Physiological Effects of Microbial Biocontrol Agents in the Maize Phyllosphere
Abstract
1. Introduction
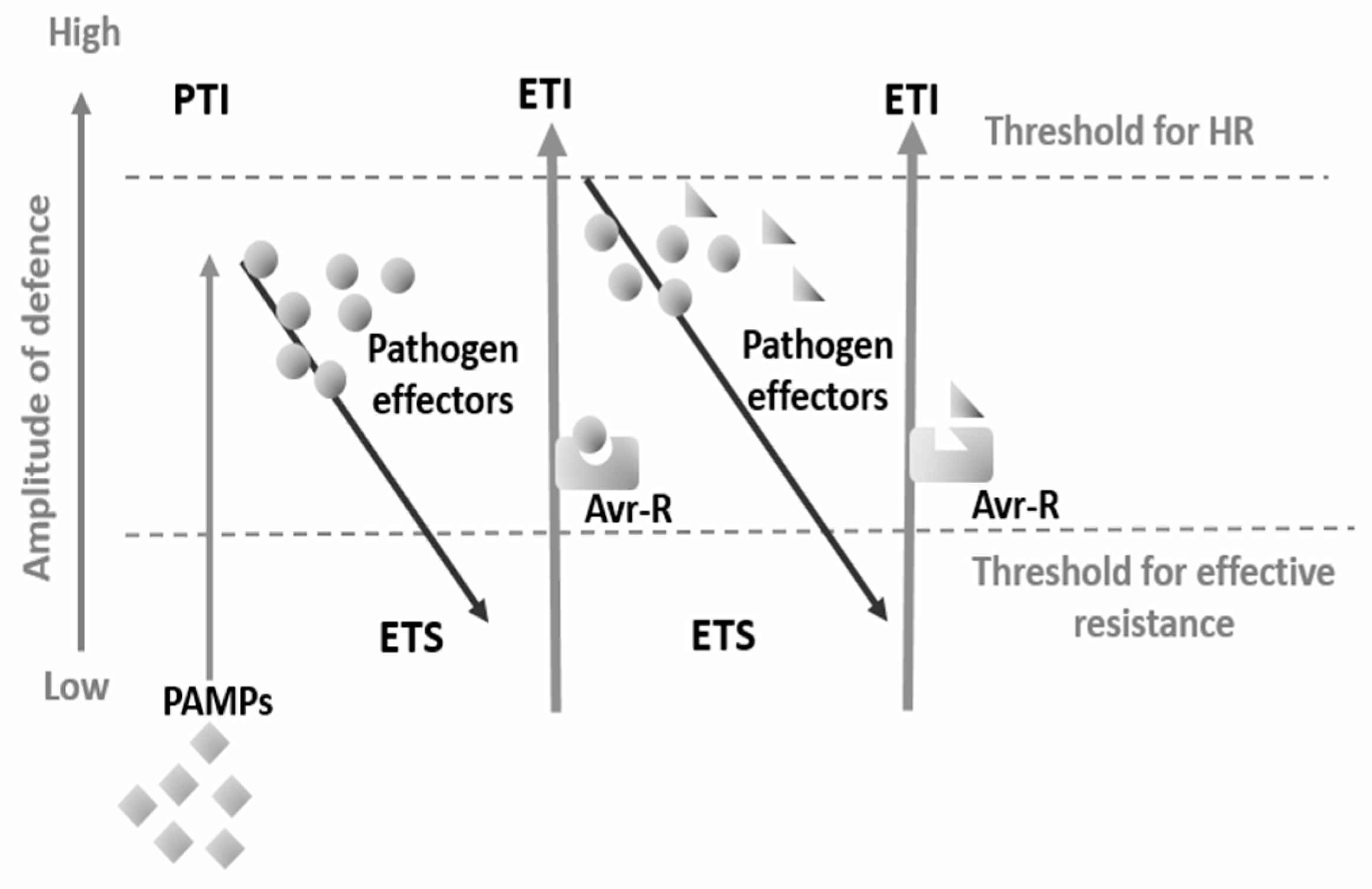
2. Plant Immune Response
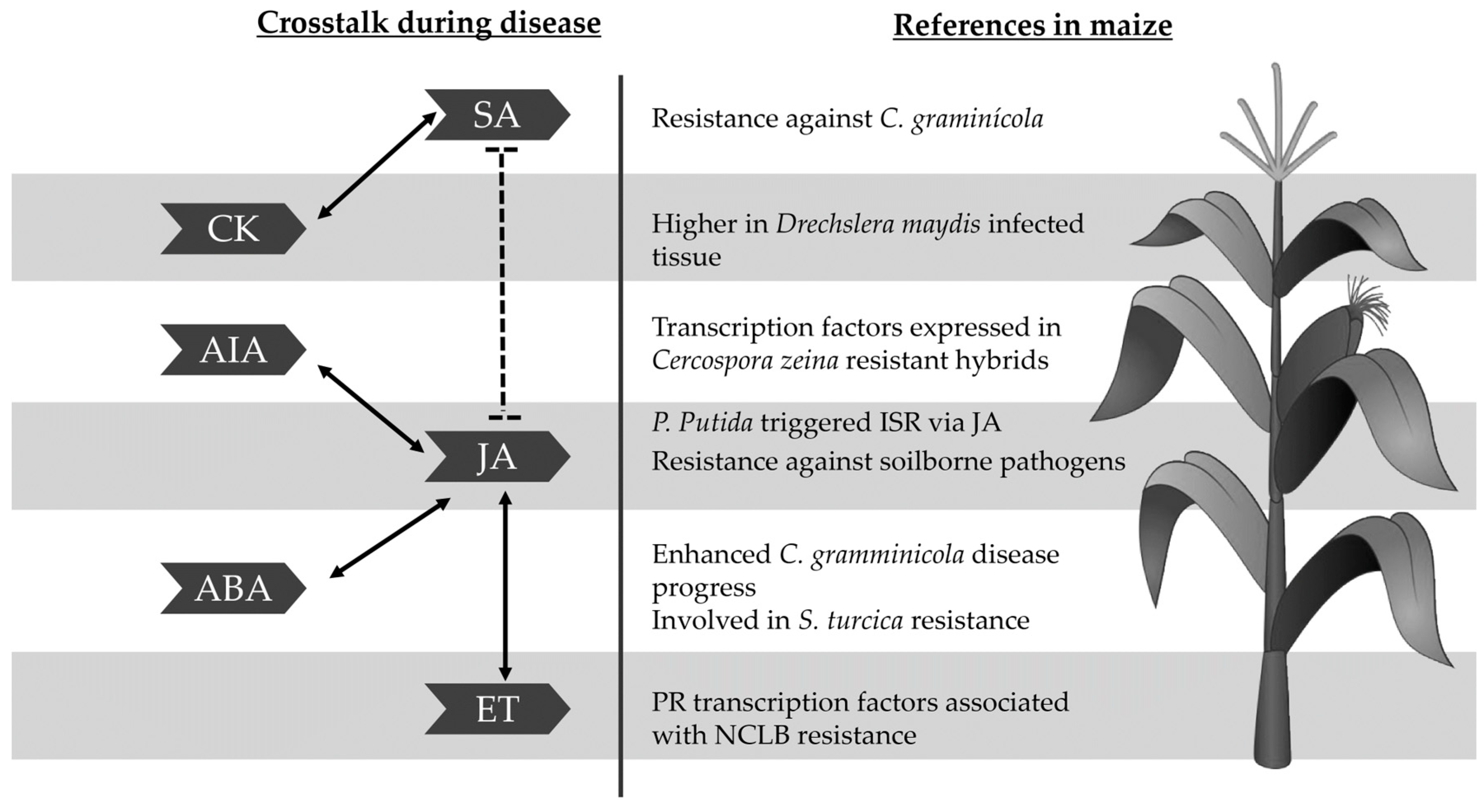
3. Phytohormones
3.1. Main Hormones Related to the Plant Infections: Salicylic Acid, Jasmonic Acid and Ethylene
3.2. Auxins
3.3. Abscisic Acid
3.4. Cytokinins
3.5. Giberellins
4. Secondary Metabolism Compounds: Phenolic Compounds and Phytoalexins
5. Lytic Enzymes: Chitinase and β-1,3-Glucanase
6. Reactive Oxygen Species
7. Research Gaps and Commercialization
8. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
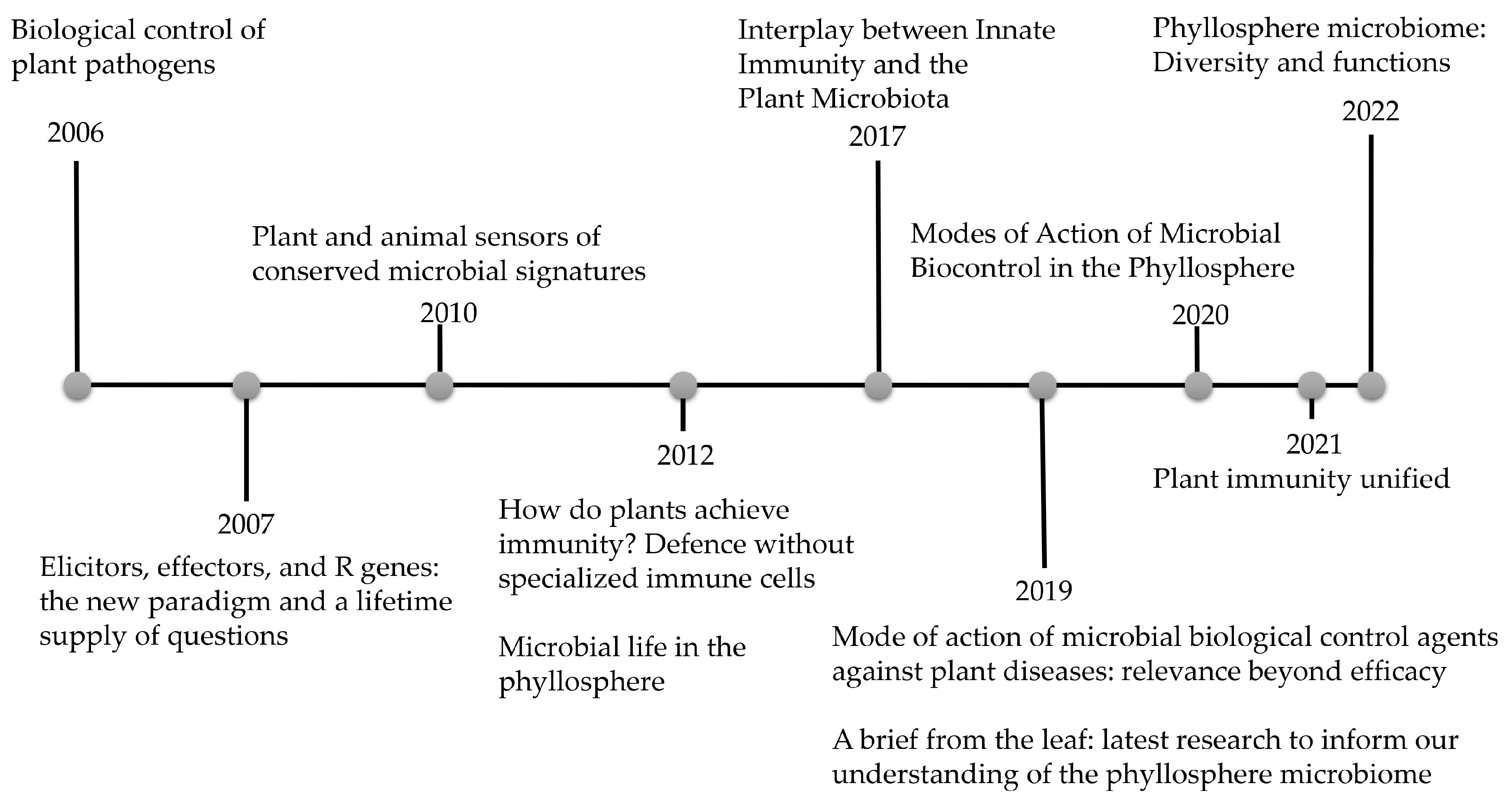
References
- Reynolds, T.L.; Nemeth, M.A.; Glenn, K.C.; Ridley, W.P.; Astwood, J.D. Natural variability of metabolites in maize grain: Differences due to genetic background. J. Agric. Food Chem. 2005, 53, 10061–10067. [Google Scholar] [CrossRef] [PubMed]
- Masisi, K.; Beta, T.; Moghadasian, M.H. Antioxidant properties of diverse cereal grains: A review on in vitro and in vivo studies. Food Chem. 2015, 196, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Bolsa de Comercio de Rosario. Available online: https://www.bcr.com.ar/es/mercados/gea/estimaciones-nacionales-de-produccion/estimaciones (accessed on 1 June 2023).
- Sweets, L.E.; Wright, S. Integrated Pest Management: Corn Diseases. Plant Prot. Programs 2008, 22. Available online: http://hdl.handle.net/10355/7284 (accessed on 1 June 2023).
- de Souza, J. Enfermedades del maíz en Entre Ríos. In Actualización Técnica Maíz, Girasol y Sorgo; INTA EEA Paraná: Paraná, Argentina, 2007; Volume 44, pp. 80–85. [Google Scholar]
- Couretot, L. Principales Enfermedades del Cultivo de Maíz en la Zona Norte de la Prov. de Bs. As. Campaña 2009/10. Available online: http://www.inta.gov.ar/pergamino (accessed on 1 June 2023).
- Díaz, C. Evolución e impacto de enfermedades foliares en el cultivo de maíz: Cercospora y Tizones. In Actas IX Congreso Nacional de Maíz; Rosario, Argentina, 2010; pp. 200–204. [Google Scholar]
- Formento, A. Enfermedades Foliares Reemergentes del Cultivo de Maíz: Royas (Puccinia sorghi y Puccinia polysora), Tizón foliar (Exserohilum turcicum) y Mancha ocular (Kabatiella zeae); INTA Paraná: Paraná, Argentina, 2010. [Google Scholar]
- De Rossi, R.; Guerra, F.; Plazas, M.; Vuletic, E.; Brücher, E.; Guerra, G.; Reis, E. Crop damage, economic losses, and the economic damage threshold for northern corn leaf blight. Crop. Prot. 2021, 154, 105901. [Google Scholar] [CrossRef]
- Pratt, R.C.; Gordon, S.G. Breeding for Resistance to Maize Foliar Pathogens. In Plant Breeding Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; Volume 27, pp. 119–173. [Google Scholar] [CrossRef]
- Hurni, S.; Scheuermann, D.; Krattinger, S.G.; Kessel, B.; Wicker, T.; Herren, G.; Fitze, M.N.; Breen, J.; Presterl, T.; Ouzunova, M.; et al. The maize disease resistance gene Htn1 against northern corn leaf blight encodes a wall-associated receptor-like kinase. Proc. Natl. Acad. Sci. USA 2015, 112, 8780–8785. [Google Scholar] [CrossRef] [PubMed]
- Sucher, J.; Boni, R.; Yang, P.; Rogowsky, P.; Büchner, H.; Kastner, C.; Kumlehn, J.; Krattinger, S.G.; Keller, B. The durable wheat disease resistance gene Lr34 confers common rust and northern corn leaf blight resistance in maize. Plant Biotechnol. J. 2016, 15, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, X.; Long, S.; Jaqueth, J.; Li, B.; Yan, J.; Ding, J. Mapping of QTL conferring resistance to northern corn leaf blight using high-density SNPs in maize. Mol. Breed. 2015, 36, 4. [Google Scholar] [CrossRef]
- Sibiya, J.; Tongoona, P.; Derera, J. Combining ability and GGE biplot analyses for resistance to northern leaf blight in tropical and subtropical elite maize inbred lines. Euphytica 2012, 191, 245–257. [Google Scholar] [CrossRef]
- Couretot, L.; Ferraris, G.; Mousegne, F.; Russian, H. Control químico de roya común del maíz (Puccinia sorghi). In Proceedings of the 1° Congreso Argentino de Fitopatología, HM-25, Córdoba, Argentino, 28–30 May 2008; p. 211. [Google Scholar]
- Carmona, M.; Melo Reis, E.; Trezzi Casa, R. Identificación y manejo de las principales enfermedades del maíz. Horiz. A 2008, 583, 44. [Google Scholar]
- Bayer. 2008. Available online: http://www.bayercropscience.com.pe/web/articulo=407 (accessed on 5 March 2023).
- Batista, A.C.; Domingues, C.E.d.C.; Costa, M.J.; Silva-Zacarin, E.C.M. Is a strobilurin fungicide capable of inducing histopathological effects on the midgut and Malpighian tubules of honey bees? J. Apic. Res. 2020, 59, 834–843. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Mohammadi, M.; Karami, A.; Tabandeh, L.; Dargahi, A.; Amirian, F. Residue Analysis of Pesticides, Herbicides, and Fungicides in Various Water Sources Using Gas Chromatography-Mass Detection. Pol. J. Environ. Stud. 2017, 26, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Baldotto, L.E.B.; Olivares, F.L. Phylloepiphytic interaction between bacteria and different plant species in a tropical agricultural system. Can. J. Microbiol. 2008, 54, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Kadivar, H.; Stapleton, A.E. Ultraviolet radiation alters maize phyllosphere bacterial diversity. Microb. Ecol. 2003, 45, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.K.; Gardener, B.M. Biological control of plant pathogens. Curr. Opin. Biotechnol. 2006, 7, 343–347. [Google Scholar] [CrossRef]
- Atehnkeng, J.; Ojiambo, P.; Ikotun, T.; Sikora, R.; Cotty, P.; Bandyopadhyay, R. Evaluation of atoxigenic isolates of Aspergillus flavus as potential biocontrol agents for aflatoxin in maize. Food Addit. Contam. Part A 2008, 25, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Etcheverry, M.G.; Scandolara, A.; Nesci, A.; Ribeiro, M.S.V.B.; Pereira, P.; Battilani, P. Biological interactions to select biocontrol agents against toxigenic strains of Aspergillus flavus and Fusarium verticillioides from maize. Mycopathologia 2009, 167, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yang, K.; Liu, Y.; Lin, Y.; Chen, C. Suppression of southern corn leaf blight by a plant growth-promoting rhizobacterium Bacillus cereus C1L. Ann. Appl. Biol. 2010, 157, 45–53. [Google Scholar] [CrossRef]
- Sartori, M.; Nesci, A.; Montemarani, A.; Barros, G.; García, J.; Etcheverry, M. Preliminary Evaluation of Biocontrol Agents against Maize Pathogens Exserohilum turcicum and Puccinia sorghi in Field Assays. Agric. Sci. 2017, 8, 1003–1013. [Google Scholar] [CrossRef]
- Sartori, M.; Nesci, A.; García, J.; Passone, M.A.; Montemarani, A.; Etcheverry, M. Efficacy of Epiphytic Bacteria to Prevent Northern Leaf Blight Caused by Exserohilum turcicum in Maize. Rev. Argent. Microbiol. 2017, 49, 75–82. [Google Scholar] [CrossRef]
- Legein, M.; Smets, W.; Vandenheuvel, D.; Eilers, T.; Muyshondt, B.; Prinsen, E.; Samson, R.; Lebeer, S. Modes of Action of Microbial Biocontrol in the Phyllosphere. Front. Microbiol. 2020, 11, 1619. [Google Scholar] [CrossRef]
- Ronald, P.C.; Beutler, B. Plant and animal sensors of conserved microbial signatures. Science 2010, 330, 1061–1064. [Google Scholar] [CrossRef]
- Bent, A.F.; Mackey, D. Elicitors, effectors, and R genes: The new paradigm and a lifetime supply of questions. Annu. Rev. Phytopathol. 2007, 45, 399–436. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.y.; Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, D.; Ianiri, G.; Del Grosso, C.; Barone, G.; De Curtis, F.; Castoria, R.; Lima, G. Advances and perspectives in the use of biocontrol agents against fungal plant diseases. Horticulturae 2022, 8, 577. [Google Scholar] [CrossRef]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Bashir, I.; War, A.F.; Rafiq, I.; Reshi, Z.A.; Rashid, I.; Shouche, Y.S. Phyllosphere microbiome: Diversity and functions. Microbiol. Res. 2021, 254, 126888. [Google Scholar] [CrossRef] [PubMed]
- Leveau, J.H. A brief from the leaf: Latest research to inform our understanding of the phyllosphere microbiome. Curr. Opin. Microbiol. 2019, 49, 41–49. [Google Scholar] [CrossRef]
- Pruitt, R.N.; Gust, A.A.; Nürnberger, T. Plant immunity unified. Nat. Plants 2021, 7, 382–383. [Google Scholar] [CrossRef]
- Hacquard, S.; Spaepen, S.; Garrido-Oter, R.; Schulze-Lefert, P. Interplay between Innate Immunity and the Plant Microbiota. Annu. Rev. Phytopathol. 2017, 55, 565–589. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Castro-Moretti, F.R.; Gentzel, I.N.; Mackey, D.; Alonso, A.P. Metabolomics as an emerging tool for the study of plant–pathogen interactions. Metabolites 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Sánchez Vallet, A.; Sánchez-Rodríguez, C. Inmunidad innata en plantas y resistencia a patógenos: Nuevos conceptos y potenciales aplicaciones en protección vegetal. Phytoma España Rev. Prof. Sanid. Veg. 2007, 192, 43–46. [Google Scholar]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Lanubile, A.; Ferrarini, A.; Maschietto, V.; Delledonne, M.; Marocco, A.; Bellin, D. Functional genomic analysis of constitutive and inducible defense responses to Fusarium verticillioides infection in maize genotypes with contrasting ear rot resistance. BMC Genom. 2014, 15, 710. [Google Scholar] [CrossRef]
- Wang, F.; Wang, C.; Yan, Y.; Jia, H.; Guo, X. Overexpression of cotton GhMPK11 decreases disease resistance through the gibberellin signaling pathway in transgenic Nicotiana benthamiana. Front. Plant Sci. 2016, 7, 689. [Google Scholar] [CrossRef]
- Serrano, I.; Audran, C.; Rivas, S. Chloroplasts at work during plant innate immunity. J. Exp. Bot. 2016, 67, 3845–3854. [Google Scholar] [CrossRef]
- Withers, J.; Dong, X. Post-translational regulation of plant immunity. Curr. Opin. Plant Biol. 2017, 38, 124–132. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Jones, J.D.; Ding, P. Plant immune networks. Trends Plant Sci. 2021, 27, 255–273. [Google Scholar] [CrossRef]
- Zeier, J. Metabolic regulation of systemic acquired resistance. Curr. Opin. Plant Biol. 2021, 62, 102050. [Google Scholar] [CrossRef]
- Shine, M.B.; Xiao, X.; Kachroo, P.; Kachroo, A. Signaling mechanisms underlying systemic acquired resistance to microbial pathogens. Plant Sci. 2019, 279, 81–86. [Google Scholar] [CrossRef]
- Groen, S.C.; Whiteman, N.K. The Evolution of Ethylene Signaling in Plant Chemical Ecology. J. Chem. Ecol. 2014, 40, 700–716. [Google Scholar] [CrossRef]
- Ton, J.; Van Pelt, J.A.; Van Loon, L.C.; Pieterse, C.M.J. Differential Effectiveness of Salicylate-Dependent and Jasmonate/Ethylene-Dependent Induced Resistance in Arabidopsis. Mol. Plant-Microbe Interact. 2002, 15, 27–34. [Google Scholar] [CrossRef]
- Van der Ent, S.; Van Wees, S.C.; Pieterse, C.M. Jasmonate signaling in plant interactions with resistance-inducing beneficial microbes. Phytochemistry 2009, 70, 1581–1588. [Google Scholar] [CrossRef]
- Denancé, N.; Sánchez-Vallet, A.; Goffner, D.; Molina, A. Disease resistance or growth: The role of plant hormones in balancing immune responses and fitness costs. Front. Plant Sci. 2013, 4, 155. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J.M. Linking development to defense: Auxin in plant–pathogen interactions. Trends Plant Sci. 2009, 14, 373–382. [Google Scholar] [CrossRef]
- Takatsuji, H.; Jiang, C.J. Plant hormone crosstalks under biotic stresses. In Phytohormones: A Window to Metabolism, Signaling and Biotechnological Applications; Springer: New York, NY, USA, 2014; Volume 9781493904, pp. 323–350. [Google Scholar] [CrossRef]
- Vos, I.A.; Pieterse, C.M.J.; van Wees, S.C.M. Costs and benefits of hormone-regulated plant defences. Plant Pathol. 2013, 62, 43–55. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.K.; Song, Q.-Q.; Li, H.-B.; Guo, D.-J.; Malviya, M.K.; Verma, K.K.; Song, X.-P.; Lakshmanan, P.; Yang, L.-T.; et al. Comparative analysis of protein and differential responses of defense-related gene and enzyme activity reveals the long-term molecular responses of sugarcane inoculated with Sporisorium scitamineum. J. Plant Interact. 2021, 16, 12–29. [Google Scholar] [CrossRef]
- Gorman, Z.; Christensen, S.A.; Yan, Y.; He, Y.; Borrego, E.; Kolomiets, M.V. Green leaf volatiles and jasmonic acid enhance susceptibility to anthracnose diseases caused by Colletotrichum graminicola in maize. Mol. Plant Pathol. 2020, 21, 702–715. [Google Scholar] [CrossRef]
- Yang, Q.; Balint-Kurti, P.; Xu, M. Quantitative Disease Resistance: Dissection and Adoption in Maize. Mol. Plant 2017, 10, 402–413. [Google Scholar] [CrossRef]
- Van Wees, S.C.; Van der Ent, S.; Pieterse, C.M. Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 2008, 11, 443–448. [Google Scholar] [CrossRef]
- Yan, Y.; Christensen, S.; Isakeit, T.; Engelberth, J.; Meeley, R.; Hayward, A.; Emery, R.N.; Kolomiets, M.V. Disruption of OPR7 and OPR8 reveals the versatile functions of jasmonic acid in maize development and defense. Plant Cell 2012, 24, 1420–1436. [Google Scholar] [CrossRef]
- Planchamp, C.; Glauser, G.; Mauch-Mani, B. Root inoculation with Pseudomonas putida KT2440 induces transcriptional and metabolic changes and systemic resistance in maize plants. Front. Plant Sci. 2015, 5, 719. [Google Scholar] [CrossRef]
- Vargas, W.A.; Martín, J.M.S.; Rech, G.E.; Rivera, L.P.; Benito, E.P.; Díaz-Mínguez, J.M.; Thon, M.R.; Sukno, S.A. Plant defense mechanisms are activated during biotrophic and necrotrophic development of Colletotrichum graminicola in maize. Plant Physiol. 2012, 158, 1342–1358. [Google Scholar] [CrossRef]
- Bräunlich, S.; Koller, T.; Glauser, G.; Krattinger, S.G.; Keller, B. Expression of the wheat disease resistance gene Lr34 in transgenic barley leads to accumulation of abscisic acid at the leaf tip. Plant Physiol. Biochem. 2021, 166, 950–957. [Google Scholar] [CrossRef]
- Choi, J.; Huh, S.U.; Kojima, M.; Sakakibara, H.; Paek, K.-H.; Hwang, I. The cytokinin-activated transcription factor ARR2 promotes plant immunity via TGA3/NPR1-dependent salicylic acid signaling in arabidopsis. Dev. Cell 2010, 19, 284–295. [Google Scholar] [CrossRef]
- Claeys, H.; De Bodt, S.; Inzé, D. Gibberellins and DELLAs: Central nodes in growth regulatory networks. Trends Plant Sci. 2014, 19, 231–239. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.M.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Dempsey, D.A.; Klessig, D.F. How does the multifaceted plant hormone salicylic acid combat disease in plants and are similar mechanisms utilized in humans? BMC Biol. 2017, 15, 23. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic acid as a safe plant protector and growth regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- van Loon, L.C.; Geraats, B.P.; Linthorst, H.J. Ethylene as a modulator of disease resistance in plants. Trends Plant Sci. 2006, 11, 184–191. [Google Scholar] [CrossRef]
- Morris, S.W.; Vernooij, B.; Titatarn, S.; Starrett, M.; Thomas, S.; Wiltse, C.C.; Frederiksen, R.A.; Bhandhufalck, A.; Hulbert, S.; Uknes, S.; et al. Induced resistance responses in maize. Mol. Plant-Microbe Interact. 1998, 11, 643–658. [Google Scholar] [CrossRef]
- Wu, X.-J.; Xu, L.; Zhao, P.-F.; Li, N.; Wu, L.; He, Y.; Wang, S.-C. Comparative transcriptome profiling of two maize near-isogenic lines differing in the allelic state for bacterial brown spot disease resistance. J. Integr. Agric. 2015, 14, 610–621. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef]
- Klessig, D.F.; Choi, H.W.; Dempsey, D.A. Systemic acquired resistance and salicylic acid: Past, present, and future. Mol. Plant-Microbe Interact. 2018, 31, 871–888. [Google Scholar] [CrossRef]
- Forchetti, G.; Masciarelli, O.; Izaguirre, M.J.; Alemano, S.; Alvarez, D.; Abdala, G. Endophytic bacteria improve seedling growth of sunflower under water stress, produce salicylic acid, and inhibit growth of pathogenic fungi. Curr. Microbiol. 2010, 61, 485–493. [Google Scholar] [CrossRef]
- Yang, Y.X.; JAhammed, G.; Wu, C.; Fan, S.Y.; Zhou, Y.H. Crosstalk among Jasmonate, Salicylate and Ethylene Signaling Pathways in Plant Disease and Immune Responses. Curr. Protein Pept. Sci. 2015, 16, 450–461. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, H.; Ren, D.; Li, Y. Activation of ZmMKK10, a maize mitogen-activated protein kinase kinase, induces ethylene-dependent cell death. Plant Sci. 2017, 264, 129–137. [Google Scholar] [CrossRef]
- Tintor, N.; Ross, A.; Kanehara, K.; Yamada, K.; Fan, L.; Kemmerling, B.; Nürnberger, T.; Tsuda, K.; Saijo, Y. Layered pattern receptor signaling via ethylene and endogenous elicitor peptides during Arabidopsis immunity to bacterial infection. Proc. Natl. Acad. Sci. USA 2013, 110, 6211–6216. [Google Scholar] [CrossRef]
- Poland, J.A.; Bradbury, P.J.; Buckler, E.S.; Nelson, R.J. Genome-wide nested association mapping of quantitative resistance to northern leaf blight in maize. Proc. Natl. Acad. Sci. USA 2011, 108, 6893–6898. [Google Scholar] [CrossRef]
- Yadav, A.N.; Saxena, A.K. Biodiversity and biotechnological applications of halophilic microbes for sustainable agriculture. J. Appl. Biol. Biotechnol. 2018, 6, 48–55. [Google Scholar] [CrossRef]
- Weller, D.M.; Mavrodi, D.V.; van Pelt, J.A.; Pieterse, C.M.J.; van Loon, L.C.; Bakker, P.A.H.M. Induced systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato by 2,4-diacetylphloroglucinol-producing Pseudomonas fluorescens. Phytopathology 2012, 102, 403–412. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Djavaheri, M.; Bakker, P.A.; Höfte, M. Pseudomonas fluorescens WCS374r-induced systemic resistance in rice against Magnaporthe oryzae is based on pseudobactin-mediated priming for a salicylic acid-repressible multifaceted defense response. Plant Physiol. 2008, 148, 1996–2012. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D.G. Hormone crosstalk in plant disease and defense: More than just JASMONATE-SALICYLATE antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- Naseem, M.; Dandekar, T. The Role of Auxin-Cytokinin Antagonism in Plant-Pathogen Interactions. PLoS Pathog. 2012, 8, e1003026. [Google Scholar] [CrossRef]
- Ding, X.; Cao, Y.; Huang, L.; Zhao, J.; Xu, C.; Li, X.; Wang, S. Activation of the indole-3-acetic acid-amido synthetase GH3-8 suppresses expansin expression and promotes salicylate- and jasmonate-independent basal immunity in rice. Plant Cell 2008, 20, 228–240. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Bacteria and fungi controlling plant growth by manipulating auxin: Balance between development and defense. J. Plant Physiol. 2015, 172, 4–12. [Google Scholar] [CrossRef]
- Park, J.-E.; Park, J.-Y.; Kim, Y.-S.; Staswick, P.E.; Jeon, J.; Yun, J.; Kim, S.-Y.; Kim, J.; Lee, Y.-H.; Park, C.-M. GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J. Biol. Chem. 2007, 282, 10036–10046. [Google Scholar] [CrossRef]
- Wang, D.; Pajerowska-Mukhtar, K.; Culler, A.H.; Dong, X. Salicylic Acid Inhibits Pathogen Growth in Plants through Repression of the Auxin Signaling Pathway. Curr. Biol. 2007, 17, 1784–1790. [Google Scholar] [CrossRef]
- Abreu, M.E.; Munné-Bosch, S. Salicylic acid deficiency in NahG transgenic lines and sid2 mutants increases seed yield in the annual plant Arabidopsis thaliana. J. Exp. Bot. 2009, 60, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Shah, L.; Rahman, S.; Riaz, M.W.; Yahya, M.; Xu, Y.J.; Liu, F.; Si, W.; Jiang, H.; Cheng, B. Plant defense mechanism and current understanding of salicylic acid and NPRs in activating SAR. Physiol. Mol. Plant Pathol. 2018, 104, 15–22. [Google Scholar] [CrossRef]
- Koornneef, A.; Pieterse, C.M. Cross Talk in Defense Signaling. Plant Physiol. 2008, 146, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Llorente, F.; Muskett, P.; Sánchez-Vallet, A.; López, G.; Ramos, B.; Sánchez-Rodríguez, C.; Jordá, L.; Parker, J.; Molina, A. Repression of the auxin response pathway increases Arabidopsis susceptibility to necrotrophic fungi. Mol. Plant 2008, 1, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Thomma, B.P.; Penninckx, I.A.; Cammue, B.P.; Broekaert, W.F. The complexity of disease signaling in Arabidopsis. Curr. Opin. Immunol. 2001, 13, 63–68. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef]
- Reineke, G.; Heinze, B.; Schirawski, J.; Buettner, H.; Kahmann, R.; Basse, C.W. Indole-3-acetic acid (IAA) biosynthesis in the smut fungus Ustilago maydis and its relevance for increased IAA levels in infected tissue and host tumour formation. Mol. Plant Pathol. 2008, 9, 339–355. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Prinsen, E.; Rolfe, S.A.; Scholes, J.D. Metabolism and Plant Hormone Action During Clubroot Disease. J. Plant Growth Regul. 2009, 28, 229–244. [Google Scholar] [CrossRef]
- Gravel, V.; Antoun, H.; Tweddell, R.J. Effect of indole-acetic acid (IAA) on the development of symptoms caused by Pythium ultimum on tomato plants. Eur. J. Plant Pathol. 2007, 119, 457–462. [Google Scholar] [CrossRef]
- Shi, F.; Zhang, Y.; Wang, K.; Meng, Q.; Liu, X.; Ma, L.; Li, Y.; Liu, J.; Ma, L. Expression profile analysis of maize in response to Setosphaeria turcica. Gene 2018, 659, 100–108. [Google Scholar] [CrossRef]
- Meyer, J.; Berger, D.K.; Christensen, S.A.; Murray, S.L. RNA-Seq analysis of resistant and susceptible sub-tropical maize lines reveals a role for kauralexins in resistance to grey leaf spot disease, caused by Cercospora zeina. BMC Plant Biol. 2017, 17, 197. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, F.; Gallei, M.; Kornienko, A.E.; Saado, I.; Khan, M.; Chia, K.-S.; Darino, M.A.; Bindics, J.; Djamei, A. TOPLESS promotes plant immunity by repressing auxin signaling and is targeted by the fungal effector Naked1. Plant Commun. 2021, 3, 100269. [Google Scholar] [CrossRef] [PubMed]
- Degani, O.; Drori, R.; Goldblat, Y. Plant growth hormones suppress the development of Harpophora maydis, the cause of late wilt in maize. Physiol. Mol. Biol. Plants 2014, 21, 137–149. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petti, C.; Reiber, K.; Ali, S.S.; Berney, M.; Doohan, F.M. Auxin as a player in the biocontrol of Fusarium head blight disease of barley and its potential as a disease control agent. BMC Plant Biol. 2012, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, N.; Sathishkumar, R.; Selvakumar, G.; Shyamkumar, R.; Arjunekumar, K. Phyllospheric Microbiomes: Diversity, Ecological Significance, and Biotechnological Applications. In Plant Microbiomes for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Ton, J.; Flors, V.; Mauch-Mani, B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009, 14, 310–317. [Google Scholar] [CrossRef]
- Anderson, J.P.; Badruzsaufari, E.; Schenk, P.M.; Manners, J.M.; Desmond, O.J.; Ehlert, C.; MacLean, D.J.; Ebert, P.R.; Kazan, K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in arabidopsis. Plant Cell 2004, 16, 3460–3479. [Google Scholar] [CrossRef]
- Yasuda, M.; Ishikawa, A.; Jikumaru, Y.; Seki, M.; Umezawa, T.; Asami, T.; Maruyama-Nakashita, A.; Kudo, T.; Shinozaki, K.; Yoshida, S.; et al. Antagonistic interaction between systemic acquired resistance and the abscisic acid-mediated abiotic stress response in Arabidopsis. Plant Cell 2008, 20, 1678–1692. [Google Scholar] [CrossRef] [PubMed]
- Balmer, D.; de Papajewski, D.V.; Planchamp, C.; Glauser, G.; Mauch-Mani, B. Induced resistance in maize is based on organ-specific defence responses. Plant J. 2013, 74, 213–225. [Google Scholar] [CrossRef]
- Erb, M.; Flors, V.; Karlen, D.; De Lange, E.; Planchamp, C.; D’alessandro, M.; Turlings, T.C.J.; Ton, J. Signal signature of aboveground-induced resistance upon belowground herbivory in maize. Plant J. 2009, 59, 292–302. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Yang, Y.; Cruz, C.V.; Höfte, M. Abscisic acid-induced resistance against the brown spot pathogen Cochliobolus miyabeanus in rice involves MAP kinase-mediated repression of ethylene signaling. Plant Physiol. 2010, 152, 2036–2052. [Google Scholar] [CrossRef]
- Asselbergh, B.; Achuo, A.E.; Höfte, M.; VAN Gijsegem, F. Abscisic acid deficiency leads to rapid activation of tomato defence responses upon infection with Erwinia chrysanthemi. Mol. Plant Pathol. 2007, 9, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, N.; Dolezal, K.; In, M.C.A.; Strnad, M.; Az, M.S.-D. Influence of mycorrhizae and rhizobium on cytokinin content in drought-stressed alfalfa. J. Exp. Bot. 1995, 46, 1543–1549. [Google Scholar] [CrossRef]
- Ko, K.-W.; Okada, K.; Koga, J.; Jikumaru, Y.; Nojiri, H.; Yamane, H. Effects of cytokinin on production of diterpenoid phytoalexins in rice. J. Pestic. Sci. 2010, 35, 412–418. [Google Scholar] [CrossRef]
- Choi, J.; Choi, D.; Lee, S.; Ryu, C.-M.; Hwang, I. Cytokinins and plant immunity: Old foes or new friends? Trends Plant Sci. 2011, 16, 388–394. [Google Scholar] [CrossRef]
- Székács, A.; Hegedűs, G.; Tóbiás, I.; Pogány, M.; Barna, B. Immunoassays for plant cytokinins as tools for the assessment of environmental stress and disease resistance. Anal. Chim. Acta 2000, 421, 135–146. [Google Scholar] [CrossRef]
- Jung, H.W.; Tschaplinski, T.J.; Wang, L.; Glazebrook, J.; Greenberg, J.T. Priming in systemic plant immunity. Science 2009, 324, 89–91. [Google Scholar] [CrossRef]
- Bean, K.M.; Kisiala, A.B.; Morrison, E.N.; Emery, R.J.N. Trichoderma Synthesizes Cytokinins and Alters Cytokinin Dynamics of Inoculated Arabidopsis Seedlings. J. Plant Growth Regul. 2021, 41, 2678–2694. [Google Scholar] [CrossRef]
- Sørensen, J.L.; Benfield, A.H.; Wollenberg, R.D.; Westphal, K.; Wimmer, R.; Nielsen, M.R.; Nielsen, K.F.; Carere, J.; Covarelli, L.; Beccari, G.; et al. The cereal pathogen Fusarium pseudograminearum produces a new class of active cytokinins during infection. Mol. Plant Pathol. 2017, 19, 1140–1154. [Google Scholar] [CrossRef]
- Behr, M.; Motyka, V.; Weihmann, F.; Malbeck, J.; Deising, H.B.; Wirsel, S.G.R. Remodeling of cytokinin metabolism at infection sites of Colletotrichum graminicola on maize leaves. Mol. Plant-Microbe Interact. 2012, 25, 1073–1082. [Google Scholar] [CrossRef]
- Cooper, S.; Ashby, A. Comparison of cytokinin and cytokinin-O-glucoside cleaving β-glucosidase production in vitro by Venturia inaequalis and other phytopathogenic fungi with differing modes of nutrition in planta. Physiol. Mol. Plant Pathol. 1998, 53, 61–72. [Google Scholar] [CrossRef]
- Angra-Sharma, R.; Sharma, D. Cytokinins in pathogenesis and disease resistance of Pyrenophora teres-barley and Dreschslera maydis-maize interactions during early stages of infection. Mycopathologia 1999, 148, 87–95. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A century of gibberellin research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef]
- Vrabka, J.; Niehaus, E.-M.; Münsterkötter, M.; Proctor, R.H.; Brown, D.W.; Novák, O.; Pěnčik, A.; Tarkowská, D.; Hromadová, K.; Hradilová, M.; et al. Production and Role of Hormones During Interaction of Fusarium Species With Maize (Zea mays L.) Seedlings. Front. Plant Sci. 2019, 9, 1936. [Google Scholar] [CrossRef]
- Navarro, L.; Bari, R.; Achard, P.; Lisón, P.; Nemri, A.; Harberd, N.P.; Jones, J.D. DELLAs Control Plant Immune Responses by Modulating the Balance of Jasmonic Acid and Salicylic Acid Signaling. Curr. Biol. 2008, 18, 650–655. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2008, 69, 473–488. [Google Scholar] [CrossRef]
- Yoshioka, H.; Asai, S.; Yoshioka, M.; Kobayashi, M. Molecular mechanisms of generation for nitric oxide and reactive oxygen species, and role of the radical burst in plant immunity. Mol. Cells 2009, 28, 321–329. [Google Scholar] [CrossRef]
- Probanza, A.; Mehouachi, J.; Tadeo, F.R.; Talon, M.; Gutiérrez-Mañero, F.J.; Ramos-Solano, B. The plant-growth-promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiol. Plant. 2001, 111, 206–211. [Google Scholar] [CrossRef]
- Shahzad, R.; Waqas, M.; Khan, A.L.; Asaf, S.; Khan, M.A.; Kang, S.-M.; Yun, B.-W.; Lee, I.-J. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol. Biochem. 2016, 106, 236–243. [Google Scholar] [CrossRef]
- Khan, A.L.; Waqas, M.; Kang, S.-M.; Al-Harrasi, A.; Hussain, J.; Al-Rawahi, A.; Al-Khiziri, S.; Ullah, I.; Ali, L.; Jung, H.-Y.; et al. Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J. Microbiol. 2014, 52, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Bidel, L.P.R.; Coumans, M.; Baissac, Y.; Doumas, P.; Jay-Allemand, C. Biological Activity of Phenolics in Plant Cells. In Recent Advances in Polyphenol Research; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2010; Volume 2. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Atanasova-Penichon, V.; Pons, S.; Pinson-Gadais, L.; Picot, A.; Marchegay, G.; Bonnin-Verdal, M.-N.; Ducos, C.; Barreau, C.; Roucolle, J.; Sehabiague, P.; et al. Chlorogenic acid and maize ear rot resistance: A dynamic study investigating Fusarium graminearum development, deoxynivalenol production, and phenolic acid accumulation. Mol. Plant-Microbe Interact. 2012, 25, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Santiago, R.; Barros-Rios, J.; Malvar, R.A. Impact of cell wall composition on maize resistance to pests and diseases. Int. J. Mol. Sci. 2013, 14, 6960–6980. [Google Scholar] [CrossRef] [PubMed]
- Ponts, N.; Pinson-Gadais, L.; Boutigny, A.-L.; Barreau, C.; Richard-Forget, F. Cinnamic-derived acids significantly affect Fusarium graminearum growth and in vitro synthesis of type B trichothecenes. Phytopathology 2011, 101, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ji, J.; Zhang, S.; Xiao, W.; Guan, C.; Wang, G.; Wang, Y. Changes in the phenolic compound content and antioxidant activity in developmental maize kernels and expression profiles of phenolic biosynthesis-related genes. J. Cereal Sci. 2020, 96, 103113. [Google Scholar] [CrossRef]
- Bernardi, J.; Stagnati, L.; Lucini, L.; Rocchetti, G.; Lanubile, A.; Cortellini, C.; De Poli, G.; Busconi, M.; Marocco, A. Phenolic Profile and Susceptibility to Fusarium Infection of Pigmented Maize Cultivars. Front. Plant Sci. 2018, 9, 1189. [Google Scholar] [CrossRef] [PubMed]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Latha, P.; Anand, T.; Ragupathi, N.; Prakasam, V.; Samiyappan, R. Antimicrobial activity of plant extracts and induction of systemic resistance in tomato plants by mixtures of PGPR strains and Zimmu leaf extract against Alternaria solani. Biol. Control 2009, 50, 85–93. [Google Scholar] [CrossRef]
- Ta, G.; Aswathanarayana, D.; Naik, M.; Kenganal, M.; Kuchanur, P.H.; Beladadi, R. Positive Role of Salicylic Acid and Trichoderma on the Enhancement of Systemic Acquired Resistance (SAR) and Induced Systemic Resistance (ISR) in Maize—Exserohilum turcicum Pathosystem under Greenhouse Condition. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1509–1522. [Google Scholar] [CrossRef]
- Djellout, H.; Raio, A.; Boutoumi, H.; Krimi, Z. Bacillus and Pseudomonas spp. strains induce a response in phenolic profile and enhance biosynthesis of antioxidant enzymes in Agrobacterium tumefaciens infected tomato plants. Eur. J. Plant Pathol. 2020, 157, 269–280. [Google Scholar] [CrossRef]
- Tuladhar, P.; Sasidharan, S.; Saudagar, P. Role of phenols and polyphenols in plant defense response to biotic and abiotic stresses. In Biocontrol Agents and Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Huffaker, A.; Kaplan, F.; Vaughan, M.M.; Dafoe, N.J.; Ni, X.; Rocca, J.R.; Alborn, H.T.; Teal, P.E.; Schmelz, E.A. Novel acidic sesquiterpenoids constitute a dominant class of pathogen-induced phytoalexins in maize. Plant Physiol. 2011, 156, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Kaplan, F.; Huffaker, A.; Dafoe, N.J.; Vaughan, M.M.; Ni, X.; Rocca, J.R.; Alborn, H.T.; Teal, P.E. Identity, regulation, and activity of inducible diterpenoid phytoalexins in maize. Proc. Natl. Acad. Sci. USA 2011, 108, 5455–5460. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Fu, J.; Liang, J.; Shen, Q.; Liu, L.; Wang, L.; Wang, Q. Promoter Variation Results in Differential Phytoalexin Accumulation in Two Maize Inbred Lines. Plant Mol. Biol. Rep. 2020, 38, 165–174. [Google Scholar] [CrossRef]
- Poloni, A.; Schirawski, J. Red card for pathogens: Phytoalexins in sorghum and maize. Molecules 2014, 19, 9114–9133. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, P.; Raja, N.U.; Gunasekaran, P. Induction and accumulation of phytoalexins in cowpea roots infected with a mycorrhizal fungus Glomus fasciculatum and their resistance to Fusarium wilt disease. J. Biosci. 1993, 18, 291–301. [Google Scholar] [CrossRef]
- Wang, M.; Ma, J.; Fan, L.; Fu, K.; Yu, C.; Gao, J.; Li, Y.; Chen, J. Biological control of southern corn leaf blight by Trichoderma atroviride SG3403. Biocontrol Sci. Technol. 2015, 25, 1133–1146. [Google Scholar] [CrossRef]
- Naumann, T.A.; Wicklow, D.T. Allozyme-specific modification of a maize seed chitinase by a protein secreted by the fungal pathogen Stenocarpella maydis. Phytopathology 2010, 100, 645–654. [Google Scholar] [CrossRef]
- Amian, A.A.; Papenbrock, J.; Jacobsen, H.-J.; Hassan, F. Enhancing transgenic pea (Pisum sativum L.) resistance against fungal diseases through stacking of two antifungal genes (Chitinase and Glucanase). GM Crop. 2011, 2, 104–109. [Google Scholar] [CrossRef]
- Hawkins, L.K.; Mylroie, J.E.; Oliveira, D.A.; Smith, J.S.; Ozkan, S.; Windham, G.L.; Williams, W.P.; Warburton, M.L. Characterization of the Maize Chitinase Genes and Their Effect on Aspergillus flavus and Aflatoxin Accumulation Resistance. PLoS ONE 2015, 10, e0126185. [Google Scholar] [CrossRef]
- Su, Y.; Xu, L.; Fu, Z.; Yang, Y.; Guo, J.; Wang, S.; Que, Y. ScChi, encoding an acidic class III chitinase of sugarcane, confers positive responses to biotic and abiotic stresses in sugarcane. Int. J. Mol. Sci. 2014, 15, 2738–2760. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xue, X.; Cui, S.; Zhang, X.; Han, Q.; Zhu, L.; Liang, X.; Wang, X.; Huang, L.; Chen, X.; et al. Cloning and characterization of a wheat b-1,3-glucanase gene induced by the stripe rust pathogen Puccinia striiformis f. sp. tritici. Mol. Biol. Rep. 2009, 37, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Mojerlou, S.; Safaie, N.; Abbasi Moghaddam, A.; Shams-Bakhsh, M. Characterizing resistance genes in wheat-stem rust interaction. J. Agric. Sci. Technol. 2020, 22, 1629–1644. [Google Scholar]
- Tran, T.M.; Ameye, M.; Landschoot, S.; Devlieghere, F.; De Saeger, S.; Eeckhout, M.; Audenaert, K. Molecular insights into defense responses of vietnamese maize varieties to Fusarium verticillioides isolates. J. Fungi 2021, 7, 724. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, G.; Kumar, A.; Sandilya, S.P.; Chutia, M.; Yadav, A.N. Diversity, Plant Growth Promoting Attributes, and Agricultural Applications of Rhizospheric Microbes. In Plant Microbiomes for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Sartori, M.; Bonacci, M.; Barra, P.; Fessia, A.; Etcheverry, M.; Nesci, A.; Barros, G. Studies on Possible Modes of Action and Tolerance to Environmental Stress Conditions of Different Biocontrol Agents of Foliar Diseases in Maize. Agric. Sci. 2020, 11, 552–566. [Google Scholar] [CrossRef]
- Li, Y.; Sun, R.; Yu, J.; Saravanakumar, K.; Chen, J. Antagonistic and Biocontrol Potential of Trichoderma asperellum ZJSX5003 Against the Maize Stalk Rot Pathogen Fusarium graminearum. Indian J. Microbiol. 2016, 56, 318–327. [Google Scholar] [CrossRef]
- Zia, M.A.; Riaz, R.; Batool, A.; Yasmin, H.; Nosheen, A.; Naz, R.; Hassan, M.N. Glucanolytic rhizobacteria associated with wheat-maize cropping system suppress the Fusarium wilt of tomato (Lycopersicum esculentum L.). Sci. Hortic. 2021, 287, 110275. [Google Scholar] [CrossRef]
- Badiaa, E.; Abdeljabbar, H.; Mohamed, R.H.; Abdellatif, B.; Najla, S.-Z. In vivo and in vitro evaluation of antifungal activities from a halotolerant Bacillus subtilis strain J9. Afr. J. Microbiol. Res. 2012, 6, 4073–4083. [Google Scholar] [CrossRef]
- Jain, S.; Vaishnav, A.; Kumari, S.; Varma, A.; Tuteja, N.; Choudhary, D.K. Chitinolytic Bacillus-Mediated Induction of Jasmonic Acid and Defense-Related Proteins in Soybean (Glycine max L. Merrill) Plant against Rhizoctonia solani and Fusarium oxysporum. J. Plant Growth Regul. 2016, 36, 200–214. [Google Scholar] [CrossRef]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 2006, 28, 1091–1101. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, D.; Pike, S.; Pallardy, S.; Gassmann, W.; Zhang, S. Chloroplast-generated reactive oxygen species are involved in hypersensitive response-like cell death mediated by a mitogen-activated protein kinase cascade. Plant J. 2007, 51, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Yang, H.; Zhang, S. Cell Death Mediated by MAPK Is Associated with Hydrogen Peroxide Production in Arabidopsis. J. Biol. Chem. 2002, 277, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Jwa, N.-S.; Hwang, B.K. Convergent evolution of pathogen effectors toward reactive oxygen species signaling networks in plants. Front. Plant Sci. 2017, 8, 1687. [Google Scholar] [CrossRef] [PubMed]
- Herrera Flores, T.S.; Ortíz Cereceres, J.; Delgado Alvarado, A.; Acosta Galleros, J.A. Contenido de osmoprotectores, ácido ascórbico y ascorbato peroxidasa en hojas de frijol sometidas a estrés por sequía. Rev. Mex. Cienc. Agrícolas 2014, 5, 859–870. [Google Scholar]
- Asmawati, L.; Widiastuti, A.; Sumardiyono, C. Proceeding of the 1st International Conference on Tropical Agriculture; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Bressan, W. Biological control of maize seed pathogenic fungi by use of actinomycetes. BioControl 2003, 48, 233–240. [Google Scholar] [CrossRef]
- Fang, R.; Lin, J.; Yao, S.; Wang, Y.; Wang, J.; Zhou, C.; Wang, H.; Xiao, M. Promotion of plant growth, biological control and induced systemic resistance in maize by Pseudomonas aurantiaca JD37. Ann. Microbiol. 2012, 63, 1177–1185. [Google Scholar] [CrossRef]
- Costa, F.G.; Zucchi, T.D.; de Melo, I.S. Biological control of phytopathogenic fungi by endophytic actinomycetes isolated from maize (Zea mays L.). Braz. Arch. Biol. Technol. 2013, 56, 948–955. [Google Scholar] [CrossRef]
- Figueiredo, M.d.L.C.; Cruz, I.; da Silva, R.B.; Foster, J.E. Biological control with Trichogramma pretiosum increases organic maize productivity by 19.4%. Agron. Sustain. Dev. 2015, 35, 1175–1183. [Google Scholar] [CrossRef]
- Degani, O.; Dor, S. Trichoderma biological control to protect sensitive maize hybrids against late wilt disease in the field. J. Fungi 2021, 7, 315. [Google Scholar] [CrossRef]
- Bejarano, A.; Puopolo, G. Bioformulation of Microbial Biocontrol Agents for a Sustainable Agriculture. In How Research Can Stimulate the Development of Commercial Biological Control against Plant Diseases; De Cal, A., Melgarejo, P., Magan, N., Eds.; Progress in Biological Control; Springer: Cham, Switzerland, 2020; Volume 21. [Google Scholar] [CrossRef]
- Sansinenea, E. Applications and patents of Bacillus spp. in agriculture. In Intellectual Property Issues in Microbiology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 133–146. [Google Scholar]
- Marrone, P.G. Pesticidal natural products—Status and future potential. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef]
- Elnahal, A.S.M.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.-S.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
| Host Plant | MBCA | Mode of Action | Pathogen/Disease | Reference |
|---|---|---|---|---|
| Arabidopsis spp. | Pseudomonas fluorescens | ISR via ET and JA | - | [82] |
| Rice | Pseudomonas fluorescens | ISR via ET and JA | Magnaporthe oryzae | [83] |
| Maize | Pseudomonas putida | ISR via JA and ABA upregulation | Colletotrichum graminicola | [63] |
| Pea | Bacillus pumilus | Phenolic compounds | Fusarium oxysporum | [139] |
| Maize | Trichoderma atroviride and SA | Phenolic compounds | Exserohilum turcicum | [141] |
| Tomato | Bacillus spp. and Pseudomonas spp. | Phenolic compounds and antioxidant enzymes | Agrobacterium tumefaciens | [142] |
| Maize | Trichoderma atroviride | Phenolic compounds and phytoalexins | Southern corn leaf blight | [143] |
| Vigna spp. | Vesicular-arbuscular mycorrhiza | Phytoalexins | Wilt | [148] |
| Soybean | Bacillus spp. | Cell-wall degrading and antioxidant enzymes and phenolic compounds | [162] | |
| Maize | Trichoderma spp. | ROS accumulation | Peronosclerospora spp. | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanacore, M.F.G.; Sartori, M.; Giordanino, F.; Barros, G.; Nesci, A.; García, D. Physiological Effects of Microbial Biocontrol Agents in the Maize Phyllosphere. Plants 2023, 12, 4082. https://doi.org/10.3390/plants12244082
Vanacore MFG, Sartori M, Giordanino F, Barros G, Nesci A, García D. Physiological Effects of Microbial Biocontrol Agents in the Maize Phyllosphere. Plants. 2023; 12(24):4082. https://doi.org/10.3390/plants12244082
Chicago/Turabian StyleVanacore, María Fiamma Grossi, Melina Sartori, Francisco Giordanino, Germán Barros, Andrea Nesci, and Daiana García. 2023. "Physiological Effects of Microbial Biocontrol Agents in the Maize Phyllosphere" Plants 12, no. 24: 4082. https://doi.org/10.3390/plants12244082
APA StyleVanacore, M. F. G., Sartori, M., Giordanino, F., Barros, G., Nesci, A., & García, D. (2023). Physiological Effects of Microbial Biocontrol Agents in the Maize Phyllosphere. Plants, 12(24), 4082. https://doi.org/10.3390/plants12244082






