Shade Effect on Phenology, Fruit Yield, and Phenolic Content of Two Wild Blueberry Species in Northwestern Ontario, Canada
Abstract
:1. Introduction
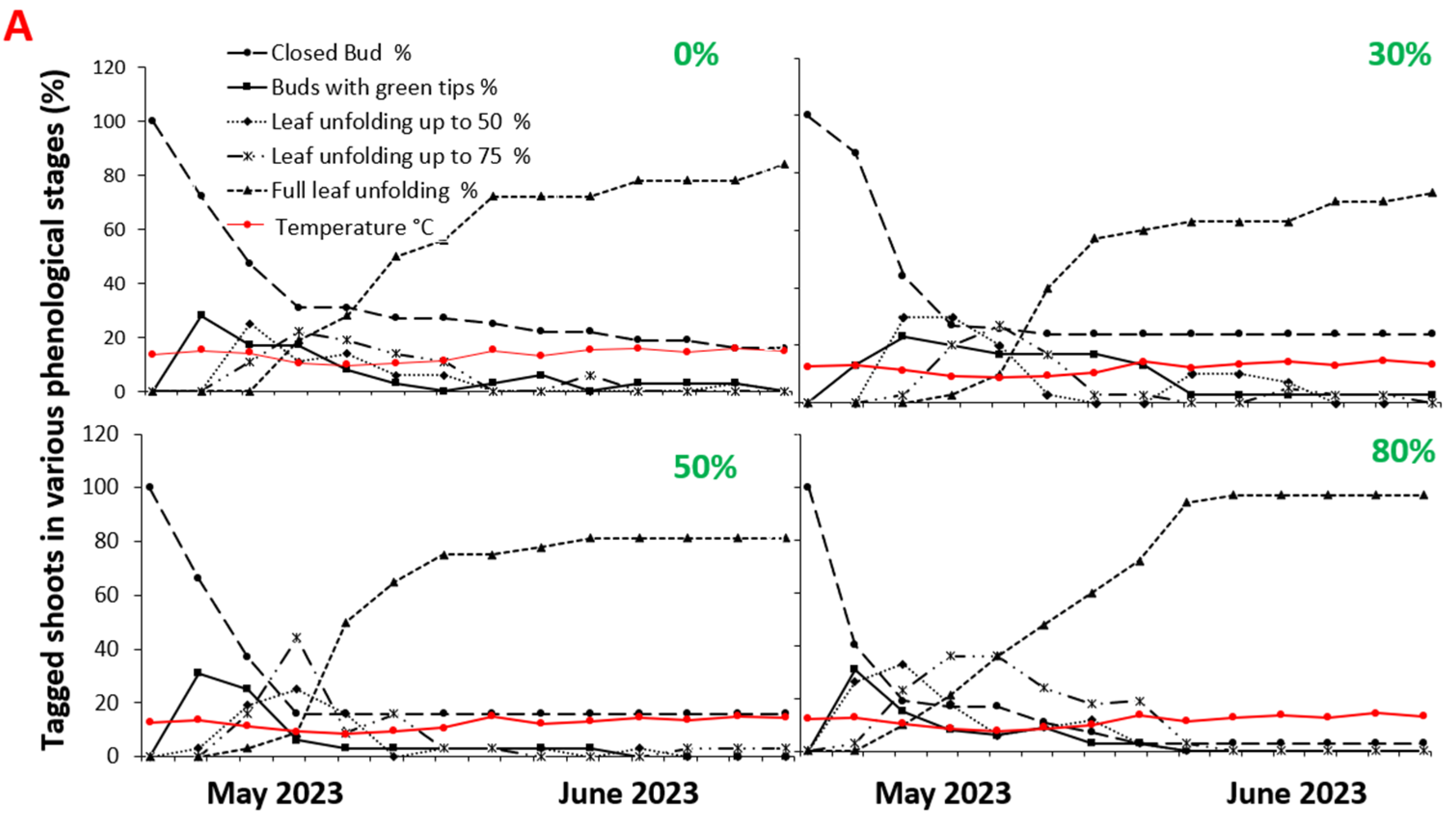
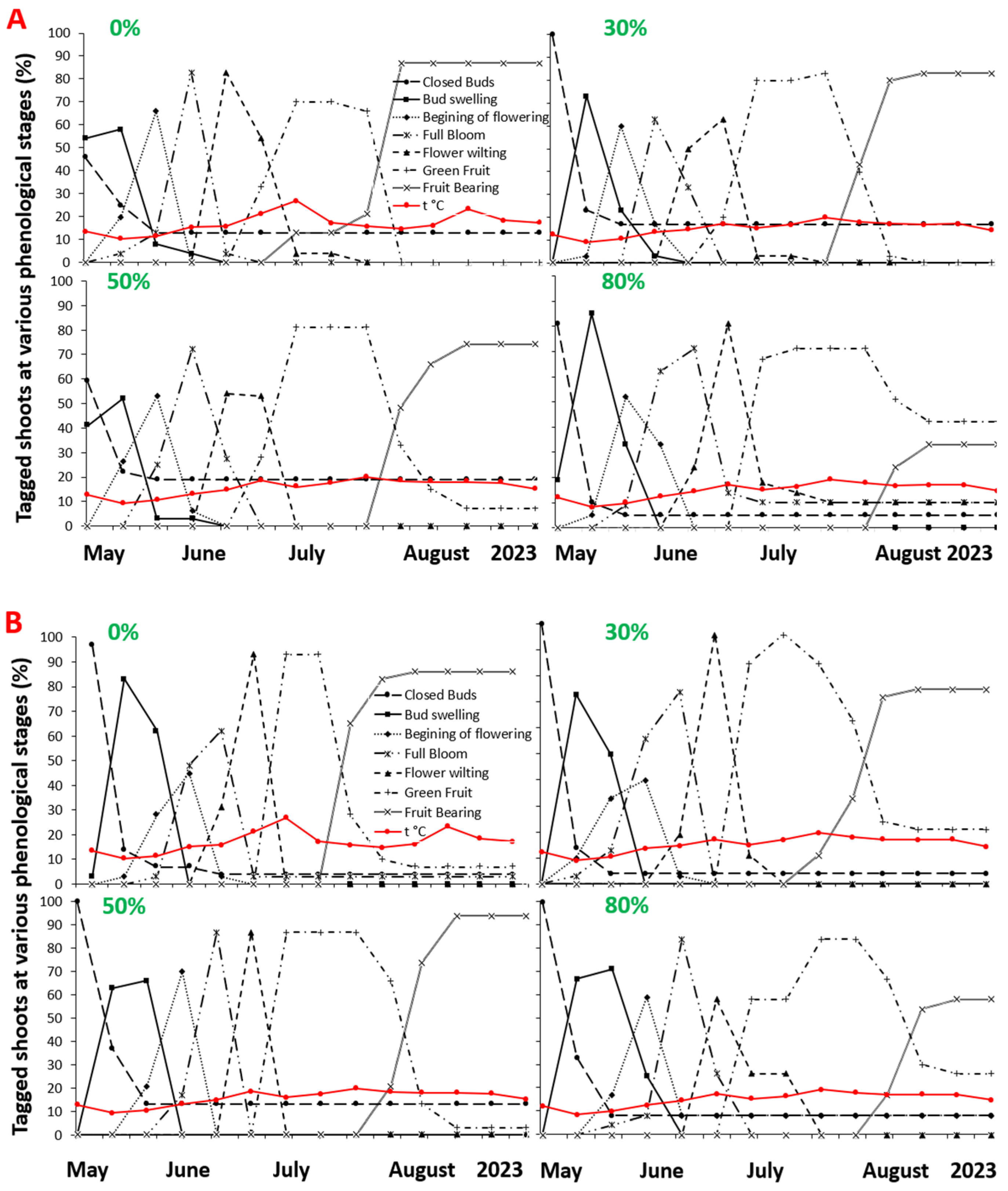
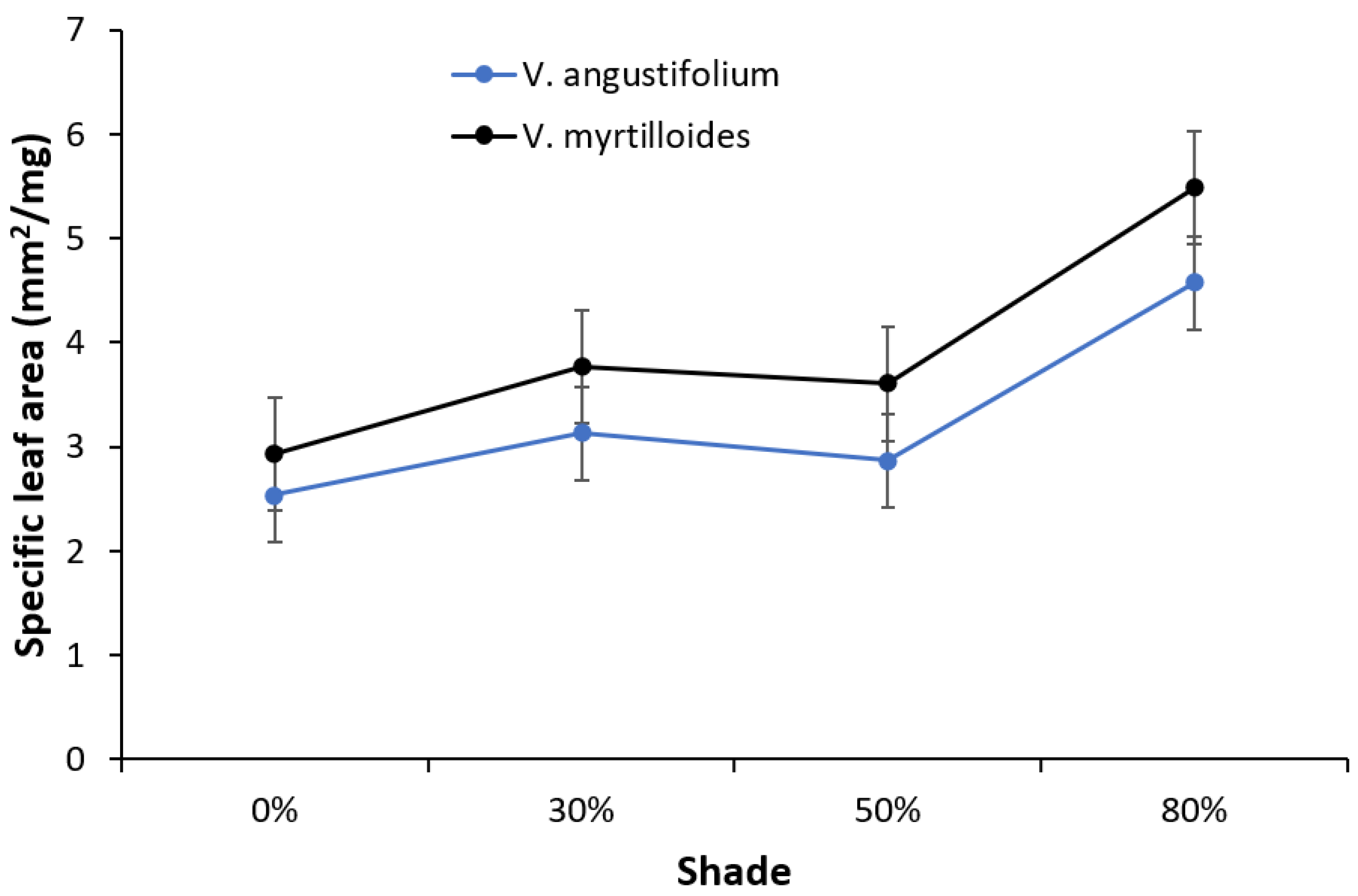
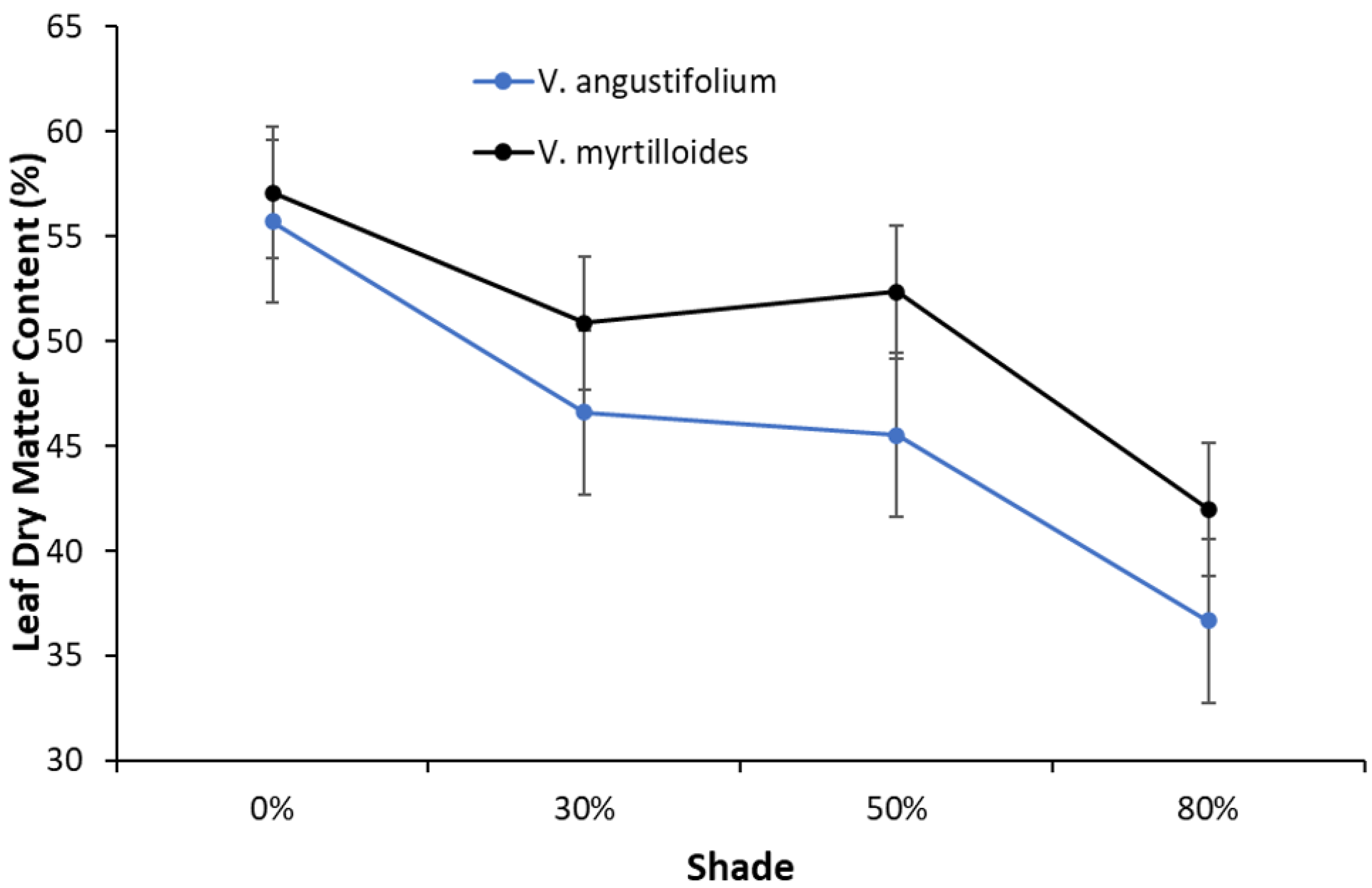
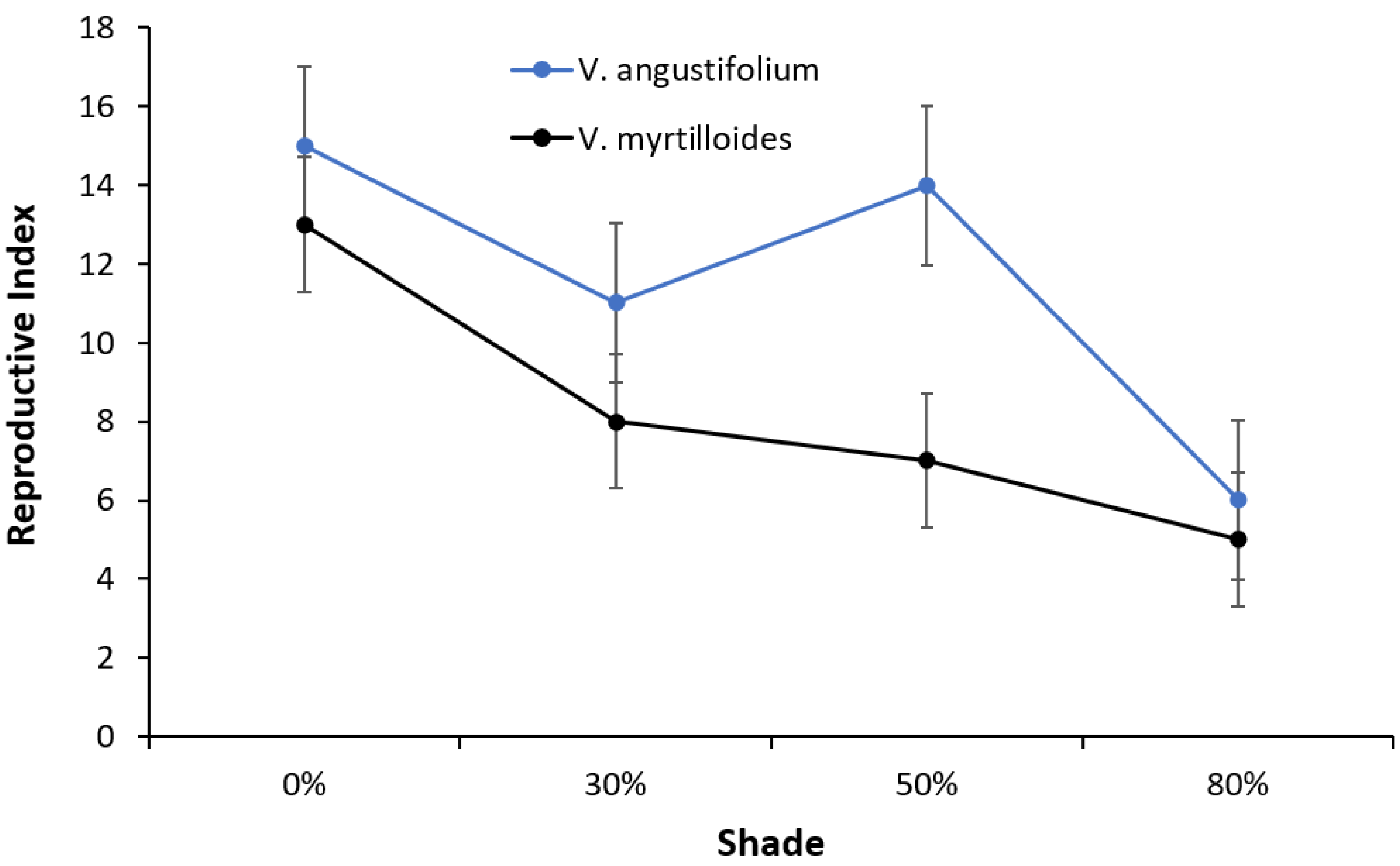
2. Results
3. Discussion
4. Materials and Methods
4.1. Shade Treatment
4.1.1. Blueberry Transplants in Pots
4.1.2. Phenology and Temperature Logging
4.1.3. Blueberry Seasonal Growth and Berry Yield
4.2. Chemical Analysis
4.2.1. Blueberry Extraction
4.2.2. Total Phenolic Content
4.2.3. Ferric Reducing Antioxidant Power Assay (FRAP)
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yavari, N.; Tripathi, R.; Wu, B.S.; MacPherson, S.; Singh, J.; Lefsrud, M. The effect of light quality on plant physiology, photosythetic, and stress response in Arabidopsis thaliana leaves. PLoS ONE 2021, 16, e0247380. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, H.; Yang, H.; Zhang, C.; Lyua, L.; Li, W.; Wu, W. A physiological and metabolomic analysis reveals the effect of shading intensity on blueberry fruit quality. Food Chem. 2022, 15, 100367. [Google Scholar] [CrossRef]
- Ligarreto, G.A.; Patiño, M.d.P.; Magnitskiy, S.V. Phenotypic plasticity of Vaccinium meridionale (Ericaceae) in wild populations of mountain forests in Colombia. Rev. Biol. Trop. 2011, 59, 569–583. [Google Scholar]
- Moola, F.M.; Mallik, A.U. Morphological plasticity and regeneration strategies of velvet leaf blueberry (Vaccinium myrtilloides Michx.) following canopy disturbance in boreal mixedwood forests. For. Ecol. Manag. 1998, 111, 35–50. [Google Scholar] [CrossRef]
- Ngoc, N.P.; Van Dang, L.; Quynh, L.N.; Hung, N.N. Enhancing soil fertility and lowbush blueberry (Vaccinium angustifolium) growth using bio-organic fertilizer. Conf. Ser. Earth Environ. Sci. 2022, 1087, 012077. [Google Scholar] [CrossRef]
- Stroink, M.L.; Nelson, C.H. Understanding traditional food behaviour and food security in rural First Nation communities: Implications for food policy. J. Rural Community Dev. 2012, 7, 65–82. [Google Scholar]
- Kalt, W.; McDonald, J.E.; Ricker, R.D.; Lu, X. Anthocyanin content and profile within and among blueberry species. Can. J. Plant Sci. 1999, 79, 617–623. [Google Scholar] [CrossRef]
- Mallik, A.U.; Hamilton, J. Harvest date and storage effect on fruit size, phenolic content, and antioxidant capacity of wild blueberries of NW Ontario, Canada. J. Food Sci. Technol. 2017, 54, 1545–1554. [Google Scholar] [CrossRef]
- Sun, Y.; Nemec-Bakk, A.S.; Mallik, A.U.; Bagchi, A.K.; Singal, P.K.; Khaper, N. Blueberry extract attenuates doxorubicin-induced damage in H9c2 cardiac cells 1. Can J. Physiol. Pharmacol. 2019, 97, 880–884. [Google Scholar] [CrossRef]
- Kalt, W.; Howell, A.; Duy, J.C.; Forney, C.F.; McDonald, J.E. Horticultural factors affecting antioxidant capacity of blueberries and other small fruit. HortTechnology 2001, 11, 523–528. [Google Scholar] [CrossRef]
- Alvarado-Raya, H.E.; Vázquez-Rodríguez, J.C.; Ramírez-Arias, A.; Calderón-Zavala, G.; Rivera-del-Río, R. Phenology and growing degree days of festival strawberry grown on red volcanic rock at two plant densities. Rev. Fitotec. Mex. 2021, 44, 349–356. [Google Scholar] [CrossRef]
- Kaur, J.; Percival, D.; Hainstock, L.J.; Jean-Pierre, P. Seasonal growth dynamics and carbon allocation of the wild blueberry plant (Vaccinium angustifolium Ait.). Can. J. Plant Sci. 2012, 92, 1145–1154. [Google Scholar] [CrossRef]
- Sakamoto, T.; Yokozawa, M.; Toritani, H.; Shibayama, M.; Ishitsuka, N.; Ohno, H. A crop phenology detection method using time-series MODIS data. Remote Sens. Environ. 2005, 96, 366–374. [Google Scholar] [CrossRef]
- Gill, D.S.; Amthor, J.S.; Bormann, F.H. Leaf phenology, photosynthesis, and the persistence of saplings and shrubs in a mature northern hardwood forest. Tree Physiol. 1998, 18, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.I. Functional Niche Differentiation in Co-Occurring Congeneric Plants. Master’s Thesis, Lakehead University, Thunder Bay, ON, Canada, 2014. [Google Scholar]
- Klady, R.A.; Henry, G.H.R.; Lemay, V. Changes in high arctic tundra plant reproduction in response to long-term experimental warming. Glob. Chang. Biol. 2011, 17, 1611–1624. [Google Scholar] [CrossRef]
- Bykova, O.; Chuine, I.; Morin, X.; Higgins, S.I. Temperature dependence of the reproduction niche and its relevance for plant species distributions. J. Biogeogr. 2012, 39, 2191–2200. [Google Scholar] [CrossRef]
- Valdes, A.; Ehrlen, J. Microclimate influences plant reproductive performance via an antagonistic interaction. Basic Appl. Ecol. 2022, 64, 13–29. [Google Scholar] [CrossRef]
- Vitasse, Y.; Ursenbacher, S.; Klein, G.; Bohnenstengel, T.; Chittaro, Y.; Delestrade, A.; Monnerat, C.; Rebetez, M.; Rixen, C.; Strebel, N.; et al. Phenological and elevational shifts of plants, animals, and fungi under climate change in the European Alps. Biol. Rev. 2021, 96, 1816–1835. [Google Scholar] [CrossRef]
- Ward, S.E.; Schulze, M.; Roy, B. A long-term perspective on microclimate and spring plant phenology in the Western Cascades. Ecosphere 2018, 9, e02451. [Google Scholar] [CrossRef]
- Liu, Y.; Dawson, W.; Prati, D.; Haeuser, E.; Feng, Y.; van Kleunen, M. Does greater specific leaf area plasticity help plants to maintain a high performance when shaded? Ann. Bot. 2016, 118, 1329–1336. [Google Scholar] [CrossRef]
- Zoratti, L.; Jaakola, L.; Häggman, H.; Giongo, L. Anthocyanin profile in berries of wild and cultivated Vaccinium spp. along altitudinal gradients in the Alps. J. Agric. Food Chem. 2015, 63, 8641–8650. [Google Scholar] [CrossRef]
- Fournier, M.-P.; Paré, M.C.; Buttò, V.; Delagrange, S.; Lafond, J.; Deslauriers, A. How plant allometry influences bud phenology and fruit yield in two Vaccinium species. Ann. Bot. 2020, 126, 825–835. [Google Scholar] [CrossRef]
- Scheepens, J.F.; Frei, E.S.; Stöcklin, J. Genotypic and environmental variation in specific leaf area in a widespread Alpine plant after transplantation to different altitudes. Oecologia 2010, 164, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Mallik, A.U.; Wang, J.R.; Siegwart-Collier, L.S.; Roberts, B.A. Morphological and ecophysiological responses of sheep laurel (Kalmia angustifolia L.) to shade. For. Int. J. For. Res. 2012, 85, 513–522. [Google Scholar] [CrossRef]
- Kim, S.J.; Yu, D.J.; Kim, T.C.; Lee, H.J. Growth, and photosynthetic characteristics of blueberry (Vaccinium corymbosum cv. bluecrop) under various shade levels. Sci. Hortic. 2011, 129, 486–492. [Google Scholar] [CrossRef]
- Jia, P.; Bayaerta, T.; Li, X.; Du, G. Relationships between flowering phenology and functional traits in eastern Tibet alpine meadow. Arct. Antarct. Alp. Res. 2011, 43, 585–592. Available online: http://www.jstor.org/stable/41416431 (accessed on 14 May 2023). [CrossRef]
- Asare, E.; Hoshide, A.K.; Drummond, F.A.; Criner, G.K.; Chen, X. Economic risk of bee pollination in Maine wild blueberry, Vaccinium angustifolium. J. Econ. Entomol. 2017, 10, 1980–1992. [Google Scholar] [CrossRef]
- Zoratti, L.; Jaakola, L.; Häggman, H.; Giongo, L. Modification of sunlight radiation through colored photo-selective nets affects anthocyanin profile in Vaccinium spp. berries. PLoS ONE 2015, 10, e0135935. [Google Scholar] [CrossRef]
- Bujor, O.C.; Tanase, C.; Popa, M.E. Phenolic antioxidants in aerial parts of wild Vaccinium species: Towards pharmaceutical and biological properties. Antioxidants 2019, 8, 649. [Google Scholar] [CrossRef]
- Jovančević, M.; Balijagić, J.; Menković, N.; Šavikin, K.; Zdunić, G.; Janković, T.; Dekić-Ivanković, M. Analysis of phenolic compounds in wild populations of bilberry (Vaccinium myrtillus L.) from Montenegro. J. Med. Plant Res 2011, 5, 910–914. [Google Scholar]
- Nishiyama, S.; Fujikawa, M.; Yamane, H.; Shirasawa, K.; Babiker, E.; Tao, R. Genomic insight into the developmental history of southern highbush blueberry populations. Heredity 2011, 126, 194–205. [Google Scholar] [CrossRef]
- Howard, L.R.; Clark, J.R.; Brownmiller, C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J. Sci. Food Agric. 2003, 83, 1238–1247. [Google Scholar] [CrossRef]
- St. Martin, P.; Mallik, A. Intraspecific trait variation as a mechanism of coexistence of congeneric blueberry (Vaccinium) species after forest harvesting. For. Ecol. Manag. 2023, 545, 121205. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent research on the health benefits of blueberries and their anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.S.; Nguyen, H.P.; Shen, S.; Schug, K.A. General method for extraction of blueberry anthocyanins and identification using high performance liquid chromatography-electrospray ionization-ion trap-time of flight-mass spectrometry. J. Chromatogr. A 2009, 1216, 4728–4735. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.H.; Xiong, J.; Esposito, D.; Ehlenfeldt, M.; Lila, M.A. Simultaneous LC-MS quantification of anthocyanins and non-anthocyanin phenolics from blueberries with widely divergent profiles and biological activities. Food Chem. 2019, 277, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.; Rossi, J. Colorimetry of total phenolic compounds with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Bolanos de la Torre, A.A.; Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2015, 174, 119–123. [Google Scholar] [CrossRef]

| Shade Intensity % | Ground Temp. (°C) | Total Phenol Content (mg GAE/100 g FW) | FRAP Index (μmol Fe2+/g FW) | ||
|---|---|---|---|---|---|
| Va | Vm | Va | Vm | ||
| 0 | 13.5 | 221.4 ± 20.3 a | 594.9 ± 47.2 a | 21.1 ± 3.2 a,A | 107.1 ± 2.2 a |
| 30 | 12.4 | 310.1 ± 29.2 | 447.2 ± 8.0 | 82.4 ± 5.5 A | 80.8 ± 5.5 |
| 50 | 12.2 | 305.1 ± 54.8 | 403.3 ± 94.2 | 74.5 ± 15.3 | 75.8 ± 9.2 |
| 80 | 11.5 | 247.6 ± 36.4 | 331.9 ± 2.6 | 67.1 ± 15.7 | 75.9 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyukaryeva, V.; Mallik, A.U. Shade Effect on Phenology, Fruit Yield, and Phenolic Content of Two Wild Blueberry Species in Northwestern Ontario, Canada. Plants 2023, 12, 4099. https://doi.org/10.3390/plants12244099
Dyukaryeva V, Mallik AU. Shade Effect on Phenology, Fruit Yield, and Phenolic Content of Two Wild Blueberry Species in Northwestern Ontario, Canada. Plants. 2023; 12(24):4099. https://doi.org/10.3390/plants12244099
Chicago/Turabian StyleDyukaryeva, Viktoriya, and Azim U. Mallik. 2023. "Shade Effect on Phenology, Fruit Yield, and Phenolic Content of Two Wild Blueberry Species in Northwestern Ontario, Canada" Plants 12, no. 24: 4099. https://doi.org/10.3390/plants12244099
APA StyleDyukaryeva, V., & Mallik, A. U. (2023). Shade Effect on Phenology, Fruit Yield, and Phenolic Content of Two Wild Blueberry Species in Northwestern Ontario, Canada. Plants, 12(24), 4099. https://doi.org/10.3390/plants12244099






