Antioxidant Response, Phenolic Compounds and Yield of Solanum tuberosum Tubers Inoculated with Arbuscular Mycorrhizal Fungi and Growing under Water Stress
Abstract
:1. Introduction
2. Result
2.1. Spores, Extraradical Hyphae, and AMF Colonization
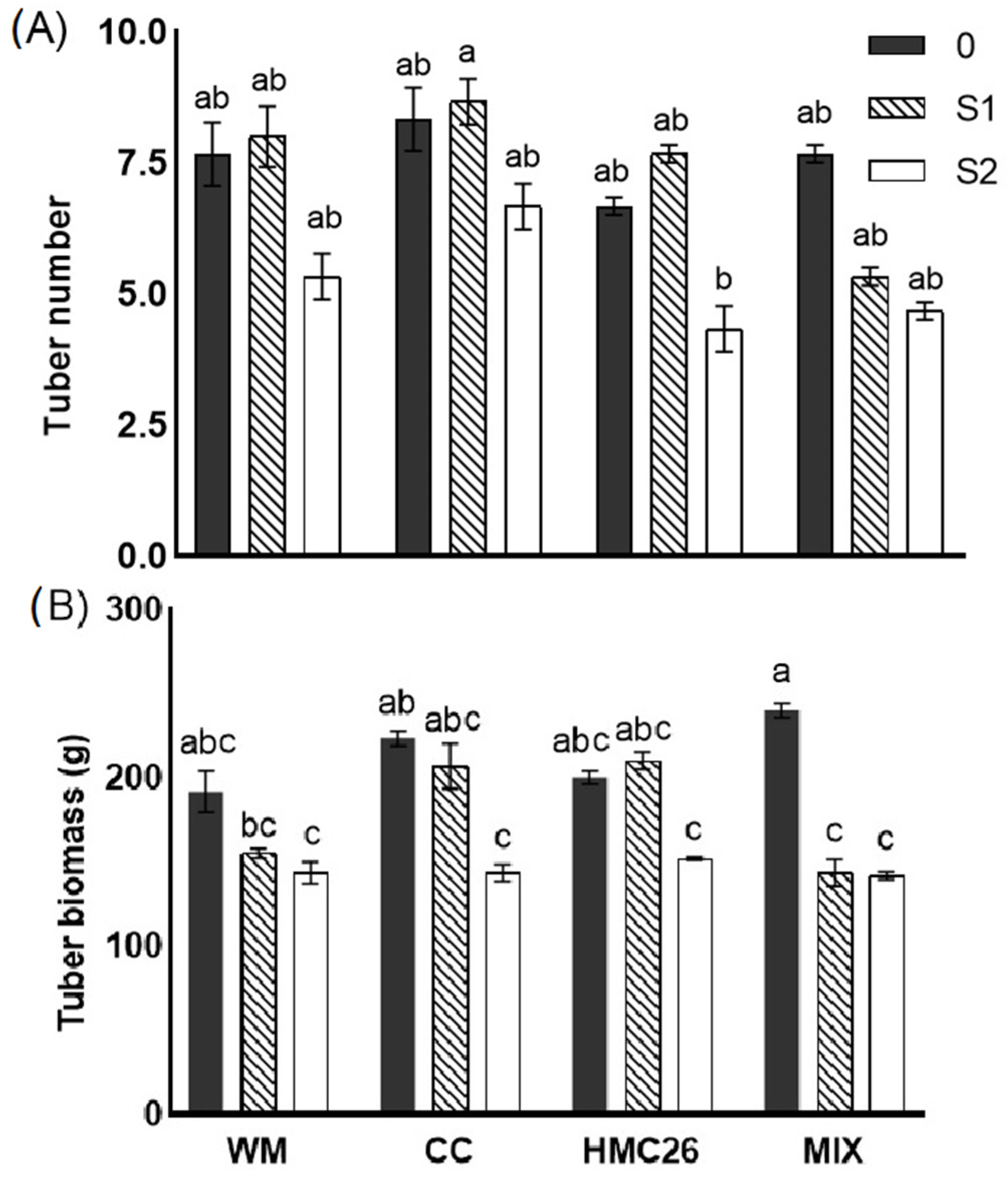
2.2. Biomass Production
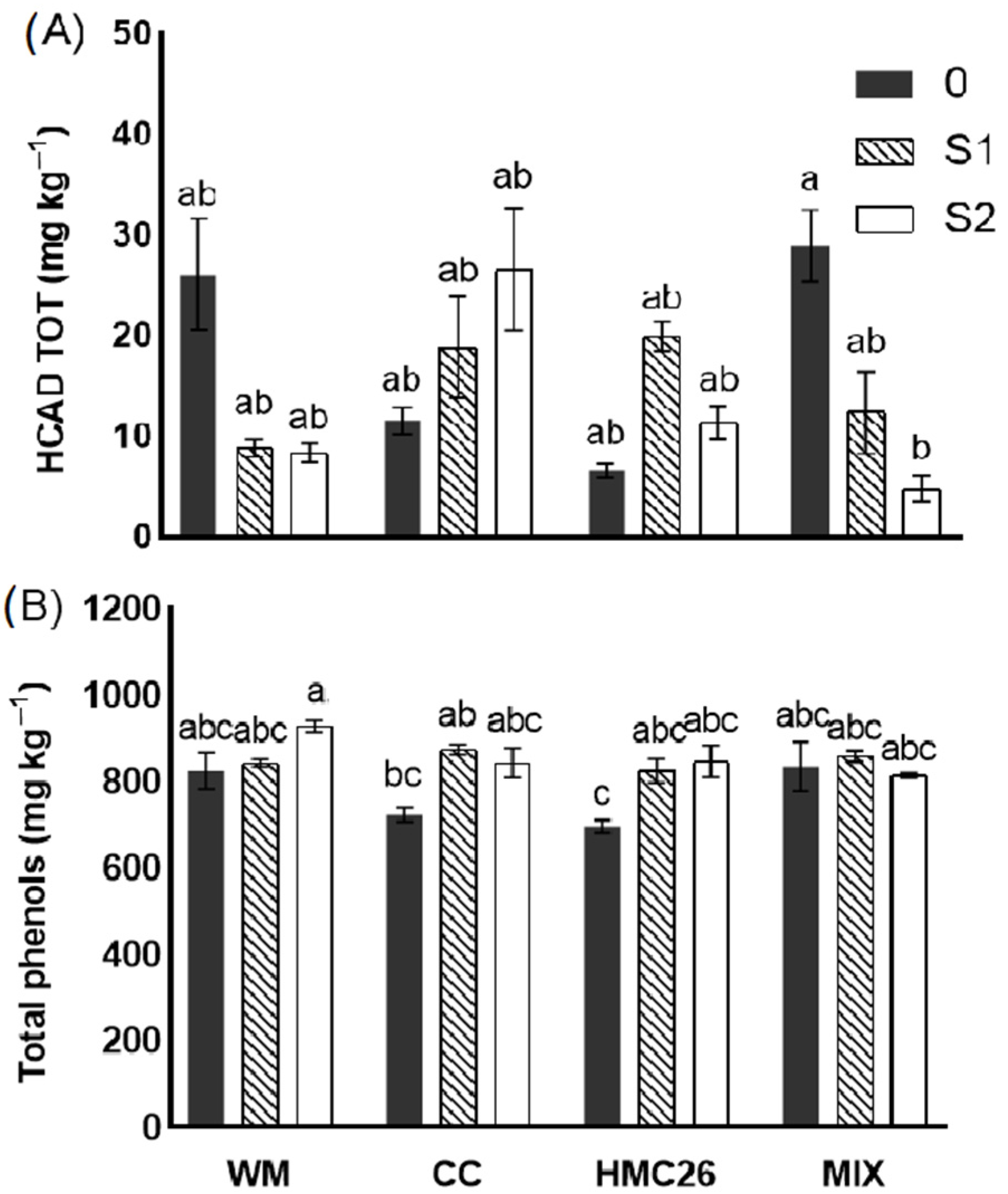
2.3. Identification and Quantification of Phenolic Compounds in Tubers
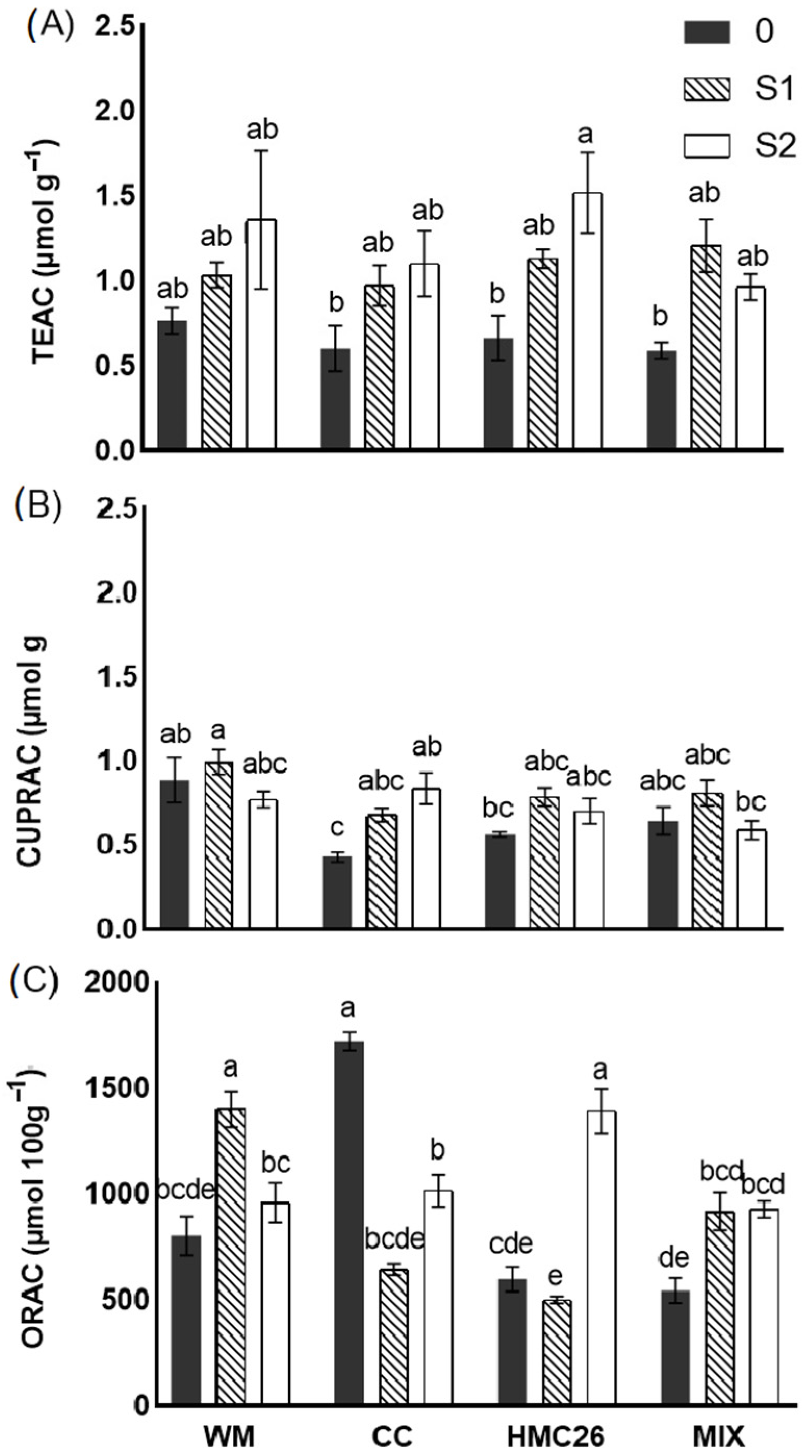
2.4. Antioxidant Activity
2.5. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Biological Material and Experimental Design
4.3. Spores, Extraradical Hyphae, and AMF Colonization
4.4. Extraction of Antioxidant Compounds
4.5. Identification and Quantification of Phenolic Compounds
4.6. Determination of Antioxidant Activity
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. World Food and Agriculture–Statistical Yearbook; FAO: Rome, Italy, 2021. [Google Scholar]
- Bhusal, N.; Lee, M.; Han, A.R.; Han, A.; Kim, H.S. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Gödecke, T.; Stein, A.J.; Qaim, M. The global burden of chronic and hidden hunger: Trends and determinants. Glob. Food Sec. 2018, 17, 21–29. [Google Scholar] [CrossRef]
- Tian, J.; Chen, J.; Ye, X.; Chen, S. Health benefits of the potato affected by domestic cooking: A review. Food Chem. 2016, 202, 165–175. [Google Scholar] [CrossRef]
- Zhou, L.; Mu, T.; Ma, M.; Zhang, R.; Sun, Q.; Xu, Y. Nutritional evaluation of different cultivars of potatoes (Solanum tuberosum L.) from China by grey relational analysis (GRA) and its application in potato steamed bread making. J. Integr. Agric. 2019, 18, 231–245. [Google Scholar] [CrossRef]
- Lal, M.K.; Tiwari, R.K.; Kumar, A.; Dey, A.; Kumar, R.; Kumar, D.; Jaiswal, A.; Changan, S.S.; Raigond, P.; Dutt, S.; et al. Mechanistic concept of physiological, biochemical, and molecular responses of the potato crop to heat and drought stress. Plants 2022, 11, 2857. [Google Scholar] [CrossRef]
- Fleisher, D.H.; Timlin, D.J.; Reddy, V.R. Climate Change and Potato: Responses to Carbon Dioxide, Temperature, and Drought. In Improving Modeling Tools to Assess Climate Change Effects on Crop; John Wiley and Sons: Hoboken, NJ, USA, 2016; pp. 69–90. [Google Scholar] [CrossRef]
- Nasir, M.W.; Toth, Z. Effect of Drought Stress on Potato Production: A Review. Agronomy 2022, 12, 635. [Google Scholar] [CrossRef]
- Alvarez-Morezuelas, A.; Barandalla, L.; Ritter, E.; Lacuesta, M.; Ruiz de Galarreta, J.I. Physiological response and yield components under greenhouse drought stress conditions in potato. J. Plant Physiol. 2022, 278, 153790. [Google Scholar] [CrossRef]
- Chang, D.C.; Jin, Y.I.; Nam, J.H.; Cheon, C.G.; Cho, J.H.; Kim, S.J.; Yu, H.S. Early drought effect on canopy development and tuber growth of potato cultivars with different maturities. Field Crops 2018, 215, 156–162. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Chen, H.Y.H.; Ruan, H. Response of plants to water stress: A meta-analysis. Front. Plant Sci. 2020, 11, 978. [Google Scholar] [CrossRef]
- Attarzadeh, M.; Balouchi, H.; Rajaie, M.; Dehnavi, M.M.; Salehi, A. Improving growth and phenolic compounds of Echinacea purpurea root by integrating biological and chemical resources of phosphorus under water deficit stress. Ind. Crops Prod. 2020, 154, 112763. [Google Scholar] [CrossRef]
- Espadas, J.L.; Castaño, E.; Marina, M.L.; Rodríguez, L.C.; Plaza, M. Phenolic compounds increase their concentration in Carica papaya leaves under drought stress. Acta Physiol. Plant 2019, 41, 180. [Google Scholar] [CrossRef]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic compounds in the potato and its byproducts: An overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef]
- Rosendahl, S. Communities, populations and individuals of arbuscular mycorrhizal fungi. New Phytol. 2008, 178, 253–266. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef]
- Tekaya, M.; Dabbaghi, O.; Guesmi, A.; Attia, F.; Chehab, H.; Khezami, L.; Algathami, F.K.; Hamadi, N.B.; Hammami, M.; Prinsen, E.; et al. Arbuscular mycorrhizas modulate carbohydrate, phenolic compounds and hormonal metabolism to enhance water deficit tolerance of olive trees (Olea europaea). Agric. Water Manag. 2022, 274, 107947. [Google Scholar] [CrossRef]
- Liu, X.Q.; Cheng, S.; Aroca, R.; Zou, Y.N.; Wu, Q.S. Arbuscular mycorrhizal fungi induce flavonoid synthesis for mitigating oxidative damage of trifoliate orange under water stress. Environ. Exp. 2022, 204, 105089. [Google Scholar] [CrossRef]
- Nahuelcura, J.; Ruiz, A.; Gomez, F.; Cornejo, P. The effect of arbuscular mycorrhizal fungi on the phenolic compounds profile, antioxidant activity and grain yields in wheat cultivars growing under hydric stress. J. Sci. Food Agric. 2022, 102, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, S.; Tereucán, G.; Cornejo, P.; Contreras, B.; Ruiz, A. Metabolic and antioxidant effects of inoculation with arbuscular mycorrhizal fungi in crops of flesh-coloured Solanum tuberosum treated with fungicides. J. Sci. Food Agric. 2022, 102, 2270–2280. [Google Scholar] [CrossRef] [PubMed]
- Fritz, V.; Tereucán, G.; Santander, C.; Contreras, B.; Cornejo, P.; Ferreira, P.A.A.; Ruiz, A. Effect of Inoculation with Arbuscular Mycorrhizal Fungi and Fungicide Application on the Secondary Metabolism of Solanum tuberosum Leaves. Plants 2022, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, D.; Yapa, N. Arbuscular mycorrhizal fungi inoculation enhances drought stress tolerance of plants. Groundw. Sustain. Dev. 2018, 7, 490–494. [Google Scholar] [CrossRef]
- Hijri, M. Analysis of a large dataset of mycorrhiza inoculation field trials on potato shows highly significant increases in yield. Mycorrhiza 2016, 26, 209–214. [Google Scholar] [CrossRef]
- Zhang, S.; Lehmann, A.; Zheng, W.; You, Z.; Rillig, M.C. Arbuscular mycorrhizal fungi increase grain yields: A meta-analysis. New Phytol. 2019, 222, 543–555. [Google Scholar] [CrossRef]
- Das, D.; Ullah, H.; Himanshu, S.K.; Tisarum, R.; Cha-um, S.; Datta, A. Arbuscular mycorrhizal fungi inoculation and phosphorus application improve growth, physiological traits, and grain yield of rice under alternate wetting and drying irrigation. J. Plant Physiol. 2022, 278, 153829. [Google Scholar] [CrossRef]
- Bhusal, N.; Han, S.G.; Yoon, T.M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Badr, M.A.; El-Tohamy, W.A.; Salman, S.R.; Gruda, N. Yield and water use relationships of potato under different timing and severity of water stress. Agric. Water Manag. 2022, 271, 107793. [Google Scholar] [CrossRef]
- Ji, X.; Shiran, B.; Wan, J.; Lewis, D.C.; Jenkins, C.L.D.; Condon, A.G.; Richards, R.A.; Dolferus, R. Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant Cell Environ. 2010, 33, 926–942. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Hann, S.; Kupriyanovich, Y.; Li, S. Timing of short period water stress determines potato plant growth, yield and tuber quality. Agric. Water Manag. 2021, 247, 106731. [Google Scholar] [CrossRef]
- Suo, H.; Peng, Z.; Guo, Z.; Wu, C.; Liu, J.; Wang, L.; Xiao, J.; Li, X. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from different potato genotypes: Comparison of free and bound phenolic profiles and antioxidant activity. Food Chem. 2022, 388, 133058. [Google Scholar] [CrossRef]
- Tereucán, G.; Ruiz, A.; Nahuelcura, J.; Oyarzún, P.; Santander, C.; Winterhalter, P.; Ademar Avelar Ferreira, P.; Cornejo, P. Shifts in biochemical and physiological responses by the inoculation of arbuscular mycorrhizal fungi in Triticum aestivum growing under drought conditions. J. Sci. Food Agric. 2022, 102, 1927–1938. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. In Plant Signaling Molecules; Woodhead Publishing: Sawston, UK, 2019; pp. 157–168. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikary, A.; Han, A.R.; Han, A.; Kim, H.S. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total Environ. 2021, 779, 146466. [Google Scholar] [CrossRef]
- Sharma, L.; Dalal, M.; Verma, R.K.; Kumar, S.V.V.; Yadav, S.K.; Pushkar, S.; Kushwaha, S.R.; Bhowmik, A.; Chinnusamy, V. Auxin protects spikelet fertility and grain yield under drought and heat stresses in rice. Environ. Exp. Bot. 2018, 150, 9–24. [Google Scholar] [CrossRef]
- Valiñas, M.A.; Lanteri, M.L.; Have, A.T.; Andreu, A.B. Chlorogenic acid, anthocyanin and flavan-3-ol biosynthesis in flesh and skin of Andean potato tubers (Solanum tuberosum subsp. andigena). Food Chem. 2017, 229, 837–846. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Valdebenito, A.; Nahuelcura, J.; Santander, C.; Cornejo, P.; Contreras, B.; Gómez-Alonso, S.; Ruiz, A. Physiological and Metabolic Effects of the Inoculation of Arbuscular Mycorrhizal Fungi in Solanum tuberosum Crops under Water Stress. Plants 2022, 11, 2539. [Google Scholar] [CrossRef]
- Prior, R.L. Oxygen radical absorbance capacity (ORAC): New horizons in relating dietary antioxidants/bioactives and health benefits. J. Funct. Foods 2015, 18, 797–810. [Google Scholar] [CrossRef]
- André, C.M.; Oufir, M.; Hoffmann, L.; Hausman, J.F.; Rogez, H.; Larondelle, Y.; Evers, D. Influence of environment and genotype on polyphenol compounds and in vitro antioxidant capacity of native Andean potatoes (Solanum tuberosum L.). J. Food Compos. Anal. 2009, 22, 517–524. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought Stress Tolerance in Plants: Interplay of Molecular, Biochemical and Physiological Responses in Important Development Stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Ruiz, A.; Bustamante, L.; Vergara, C.; von Baer, D.; Hermosín-Gutiérrez, I.; Obando, L.; Mardones, C. Hydroxycinnamic acids and flavonols in native edible berries of South Patagonia. Food Chem. 2015, 167, 84–90. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Sieverding, E. Vesicular–Arbuscular Mycorrhiza Management in Tropical Agrosystems; GTZ: Eschborn, Germany, 1991. [Google Scholar]
- Borie, F.; Rubio, R. Total and organic phosphorus in Chilean volcanic soils. Gayana Bot. 2003, 60, 69–73. [Google Scholar] [CrossRef]
- Newman, E.I. A method of estimating the total lenght of root in a sample. J. Appl. Ecol. 1966, 3, 139–145. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Parada, J.; Valenzuela, T.; Gómez, F.; Tereucán, G.; García, S.; Cornejo, P.; Winterhalter, P.; Ruiz, A. Effect of fertilization and arbuscular mycorrhizal fungal inoculation on antioxidant profiles and activities in Fragaria ananassa fruit. J. Sci. Food Agric. 2019, 99, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Chang, T.; Huang, D.; Prior, R.L. Determination of total antioxidant capacity by oxygen radical absorbance capacity (ORAC) using fluorescein as the fluorescence probe. J. AOAC Int. 2013, 96, 1372–1376. [Google Scholar] [CrossRef] [PubMed]


| Source of Variation | HCAD1 | HCAD2 | HCAD TOT | Phenols | TEAC | CUPRAC | ORAC | Tubers | Biomass | Mycelium | Spores | Colonization |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMF | 0.94 ns | 3.49 * | 0.49 ns | 1.27 ns | 1.19 ns | 2.48 ns | 5.39 ** | 3.62 * | 2.23 ns | 1041 *** | 2649 *** | 316 *** |
| Water stress | 1.25 ns | 0.26 ns | 0.70 ns | 3.82 * | 13.95 *** | 2.50 ns | 3.16 ns | 10.31 ** | 25.57 *** | 718 *** | 732 *** | 33.3 *** |
| AMF x Stress | 2.78 * | 2.39 ns | 2.46 ns | 0.93 ns | 0.93 ns | 1.13 ns | 14.2 *** | 0.96 ns | 2.76 * | 256 *** | 990 *** | 33.1 *** |
| Method | Standard | Equation | R2 | DL | QL | LR |
|---|---|---|---|---|---|---|
| HCAD TOT | Chlorogenic acid | y = 73284x + 6553.5 | 1.000 | 0.042 mg L−1 | 0.140 mg L−1 | 0.140–100 mg L−1 |
| FOLIN | Gallic acid | y = 0.0006x + 0.0008 | 0.996 | 3.600 mg L−1 | 12.001 mg L−1 | 12.001–500 mg L−1 |
| TEAC | Trolox | y = 0.4094x + 0.013 | 0.991 | 0.024 mmol L−1 | 0.078 mmol L−1 | 0.045–0.7 mmol L−1 |
| CUPRAC | Trolox | y = 3.2224x + 0.1266 | 0.996 | 0.008 mmol L−1 | 0.027 mmol L−1 | 0.027–0.6 mmol L−1 |
| DPPH | Trolox | y = 0.7608x + 0.0272 | 0.990 | 0.036 mmol L−1 | 0.119 mmol L−1 | 0.119–0.7 mmol L−1 |
| ORAC | Trolox | y = 0.38x + 6.7092 | 0.995 | 0.870 umol L−1 | 2.899 umol L−1 | 2.899–80 umol L−1 |
| Treatment | 5-Caffeoylquinic Acid (mg kg−1) | Caffeoylquinic Acid Isomer (mg kg−1) |
|---|---|---|
| WM 0 | 21.06 ± 15.94 ab | 5.00 ± 3.49 ab |
| CC 0 | 10.20 ± 5.32 abc | 1.36 ± 0.66 b |
| HMC26 0 | 4.73 ± 2.22 abc | 1.83 ± 0.35 ab |
| MIX 0 | 21.53 ± 12.27 a | 7.35 ±1.39 a |
| WM S1 | 2.40 ± 1.15 bc | 6.44 ± 2.08 ab |
| CC S1 | 18.02 ± 16.78 abc | 0.80 ± 0.74 b |
| HMC26 S1 | 18.98 ± 4.81 abc | 0.90 ± 0.56 b |
| MIX S1 | 5.09 ± 6.66 abc | 7.25 ± 7.59 a |
| WM S2 | 3.29 ± 1.77 abc | 5.02 ± 1.48 ab |
| CC S2 | 19.18 ± 17.73 abc | 7.40 ± 3.80 a |
| HMC26 S2 | 8.13 ± 4.88 abc | 3.27 ± 2.12 ab |
| MIX S2 | 1.79 ± 1.94 c | 2.95 ± 2.60 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahuelcura, J.; Ortega, T.; Peña, F.; Berríos, D.; Valdebenito, A.; Contreras, B.; Santander, C.; Cornejo, P.; Ruiz, A. Antioxidant Response, Phenolic Compounds and Yield of Solanum tuberosum Tubers Inoculated with Arbuscular Mycorrhizal Fungi and Growing under Water Stress. Plants 2023, 12, 4171. https://doi.org/10.3390/plants12244171
Nahuelcura J, Ortega T, Peña F, Berríos D, Valdebenito A, Contreras B, Santander C, Cornejo P, Ruiz A. Antioxidant Response, Phenolic Compounds and Yield of Solanum tuberosum Tubers Inoculated with Arbuscular Mycorrhizal Fungi and Growing under Water Stress. Plants. 2023; 12(24):4171. https://doi.org/10.3390/plants12244171
Chicago/Turabian StyleNahuelcura, Javiera, Tiare Ortega, Fabiola Peña, Daniela Berríos, Analía Valdebenito, Boris Contreras, Christian Santander, Pablo Cornejo, and Antonieta Ruiz. 2023. "Antioxidant Response, Phenolic Compounds and Yield of Solanum tuberosum Tubers Inoculated with Arbuscular Mycorrhizal Fungi and Growing under Water Stress" Plants 12, no. 24: 4171. https://doi.org/10.3390/plants12244171
APA StyleNahuelcura, J., Ortega, T., Peña, F., Berríos, D., Valdebenito, A., Contreras, B., Santander, C., Cornejo, P., & Ruiz, A. (2023). Antioxidant Response, Phenolic Compounds and Yield of Solanum tuberosum Tubers Inoculated with Arbuscular Mycorrhizal Fungi and Growing under Water Stress. Plants, 12(24), 4171. https://doi.org/10.3390/plants12244171







