat, T.A.; Bhat, A.H.; Bhat, M.A. Commercial biocontrol agents and their mechanism of action in the management of plant pathogens. Int. J. Mod. Plant Anim. Sci. 2013, 1, 39–57. [Google Scholar]
- Roberts, D.P.; Lohrke, S.M.; Meyer, S.L.; Buyer, J.S.; Bowers, J.H.; Baker, C.J.; Chung, S. Biocontrol agents applied individually and in combination for suppression of soilborne diseases of cucumber. Crop Prot. 2005, 24, 141–155. [Google Scholar] [CrossRef]
- Murslain, M.; Javed, N.; Khan, S.A.; Khan, H.U.; Abbas, H.; Kamran, M. Combined efficacy of Moringa oleifera leaves and a fungus, Trichoderma harzianum against Meloidogyne javanica on eggplant. Pak. J. Zool. 2014, 46, 827–832. [Google Scholar]
- Haque, Z.; Khan, M.R.; Ahamad, F. Relative antagonistic potential of some rhizosphere biocontrol agents for the management of rice root-knot nematode(Meloidogyne graminicola). Biol. Control 2018, 126, 109–116. [Google Scholar] [CrossRef]
- Askary, T.H.; Martinelli, P.R.P. Biocontrol Agents Phytonematodes; CABI: Wallingford, UK, 2015; pp. 81–125. [Google Scholar]
- Forghani, F.; Hajihassani, A. Recent advances in the development of environmentally benign treatments to control root-knot nematodes. Front. Plant Sci. 2020, 11, 1125. [Google Scholar] [CrossRef]
- Miliute, I.; Buzaite, O.; Baniulis, D.; Stanys, V. Bacterial endophytes in agricultural crops and their role in stress tolerance: A review. Zemdirbyste 2015, 102, 465–478. [Google Scholar] [CrossRef]
- Glick, B.R. Beneficial Plant-Bacterial Interactions; Springer: Berlin/Heidelberg, Germany, 2015; pp. 139–180. [Google Scholar]
- Zhao, J.; Wang, S.; Zhu, X.; Wang, Y.; Liu, X.; Duan, Y.; Chen, L. Isolation, and characterization of nodules endophytic bacteria Pseudomonas protegens Sneb 1997and Serratia plymuthica Sneb 2001 for the biological control of root-knot nematode. Appl. Soil Ecol. 2021, 164, 103924. [Google Scholar] [CrossRef]
- Tariq, M.; Khan, A.; Asif, M.; Khan, F.; Ansari, T.; Shariq, M.; Siddiqui, M.A. Biological control: A sustainable and practical approach for plant disease management. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2020, 70, 507–524. [Google Scholar] [CrossRef]
- Migunova, V.D.; Sasanelli, N. Bacteria as biocontrol tool against phytoparasitic nematodes. Plants 2021, 10, 389. [Google Scholar] [CrossRef]
- Mhatre, P.H.; Karthik, C.; Kadirvelu, K.; Divya, K.L.; Venkatasalam, E.P.; Srinivasan, S.; Shanmuganathan, R. Plant growth promoting rhizobacteria(PGPR): A potential alternative tool for nematodes biocontrol. Biocatal. Agric. Biotechnol. 2019, 17, 119–128. [Google Scholar] [CrossRef]
- Panpatte, D.G.; Jhala, Y.K.; Shelat, H.N.; Vyas, R.V. Pseudomonas fluorescens: A promising biocontrol agent and PGPR for sustainable agriculture. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: New Delhi, India, 2016; pp. 257–270. [Google Scholar]
- Sharma, I.P.; Sharma, A.K. Effective control of root-knot nematode disease with Pseudomonad rhizobacteria filtrate. Rhizosphere 2017, 3, 123–125. [Google Scholar] [CrossRef]
- Chinheya, C.C.; Yobo, K.S.; Laing, M.D. Biological control of the root-knot nematode, Meloidogyne javanica (Chitwood) using Bacillus isolates, on soybean. Biol. Control 2017, 109, 37–41. [Google Scholar] [CrossRef]
- Mehtab, A.; Javed, N.; Khan, S.A.; Gondal, A.S. Combined effect of Pasteuria penetrans and neem extract on the development of root-knot nematode in medicinal plants. Pak. J. Nematol. 2013, 31, 55–59. [Google Scholar]
- Abd-El-Khair, H.; El-Nagdi, W.; Youssef, M.; Abd-Elgawad, M.M.; Dawood, M.G. Protective effect of Bacillus subtilis, B. pumilus, and Pseudomonas fluorescens isolates against root-knot nematode Meloidogyne incognita on cowpea. Bull. Natl. Res. Cent. 2019, 43, 1–7. [Google Scholar] [CrossRef]
- Khalil, M.S.; Kenawy, A.; Gohrab, M.A.; Mohammed, E.E. Impact of microbial agents on Meloidogyne incognita management and morphogenesis of tomato. J. Biopestic. 2012, 5, 28–35. [Google Scholar]
- Wang, J.Y.; Guo, C.; Zhao, P.; Yu, F.Y.; Su, Y.; Qu, J.P.; Zhou, B. Biocontrol potential of Bacillus altitudinis AMCC1040 against root-knot nematode disease of ginger and its impact on rhizosphere microbial community. Biol. Control 2021, 158, 104598. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, K.Y. Antagonistic potential of Bacillus pumilus L1 against root-knot nematode(Meloidogyne arenaria). J. Phytopathol. 2016, 164, 29–39. [Google Scholar] [CrossRef]
- Abbasi, M.W.; Ahmed, N.; Zaki, M.J.; Shuakat, S.S.; Khan, D. Potential of Bacillus species against Meloidogyne javanica parasitizing eggplant (Solanum melongena L.) and induced biochemical changes. Plant Soil 2014, 375, 159–173. [Google Scholar] [CrossRef]
- Sivasakthi, S.; Usharani, G.; Saranraj, P. Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: A review. Afr. J. Agric. Res. 2014, 9, 1265–1277. [Google Scholar]
- Rao, M.S.; Kamalnath, M.; Umamaheswari, R.; Rajinikanth, R.; Prabu, P.; Priti, K.; Gopalakrishnan, C. Bacillus subtilis IIHRBS-2 enriched vermicompost controls root-knot nematode and soft rot disease complex in carrot. Sci. Hortic. 2017, 218, 56–62. [Google Scholar] [CrossRef]
- Bhuiyan, S.A.; Garlick, K.; Anderson, J.M.; Wickramasinghe, P.; Stirling, G.R. Biological control of root-knot nematode on sugarcane in soil naturally or artificially infested with Pasteuria penetrans. Australas. Plant Pathol. 2018, 47, 45–52. [Google Scholar] [CrossRef]
- Cetintas, R.; Dickson, D.W. Persistence and Suppressiveness of Pasteuria penetrans to Meloidogyne arenaria Race. J. Nematol. 2004, 36, 540. [Google Scholar] [PubMed]
- Cho, M.R.; Na, S.Y.; Yiem, M.S. Biological control of Meloidogyne arenaria by Pasteuria penetrans. J. Asia-Pac. Entomol. 2000, 3, 71–76. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.; Kannangara, S.D.; Promputtha, I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef]
- Stirling, G.R.; West, L.M. Fungal parasites of root-knot nematode eggs from tropical and subtropical regions of Australia. Australas. Plant Pathol. 1991, 20, 149–154. [Google Scholar] [CrossRef]
- Li, J.; Zou, C.; Xu, J.; Ji, X.; Niu, X.; Yang, J.; Zhang, K.Q. Molecular mechanisms of nematode-nematophagous microbe interactions: Basis for biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2015, 53, 67–95. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Yin, Y.; Cui, F.; Cai, J.; Chen, Z.; Hu, R. Neurological effects of pesticide use among farmers in China. Int. J. Environ. Res. Public Health 2014, 11, 3995–4006. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, G.; Fang, M.; Deng, C.; Zhang, K.Q.; Yu, Z.; Xu, J. Comparative analyses of mitochondrial genomes provide evolutionary insights into nematode-trapping fungi. Front. Microbiol. 2020, 11, 617. [Google Scholar] [CrossRef]
- Abd Elgawad, M.M.; Askary, T.H. Fungal and bacterial nematicides in integrated nematode management strategies. Egypt. J. Biol. Pest Control 2018, 28, 1–24. [Google Scholar] [CrossRef]
- Ahmed, S.; Monjil, M.S. Effect of Paecilomyceslilacinus ontomatoplantsandthemanagement of root-knot nematodes: Paecilomyces lilacinus on tomato root-knot disease. J. Bangladesh Agric. Univ. 2019, 17, 9–13. [Google Scholar] [CrossRef]
- Khalil, M.S.E.D.H.; Allam, A.F.G.; Barakat, A.S.T. Nematicidal activity of some biopesticide agents and microorganisms against root-knot nematode on tomato plants under green houseconditions. J. Plant Prot. Res. 2012, 52, 47–52. [Google Scholar]
- Singh, S.; Mathur, N. Biological control of root-knot nematode (Meloidogyne incognita) infesting tomato. Biocontrol Sci. Technol. 2010, 20, 865–874. [Google Scholar] [CrossRef]
- Tian, X.; Yao, Y.; Chen, G.; Mao, Z.; Wang, X.; Xie, B. Suppression of Meloidogyne incognita by the endophytic fungus Acremonium implicatum from tomato root galls. Int. J. Pest Manag. 2014, 60, 239–245. [Google Scholar] [CrossRef]
- Yao, Y.R.; Tian, X.L.; S hen, B.M.; Mao, Z.C.; Chen, G.H.; Xie, B.Y. Transformation of the endophytic fungus Acremonium implicatum with GFP and evaluation of its biocontrol effect against Meloidogyne incognita. World J. Microbiol. Biotechnol. 2015, 31, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Goswami, J.; Pandey, R.K.; Tewari, J.P.; Goswami, B.K. Management of root-knot nematode on tomato through application of fungal antagonists, Acremonium strictum and Trichoderma harzianum. J. Environ. Sci. Health Part B 2008, 43, 237–240. [Google Scholar] [CrossRef]
- Noweer, E.M.A. A field trial to use the nematode-trapping fungus (Arthrobotrys dactyloides) to control the root-knot nematode(Meloidogyne incognita) infesting bean plants. Comm. Appl. Biol. Sci. Ghent. Univ. 2017, 82, 275–280. [Google Scholar]
- Soliman, M.S.; El-Deriny, M.M.; Ibrahim, D.S.S.; Zakaria, H.; Ahmed, Y. Suppression of root-knot nematode Meloidogyne incognita on tomato plants using the nematode trapping fungus Arthrobotrys oligospora Fresenius. J. Appl. Microbiol. 2021, 131, 2402–2415. [Google Scholar] [CrossRef] [PubMed]
- Bakr, R.A.; Mahdy, M.E.; Mousa, E.S.M. Biological control of root-knot nematode (Meloidogyne incognita) by Arthrobotrys oligospora. Egypt. J. Crop Prot. 2014, 9, 1–11. [Google Scholar] [CrossRef]
- Mostafanezhad, H.; Sahebani, N.; Nourinejhad Zarghani, S. Control of root-knot nematode (Meloidogyne javanica) with combination of Arthrobotrys oligospora and salicylic acid and study of some plant defence responses. Biocontrol. Sci. Technol. 2014, 24, 203–215. [Google Scholar] [CrossRef]
- Cui, R.; Fan, C.; Sun, X. Isolation, and characterisation of Aspergillus awamori BS05, a root-knot nematode trapping fungus. Biocontrol. Sci. Technol. 2015, 25, 1233–1240. [Google Scholar] [CrossRef]
- He, Q.; Wang, D.; Li, B.; Maqsood, A.; Wu, H. Nematicidal evaluation and active compounds isolation of Aspergillus japonicus ZW1 against root-knot nematodes (Meloidogyne incognita). Agronomy 2020, 10, 1222. [Google Scholar] [CrossRef]
- Ying, L.I.U.; Zhong, D.; Peng, D.L.; Liu, S.M.; Kong, L.A.; Huan, P.; Huang, W.K. Evaluation of the biocontrol potential of Aspergillus welwitschiae against the root-knot nematode (Meloidogyne graminicola)in rice(Oryza sativa L.). J. Integr. Agric. 2019, 18, 2561–2570. [Google Scholar]
- Le, H.T.; Padgham, J.L.; Sikora, R.A. Biological control of the rice root-knot nematode (Meloidogyne graminicola) on rice, using endophytic and rhizosphere fungi. Int. J. Pest Manag. 2009, 55, 31–36. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, W.; Zhang, P.; Ruan, W.; Zhu, X. Nematicidal activity of chaetoglobosin A produced by Chaetomium globosum NK102 against Meloidogyne incognita. J. Agric. Food Chem. 2013, 61, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Yan, W.; Wei, S.; Wang, Z.; Zhao, S.; Cao, L.; Ye, Y. Nematicidal metabolites from endophytic fungus (Chaetomium globosum YSC5). FEMS Microbiol. Lett. 2019, 366, fnz169. [Google Scholar] [CrossRef]
- Noweer, E.M.A.; Elkelany, U.S. Biological control of root-knot nematode (Meloidogyne incognita) infesting eggplant by the nematode-trapping fungus (Dactylaria brochopaga) and the nematode egg parasitic fungus (Verticilium chlamydosporium) under field conditions. J. Innov. Pharm. Biol. Sci. 2019, 6, 1–6. [Google Scholar]
- Noweer, E.M.A.; Aboul-Eid, H.Z. Biological control of root-knot nematode Meloidogyne incognita infesting cucumber Cucumis sativus L. cvs. Alfa by the nematode-trapping fungus (Dactylaria brochopaga) under field conditions. Agric. Biol. J. North Am. 2013, 4, 435–440. [Google Scholar] [CrossRef]
- Youssef, M.M.; El-Nagdi, W.M. New approach for biocontrolling root-knot nematode (Meloidogyne incognita) on cowpea by commercial fresh oyster mushroom (Pleurotus ostreatus). Jordan J. Biol. Sci. 2021, 14, 173–177. [Google Scholar]
- Amin, N. The use of fungal endophytes Gliocladium spp. In different concentration to control of root-knot nematode(Meloidogyne spp.). Acad. Res. Int. 2014, 5, 91. [Google Scholar]
- Hussain, M.; Zouhar, M.; Rysanek, P. Population dynamics of a nematophagous fungus Lecanicillium muscarium, and root-knot nematode (Meloidogyne incognita) to assess the disease pressure and its management. Pak. J. Zool. 2017, 49, 197–204. [Google Scholar] [CrossRef]
- Bawa, N.; Kaur, S.; Dhillon, N.K. Integrated management of root-knot nematode (M. incognita) in capsicum, using Paecilomyces lilacinus and organic amendments. J. Entomol. Zool. Stud. 2020, 8, 1693–1701. [Google Scholar]
- Liu, T.; Wang, L.; Duan, Y.X.; Wang, X. Nematicidal activity of culture filtrate of Beauveria bassiana against Meloidogyne hapla. World J. Microbiol. Biotechnol. 2008, 24, 113–118. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, K.P. Assessment of predacity and efficacy of Arthrobotrys dactyloides for biological control of root-knot disease of tomato. J. Phytopathol. 2006, 154, 1–5. [Google Scholar] [CrossRef]
- Youssef, M.; El-Nagdi, W.; Lotfy, D.E. Evaluation of the fungal activity of Beauveria bassiana, Metarhizium anisopliae and Paecilomyces lilacinus as biocontrol agents against root-knot nematode (Meloidogyne incognita) on cowpea. Bull. Natl. Res. Cent. 2020, 44, 1–11. [Google Scholar] [CrossRef]
- Anke, H.; Stadler, M.; Mayer, A.; Sterner, O. Secondary metabolites with nematicidal and antimicrobial activity from nematophagous fungi and Ascomycetes. Can. J. Bot. 1995, 73 (Suppl. S1), 932–939. [Google Scholar] [CrossRef]
- Ghayedi, S.; Abdollahi, M. Biocontrol potential of Metarhizium anisopliae (Hypocreales: Clavicipitaceae), isolated from suppressive soils of the Boyer-Ahmad region, Iran, against J2s of Heterodera avenae. J. Plant Prot. Res. 2013, 53, 2. [Google Scholar] [CrossRef]
- Kershaw, M.J.; Moorhouse, E.R.; Bateman, R.; Reynolds, S.E.; Charnley, A.K. The role of destruxins in the pathogenicity of Metarhizium anisopliae for three species of insect. J. Invertebr. Pathol. 1999, 74, 213–223. [Google Scholar] [CrossRef]
- Satou, T.; Kaneko, K.; Li, W.; Koike, K. The toxin produced by Pleurotus ostreatus reduces the head size of nematodes. Biol. Pharm. Bull. 2008, 31, 574–576. [Google Scholar] [CrossRef]
- Kwock, O.C.H.; Plattner, R.; Weisleder, D.; Wicklow, D.T. A nematicidal toxin from Pleurotus ostreatus NRRL3526. J. Chem. Ecol. 1992, 18, 127–136. [Google Scholar]
- Heydari, R.; Pourjam, E.; Goltapeh, E.M. Antagonistic effect of some species of Pleurotus on the root-knot nematode(Meloidogyne javanica) in-vitro. Plant Pathol. J. 2006, 5, 173–177. [Google Scholar] [CrossRef]
- Jiang, X.; Xiang, M.; Liu, X. Nematode-trapping fungi. Microbiol. Spectr. 2017, 5, 1–12. [Google Scholar] [CrossRef]
- Al Kader, A. In-Vitro Studies on Nematode Interactions with Their Antagonistic Fungi in the Rhizosphere of Various Plants. Ph.D. Thesis, University of Freiburg, Freiburg im Breisgau, Germany, 2009. [Google Scholar]
- Nourani, S.L.; Mohammadi-Goltapeh, E.; Safaie, N.; JalaliJavaran, M.; Pourjam, E.; Shams-Bakhsh, M.; Jahanshahi Afshar, F. The effects of Arthrobotrys oligospora and Arthrobotrys conoides culture filtrates on second stage juvenile mortality and egg hatching of Meloidogyne incognita and Meloidogyne javanica. J. Crop Prot. 2015, 4, 667–674. [Google Scholar]
- Yang, J.; Li, J.; Liang, L.; Tian, B.; Zhang, Y.; Cheng, C.; Zhang, K.Q. Cloning and characterization of an extracellular serine protease from the nematode-trapping fungus Arthrobotrys conoides. Arch. Microbiol. 2007, 188, 167–174. [Google Scholar] [CrossRef]
- Aboul-Eid, H.Z.; Abdel-Bari, N.A.; Ameen, H.H.; Noweer, E.M.A. The morphological features of twelve nematode-antagonistic fungi and the bacterium Pasteuria penetrans isolated from El-Mansouria region soils(Giza, Egypt). Egypt. J. Agronematology 1997, 1, 59–76. [Google Scholar]
- Aboul-Eid, H.Z.; Noweer, E.M.A.; Ashour, N.E. Impact of the nematode-trapping fungus(Dactylaria brochopagaas) a biocontrol agent against Meloidogyne incognita infesting Superior) grapevine. Egypt. J. Biol. Pest Control 2014, 24, 477. [Google Scholar]
- Kumar, P.; Chand, R. Studies on the bio-efficacy of Monacrosporium eudermatum against root-knot nematode(Meloidogyne incognita) on brinjal. J. Pharmacogn. Phytochem. 2017, 6, 2427–2430. [Google Scholar]
- Aguilar-Marcelino, L.; Mendoza-de-Gives, P.; Al-Ani, L.K.T.; López-Arellano, M.E.; Gómez-Rodríguez, O.; Villar-Luna, E.; Reyes-Guerrero, D.E. Using molecular techniques applied to beneficial microorganisms as biotechnological tools for controlling agricultural plant pathogens and pest. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Academic Press: Cambridge, MA, USA, 2020; pp. 333–349. [Google Scholar]
- Wan, J.; Dai, Z.; Zhang, K.; Li, G.; Zhao, P. Pathogenicity and metabolites of Endoparasitic nematophagous fungus(Drechmeria coniospora) YMF1.01759 against nematodes. Microorganisms 2021, 9, 1735. [Google Scholar] [CrossRef] [PubMed]
- De Freitas Soares, F.E.; Sufiate, B.L.; de Queiroz, J.H. Nematophagous fungi: Far beyond the endoparasite, predator and ovicidal groups. Agric. Nat. Resour. 2018, 52, 1–8. [Google Scholar]
- Singh, S.; Pandey, R.K.; Goswami, B.K. Bio-control activity of Purpureocillium lilacinum strains in managing root-knot disease of tomato caused by Meloidogyne incognita. Biocontrol Sci. Technol. 2013, 23, 1469–1489. [Google Scholar] [CrossRef]
- Hano, P.; Khan, M.R. Evaluation of fungal (Paecilomyces lilacinus) formulations against root-knot nematode infecting tomato. Bangladesh J. Bot. 2016, 45, 1003–1013. [Google Scholar]
- Sivakumar, T.; Renganathan, P.B.P.; Sanjeev kumar, K. Bio efficacy of bio-nematon(Paecilomyces lilacinus 1.15%wp) against root-knot nematode(Meloidogyne incognita) in cucumber crop. Plant Arch. 2020, 20, 3805–3810. [Google Scholar]
- Hore, J.; Roy, K.; Maiti, A.K. Evaluation of Bio-Nematon(Purpureocillium lilacinum 1.15%WP) against root-knot nematode(Meloidogyne incognita) in tomato. J. Entomol. Zool. Stud. 2018, 6, 1700–1704. [Google Scholar]
- Pandey, R.K.; Singh, S.R.; Gupta, P.K.; Goswami, B.K.; Singh, D.V.; Gharde, Y. Effect of different bioformulations of Paecilomyces lilacinus against root-knot nematode (Meloidogyne incognita) infecting tomato (Solanum esculentum). Indian J. Agric. Sci. 2011, 81, 261–267. [Google Scholar]
- Sharma, A.; Sharma, S.; Dalela, M. Nematicidal activity of Paecilomyces lilacinus 6029 cultured on Karanja cake medium. Microb. Pathog. 2014, 75, 16–20. [Google Scholar] [CrossRef]
- Degenkolb, T.; Vilcinskas, A. Metabolites from nematophagous fungi and nematicidal natural products from fungi as an alternative for biological control. PartI: Metabolites from nematophagous ascomycetes. Appl. Microbiol. Biotechnol. 2016, 100, 3799–3812. [Google Scholar] [CrossRef]
- Jang, J.Y.; Choi, Y.H.; Shin, T.S.; Kim, T.H.; Shin, K.S.; Park, H.W.; Kim, J.C. Biological control of Meloidogyne incognita by Aspergillus niger F22 producing oxalic acid. PLoS ONE 2016, 11, e0156230. [Google Scholar] [CrossRef]
- Xiang, C.; Liu, Y.; Liu, S.M.; Huang, Y.F.; Kong, L.A.; Peng, H.; Huang, W.K. αβ-Dehydrocurvularin isolated from the fungus Aspergillus welwitschiae effectively inhibited the behaviour and development of the root-knot nematode (Meloidogyne graminicola) in rice roots. BMC Microbiol. 2020, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Campos, V.P.; Pinho, R.S.C.D.; Freire, E.S. Volatiles produced by interacting microorganisms potentially useful for the control of plant pathogens. Ciência e Agrotecnologia 2010, 34, 525–535. [Google Scholar] [CrossRef]
- Terra, W.C.; Campos, V.P.; Martins, S.J.; Costa, L.S.A.S.; daSilva, J.C.P.; Barros, A.F.; Oliveira, D.F. Volatile organic molecules from Fusarium oxysporum strain 21 with nematicidal activity against Meloidogyne incognita. Crop Prot. 2018, 106, 125–131. [Google Scholar] [CrossRef]
- Liarzi, O.; Bucki, P.; Braun Miyara, S.; Ezra, D. Bioactive volatiles from an endophytic Daldinia cf. concentrica isolate affect the viability of the plant-parasitic nematode (Meloidogyne javanica). PloS one 2016, 11, e0168437. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Wang, X.; Li, G. Pathogenicity and Volatile Nematicidal Metabolites from Duddingtonia flagrans against Meloidogyne incognita. Microorganisms 2021, 9, 2268. [Google Scholar] [CrossRef]
- Khan, A.; Williams, K.; Molloy, M.P.; Nevalainen, H. Purification and characterization of a serine protease and chitinases from Paecilomyces lilacinus and detection of chitinase activity on 2D gels. Protein Expr. Purif. 2003, 32, 210–220. [Google Scholar] [CrossRef]
- Sugimoto, M.; Koike, M.; Hiyama, N.; Nagao, H. Genetic, morphological, and virulence characterization of the entomopathogenic fungus Verticillium lecanii. J. Invertebr. Pathol. 2003, 82, 176–187. [Google Scholar] [CrossRef]
- Khan, A.; Williams, K.L.; Nevalainen, H.K. Effects of Paecilomyces lilacinus protease and chitinase on the eggshell structures and hatching of Meloidogyne javanica juveniles. Biol. Control 2004, 31, 346–352. [Google Scholar] [CrossRef]
- Tikhonov, V.E.; Lopez-Llorca, L.V.; Salinas, J.; Jansson, H.B. Purification and characterization of chitinases from the nematophagous fungi Verticillium chlamydosporium and V. Suchlasporium. Fungal Genet. Biol. 2002, 35, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Liu, L.J.; Shi, M.; Song, X.Y.; Zheng, C.Y.; Chen, X.L.; Zhang, Y.Z. Characterization and gene cloning of a novel serine protease with nematicidal activity from Trichoderma pseudokoningii SMF2. FEMS Microbiol. Lett. 2009, 299, 135–142. [Google Scholar] [CrossRef]
- Wang, Y.L.; Li, L.F.; Li, D.X.; Wang, B.; Zhang, K.; Niu, X. Yellow pigment aurovertins mediate interactions between the pathogenic fungus Pochonia chlamydosporia and its nematode host. J. Agric. Food Chem. 2015, 63, 6577–6587. [Google Scholar] [CrossRef]
- Kim, T.Y.; Jang, J.Y.; Yu, N.H.; Chi, W.J.; Bae, C.H.; Yeo, J.H.; Kim, J.C. Nematicidal activity of grammicin produced by Xylaria grammica KCTC13121BP against Meloidogyne incognita. Pest Manag. Sci. 2018, 74, 384–391. [Google Scholar] [CrossRef]
- Yang, J.; Huang, X.; Tian, B.; Wang, M.; Niu, Q.; Zhang, K. Isolation and characterization of a serine protease from the nematophagous fungus (Lecanicillium psalliotae) displaying nematicidal activity. Biotechnol. Lett. 2005, 27, 1123–1128. [Google Scholar] [CrossRef]
- Gan, Z.; Yang, J.; Tao, N.; Liang, L.; Mi, Q.; Li, J.; Zhang, K.Q. Cloning of the gene Lecanicillium psalliotae chitinase Lpchi1 and identification of its potential role in the biocontrol of root-knot nematode (Meloidogyne incognita). Appl. Microbiol. Biotechnol. 2007, 76, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, N.; Kim, Y.J.; Oh, K.T.; Jung, W.J.; Park, R.D. The role of chitinase from Lecanicillium antillanum B-3 in parasitism to root-knot nematode (Meloidogyne incognita) eggs. Biocontrol. Sci. Technol. 2007, 17, 1047–1058. [Google Scholar] [CrossRef]
- De Souza Gouveia, A.; de Freitas Soares, F.E.; Morgan, T.; Sufiate, B.L.; Tavares, G.P.; Braga, F.R.; de Queiroz, J.H. Enhanced production of Monacrosporium thaumasium protease and destruction action on root-knot nematode (Meloidogyne javanica) eggs. Rhizosphere 2017, 3, 13–15. [Google Scholar] [CrossRef]
- Qin, J.C.; Zhang, Y.M.; Gao, J.M.; Bai, M.S.; Yang, S.X.; Laatsch, H.; Zhang, A.L. Bioactive metabolites produced by Chaetomium globosum, an endophytic fungus isolated from Ginkgo biloba. Bioorganic Med. Chem. Lett. 2009, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Natori, S. Chemical surveys on mycotoxins using cytotoxicity testing with special reference to cytochalasans. Yakugakuzasshi: J. Pharm. Soc. Jpn. 1983, 103, 1109–1128. [Google Scholar]
- Grainge, M.; Ahmed, S. Handbook of Plants with Pest-Control Properties; John Wiley & Sons Limited: Hoboken, NJ, USA, 1988; p. 470. [Google Scholar]
- Mwamula, A.O.; Kabir, M.F.; Lee, D. A Review of the Potency of Plant Extracts and Compounds from Key Families as an Alternative to Synthetic Nematicides: History, Efficacy, and Current Developments. Plant Pathol. J. 2022, 38, 53–77. [Google Scholar] [CrossRef]
- Gommers, F.J. Biochemical interactions between nematodes and plants and the irrelevance to control. Helminthol. Abstr. 1981, 50, 9–24. [Google Scholar]
- Chitwood, D.J. Phytochemical based strategies for nematode control. Annu. Rev. Phytopathol. 2002, 40, 221–249. [Google Scholar] [CrossRef]
- Orisajo, S.B.; Dongo, L.N. Nematicidal potential of some indigenous plant extracts against root-knot nematode on cacao. Afr. Sci. 2022, 6, 129–134. [Google Scholar]
- Fabiyi, O.A. Evaluation of plant materials as root-knot nematode (Meloidogyne incognita) suppressant in okra(Abelmoschus esculentus). Agric. Conspec. Sci. 2021, 86, 51–56. [Google Scholar]
- De Kesel, J.; Degroote, E.; Nkurunziza, R.; Singh, R.R.; Demeestere, K.; DeKock, K.; Kyndt, T. Cucurbitaceae cold Peeling Extracts (CCOPEs) protect plants from root-knot nematode infections through induced resistance and nematicidal effects. Front. Plant Sci. 2022, 12, 785699. [Google Scholar] [CrossRef] [PubMed]
- Arshad, U.; Jabran, M.; Ahmed, S.; Abbas, A.; Jabbar, A.; Zahid, M.S.; Ali, M.A. Seed-Priming: A novel approach for improving growth performance and resistance against root-knot nematode (Meloidogyne incognita) in bread wheat(Triticum aestivum L.). Gesunde Pflanzen. 2022, 74, 1041–1051. [Google Scholar] [CrossRef]
- Khan, F.; Asif, M.; Khan, A.; Tariq, M.; Ansari, T.; Shariq, M.; Siddiqui, M.A. Evaluation of the nematicidal potential of some botanicals against root-knot nematode, Meloidogyne incognita infected carrot: In in-vitro and greenhouse study. Curr. Plant Biol. 2019, 20, 100115. [Google Scholar] [CrossRef]
- Lynn, O.M.; Song, W.G.; Shim, J.K.; Kim, J.E.; Lee, K.Y. Effects of azadirachtin and neem-based formulations for the control of sweet potato whitefly and root-knot nematode. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 598–604. [Google Scholar] [CrossRef]
- Javed, N.; Gowen, S.R.; El-Hassan, S.A.; Inam-ul-Haq, M.; Shahina, F.; Pembroke, B. Efficacy of neem (Azadirachta indica) formulations on biology of root-knot nematodes (Meloidogyne javanica) on tomato. Crop Prot. 2008, 27, 36–43. [Google Scholar] [CrossRef]
- Qamar, F.; Begum, S.; Raza, S.M.; Wahab, A.; Siddiqui, B.S. Nematicidal natural products from the aerial parts of Lantana camara Linn. Nat. Prod. Res. 2005, 19, 609–613. [Google Scholar] [CrossRef]
- Naz, I.; Khan, M.R. Nematicidal activity of nonacosane-10-ol and 23a-homostigmast-5-en-3β-ol isolated from the roots of Fumaria parviflora (Fumariaceae). J. Agric. Food Chem. 2013, 61, 5689–5695. [Google Scholar] [CrossRef]
- Adaka, P.; Singh, A.; Dhiman, P.; Chandrika, K.P.; Walia, S.; Sirohi, A.; Parmar, B.S. Hydrogel based formulations of Tagetes patula root extract and MgSO4 to control Meloidogyne incognita in cucumber. Allelopathy J. 2017, 40, 173–186. [Google Scholar] [CrossRef]
- Das, S.; Wadud, A.; Khokon, M.A.R. Evaluation of the effect of different concentrations of organic amendments and botanical extracts on the mortality and hatching of Meloidogyne javanica. Saudi J. Biol. Sci. 2021, 28, 3759–3767. [Google Scholar] [CrossRef]
- Arshad, U.; Butt, H.; Ali, M.A.; Jabran, M.; Zahid, M.S.; Sarfraz, S. Exploring the nematicidal activity of plant extracts for management of Meloidogyne incognita in local cultivars of eggplant (Solanum melongena L.) in Pakistan. Arch. Phytopathol. Plant Prot. 2021, 54, 2333–2344. [Google Scholar] [CrossRef]
- Khairy, D.; Refaei, A.; Mostafa, F. Management of Meloidogyne incognita infecting eggplant using Moringa extracts, vermicompost, and two commercial bio-products. Egypt. J. Agronematology 2021, 20, 1–16. [Google Scholar] [CrossRef]
- Seo, D.J.; Kim, K.Y.; Park, R.D.; Kim, D.H.; Han, Y.S.; Kim, T.H.; Jung, W.J. Nematicidal activity of 3,4-dihydroxy benzoic acid purified from Terminalia nigrovenulosa bark against Meloidogyne incognita. Microb. Pathog. 2013, 59, 52–59. [Google Scholar]
- Bakr, R.A. Nematicidal activity of Jimson weed (Datura spp.) for management of plant-parasitic nematodes with emphasis on root-knot nematode: A review. Pak. J. Phytopathol. 2021, 33, 183–204. [Google Scholar] [CrossRef]
- Maleita, C.; Esteves, I.; Braga, M.E.; Figueiredo, J.; Gaspar, M.C.; Abrantes, I.; de Sousa, H.C. Juglone and 1,4-Naphthoquinone promising nematicides for sustainable control of the root-knot nematode Meloidogyne luci. Front. Plant Sci. 2022, 13, 867803. [Google Scholar] [CrossRef] [PubMed]
- Maleita, C.; Esteves, I.; Chim, R.; Fonseca, L.; Braga, M.E.; Abrantes, I.; de Sousa, H.C. Naphthoquinones from walnut husk residues show strong nematicidal activities against the root-knot nematode Meloidogyne hispanica. ACS Sustain. Chem. Engineering 2017, 5, 3390–3398. [Google Scholar] [CrossRef]
- Jang, J.; Le Dang, Q.; Choi, G.J.; Park, H.W.; Kim, J.C. Control of root-knot nematodes using Waltheria indica producing 4-quinolone alkaloids. Pest Manag. Sci. 2019, 75, 2264–2270. [Google Scholar] [PubMed]
- D’Addabbo, T.; Tava, A.; Argentieri, M.P.; Biazzi, E.; Candido, V.; Avato, P. Nematicidal Potential of Sulla (Hedysarum coronarium, L.) against the root-knot nematode Meloidogyne incognita. Plants 2022, 11, 2550. [Google Scholar] [CrossRef]
- Khairan, K.; Yusra, N.; Eriana, C.N.; Bahi, M.; Syaukani, S.; Sriwati, R.; Jacob, C. Termiticidal and Nematicidal activities of five extracts from Garlic (Allium sativum). In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2021; Volume 1882, No.1; p. 012121. [Google Scholar]
- Jacob, C. A scent of therapy: Pharmacological implications of natural products containing redox-active sulphur atoms. Nat. Prod. Rep. 2006, 23, 851–863. [Google Scholar] [CrossRef]
- Ajith, M.; Pankaj; Shakil, N.A.; Kaushik, P.; Rana, V.S. Chemical composition and nematicidal activity of essential oils and their major compounds against Meloidogyne graminicola (rice root-knot nematode). J. Essent. Oil Res. 2020, 32, 526–535. [Google Scholar] [CrossRef]
- Ntalli, N.; Caboni, P. A review of isothiocyanates biofumigation activity on plant-parasitic nematodes. Phytochem. Rev. 2017, 16, 827–834. [Google Scholar] [CrossRef]
- Hajji-Hedfi, L.; Larayedh, A.; Hammas, N.C.; Regaieg, H.; Horrigue-Raouani, N. Biological activities and chemical composition of Pistacia lentiscus in controlling Fusarium wilt and root-knot nematode disease complex on tomato. Eur. J. Plant Pathol. 2019, 155, 281–291. [Google Scholar] [CrossRef]

| Species Name | Concentration Used | Reduction in Diseases/Result | Crop | Nematode Managed | References |
|---|---|---|---|---|---|
| Pseudomonas jessenii Verhille, et al. 1999, and Pseudomonas synxantha (Ehrenberg 1840) Holland 1920 | 25%, 50%, 75%, 100% | All concentrations greater than 75% resulted in 100% mortality of J2. | Tomato (Solanum lycopersicum L.) | Meloidogyne incognita | [40] |
| Bacillus isolates (BC 27, BC 29, and BC 31) | 108 spores mL−1 | BC 27 and BC 29 caused 100% mortality after 24 h. BC 31 was less effective compared to BC 27 and BC 29, as it caused only 84% mortality after 24 h. | Soybean (Glycine max (L.) Merr.) | Meloidogyne javanica | [41] |
| Pasteuria penetrans (ex Thorne 1940) Sayre and Starr 1986 | 50% spore suspension | Number of J2/100 cm3 was reduced to 9.2 in soil compared to 16.6 in control. | Babchi (Psoralea corylifolia L.) | Meloidogyne incognita | [42] |
| Pseudomonas fluorescens (pf1) (Flugge 1886) Migula, 1895 | 107–109 CFU/mL | 69.8% reduction of Meloidogyne incognita | Cowpea (Vigna unguiculata (L.) Walp.) | Meloidogyne incognita | [43] |
| Bacillus subtilis (bs2) (Ehrenberg 1835) Cohn 1872 | 107–109 CFU/mL | 82% reduction of total nematode population | Cowpea | Meloidogyne incognita | [43] |
| Bacillus pumilis (bp2) Meyer and Gottheli 1901 | 107–109 CFU/mL | 81.8% reduction of nematode population | Cowpea | Meloidogyne incognita | [43] |
| Bacillus thuringiensis Berliner 1915 | 108 CFU/mL/2 | 80.5% reduction of root-knot nematode | Tomato | Meloidogyne incognita | [44] |
| Bacillus altitudinis (AMCC1040) Shivaji et al. 2006 | 108 CFU/mL | Numbers of J2s in roots and soil were reduced by 93.68% and 84.48%, respectively. | Ginger (Zingiber officinale Rosc.) | Meloidogyne incognita | [45] |
| Pseudomonas protegens Ramette et al. 2011 | 1 × 109 | Mortality rate of 87.76% was observed in J2 24 h after treatment (in-vitro), and Gall index was reduced to 30.67% compared to 49.33% in control, and biocontrol efficacy of 37.84 was observed | Tomato | Meloidogyne incognita | [35] |
| Serratia plymuthica (Lehmann and Neumann 1896) Breed et al. 1948 | 1 × 109 | Mortality rate of 92.67% was observed in J2 24 h after treatment (in-vitro), and Gall index lowered to 38.67% compared to 49.33% in control, and biocontrol efficacy of 21.62% was observed | Tomato | M. incognita | [35] |
| Species Name | Concentration Used | Reduction in Diseases/Result | Crop | Nematode Managed | References |
|---|---|---|---|---|---|
| Purpureocillium lilacinus Luangsa-ard, Houbraken, van Doom Hong Borman, Hywel-Jones, and Samson, 2011 | Spore suspension, 10 × 105 concentration | 85% reduction of egg masses of Meloidogyne spp. | Tomato | Meloidogyne spp. | [59] |
| Purpureocillium lilacinus | 1 × 109 cfu/mL | 76.24% reduction of root-knot diseases. | Tomato | Meloidogyne incognita | [60] |
| Aspergillus terreus Thom 1918, Acremonium strictum W. Gams 1971 | 2% (w/w) spore load, 2.3 × 106 to 2.3 × 108 | 76% and 73% reduction in eggs/egg mass and the number of hatching egg/egg mass by A. Terrus, and 71% and 68% by A. Strictum, respectively. | Tomato | Meloidogyne incognita | [61] |
| Acremonium implicatum (J.C. Gilman and E.V. Abbott) W. Gams, 1975 | Spore suspension, 1 × 106 CFU/mL | Reduction in galls with 40.6 galls/treated plant as compared with 121.6 on control plant. | Tomato | Meloidogyne incognita | [62] |
| Acremonium implicatum | 1 × 106 CFU/mL conidial suspension | Reduction of 60% root galls | Tomato | Meloidogyne incognita | [63] |
| Acremonium strictum, Aspergillus niger van Tieghem 1867, Purpureocillium lilacinus and Trichoderma harzianum Rifai 1969 | Talc-based formulation with spore load 2 × 108 CFU | Combined effect of Trichoderma harzianum and Acremonium strictum resulted in greater reduction of disease and high yield. | Tomato | Meloidogyne incognita | [64] |
| Arthrobotrys dactyloides Drechsler 1937 | 2 g (10,000 spore concentration) + 1 g yeast + 3 g vermiculite + 1 mL molasses | Reduction of 94.1% of root galls per plant | Snap bean (Phaseolus vulgaris L.) | Meloidogyne incognita | [65] |
| Arthrobotrys oligospora Fresen 1850 | Spore suspension with 105 conidia/mL | Reduced number of galls, females and nematodes and enhanced plant growth. | Tomato | Meloidogyne incognita | [66] |
| Arthrobotrys oligospora | Fungal suspension at 10, 30 and 50 mL/plant (1 × 104 spore/mL) | 50 mL/plant resulted in reduction of females, eggs/egg-mass and no. of J2 in soil. | Tomato | Meloidogyne incognita | [67] |
| Arthrobotrys oligospora | 106 spores/mL | Application of fungus with salicylic acid reduced root galls and nematode population and increased plant growth. | Tomato | Meloidogyne javanica | [68] |
| Aspergillus awamori Nakaz | 108 CFU/mL | Resulted in 44.9% reduction of nematode infection. | Tomato | M. incognita | [69] |
| Aspergillus japonicus ZW1 Saito 1906 | 20% fermentation broth | Resulted in 51.8 and 47.3% reduction of eggs and galls, respectively. | Tomato | M. incognita | [70] |
| Aspergillus welwitschiae AW2017 (Bres.) Henn. | 2 × 108 conidia/mL (5× AW2017) | Reduction by 40.5% and 24.5% of root galls and juveniles, respectively. | Rice (Oryza sativa L.) | M. graminicola | [71] |
| Fusarium and Trichoderma isolates | 5 × 106 conidial suspension per pot | 29–42% of root galling was reduced by application of conidia of rhizosphere Fusarium isolates and 38% reduction of root galls by treatment with Trichoderma. | Rice | M. graminicola | [72] |
| Chaetomium globosum Kunze 1817 | 30 mg ChA/kg soil (Chaetoglobosin A-ChA) | Resulted in reduction of 63% of eggs per plant | Cucumber (Cucumis sativus L.) | M. incognita | [73] |
| Chaetomium globosum YSC5 | 200 μg/Ml of chaetoglobosin B and chaetoglobosin A | 59.0–61.5% reduction in number of galls and 71.1–72.4% reduction in number of egg masses | Tomato | M. javanica | [74] |
| Dactylaria brochopaga Drechsler 1937 & Verticilium chlamydosporium Goddard 1913 | 2 g (Dactylaria + Verticilium chlamydosporium) + 3 g (vermiculite) + 1 mL (molasses) + 1 g yeast | 93.1% reduction of root galls per plant | Eggplant (Solanum melongena L.) | M. incognita | [75] |
| Dactylaria brochopaga | 2 g (fungus) + 1 g (yeast) + 1 mL (molasses) + 3 gm (vermiculite) | Resulted in 94.1% mean reduction in the number of root galls | Cucumber | M. incognita | [76] |
| Pleurotus ostreatus (Jacq.) P. Kumm. 1871 | 5, 10, 15 g fresh mashed mushroom | 15 g mushroom residue resulted in an 86.4% reduction of nematode reproduction and gall reduction by 92.4%. | cowpea | M. incognita | [77] |
| Gliocladium spp. | 104 mL−1, 105 mL−1, 10−6 mL−1 conidia suspension | 106 mL−1 conidia suspension significantly decreased intensity of damage by 33%. | Tomato | Meloidogyne spp. | [78] |
| Lecanicillium muscarium R. Zare and W. Gams 2001 | 103, 104, 105 and 106 conidia levels with different inoculum densities of M. incognita (500, 1000, 1500, 2000) | Higher density 1 × 106 decreased nematode population, and plant growth parameters improved with increasing fungus inoculum. | Tomato | M. incognita | [79] |
| Purpureocillium lilacinus | P. lilacinus WP 1.15% (1 × 108 CFU/g), P. lilacinus liquid 1.50% (1 × 109 CFU/mL) and P. lilacinus AS 1.0% (2 × 106 CFU/g) | P. lilacinus liquid 1.50% resulted in 48.72% reduction in average root gall index; average number of egg masses per root system and average soil nematode population reduced by 60.15% and 61.10%, respectively. | Capsicum (Capsicum annuum L.) | M. incognita | [80] |
| Beauveria bassiana (Bals. Criv.) Vuill. 1912 | 1×, 5×, 10×, 20×, 50× dilution of culture filtrate | Resulted in 98.61% and 76.39% rates of inhibition of nematodes at 1× and 5× solutions | Tomato | M. hapla | [81] |
| Arthrobotrys dactyloides Drechsler 1937 | 4 × 106 CFU/kg of soil | Resulted in reduction of 37.9–81.8% of juveniles and 44.5–51.3% of egg masses | Tomato | M. incognita | [82] |
| Plant Species | Family | Plant Parts Used | Active Compounds | Nematodes Targeted | References |
|---|---|---|---|---|---|
| Azadirachta indica | Meliaceae | Leaf, Seed, Fruit, Root, Bark | Azadirachtin | Meloidogyne incognita, M. javanica | [135,136] |
| Lantana camara | Verbenaceae | Aerial part | Lantanilic acid, camaric acid and oleanolic acid | M. incognita | [137] |
| Fumaria parviflora | Papaveraceae | Root | Nonacosane-10-ol and 23a-homostigmast-5-en-3β-ol | M. incognita | [138] |
| Tageteserecta L. | Asteraceae | Leaves | Alpha-terthienyl | M. incognita, M. javanica | [139,140,141] |
| Moringa oleifera | Moringaceae | Leaves | Flavonoids, glycosides, saponin | M. incognita | [142] |
| Terminalia nigrovenulosa | Combretaceae | Bark | 3,4-dihydroxybenzoic acid (3,4-DHBA) | M. incognita | [143] |
| Datura spp. | Solanaceae | Leaves, inflorescence, roots | Atropine, scopolamine, hyoscyamine | Meloidogyne spp. | [144] |
| Juglans regia L. | Juglandaceae | Leaves, husk | Beta 1, 4 naphthoquinones | M. hispanica, M. luci | [145,146] |
| Waltheria indica L. | Malvaceae | Roots | 5-methoxywaltherione A, waltherione A and waltherione C | M. incognita, M. hapla, M. arenaria | [147] |
| Hedysarum coronarium L. | Fabaceae | Leaves, flower | Saponins, flavonoids and tannins | M. incognita | [148] |
| Allium sativum L. | Alliaceae | Bulb, leaves | Organosulfur compound, Allicin | Meloidogyne spp. | [149,150] |
| Cymbopogon martini (Roxb.) Watsand C. flexuosus (Nees ex Steud) W. Watson | Poaceae | Leaves | Eugenol and citral | M. incognita | [151] |
| Brassica spp. | Brassicaceae | Shoot, roots, seed | Isothiocynates | Meloidogyne spp. | [152] |
| Pistacia lentiscus L. | Anacardiaceae | Leaves | Quercetin, quinic and gallic acid | M. javanica | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, A.A.; Shakeel, A.; Waqar, S.; Handoo, Z.A.; Khan, A.A. Microbes vs. Nematodes: Insights into Biocontrol through Antagonistic Organisms to Control Root-Knot Nematodes. Plants 2023, 12, 451. https://doi.org/10.3390/plants12030451
Bhat AA, Shakeel A, Waqar S, Handoo ZA, Khan AA. Microbes vs. Nematodes: Insights into Biocontrol through Antagonistic Organisms to Control Root-Knot Nematodes. Plants. 2023; 12(3):451. https://doi.org/10.3390/plants12030451
Chicago/Turabian StyleBhat, Adil Ameen, Adnan Shakeel, Sonia Waqar, Zafar Ahmad Handoo, and Abrar Ahmed Khan. 2023. "Microbes vs. Nematodes: Insights into Biocontrol through Antagonistic Organisms to Control Root-Knot Nematodes" Plants 12, no. 3: 451. https://doi.org/10.3390/plants12030451
APA StyleBhat, A. A., Shakeel, A., Waqar, S., Handoo, Z. A., & Khan, A. A. (2023). Microbes vs. Nematodes: Insights into Biocontrol through Antagonistic Organisms to Control Root-Knot Nematodes. Plants, 12(3), 451. https://doi.org/10.3390/plants12030451







