Impact of Harvest Time on the Dry Matter Content, and Nutritional Parameters Related to Forage Quality of Maralfalfa (Cenchrus purpureus (Schumach.) Morrone, Poaceae) under Mediterranean Climate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Crops and Plant Sampling
2.2. Ash
2.3. Ether Extract (EE)
2.4. Crude Protein (CP)
2.5. Fibers (Neutral, and Acid Detergent Fibers)
2.6. Plant Extraction and Crude Extract Purification
2.7. Amino Acids Profile
2.8. Determination of Tryptophan
2.9. Experimental Design and Data Treatments
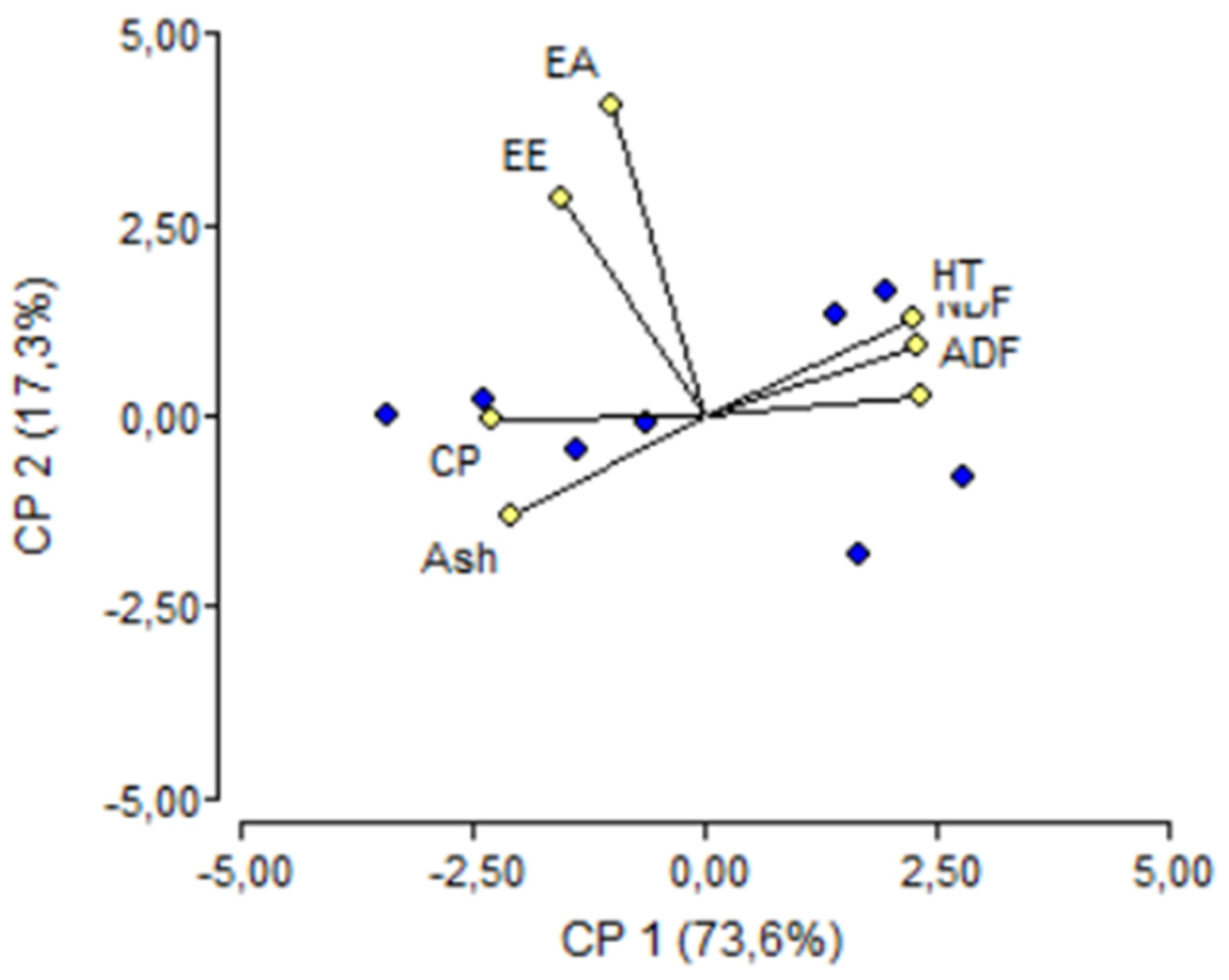
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macoon, B.; Sollenberger, L.E.; Moore, J.E. Defoliation Effects on Persistence and Productivity of Four spp. Genotypes. Agron. J. 2002, 94, 541–548. [Google Scholar] [CrossRef]
- Negawo, A.T.; Teshome, A.; Kumar, A.; Hanson, J.; Jones, C.S. Opportunities for Napier Grass (Pennisetum purpureum) Improvement Using Molecular Genetics. Agronomy 2017, 7, 28. [Google Scholar] [CrossRef]
- Gupta, S.K.; Govintharaj, P.; Bhardwaj, R. Three-Way Top-Cross Hybrids to Enhance Production of Forage with Improved Quality in Pearl Millet (Pennisetum glaucum (L.) R. Br.). Agriculture 2022, 12, 1508. [Google Scholar] [CrossRef]
- Ukanti, A.K.; Gowda, C.L.L.; Rai, K.N.; Manga, V.K.; Bhatt, R.K. Crops that feed the world 11. Pearl Millet (Pennisetum glaucum L.): An important source of food security, nutrition and health in the arid and semi-arid tropics. Food Sec. 2016, 8, 307–329. [Google Scholar] [CrossRef]
- Rosales-Serna, R.; Ríos-Saucedo, J.C.; Martínez-Galindo, J.A.; Carrillo-Parra, A.; Santana-Espinoza, S.; Jiménez-Ocampo, R.; Domínguez-Martínez, P.A. Yield and Bioenergy Quality of Maralfalfa Biomass Obtained at Different Plant Strata and Cutting Dates. Energies 2023, 16, 448. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture. Livestock in the Balance; FAO: Rome, Italy, 2009. [Google Scholar]
- Iannucci, A.; Carroni, A.M.; Martiniello, P. Performances of legume-grass mixtures under different cutting managements in Mediterranean environments. Ital. J. Agron. 2006, 1, 359–368. [Google Scholar] [CrossRef]
- Tariq, M.; Ayub, M.; Elahi, M.; Ahmad, A.H.; Chaudhary, M.N.; Nadeem, M.A. Forage yield and some quality attributes of millet (Pennisetum americannum L.) hybrid under various regimes of nitrogen fertilization and harvesting dates. Afr. J. Agric. Res. 2011, 6, 3883–3890. [Google Scholar]
- Larsen, S.U.; Jørgensen, H.; Bukh, C.; Schjoerring, J.K. Green biorefining: Effect of nitrogen fertilization on protein yield, protein extractability and amino acid composition of tall fescue biomass. Ind. Crops Prod. 2019, 130, 642–652. [Google Scholar] [CrossRef]
- Fahey, G.C., Jr. (Ed.) Forage Quality, Evaluation, and Utilization; American Society of Agronomy, Inc. Crop Science Society of America, Inc. Soil Science Society of America, Inc.: Madison, WI, USA, 1994. [Google Scholar] [CrossRef]
- Fulgueira, C.L.; Amigot, S.L.; Gaggiotti, M.; Romero, L.A.; Basílico, J.C. Forage Quality: Techniques for Testing; Global Science Books, Ltd.: UK, 2007. Available online: http://www.globalsciencebooks.info/Online/GSBOnline/images/0712/FP_1 (accessed on 30 September 2023).
- Sidari, M.; Panuccio, M.; Muscolo, A. Influence of Acidity on Growth and Biochemistry of Pennisetum clandestinum. Biol. Plant. 2004, 48, 133–136. [Google Scholar] [CrossRef]
- Mandić, V.; Bijelić, Z.; Krnjaja, V.; Simić, A.; Petričević, M.; Mićić, N.; Petrović, V.C. Effect of harvesting time on forage yield and quality of maize. Biotechnol. Anim. Husb. 2018, 34, 345–353. [Google Scholar] [CrossRef]
- Ronga, D.; Dal Prà, A.; Immovilli, A.; Ruozzi, F.; Davolio, R.; Pacchioli, M.T. Effects of Harvest Time on the Yield and Quality of Winter Wheat Hay Produced in Northern Italy. Agronomy 2020, 10, 917. [Google Scholar] [CrossRef]
- Åman, P.; Graham, H. Whole crop peas. Changes in botanical and chemical composition and rumen in vitro degradability during maturation. Animal Feed Sci. Tech. 1987, 17, 15–31. [Google Scholar] [CrossRef]
- Buxton, D.R. Quality-related characteristics of forages as influenced by plant environment and agronomic factors. Anim. Feed Sci. Tech. 1996, 59, 3749. [Google Scholar] [CrossRef]
- Szuba-Trznadel, A.; Hikawczuk, T.; Jama-Rodzeńska, A.; Król, Z.; Fuchs, B. The Effect of Harvest Date on the Chemical Composition and Fodder Yield of Guizotia abyssinica (Guizotia abyssinica (L.f.) Cass.) under the Climatic Conditions of South-West Poland. Agriculture 2022, 12, 481. [Google Scholar] [CrossRef]
- Márquez, F.; Sánchez, J.; Urbano, D.; Dávila, D. Evaluación de la frecuencia de corte y tipos de fertilización sobre tres genotipos de pasto elefante (Pennisetum purpureum). 1. Rendimiento y contenido de proteína. Zootec. Trop. 2007, 25, 253–259. [Google Scholar]
- Palacios-Diaz, M.D.P.; Mendoza-Grimón, V.; Garcia, A.D.V. Influence of Policy Making in the Profitability of Forage Production Irrigated with Reclaimed Water. Water 2015, 7, 4274–4282. [Google Scholar] [CrossRef]
- Nava-Berumen, C.A.; Carrete-Carreón, F.O.; Rosales-Serna, R.; Reyes-Estrada, O.; Domínguez-Martínez, P.; Herrera-Torres, E. Rendimiento y calidad de forraje obtenido con el pasto maralfalfa cosechado a diferentes edades de rebrote en Durango, México. Investig. Cienc. Univ. Autónoma Aguascalientes 2021, 29, e3070. [Google Scholar]
- de Torres, J.; Garzón, E.; Ryan, J.; González-Andrés, F. Organic Cereal/Forage Legume Rotation in a Mediterranean Calcareous Soil: Implications for Soil Parameters. Agroecol. Sustain. Food Syst. 2013, 37, 215–230. [Google Scholar] [CrossRef]
- Criscioni, P.; Marti, J.V.; Pérez-Baena, I.; Palomares, J.L.; Larsen, T.; Fernández, C. Replacement of alfalfa hay (Medicago sativa) with maralfalfa hay (Pennisetum sp.) in diets of lactating dairy goats. Anim. Feed Sci. Technol. 2016, 219, 1–12. [Google Scholar] [CrossRef]
- Calzada-Marín, J.M.; Enríquez Quiroz, J.F.; Hernández Garay, A.; Ortega Jiménez, E.; Mendoza Pedrosa, S.I. Análisis de crecimiento del pasto maralfalfa (Pennisetum sp.) en clima cálido subhúmedo. Rev. Mex. De Cienc. Pecu. 2014, 5, 247–260. [Google Scholar] [CrossRef]
- Gamón, M.; Sáez, E.; Gil, J.; Boluda, R. Direct and Indirect Exogenous Contamination by Pesticides of Rice-Farming Soils in a Mediterranean Wetland. Arch. Environ. Contam. Toxicol. 2003, 44, 0141–0151. [Google Scholar] [CrossRef]
- Boluda, R.; Andreu, V.; Gilabert, M.A.; Sobrino, P. Relation between reflectance of rice crop and indices of pollution by heavy metals in soils of Albufera Natural Park (Valencia, Spain). Soil Technol. 1993, 6, 351–363. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Nielsen, S. Food Analysis Laboratory Manual; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003. [Google Scholar]
- García-Martínez, E.M.; Fernández-Segovia, I.; Fuentes-López, A. Aplicación de la determinación de proteínas de un alimento por el método Kjeldahl. Valoración con una base fuerte. In Publicaciones de la Universitat Politècnica de València; Editorial Universitat Politècnica de València: València, Spain, 2013; Available online: http://hdl.handle.net/10251/29832 (accessed on 30 September 2023).
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976, 7, 248–254. [Google Scholar] [CrossRef]
- Urribarrí, C.; Ferrer, A.; Colina, A. Extracción y precipitación de las proteínas solubles del pasto elefante enano (Pennisetum purpureum Schum cv. Mott). Rev. Fac. Agron. 2004, 21, 268–279. [Google Scholar]
- Otter, D.E. Standardised methods for amino acid analysis of food. Br. J. Nutr. 2012, 108, S230–S237. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat, Versión 2008; Grupo InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2008. [Google Scholar]
- Jung, H.G.; Allen, M.S. Characteristics of plant cell walls affecting intake and digestibility of forages by ruminants. J. Anim. Sci. 1995, 73, 2774–2790. [Google Scholar] [CrossRef]
- Murphy, J.D.; Braun, R.; Weiland, P.; Wellinger, A. Biogas from crop digestion. In Task 37—Energy from Biogas; IEA Bioenergy: Paris, France, 2011. [Google Scholar]
- King, C.; McEniry, J.; Richardson, M.; O’Kiely, P. Yield and chemical composition of five common grassland species in response to nitrogen fertiliser application and phenological growth stage. Acta Agric. Scand. Sect. 2012, 62, 644–658. [Google Scholar] [CrossRef]
- Maleko, D.; Mwilawa, A.; Msalya, G.; Pasape, L.; Mtei, K. Forage growth, yield and nutritional characteristics of four varieties of napier grass (Pennisetum purpureum Schumach) in the west Usambara highlands, Tanzania. Sci. Afr. 2019, 6, e00214. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Ahmad, A.; Sohail, A.; Asad, M.J. Nutritional and functional characterization of different oat (Avena sativa L.) cultivars. Int. J. Food Prop. 2020, 23, 1373–1385. [Google Scholar] [CrossRef]
- Beck, P.A.; Stewart, C.B.; Gray, H.C.; Smith, J.L.; Gunter, S.A. Effect of wheat forage maturity and preservation method on forage chemical composition and performance of growing calves fed mixed diets. J. Anim. Sci. 2009, 87, 4133–4142. [Google Scholar] [CrossRef]
- MacKown, C.T.; Heitholt, J.J.; Rao, S.C. Agronomic feasibility of a continuous double crop of winter wheat and soybean forage in the southern Great Plains. Crop Sci. 2007, 47, 1652–1660. [Google Scholar] [CrossRef]
- Borreani, G.; Cavallarin, L.; Antoniazzi, S.; Tabacco, E. Effect of the stage of growth, wilting and inoculation in field pea (Pisum sativum L.) silages. I. Herbage composition and silage fermentation. J. Sci. Food Agric. 2006, 86, 1377–1382. [Google Scholar] [CrossRef]
- Arambel, M.J.; Coon, C.N. Effect of Dietary Protein on Amino Acids and Microbial Protein of Duodenal Digesta. J. Dairy Sci. 1981, 64, 2201–2208. [Google Scholar] [CrossRef]
- Schwab, C.G.; Broderick, G.A. A 100-Year Review: Protein and amino acid nutrition in dairy cows. J. Dairy Sci. 2017, 100, 10094–10112. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Schwab, C.G.; Whitehouse, N.L. Feed supplements: Ruminally protected amino acids. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 540–547. ISBN 9780128187678. [Google Scholar] [CrossRef]
- Glew, R.H.; Vanderjagt, D.J.; Lockett, C.; Grivetti, L.E.; Smith, G.C.; Pastuszyn, A.; Millson, M. Amino Acid, Fatty Acid, and Mineral Composition of 24 Indigenous Plants of Burkina Faso. J. Food Compos. Anal. 1997, 10, 205–217. [Google Scholar] [CrossRef]
- Mazinani, M.; Naserian, A.A.; Rude, B.J.; Tahmasbi, A.M.; Valizadeh, R. Effects of feeding rumen-protected amino acids on the performance of feedlot calves. J. Adv. Vet. Anim. Res. 2020, 13, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Varastegani, A.; Dahlan, I. Influence of dietary fiber levels on feed utilization and growth performance in poultry. J. Anim. Prod. Adv. 2014, 4, 422–429. [Google Scholar] [CrossRef]
- Peyraud, J.; Astigarraga, L. Review of the effect of nitrogen fertilization on the chemical composition, intake, digestion and nutritive value of fresh herbage: Consequences on animal nutrition and N balance. Anim. Feed Sci. Technol. 1998, 72, 235–259. [Google Scholar] [CrossRef]
- Yadav, A.K.; Chauhan, S.K.; Shroti, S.K. Effect of sowing dates and nitrogen levels on yield and economics of vegetable pea-wheatmaize cropping system in central part of Uttar Pradesh. Ann. Plant Soil Res. 2012, 14, 159–162. [Google Scholar]
- Biel, W.; Podsiadło, C.; Witkowicz, R.; Kępińska-Pacelik, J.; Stankowski, S. Effect of Irrigation, Nitrogen Fertilization and Amino Acid Biostimulant on Proximate Composition and Energy Value of Pisum sativum L. Seeds. Agriculture 2023, 13, 376. [Google Scholar] [CrossRef]
- Kishorekumar, R.; Bulle, M.; Wany, A.; Gupta, K.J. An overview of important enzymes involved in nitrogen assimilation of plants. Methods Mol. Biol. 2020, 2057, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Leite, R.G.; Cardoso, A.D.; Fonseca, N.V.B.; Curvelo Silva, M.L.; Orlindo Tedeschi, L.; Maneck Delevatti, L.; Ruggieri, A.C.; Reis, R.A. Effects of nitrogen fertilization on protein and carbohydrate fractions of Marandu palisadegrass. Sci. Rep. 2021, 11, 14786. [Google Scholar] [CrossRef] [PubMed]
- Viegi, L.; Pieroni, A.; Guarrera, P.M.; Vangelisti, R.A. A review of plants used in folk veterinary medicine in Italy as basis for a databank. J. Ethnopharmacol. 2003, 89, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Gaikwad, D.S. Phytogenic Feed Additives in Animal Nutrition; Singh, J., Yadav, A., Eds.; Natural Bioactive Products in Sustainable Agriculture; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Kuralkar, P.; Kuralkar, S.V. Role of herbal products in animal production—An updated review. J. Ethnopharmacol. 2021, 278, 114246. [Google Scholar] [CrossRef] [PubMed]
- Al-Tabini, R.; Al-Khalidi, K.; Al-Shudiefat, M. Livestock, medicinal plants and rangeland viability in Jordan’s Badia: Through the lens of traditional and local knowledge. Pastoralism 2012, 2, 4. [Google Scholar] [CrossRef]
- Chakale, M.V.; Mwanza, M.; Aremu, A.O. Ethnoveterinary Knowledge and Biological Evaluation of Plants Used for Mitigating Cattle Diseases: A Critical Insight into the Trends and Patterns in South Africa. Front. Vet. Sci. 2021, 8, 710884. [Google Scholar] [CrossRef]
- Willer, J.; Zidorn, C.; Juan-Vicedo, J. Ethnopharmacology, phytochemistry, and bioactivities of Hieracium L. and Pilosella Hill (Cichorieae, Asteraceae) species. J. Ethnopharmacol. 2021, 281, 114465. [Google Scholar] [CrossRef]
- Haq, S.M.; Yaqoob, U.; Majeed, M.; Amjad, M.S.; Hassan, M.; Ahmad, R.; Waheed, M.; Bussmann, R.W.; Calixto, E.S.; Procków, J.; et al. Quantitative ethnoveterinary study on plant resource utilization by indigenous communities in high-altitude regions. Front. Vet. Sci. 2022, 9, 944046. [Google Scholar] [CrossRef]
- Juan-Vicedo, J.; Ramírez-Luna, J.E.; Piqueras, A.; Casas, J.L. Micropropagation and cryopreservation by vitrification of the Spanish endemic medicinal plant Sideritis leucantha Cav. subsp. leucantha (Lamiaceae). In Vitro Cell. Dev. Biol. Plant 2021, 57, 1057–1065. [Google Scholar] [CrossRef]
- Hasnain, A.; Naqvi, S.A.H.; Ayesha, S.I.; Khalid, F.; Ellahi, M.; Iqbal, S.; Hassan, M.Z.; Abbas, A.; Adamski, R.; Markowska, D.; et al. Plants in vitro propagation with its applications in food, pharmaceuticals and cosmetic industries; current scenario and future approaches. Front. Plant Sci. 2022, 13, 1009395. [Google Scholar] [CrossRef]
- Asensio, E.; de Medinacelli Juan-Méndez, R.; Juan-Vicedo, J. In Vitro Propagation and Phytochemistry of Thymol-Producing Plants from a Horticultural Form of Thymus × josephi-angeli Mansanet & Aguil. (Lamiaceae). Horticulturae 2022, 8, 1188. [Google Scholar] [CrossRef]
| Temperature 2017 (°C) | Rainfall 2017 (mm) | Temperature 2018 (°C) | Rainfall 2018 (mm) | |
|---|---|---|---|---|
| January | 9.3 | 92.1 | 14.0 | 29.1 |
| February | 11.8 | 14.7 | 12.5 | 58.8 |
| March | 18.3 | 48.1 | 16.8 | 13.6 |
| April | 17.6 | 34.6 | 18.1 | 18.3 |
| May | 20.8 | 4.2 | 20.3 | 21.4 |
| June | 25.2 | 42.8 | 24.4 | 90.9 |
| July | 26.0 | 0.6 | 27.2 | 2.4 |
| August | 27.5 | 18.1 | 27.4 | 2.2 |
| September | 26.2 | 13.4 | 24.2 | 186.3 |
| October | 21.4 | 21.7 | 18.9 | 227.9 |
| November | 14.6 | 7.4 | 15.0 | 154.7 |
| December | 9.3 | 3.8 | 13.1 | 12.0 |
| HTs (Days) | DM (%) | Ash (%) | EE (%) | CP (%) | NDF (%) | ADF (%) |
|---|---|---|---|---|---|---|
| 16 | 13.00 | 13.13 ± 0.32 ab | 0.019 ± 0.003 a | 21.57 ± 0.98 a | 50.94 ± 0.42 g | 30.12 ± 1.05 f |
| 28 | 16.40 | 13.07 ± 0.17 ab | 0.016 ± 0.002 a | 15.53 ± 0.71 b | 57.13 ± 1.11 f | 34.76 ± 0.38 e |
| 42 | 15.40 | 14.48 ± 0.21 a | 0.014 ± 0.012 a | 8.84 ± 0.12 c | 63.12 ± 0.62 e | 37.52 ± 1.03 d |
| 58 | 19.40 | 13.38 ± 0.12 ab | 0.018 ± 0.01 b | 6.49 ± 1.33 d | 67.32 ± 0.88 d | 41.86 ± 0.69 c |
| 75 | 16.60 | 09.24 ± 0.39 bc | 0.001 ± 0.0002 a | 3.91 ± 0.12 e | 71.35 ± 0.59 c | 49.16 ± 0.48 b |
| 87 | 13.80 | 7.08 ± 2.90 c | 0.017 ± 0.010 a | 3.88 ± 0.14 e | 75.72 ± 0.59 b | 52.87 ± 0.16 a |
| 124 | 25.20 | 6.42 ± 0.14 c | 0.008 ± 0.001 ab | 2.19 ± 0.11 ef | 78.24 ± 0.64 a | 54.42 ± 0.41 a |
| 150 | 16.50 | 6.80 ± 0.16 c | 0.009 ± 0.002 ab | 3.08 ± 0.01 f | 78.94 ± 0.26 a | 49.06 ± 0.80 b |
| Amino Acid | HTs (Days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 16 | 28 | 42 | 58 | 75 | 87 | 124 | 150 | |
| Alanine | 7.29 ± 0.70 | 8.84 ± 1.47 | 7.50 ± 0.85 | 16.77 ± 2.12 | 17.33 ± 2.92 | 13.24 ± 4.09 | 31.57 ± 9.21 | 8.60 ± 2.49 |
| Arginine | 10.12 ± 0.31 | 9.72 ± 2.47 | 11.18 ± 1.76 | 7.59 ± 0.49 | 20.65 ± 4.83 | 11.37 ± 1.24 | 6.47 ± 1.31 | 8.92 ± 1.18 |
| Aspartate | 11.21 ± 0.09 | 10.67 ± 0.24 | 10.46 ± 0.26 | 11.95 ± 0.57 | 8.69 ± 1.82 | 8.94 ± 1.18 | 8.63 ± 1.82 | 11.53 ± 0.37 |
| Cysteine | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Glutamic acid | 12.13 ± 0.08 | 12.23 ± 0.24 | 12.49 ± 0.20 | 11.00 ± 0.45 | 4.34 ± 0.30 | 5.26 ± 0.60 | 5.29 ± 0.90 | 7.34 ± 0.24 |
| Glycine | 4.51 ± 0.07 | 4.80 ± 0.19 | 3.78 ± 0.04 | 4.14 ± 0.08 | 4.02 ± 1.43 | 4.68 ± 0.67 | 3.96 ± 0.64 | 4.58 ± 0.33 |
| Histidine | 2.43 ± 0.09 | 1.71 ± 0.15 | 1.68 ± 0.22 | 1.48 ± 0.11 | 2.18 ± 0.30 | 3.64 ± 0.13 | 2.20 ± 0.44 | 3.37 ± 0.39 |
| Isoleucine * | 4.82 ± 0.12 | 4.95 ± 0.10 | 5.13 ± 0.08 | 4.80 ± 0.13 | 4.07 ± 0.37 | 4.96 ± 0.60 | 4.22 ± 0.87 | 5.21 ± 0.18 |
| Leucine * | 7.82 ± 0.15 | 8.17 ± 0.14 | 8.53 ± 0.12 | 7.80 ± 0.23 | 7.16 ± 1.58 | 7.83 ± 0.97 | 6.45 ± 1.35 | 8.98 ± 0.05 |
| Lysine * | 5.90 ± 0.01 | 6.00 ± 0.09 | 6.23 ± 0.24 | 5.39 ± 0.15 | 3.29 ± 1.38 | 6.14 ± 0.62 | 3.39 ± 1.49 | 5.65 ± 0.46 |
| Methionine * | 0.61 ± 0.06 | 0.02 ± 0.04 | 0.57 ± 0.42 | 0.21 ± 0.03 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Phenylalanine * | 4.93 ± 0.17 | 5.18 ± 0.07 | 5.18 ± 0.07 | 4.95 ± 0.15 | 4.39 ± 0.64 | 5.08 ± 0.37 | 4.47 ± 0.96 | 5.79 ± 0.14 |
| Proline | 5.93 ± 0.15 | 5.98 ± 0.20 | 5.45 ± 0.25 | 6.17 ± 0.37 | 5.15 ± 0.40 | 5.86 ± 1.03 | 6.11 ± 1.31 | 7.45 ± 2.58 |
| Serine | 4.62 ± 0.59 | 3.77 ± 0.01 | 3.73 ± 0.18 | 3.35 ± 0.21 | 3.27 ± 0.36 | 3.95 ± 0.46 | 3.53 ± 0.73 | 4.66 ± 0.12 |
| Threonine * | 7.89 ± 0.07 | 7.81 ± 0.04 | 7.73 ± 0.06 | 6.08 ± 0.27 | 7.00 ± 0.17 | 8.03 ± 1.17 | 5.45 ± 1.23 | 7.47 ± 1.20 |
| Tryptophan * | 0.52 ± 0.09 | 0.49 ± 0.09 | 0.38 ± 0.07 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.03 ± 0.02 | 0.07 ± 0.03 | 0.09 ± 0.04 |
| Tyrosine | 2.37 ± 0.10 | 2.29 ± 0.97 | 2.75 ± 0.91 | 1.43 ± 0.15 | 2.13 ± 0.31 | 3.78 ± 0.77 | 2.04 ± 0.78 | 2.03 ± 0.22 |
| Valine * | 6.91 ± 0.17 | 7.37 ± 0.23 | 7.24 ± 0.24 | 6.86 ± 0.27 | 6.43 ± 0.76 | 7.21 ± 0.42 | 6.16 ± 1.48 | 8.39 ± 0.27 |
| essential aa/ total aa | 0.39 | 0.4 | 0.36 | 0.36 | 0.32 | 0.39 | 0.3 | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayos-Febrer, J.; Juan-Vicedo, J.; Rodríguez-Mengod, A.; Mazón, J.; Gardón, J.C. Impact of Harvest Time on the Dry Matter Content, and Nutritional Parameters Related to Forage Quality of Maralfalfa (Cenchrus purpureus (Schumach.) Morrone, Poaceae) under Mediterranean Climate. Plants 2023, 12, 4045. https://doi.org/10.3390/plants12234045
Fayos-Febrer J, Juan-Vicedo J, Rodríguez-Mengod A, Mazón J, Gardón JC. Impact of Harvest Time on the Dry Matter Content, and Nutritional Parameters Related to Forage Quality of Maralfalfa (Cenchrus purpureus (Schumach.) Morrone, Poaceae) under Mediterranean Climate. Plants. 2023; 12(23):4045. https://doi.org/10.3390/plants12234045
Chicago/Turabian StyleFayos-Febrer, Joaquín, Jorge Juan-Vicedo, Alba Rodríguez-Mengod, Javier Mazón, and Juan Carlos Gardón. 2023. "Impact of Harvest Time on the Dry Matter Content, and Nutritional Parameters Related to Forage Quality of Maralfalfa (Cenchrus purpureus (Schumach.) Morrone, Poaceae) under Mediterranean Climate" Plants 12, no. 23: 4045. https://doi.org/10.3390/plants12234045
APA StyleFayos-Febrer, J., Juan-Vicedo, J., Rodríguez-Mengod, A., Mazón, J., & Gardón, J. C. (2023). Impact of Harvest Time on the Dry Matter Content, and Nutritional Parameters Related to Forage Quality of Maralfalfa (Cenchrus purpureus (Schumach.) Morrone, Poaceae) under Mediterranean Climate. Plants, 12(23), 4045. https://doi.org/10.3390/plants12234045






