Abstract
CLE peptides are well-known hormonal regulators of plant development, but their role in somatic embryogenesis remains undetermined. CLE genes are often regulated by WOX transcription factors and, in their turn, regulate the expression level of WOX genes. In this study, we used in vitro cultivation, as well as qPCR and transcriptomic analysis, to find CLE peptides which could regulate the MtWOX9-1 gene, stimulating somatic embryogenesis in Medicago truncatula. Three CLE peptides were found which could probably be such regulators, but none of them was found to influence MtWOX9-1 expression in the embryogenic calli. Nevertheless, overexpression of one of CLE genes under study, MtCLE16, decreased somatic embryogenesis intensity. Additionally, overexpression of MtCLE08 was found to suppress expression of MtWOX13a, a supposed antagonist of somatic embryo development. Our findings can be helpful for the search for new regeneration regulators which could be used for plant transformation.
1. Introduction
CLAVATA3/EMBRYO SURROUNDING REGION-RELATED (CLE) peptides are small peptide hormones regulating many aspects of plant development. The genes from the CLE family encode pre-propeptides, which, after proteolytic processing and post-translational modifications, turn into functional peptide hormones: CLE peptides. CLE peptides are 12–14 amino acids long and, as with other hormones, are used for intercellular communication [1]. Reception of CLE-peptides is carried out through leucine-rich repeat-receptor-like kinases from the CLAVATA1 (CLV1) and BARELY ANY MERISTEM (BAM) groups, but further regulatory pathways, by which CLE peptides may influence gene transcription, are unclear [2].
CLE peptides represent one of the most diverse groups of plant hormones. In Medicago truncatula, for example, 52 CLE genes were found [3]. Functions of CLE peptides are related to variable developmental processes, including meristem maintenance, embryogenesis, leaf development, nodulation, etc. [4]. The most well-known CLE peptide, CLAVATA3 (CLV3), restricts the proliferation of stem cells in the shoot apical meristem (SAM). The CLV3 gene is expressed in the central zone of SAM, maintaining SAM size together with the WUSCHEL (WUS) transcription factor from the WUSCHEL-related homeobox (WOX) family. WUS is stably expressed in the organizing center. The protein it encodes migrates to the upper layers of the SAM and inhibits the differentiation of its cells, due to the activation and repression of different targets related to auxin and cytokinin signaling [5,6]. WUS also directly stimulates CLV3 expression in the central zone of SAM, binding to the sequences located in a 3′-regulatory region of the CLV3 gene [7]. CLV3, in its turn, represses WUS expression through LRR-kinases and similar proteins, including CLAVATA1 (CLV1) and RECEPTOR-LIKE PROTEIN KINASE 2 (RPK2). This repression makes it possible for SAM cells to begin differentiation [8,9].
Apart from the WUS-CLV3 regulatory loop, there are several other examples of WOX and CLE genes interacting. In the root apical meristem (RAM), WOX5 transcription factor inhibits the differentiation of columella stem cells. The CLE40 peptide acts antagonistically to WOX5, repressing the expression of quiescent center markers in the distal domain of RAM and activating WOX5 expression in the proximal domain [10]. TDIF (TRACHEARY ELEMENT DIFFERENTIATION INHIBITORY FACTOR) CLE peptide, encoded by CLE41 and CLE44 genes, stimulates the expression of WOX4 and WOX14 genes in the lateral meristem, which is important for cell proliferation in this tissue [11].
Although there are a lot of studies elucidating the functions of CLE peptides in different plant organs, the role of these hormones in embryogenesis is poorly understood. Several CLE genes were shown to function in the embryo, including CLV3 [12], CLE25 [13] and others, but the only known CLE peptide with specific functions during embryogenesis is CLE8. The CLE8 gene is expressed in the embryo and in the endosperm. In plants with a loss of CLE8 function, the endosperm is underdeveloped, and the embryo and suspensor develop abnormally. CLE8 is required for the activation of the WOX8 gene in the suspensor and endosperm; overexpression of CLE8 leads to an increase in seed size, but loss of WOX8 function neutralizes this effect, suggesting that these two genes act together during embryogenesis regulation [14].
Many embryogenesis regulators also function during the similar process of somatic embryogenesis. Somatic embryogenesis (SE) is a process during which plant somatic cells develop into embryos which eventually can form whole plants. Usually, SE is observed in vitro, although for some plants (for example, some Kalanchoe species) it is also characteristic in vivo. It has been established that in vitro SE can be induced mostly by phytohormones and stress conditions, although many varied specific factors may exert an impact on the intensity and rate of this process. Typical protocols for SE induction include the cultivation of explants on a medium, containing auxins and cytokinins. As a way of plant regeneration, SE is widely used for transformation and propagation of plants, therefore, the search for regulators of this process is an important task for biotechnology.
Recently, a WOX transcription factor MtWOX9-1 was shown to stimulate SE in M. truncatula [15]. In this study, we found 3 CLE peptides possibly related with MtWOX9-1 and investigated their functions.
2. Results
2.1. Search for CLE Peptides Related with MtWOX9-1
MtWOX9-1 was found to be expressed during SE [16] and to stimulate this process [15]. Recently, transcriptomic analysis of calli with MtWOX9-1 overexpression was performed [17]. We analyzed these data and found that MtCLE18 gene expression level increases substantially in the calli with MtWOX9-1 overexpression in comparison with the wildtype calli (Figure 1A, Table S1).
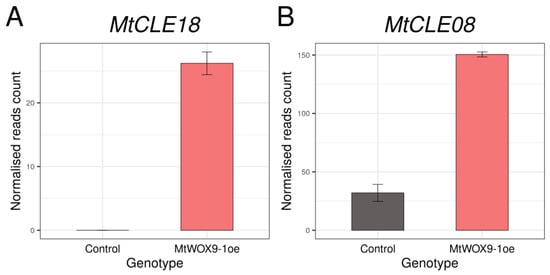
Figure 1.
Expression levels of MtCLE18 (A) and MtCLE08 (B) in control (R108) calli and calli with MtWOX9-1 overexpression according to the transcriptomic analysis [17]. Error bars represent the standard error for two biological replicates.
It was found by Fiume and Fletcher [14] that, during zygotic embryogenesis in A. thaliana, the CLE8 peptide stimulates expression of the WOX8 gene. Interestingly, the MtWOX9-1 gene is closely related with WOX8 and WOX9 genes of A. thaliana (Figure 2A), whereas MtCLE18 has a CLE domain identical to the CLE domain of the CLE8 peptide (Figure 2B). Therefore, we supposed that MtCLE18, whose expression level is affected by MtWOX9-1, may also have influence on MtWOX9-1 expression, and, thereby, on SE intensity. We also found that, among MtCLE peptides, two other CLE exist—MtCLE08 and MtCLE16—with CLE domains similar to MtCLE18: their CLE domains differ from MtCLE18′s in one amino acid only (Figure 2B). Moreover, MtWOX9-1 overexpression also increases the expression level of MtCLE08 according to the transcriptomic analysis (Figure 1B, Table S1). Thus, in this study, we tried to check the expression patterns of MtCLE08, 16, and 18, as well as to evaluate the influence of their overexpression on SE capacity and MtWOX9-1 expression.
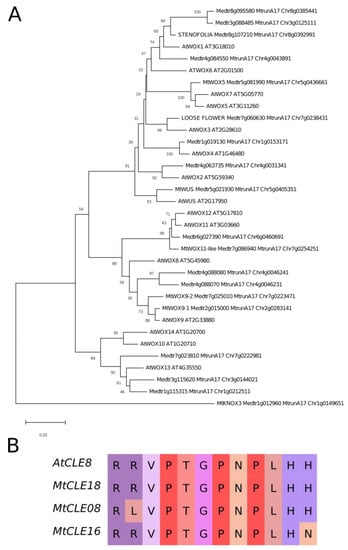
Figure 2.
(A) Phylogenetic tree of A. thaliana and M. truncatula WOX proteins, based on homeodomain sequences. The tree was inferred using the Neighbor-Joining method [18]. The percentages of replicate trees in which the associated proteins are clustered together in the bootstrap test (500 replicates) are shown next to the branches [19]. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. Evolutionary analyses were conducted in MEGA X [20]; (B) Alignment of CLE domains of A. thaliana CLE8 peptide and M. truncatula MtCLE18, 08 and 16 peptides. Different colors represent different amino acids.
2.2. MtCLE08, 16 and 18 Expression Patterns in Different Organs
To investigate the possible functions of MtCLE08, 16, and 18, we evaluated their expression patterns, as well as the expression pattern of MtWOX9-1 gene, in different organs using the Medicago truncatula Gene Expression Atlas [21] (Figure 3). According to this database, MtWOX9-1 is mostly expressed in generative organs, seeds, pods and flowers, which is consistent with its high expression level in ovules as evaluated with qPCR [16]. Among the MtCLE genes investigated, only MtCLE16 has a high expression level in seeds, whereas MtCLE18 is active in the stem, roots and vegetative buds, and MtCLE08 is expressed in nodules, roots, stem, and petioles, with low expression levels in other organs. Therefore, according to the expression patterns in vivo, MtCLE16 is more likely to be involved in the embryogenesis process than MtCLE08 and 18.
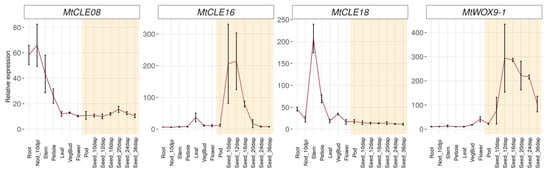
Figure 3.
Expression patterns of MtCLE08, MtCLE16, MtCLE18 and MtWOX9-1 genes in different organs and during different stages of seed development according to the Medicago truncatula Gene Expression Atlas [21]. The error bars represent SD of three biological replicates. Stages of seed development are highlighted in yellow.
2.3. Evaluation of the Expression Dynamics of MtCLE08, 16, and 18 during Somatic Embryogenesis
We also evaluated the expression of the MtCLE genes under study in embryogenic and non-embryogenic calli. For that purpose, we used calli of the embryogenic 2HA M. truncatula line and its predecessor, non-embryogenic A17 line (Figure S1A,B). We measured the expression of the MtCLE genes in explants and calli of both lines, which were cultured as described in Section 4, in the conditions, inducing SE in the 2HA line. As a result, only MtCLE08 and 18 were expressed in these in vitro conditions.
The MtCLE08 gene expression increased during the later stages of cultivation (4–6th week), when somatic embryos developed on the calli of the embryogenic 2HA line. The expression increase was found in both the embryogenic and non-embryogenic lines at these stages, although it occurred earlier and more prominently in the 2HA line (Figure 4A). At the same time, MtCLE18 was expressed predominantly at the early stages of callus development in both lines (Figure 4B). We did not observe the expression of MtCLE16 in calli of the A17 and 2HA lines (data not shown), which is consistent with transcriptome analysis results, showing an almost zero expression level of MtCLE16 in the calli of another embryogenic M. truncatula line, R108 (Table S1).
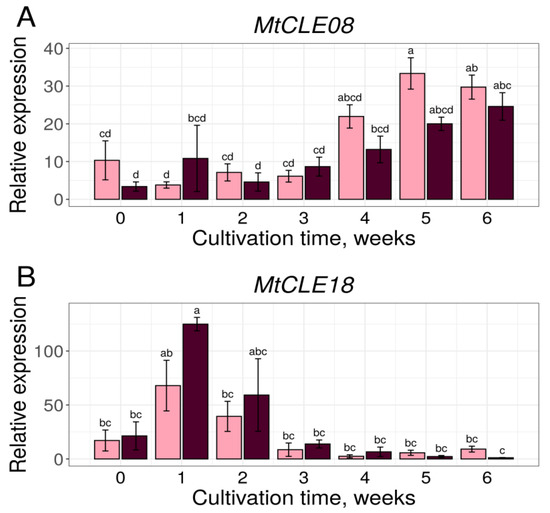
Figure 4.
MtCLE08 (A) and MtCLE18 (B) genes expression dynamics during in vitro cultivation of explants of embryogenic 2HA (pink) and non-embryogenic A17 (dark-red) lines. Error bars represent the standard error. Data are obtained from three biological replicates. To assess the statistical significance of the observed differences, one-way analysis of variance (one-way ANOVA) with Tukey’s post hoc test was used, with confidence level 0.95. Different lowercase letters represent expression levels with statistically significant differences (p-value < 0.05).
2.4. Evaluation of the Influence of MtCLE08, 16, and 18 Overexpression on MtWOX Expression Levels
To check if chosen CLE peptides have any influence on MtWOX9-1 expression, we transformed leaf explants of the M. truncatula R108 line with constructions for MtCLE08, 16, or 18 overexpression, as well as the construction for GUS overexpression, which was used as a control. After 30–50 days of cultivation on the selective medium with hormones (BAP and 2.4-D), transgenic calli developed, and we transferred them onto the hormone-free selective medium. After several days of cultivation on the hormone-free medium, we extracted the material for expression analysis. According to our qPCR analysis, T0 calli had elevated expression levels of corresponding CLE genes, but none of these genes was found to have any influence on the MtWOX9-1 expression level (Figure 5A,B,D,E,G,H). Due to the heterogeneous nature of transgenic T0 calli, individual calli transformed with construction for some CLE gene overexpression may have different levels of expression for this CLE gene. Therefore, we also evaluated the correlation between MtWOX9-1 and the analyzed CLE gene expression in transgenic calli, but no significant correlation was found (Figure 5C,F,I).
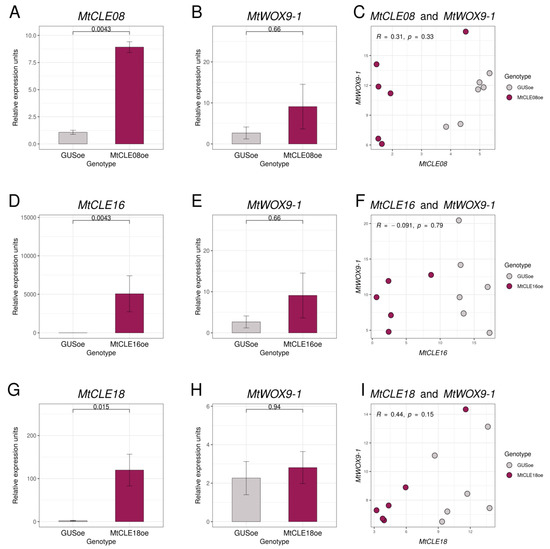
Figure 5.
Expression levels of MtCLE08 (A), MtCLE16 (D), MtCLE18 (G), and MtWOX9-1 (B,E,H) genes in transgenic calli with GUS and MtCLE08 (A,B), MtCLE16 (D,E), or MtCLE18 (F,G) overexpression. Expression levels of the MtCLE08 (C), MtCLE16 (F), or MtCLE18 (I) gene and the MtWOX9-1 gene in individual callus samples. Error bars represent the standard error. Data are obtained from six biological repeats per genotype. To assess the statistical significance of the observed differences between different genotypes of calli, the Wilcoxon signed-rank test was used. To assess the statistical significance of correlation between expression levels of different genes, Spearman’s rank correlation test was used. Material for gene expression analysis in T0 calli was taken at 43rd–56th day after transformation, after 33–50 days of cultivation on the callus induction medium, and 7–14 days of cultivation on the hormone-free medium.
We also suggested that MtCLE08, 16, or 18 could change expression of other WOX genes, on which MtWOX9-1 overexpression has influence. According to the transcriptomic analysis, WOX genes which changed expression in MtWOX9-1-overexpressing calli (p-value < 0.05) included MtWOX2, MtWOX4, MtWOX11-1, and MtWOX13a (Table S1). We also analyzed MtWOX11-2 and MtWOX13b, which had rather high expression levels in both R108 (wildtype) and MtWOX9-1-overexpressing calli (DESeq2 normalized reads mean >50). Using qPCR, we checked the expression levels of these WOX genes in control calli and calli with MtCLE08, 16, or 18 overexpression. We did not find any significant influence in most cases, but MtWOX13a gene expression level was lower in the calli with MtCLE08 overexpression in comparison with control GUS overexpressing calli (p-value = 0.0043, Wilcoxon test) (Figure 6, Figures S2 and S3).
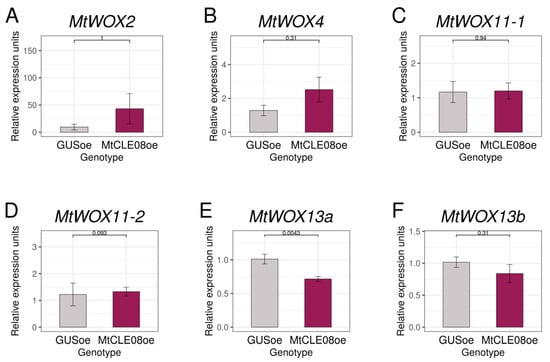
Figure 6.
Expression levels of the MtWOX2 (A), MtWOX4 (B), MtWOX11-1 (C), MtWOX11-2 (D), MtWOX13a (E) and MtWOX13b (F) genes in transgenic calli with GUS and MtCLE08 overexpression. Error bars represent the standard error. Data are obtained from 6 biological repeats per genotype. To assess the statistical significance of the observed differences between different genotypes of calli, the Wilcoxon signed-rank test was used. Material for gene expression analysis in T0 calli was taken at 43rd–56th day after transformation, after 33–50 days of cultivation on the callus induction medium and 7–14 days of cultivation on the hormone-free medium.
Given that in this experiment 18 different expression comparisons were made, as the effect of three CLE genes was evaluated for six WOX genes, the Bonferroni adjusted p-value for the difference between MtWOX13a expression in control and MtCLE08oe calli is 0.0043 × 18 = 0.0774, which exceeds the traditionally accepted threshold level (0.05). To check if the relation between MtCLE08 and MtWOX13a genes exists, we evaluated their expression in different M. truncatula organs using the Medicago truncatula Expression Atlas [21]. Interestingly, expression of these two genes had a significant positive correlation when all organs were analyzed (R = 0.6, p-value = 1.3 × 10−0.6), but also a significant negative correlation in seeds only (R = −0.63, p-value = 0.0057) (Figure 7), suggesting the existence of complex regulatory intercourse between these two genes.
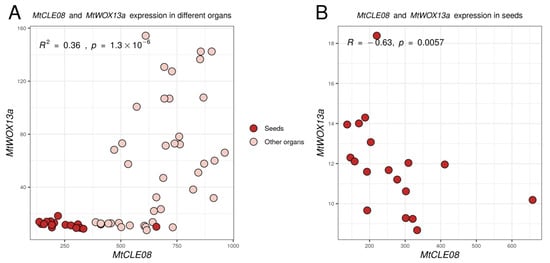
Figure 7.
Expression levels of the MtWOX13a and MtCLE08 genes in different organs and during different stages of seed development according to the Medicago truncatula Gene Expression Atlas [21]. To assess the statistical significance of correlation between expression levels of different genes, Spearman’s rank correlation test was used.
2.5. Evaluation of the Influence of MtCLE08, 16, and 18 Overexpression on Somatic Embryogenesis
Although the overexpression of MtCLE08, 16, or 18 appeared not to have any influence on the MtWOX9-1 expression level, we checked whether overexpression of these genes had any effect on the SE and callus formation capacity of T0 calli (Figure S1C,D). No influence of the overexpression of any gene analyzed on callus weight was found (data not shown). We also did not detect any effect of MtCLE08 or MtCLE18 overexpression on embryogenic calli development, but MtCLE16 overexpression significantly reduced the number of somatic embryos per callus (Figure 8).
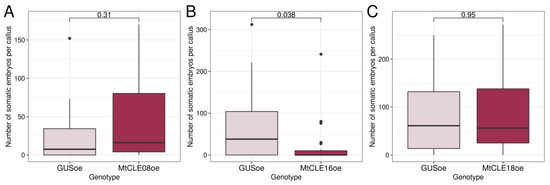
Figure 8.
Number of somatic embryos per callus in transgenic calli with GUS and MtCLE08 (A), MtCLE16 (B), or MtCLE18 (C) overexpression. Data are obtained from 10–24 calli for different samples. For MtCLE16 overexpressing calli, two experiments were performed with similar results. To assess the statistical significance of the observed differences, the Wilcoxon signed-rank test was used. Analysis of M. truncatula SE capacity and weight measurement of T0 calli were performed on the 72nd–86th day after transformation, after 33–50 days of cultivation on the callus induction medium and 30–43 days of cultivation on the hormone-free medium.
3. Discussion
Transcription factors from the WOX family regulate cell proliferation and differentiation, and different WOX genes have been found to stimulate regeneration processes. For example, WUS or its orthologs have been shown to induce regeneration in many plant species and can be applied as morphogenic regulators to speed up the transformation process or to make it possible [22,23]. WOX2 and 8 induce regeneration in tobacco [24], and MtWOX9-1 has been shown to stimulate SE in M. truncatula [15]. In this study, using M. truncatula, we tried to find new participants of SE among the family of CLE peptides, well-known regulators of the expression of the WOX genes. As WOX and CLE genes can be related to each other through feedback regulatory loops, we supposed that CLE genes regulated by MtWOX9-1 may possibly, in their turn, have an influence on MtWOX9-1 expression level. Using transcriptomic data, we found that MtWOX9-1 overexpression led to the increase in expression levels in MtCLE18 and MtCLE08 genes. MtCLE18 and MtCLE08 CLE domains, as well as the CLE domain of another peptide, MtCLE16, are similar to the CLE domain of the A. thaliana CLE8 peptide, for which functions in embryogenesis have been shown, as well as its stimulatory effect on the expression of WOX8, a close homolog of MtWOX9-1 [14]. We supposed that MtCLE08, MtCLE16 and/or MtCLE18 may participate in zygotic or somatic embryogenesis and influence MtWOX9-1 expression. To check our hypotheses, we evaluated the expression levels of chosen CLE peptides in different plant organs, as well as in callus cultures. Interestingly, the MtCLE16 gene, having specific expression increase in developing seeds, did not demonstrate any significant expression during somatic embryogenesis. Localization of its expression in seeds as well as mutant analysis may help to elucidate the functions of this gene. At the same time, MtCLE18 was specifically expressed in the stem as well as at the early stages of callus development in both embryogenic and non-embryogenic lines. Possibly, its functions may be related to the activity of lateral meristems, which play a significant part in callus development [25]. In its turn, MtCLE08 demonstrated a less specific expression pattern, being activated in different plant organs including roots, nodules, and the stem, as well as in the calli of both embryogenic and non-embryogenic lines at different stages. Nevertheless, the increase in its expression level during SE suggests its participation in this process.
We did not find any influence of overexpression of the CLE genes under study on the MtWOX9-1 expression level. This result suggests that the relations between CLE8 and WOX8 homologs are not very conservative. It is also worth mentioning that there are several WOX genes from the WOX8/9 group in M. truncatula, and all of them, including MtWOX9-1, are more closely related to WOX9 than to WOX8 (Figure 2). It would be interesting to check if MtCLE08, 16, or 18 peptides have any influence on the expression of other WOX genes from this group, although these WOX genes are not expressed in wild type embryogenic calli (Table S1).
We also suggest that the effect of MtCLE08 and 18 overexpression could have been missed because their expression level was rather high even in control calli. It is also possible that these peptides can stimulate MtWOX9-1 expression in different conditions. Probably, it is worth investigating whether the induction of MtCLE08 and 18 in the tissues where they are normally not expressed has any effect on MtWOX9-1 expression or other processes.
The effect of MtCLE08 overexpression on MtWOX13a expression level was found. MtWOX13a is a member of an ancient WOX family branch. Its ortholog in A. thaliana is expressed in many tissues and was studied primarily as the regulator of fruit development [26]. Its functions in M. truncatula have yet to be discovered, but the expression level of MtWOX13a is lower in MtWOX9-1-overexpressing highly embryogenic calli than in control calli (Table S1). This may suggest that MtWOX13a acts antagonistically to SE.
We did not find a SE stimulator among MtCLE08, 16 and 18; on the contrary, MtCLE16 overexpression repressed SE. A recent study shows that several CLE peptides (CLE1-7) repress shoot regeneration in A. thaliana through the inhibition of WUS expression [27], but the sequences of their CLE domains differ significantly from the CLE domain of MtCLE16. In another study, MtCLV3 expression was found to be associated with SE in M. truncatula [28], but, so far, no regeneration stimulator has been found among CLE peptides.
The mechanisms by which MtCLE16 overexpression represses embryogenesis are yet to be discovered, but one can suppose that MtCLE16 acts antagonistically to another, as yet unknown, CLE peptide, which stimulates regeneration. If such a peptide was found, it could probably be used as a morphogenic regulator to enhance transformation efficiency. The MtCLE16 CLE domain has an asparagine residue in the 12th position, whereas the MtCLE08 and 18 CLE domains have a histidine residue. The MtCLE16 overexpression effect and the absence of significant effects of MtCLE08 and 18 overexpression allow us to suggest that this amino acid position is important in SE regulation by CLE peptides. It would be interesting to investigate whether overexpression of a mutated MtCLE18 gene encoding peptide with asparagine residue at the 12th position could have repressive effect on SE.
Taken together, our results specify regulatory relationships between WOX and CLE genes in the embryogenic calli and suppose several directions for the search of new morphogenic regulators.
4. Materials and Methods
4.1. Plants and Microorganisms
M. truncatula R108, A17 and 2HA lines were used. Seeds of the R108 line were provided by the Samuel Roberts Institute (OK, USA). Seeds of the 2HA line were provided by Dr. Mireille Chabaud (Laboratory of Plant-Microbe Interactions, Ausville-Tolozan, France). Seeds of the A17 line were provided by colleagues from Wageningen University (Netherlands).
Prior to germination, M. truncatula seeds were submerged in sulfuric acid (95–97%) for 10 min, rinsed 10 times with distilled water, and put on the 1% agar at 4 °C. Plants were grown in in vivo conditions in soil (Terra Vita, Nord Pulp, Russia) mixed with vermiculite (3:1).
Transformation of R108 plants, induction of SE in the 2HA line, and in vitro cultivation of the A17 line were carried out as in [15]. Evaluation of SE capacity was performed in the R108 line T0 calli, which were developed after leaf explant transformation, without obtaining transgenic plants. Embryo number per callus and callus weight were evaluated for 10–24 T0 calli per genotype, depending on the experiment. Analysis of M. truncatula SE capacity and weight measurement for T0 calli were performed on the 72nd–86th day after transformation, after 33–50 days of cultivation on the callus induction medium and 30–43 days of cultivation on the hormone-free medium. Material for gene expression analysis in T0 calli was taken on the 43rd–56th day after transformation, after 33–50 days of cultivation on the callus induction medium and 7–14 days of cultivation on the hormone-free medium.
Escherichia coli DH10B strain was used for cloning. For M. truncatula transformation, Rhizobium radiobacter (Agrobacterium tumefaciens) AGL1 strain was used. Microorganisms cultivation conditions and transformation methods are described in [29].
4.2. Molecular Cloning and qPCR Analysis
To obtain the constructions for MtCLE genes overexpression, the CDS of MtCLE genes under study were cloned in the pDONR207 vector (Invitrogen) and then to the pMDC32 vector [30], using the Gateway method (Invitrogen).
For the expression analysis, RNA was isolated from plant calli using RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) or TRIzol (Thermo Fisher Scientific, MA, USA), according to the manufacturer’s instructions. For DNAse treatment and DNAse removal, RapidOut DNA Removal Kit (Thermo Fisher Scientific) was used. Depending on the experiment, 50–500 ng of RNA was used for cDNA synthesis. For reverse transcription, RevertAid reverse transcriptase, RiboLock RNase Inhibitor (Thermo Fisher Scientific), and oligo-dT18 primer were used according to manufacturer’s instructions. Reverse transcription was performed in 20 μL volume; cDNA samples were diluted 5-fold with water. For each qPCR reaction, we used 1/100th of cDNA, synthesized in each reverse transcription reaction.
The kit with Eva Green (Syntol, Moscow, Russia) was used to perform qPCR, according to the manufacturer’s instructions. qPCR was performed in the CFX96 Real-Time PCR Detection System (Bio-Rad, CA, USA). The CFX-Manager software (Bio-Rad) was used for threshold cycle estimation. Quantitative analysis of differences in gene expression between samples was performed using the delta delta Ct method [31]. For gene expression analysis in T0 calli, 6 biological replicates (6 separate callus samples) per genotype were analyzed with qPCR. For the evaluation of gene expression dynamics in 2HA and A17 calli, 3 biological replicates (3 separate callus samples) were analyzed per each cultivation stage for each line.
For correlation analysis, delta Ct values of two analyzed genes were estimated for each sample, and then Spearman’s rank correlation coefficient was calculated. The MtH3L gene was used as a reference. Primers (Evrogen, Moscow, Russia) used in the study are shown in Table S2.
4.3. Software
For sequence analysis, Ugene [32], SnapGene (from GSL Biotech; available at snapgene.com) and ApE (M. Wayne Davis) were used. For statistical analysis and diagram drawing, Rstudio (RStudio Team, 2020) was used. MEGA X software [20] was used for alignment and phylogeny analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12030435/s1, Table S1: Results of transcriptomic analysis of calli with MtWOX9-1 overexpression for chosen MtCLE and MtWOX genes. WOX genes chosen for analysis are highlighted with red. Table S2: Primers used in the study. Gene IDs in tables correspond to the versions MedtrA17_4.0 [33] and MtrunA17r5.0-ANR [34,35] of M. truncatula genome. Figure S1: (A,B) Representative photos of A17 (A) and 2HA (B) calli at the sixth week of cultivation. Somatic embryos on 2HA calli appear as green-colored structures. (C,D) Representative photos of T0 control calli with GUS overexpression, taken when the weighing and counting of embryos was performed (83th day of cultivation). Figure S2: Expression levels of the MtWOX2 (A), MtWOX4 (B), MtWOX11-1 (C), MtWOX11-2 (D), MtWOX13a (E) and MtWOX13b (F) genes in transgenic calli with GUS and MtCLE16 overexpression. Error bars represent the standard error. Data are obtained from 6 biological repeats per genotype. To assess the statistical significance of the observed differences between different genotypes of calli, the Wilcoxon signed-rank test was used. Figure S3: Expression levels of the MtWOX2 (A), MtWOX4 (B), MtWOX11-1 (C), MtWOX11-2 (D), MtWOX13a (E) and MtWOX13b (F) genes in transgenic calli with GUS and MtCLE18 overexpression. Error bars represent the standard error. Data are obtained from 4–6 biological repeats per genotype. To assess the statistical significance of the observed differences between different genotypes of calli, the Wilcoxon signed-rank test was used.
Author Contributions
Conceptualization, V.E.T. and L.A.L.; methodology, A.A.K.; software, V.E.T.; validation, E.P.E. and V.E.T.; investigation, A.A.K., N.S.Z. and E.P.E.; writing—original draft preparation, V.E.T.; writing—review and editing, A.A.K., N.S.Z., E.P.E., V.E.T. and L.A.L.; visualization, V.E.T.; supervision, L.A.L.; project administration, L.A.L.; funding acquisition, L.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 21-66-00012 (Section 2.1 and Section 2.2), by the Russian Foundation for Basic Research, grant number 20-016-00124 (Section 2.3) and by the Sirius University of Science and Technology project: PBB-RND-2243 (Section 2.4).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data files and gene expression data on the transcriptome analysis of wild type calli and calli with MtWOX9-1 overexpression are available via the Gene Expression Omnibus (GEO) with identifier GSE201314.
Acknowledgments
Authors acknowledge the Research Resource Center for Molecular and Cell Technologies of Saint-Petersburg State University for DNA sequencing.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Fletcher, J.C. Recent Advances in Arabidopsis CLE Peptide Signaling. Trends Plant Sci. 2020, 25, 1005–1016. [Google Scholar] [CrossRef]
- Willoughby, A.C.; Nimchuk, Z.L. WOX Going on: CLE Peptides in Plant Development. Curr. Opin. Plant Biol. 2021, 63, 102056. [Google Scholar] [CrossRef] [PubMed]
- Hastwell, A.H.; de Bang, T.C.; Gresshoff, P.M.; Ferguson, B.J. CLE Peptide-Encoding Gene Families in Medicago Truncatula and Lotus Japonicus, Compared with Those of Soybean, Common Bean and Arabidopsis. Sci. Rep. 2017, 7, 9384. [Google Scholar] [CrossRef]
- Song, X.-F.; Hou, X.-L.; Liu, C.-M. CLE Peptides: Critical Regulators for Stem Cell Maintenance in Plants. Planta 2021, 255, 5. [Google Scholar] [CrossRef]
- Leibfried, A.; To, J.P.C.; Busch, W.; Stehling, S.; Kehle, A.; Demar, M.; Kieber, J.J.; Lohmann, J.U. WUSCHEL Controls Meristem Function by Direct Regulation of Cytokinin-Inducible Response Regulators. Nature 2005, 438, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Miotk, A.; Šutiković, Z.; Ermakova, O.; Wenzl, C.; Medzihradszky, A.; Gaillochet, C.; Forner, J.; Utan, G.; Brackmann, K.; et al. WUSCHEL Acts as an Auxin Response Rheostat to Maintain Apical Stem Cells in Arabidopsis. Nat. Commun. 2019, 10, 5093. [Google Scholar] [CrossRef] [PubMed]
- Perales, M.; Rodriguez, K.; Snipes, S.; Yadav, R.K.; Diaz-Mendoza, M.; Reddy, G.V. Threshold-Dependent Transcriptional Discrimination Underlies Stem Cell Homeostasis. Proc. Natl. Acad. Sci. USA 2016, 113, E6298–E6306. [Google Scholar] [CrossRef]
- Brand, U.; Fletcher, J.C.; Hobe, M.; Meyerowitz, E.M.; Simon, R. Dependence of Stem Cell Fate in Arabidopsis on a Feedback Loop Regulated by CLV3 Activity. Science 2000, 289, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Poliushkevich, L.O.; Gancheva, M.S.; Dodueva, I.E.; Lutova, L.A. Receptors of CLE Peptides in Plants. Russ. J. Plant Physiol. 2020, 67, 1–16. [Google Scholar] [CrossRef]
- Berckmans, B.; Kirschner, G.; Gerlitz, N.; Stadler, R.; Simon, R. CLE40 Signaling Regulates Root Stem Cell Fate. Plant Physiology 2020, 182, 1776–1792. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Kondo, Y.; Fukuda, H. TDIF Peptide Signaling Regulates Vascular Stem Cell Proliferation via the WOX4 Homeobox Gene in Arabidopsis. Plant Cell 2010, 22, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tucker, E.; Hermann, M.; Laux, T. A Molecular Framework for the Embryonic Initiation of Shoot Meristem Stem Cells. Dev. Cell 2017, 40, 264–277.e4. [Google Scholar] [CrossRef]
- Ren, S.-C.; Song, X.-F.; Chen, W.-Q.; Lu, R.; Lucas, W.J.; Liu, C.-M. CLE25 Peptide Regulates Phloem Initiation in Arabidopsis through a CLERK-CLV2 Receptor Complex. J. Integr. Plant Biol. 2019, 61, 1043–1061. [Google Scholar] [CrossRef] [PubMed]
- Fiume, E.; Fletcher, J.C. Regulation of Arabidopsis Embryo and Endosperm Development by the Polypeptide Signaling Molecule CLE8. Plant Cell 2012, 24, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Tvorogova, V.E.; Fedorova, Y.A.; Potsenkovskaya, E.A.; Kudriashov, A.A.; Efremova, E.P.; Kvitkovskaya, V.A.; Wolabu, T.W.; Zhang, F.; Tadege, M.; Lutova, L.A. The WUSCHEL-Related Homeobox Transcription Factor MtWOX9-1 Stimulates Somatic Embryogenesis in Medicago Truncatula. Plant Cell Tissue Organ Cult. 2019, 138, 517–527. [Google Scholar] [CrossRef]
- Tvorogova, V.E.; Lebedeva, M.A.; Lutova, L.A. Expression of WOX and PIN Genes during Somatic and Zygotic Embryogenesis in Medicago Truncatula. Russ. J. Genet. 2015, 51, 1189–1198. [Google Scholar] [CrossRef]
- Tvorogova, V.E.; Krasnoperova, E.Y.; Kudriashov, A.A.; Kuznetsova, K.A.; Potsenkovskaya, E.A.; Fedorova, Y.A.; Lutova, L.A. Transcriptomic Analysis of Medicago Truncatula Calli with MtWOX9-1 Overexpression. Vavilov J. Genet. Breed. 2019, 23, 691–699. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Benedito, V.A.; Torres-Jerez, I.; Murray, J.D.; Andriankaja, A.; Allen, S.; Kakar, K.; Wandrey, M.; Verdier, J.; Zuber, H.; Ott, T.; et al. A Gene Expression Atlas of the Model Legume Medicago Truncatula. Plant J. 2008, 55, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Lowe, K.; La Rota, M.; Hoerster, G.; Hastings, C.; Wang, N.; Chamberlin, M.; Wu, E.; Jones, T.; Gordon-Kamm, W. Rapid Genotype “Independent” Zea mays L. (Maize) Transformation via Direct Somatic Embryogenesis. Vitr. Cell Dev. Biol. Plant 2018, 54, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Kadri, A.; Grenier De March, G.; Guerineau, F.; Cosson, V.; Ratet, P. WUSCHEL Overexpression Promotes Callogenesis and Somatic Embryogenesis in Medicago Truncatula Gaertn. Plants 2021, 10, 715. [Google Scholar] [CrossRef] [PubMed]
- Kyo, M.; Maida, K.; Nishioka, Y.; Matsui, K. Coexpression of WUSCHEL Related Homeobox (WOX) 2 with WOX8 or WOX9 Promotes Regeneration from Leaf Segments and Free Cells in Nicotiana tabacum L. Plant Biotechnol. 2018, 35, 23–30. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Iwase, A.; Rymen, B.; Lambolez, A.; Kojima, M.; Takebayashi, Y.; Heyman, J.; Watanabe, S.; Seo, M.; De Veylder, L.; et al. Wounding Triggers Callus Formation via Dynamic Hormonal and Transcriptional Changes1[OPEN]. Plant Physiol. 2017, 175, 1158–1174. [Google Scholar] [CrossRef]
- Tvorogova, V.E.; Krasnoperova, E.Y.; Potsenkovskaia, E.A.; Kudriashov, A.A.; Dodueva, I.E.; Lutova, L.A. What Does the WOX Say? Review of Regulators, Targets, Partners. Mol. Biol. 2021, 55, 311–337. [Google Scholar] [CrossRef]
- Kang, J.; Wang, X.; Ishida, T.; Grienenberger, E.; Zheng, Q.; Wang, J.; Zhang, Y.; Chen, W.; Chen, M.; Song, X.-F.; et al. A Group of CLE Peptides Regulates de Novo Shoot Regeneration in Arabidopsis Thaliana. New Phytologist 2022, 235, 2300–2312. [Google Scholar] [CrossRef]
- Chen, S.-K.; Kurdyukov, S.; Kereszt, A.; Wang, X.-D.; Gresshoff, P.M.; Rose, R.J. The Association of Homeobox Gene Expression with Stem Cell Formation and Morphogenesis in Cultured Medicago Truncatula. Planta 2009, 230, 827–840. [Google Scholar] [CrossRef]
- Potsenkovskaia, E.; Tvorogova, V.; Yakovleva, D.; Zlydneva, N.; Lutova, L. Novel NF-Y Genes Expressed during Somatic Embryogenesis in Medicago Truncatula. Plant Gene 2022, 31, 100364. [Google Scholar] [CrossRef]
- Curtis, M.D.; Grossniklaus, U. A Gateway Cloning Vector Set for High-Throughput Functional Analysis of Genes in Planta. Plant Physiol. 2003, 133, 462–469. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M. UGENE team Unipro UGENE: A Unified Bioinformatics Toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Pecrix, Y.; Staton, S.E.; Sallet, E.; Lelandais-Brière, C.; Moreau, S.; Carrère, S.; Blein, T.; Jardinaud, M.-F.; Latrasse, D.; Zouine, M.; et al. Whole-Genome Landscape of Medicago Truncatula Symbiotic Genes. Nat. Plants 2018, 4, 1017. [Google Scholar] [CrossRef]
- Young, N.D.; Debellé, F.; Oldroyd, G.E.D.; Geurts, R.; Cannon, S.B.; Udvardi, M.K.; Benedito, V.A.; Mayer, K.F.X.; Gouzy, J.; Schoof, H.; et al. The Medicago Genome Provides Insight into the Evolution of Rhizobial Symbioses. Nature 2011, 480, 520–524. [Google Scholar] [CrossRef]
- Tang, H.; Krishnakumar, V.; Bidwell, S.; Rosen, B.; Chan, A.; Zhou, S.; Gentzbittel, L.; Childs, K.L.; Yandell, M.; Gundlach, H.; et al. An Improved Genome Release (Version Mt4.0) for the Model Legume Medicago Truncatula. BMC Genom. 2014, 15, 312. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).