A Review of the Role of an Anthocyanin, Cyanidin-3-O-β-glucoside in Obesity-Related Complications
Abstract
:1. Introduction
2. The Occurrence and Color Characteristics of C3G
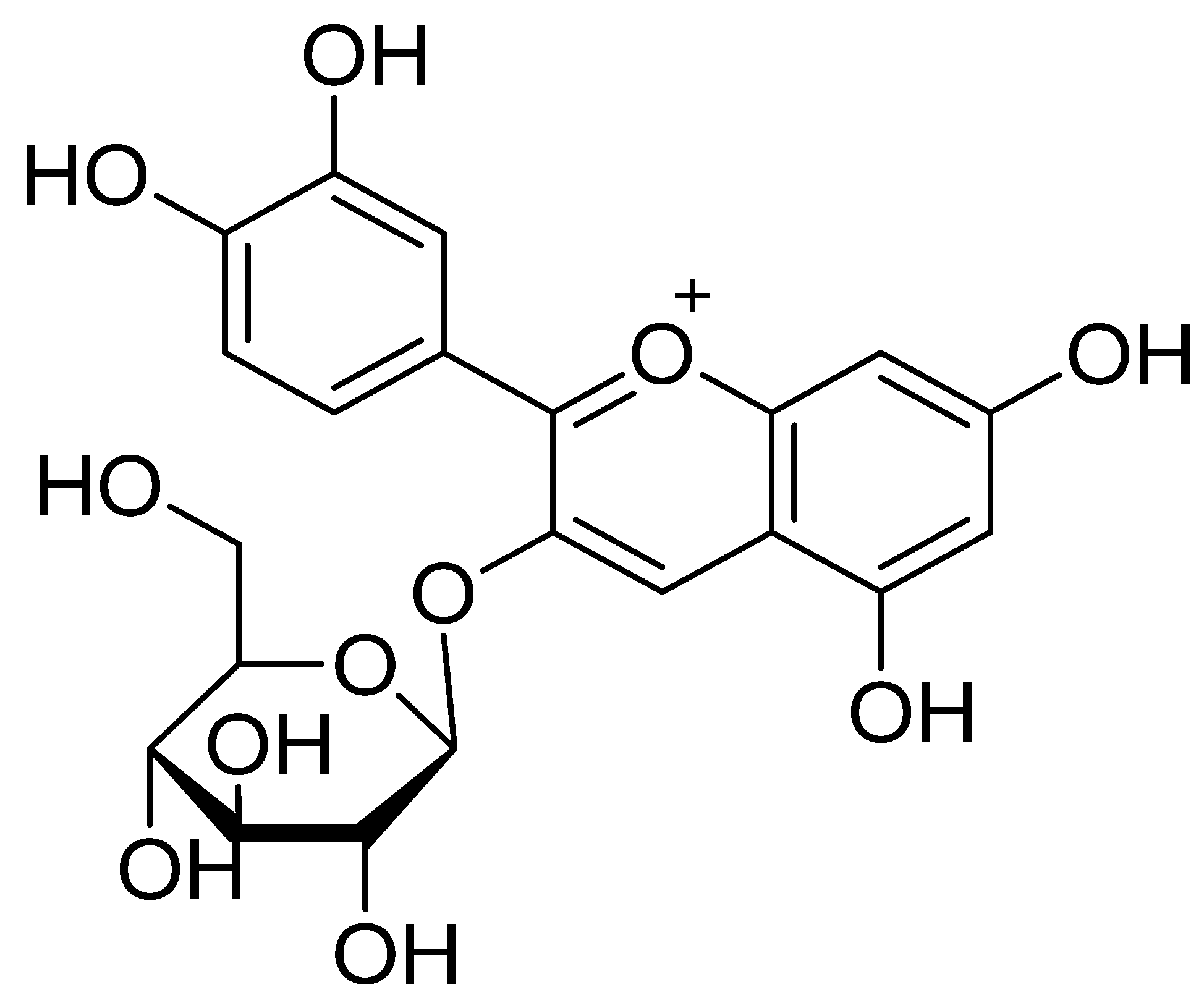
3. The Chemistry of C3G
4. In Vitro Studies on the Role of C3G in Obesity-Related Complications
5. In Vivo Studies on the Role of C3G in Obesity-Related Complications
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| AGTR-1 | Angiotensin II Receptor Type 1 |
| Akt | Protein Kinase B |
| AMPK | Adenosine monophosphate-activated protein kinase |
| aP2 | Adipocyte Protein 2 |
| ATP | Adenosine triphosphate |
| BMP-2 | Bone Morphogenetic Protein-2 |
| C/EBPα | CCAAT/enhancer-binding protein α |
| C3G | Cyanidin-3-O-β-glucoside |
| C3R | Cyanidin-3-rutinoside |
| cAMP | Cyclic adenosine 3′,5′-monophosphate |
| ChREBP | Carbohydrate response element-binding protein |
| COX-2 | Cyclooxygenase 2 |
| CPT-1 | Carnitine palmitoyltransferase I |
| FABP4 | Fatty acid-binding protein 4 |
| FAS | Fatty acid synthase |
| FGF21 | Fibroblast growth factor 21 i |
| FoxO1 | Forkhead box protein O1 |
| GFAT | Glutamine:fructose-6-phosphate aminotransferase |
| GLUT4 | Glucose transporter type 4 |
| GPx | Glutathione peroxidase |
| H2O2 | Hydrogen peroxide |
| hAECs | Human aortic endothelial cells |
| HFD | High-fat diet |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| IRS-1 | Insulin receptor substrate 1 |
| IκBα | Inhibitor of nuclear factor kappa B |
| JNK | c-Jun N-terminal kinase |
| LPL | Lipoprotein lipase |
| malonyl CoA | Malonyl coenzyme A |
| MCP-1 | Monocyte chemoattractant protein-1 |
| mRNA | Messenger RNA |
| MRP-2 | Macrophage inflammatory protein-related protein-2 |
| NF-κB | Nuclear factor kappa B |
| NPC1L1 | Niemann-Pick C1-Like 1 |
| OVX | Ovariectomy |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PGC1α | Peroxisome proliferator-activated receptor-γ coactivator 1-α |
| PI3K | Phosphoinositide 3-kinase |
| PPARs | Peroxisome proliferator-activated receptors |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| PPARγ | Peroxisome proliferator-activated receptor gammaa |
| PRDM16 | PR/SET Domain 16 |
| PTP1B | Protein tyrosine phosphatase 1B |
| ROS | Reactive oxygen species |
| Runx-2 | Runt-related transcription factor 2 |
| SIRT1 | Sirtuin1 |
| SOD | Superoxide dismutase |
| SOD2 | Superoxide dismutase-2 |
| SREBP-1c | Sterol regulatory element-binding protein 1c |
| STAT3 | Signal transducer and activator of transcription 3 |
| TFAM | Mitochondrial transcription factor A |
| TNF-α | Tumor necrosis factor alpha |
| UCP | Uncoupling protein |
| WHO | World Health Organization |
References
- Wharton, S.; Lau, D.C.W.; Vallis, M.; Sharma, A.M.; Biertho, L.; Campbell-Scherer, D. Obesity in adults: A clinical practice guideline. Can. Med. Assoc. J. 2020, 192, E875–E891. [Google Scholar] [CrossRef]
- Zhang, Q.; Vital, D.L.; Mejia, E.G.D. Anthocyanins from colored maize ameliorated the inflammatory paracrine interplay between macrophages and adipocytes through regulation of NF-κB and JNK-dependent MAPK pathways. J. Funct. Foods 2019, 54, 175–186. [Google Scholar] [CrossRef]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef]
- Xu, H.; Liu, M.; Liu, H.; Zhao, B.; Zheng, M.; Liu, J. Anthocyanins from purple corn ameliorated obesity in high fat diet-induced obese mice through activating hepatic AMPK. J. Funct. Foods 2021, 84, 104582. [Google Scholar] [CrossRef]
- Gomes, J.V.P.; Rigolon, T.C.B.; Souza, M.S.D.S. Antiobesity effects of anthocyanins on mitochondrial biogenesis, inflammation, and oxidative stress: A systematic review. Nutrition 2019, 66, 192–202. [Google Scholar] [CrossRef]
- Wolfenden, L.; Ezzati, M.; Larijani, B.; Dietz, W. The challenge for global health systems in preventing and managing obesity. Obes. Rev. 2019, 20, 185–193. [Google Scholar] [CrossRef]
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Palou, A.; Bonet, M.L. Challenges in obesity research. Nutr. Hosp. 2013, 5, 144–153. [Google Scholar]
- Lao, F.; Sigurdson, G.T.; Giusti, M.M. Health benefits of purple corn (Zea mays L.) phenolic compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 234–246. [Google Scholar] [CrossRef]
- Cuevas Montilla, E.; Hillebrand, S.; Antezana, A.; Winterhalter, P. Soluble and bound phenolic compounds in different Bolivian purple corn (Zea mays L.) cultivars. J. Agric. Food Chem. 2011, 59, 7068–7074. [Google Scholar] [CrossRef]
- Yang, Z.; Zhai, W. Identification and antioxidant activity of anthocyanins extracted from the seed and cob of purple corn (Zea mays L.). Innov. Food Sci. Emerg. Technol. 2010, 11, 169–176. [Google Scholar] [CrossRef]
- You, Y.; Zhou, F.; Huang, D. Eating the right color: Dietary anthocyanins and obesity control. Food Beverage Asia 2018, 12, 57–59. [Google Scholar]
- Overall, J.; Bonney, S.A.; Wilson, M.; Beermann, A.; Grace, M.H.; Esposito, D.; Lila, M.A.; Komarnytsky, S. Metabolic Effects of Berries with Structurally Diverse Anthocyanins. Int. J. Mol. Sci. 2017, 18, 422. [Google Scholar] [CrossRef]
- Acquaviva, R.; Russo, A.; Galvano, F.; Galvano, G.; Barcellona, M.L.; Li Volti, G.; Vanella, A. Cyanidin and cyanidin 3-O-beta-D-glucoside as DNA cleavage protectors and antioxidants. Cell Biol. Toxicol. 2003, 19, 243–252. [Google Scholar] [CrossRef]
- Galvano, F.; La Fauci, L.; Lazzarino, G.; Fogliano, V.; Ritieni, A.; Ciappellano, S.; Battistini, N.C.; Tavazzi, B.; Galvano, G. Cyanidins: Metabolism and biological properties. J. Nutr. Biochem. 2004, 15, 2–11. [Google Scholar] [CrossRef]
- Zhu, W.; Jia, Q.; Wang, Y.; Zhang, Y.; Xia, M. The anthocyanin cyanidin-3-O-β-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP-PKA-dependent signaling pathway. Free Radic. Biol. Med. 2012, 52, 314–327. [Google Scholar] [CrossRef]
- You, Y.; Yuan, X.; Liu, X.; Liang, C.; Meng, M.; Huang, Y.; Han, X.; Guo, J.; Guo, Y.; Ren, C.; et al. Cyanidin-3-glucoside increases whole body energy metabolism by upregulating brown adipose tissue mitochondrial function. Mol. Nutr. Food Res. 2017, 61, 1700261. [Google Scholar] [CrossRef]
- Zannou, O.; Oussou, K.F.; Chabi, I.B.; Awad, N.M.H.; Aïssi, M.V.; Goksen, G.; Mortas, M.; Oz, F.; Proestos, C.; Kayodé, A.P.P. Nanoencapsulation of Cyanidin 3-O-Glucoside: Purpose, technique, bioavailability, and stability. Nanomaterials 2023, 13, 617. [Google Scholar] [CrossRef]
- Jia, Y.; Wu, C.; Kim, Y.S.; Yang, S.O.; Kim, Y.; Kim, J.S.; Jeong, M.Y.; Lee, J.H.; Kim, B.; Lee, S.; et al. A dietary anthocyanin cyanidin-3-O-glucoside binds to PPARs to regulate glucose metabolism and insulin sensitivity in mice. Commun. Biol. 2020, 3, 514. [Google Scholar] [CrossRef]
- Spinardi, A.; Cola, G.; Gardana, C.S.; Mignani, I. Variation of Anthocyanin Content and Profile Throughout Fruit Development and Ripening of Highbush Blueberry Cultivars Grown at Two Different Altitudes. Front. Plant Sci. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, B.; Liu, C.; Zong, Y.; Shao, Y.; Liu, B.; Yue, H. Variation of anthocyanin content in fruits of wild and cultivated Lycium ruthenicum. Ind. Crops Prod. 2020, 146, 11220. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Cheng, Z.; Si, X.; Tan, H.; Zang, Z.; Tian, J.; Shu, C.; Sun, X.; Li, Z.; Jiang, Q.; Meng, X.; et al. Cyanidin-3-O-glucoside and its phenolic metabolites ameliorate intestinal diseases via modulating intestinal mucosal immune system: Potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. Nutr. 2023, 63, 1629–1647. [Google Scholar] [CrossRef]
- Frountzas, M.; Karanikki, E.; Toutouza, O.; Sotirakis, D.; Schizas, D.; Theofilis, P.; Tousoulis, D.; Toutouzas, K.G. Exploring the Impact of Cyanidin-3-Glucoside on Inflammatory Bowel Diseases: Investigating New Mechanisms for Emerging Interventions. Int. J. Mol. Sci. 2023, 24, 9399. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, Z.; Feng, L.; Fang, Y. Cloning and expression of anthocyanin biosynthetic genes in red and white pomegranate. J. Plant Res. 2015, 128, 687–696. [Google Scholar] [CrossRef]
- Tan, J.; Li, Y.; Hou, D.X.; Wu, S. The Effects and Mechanisms of Cyanidin-3-Glucoside and Its Phenolic Metabolites in Maintaining Intestinal Integrity. Antioxidants 2019, 8, 479. [Google Scholar] [CrossRef]
- Tsuda, T.; Ueno, Y.; Aoki, H.; Koda, T.; Horio, F.; Takahashi, N.; Kawada, T.; Osawa, T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun. 2004, 316, 149–157. [Google Scholar] [CrossRef]
- Tsuda, T.; Ueno, Y.; Yoshikawa, T.; Kojo, H.; Osawa, T. Microarray profiling of gene expression in human adipocytes in response to anthocyanins. Biochem. Pharmacol. 2006, 71, 1184–1197. [Google Scholar] [CrossRef]
- Choe, M.R.; Kang, J.H.; Yoo, H.; Choe, S.Y.; Yang, C.H.; Kim, M.O.; Yu, R. Cyanidin and Cyanidin-3-O-β-D-glucoside suppress the inflammatory responses of obese adipose tissue by inhibiting the release of chemokines MCP-1 and MRP-2. J. Food Sci. Nutr. 2007, 12, 148–153. [Google Scholar] [CrossRef]
- Guo, H.; Ling, W.; Wang, Q.; Liu, C.; Hu, Y.; Xia, M. Cyanidin 3-glucoside protects 3T3-L1 adipocytes against H2O2- or TNF-alpha-induced insulin resistance by inhibiting c-Jun NH2-terminal kinase activation. Biochem. Pharmacol. 2008, 75, 1393–1401. [Google Scholar] [CrossRef]
- Titta, L.; Trinei, M.; Stendardo, M.; Berniakovich, I.; Petroni, K.; Tonelli, C.; Riso, P.; Porrini, M.; Minucci, S.; Pelicci, P.G.; et al. Blood orange juice inhibits fat accumulation in mice. Int. J. Obes. 2010, 34, 578–588. [Google Scholar] [CrossRef]
- Inaguma, T.; Han, J.; Isoda, H. Improvement of insulin resistance by Cyanidin 3-glucoside, anthocyanin from black beans through the up-regulation of GLUT4 gene expression. BMC Proc. 2011, 5 (Suppl. S8), P21. [Google Scholar] [CrossRef]
- Wei, X.; Wang, D.; Yang, Y.; Xia, M.; Li, D.; Li, G.; Zhu, Y.; Xiao, Y.; Ling, W. Cyanidin-3-O-β-glucoside improves obesity and triglyceride metabolism in KK-Ay mice by regulating lipoprotein lipase activity. J. Sci. Food Agric. 2011, 91, 1006–1013. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Vari, R.; Filesi, C.; Archivio, M.D.; Santangelo, C.; Giovannini, C.; Lacovelli, A.; Silecchia, G.; Volti, G.L.; Galvano, F.; et al. Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARγ activity in human omental adipocytes. Diabetes 2011, 60, 2234–2244. [Google Scholar] [CrossRef]
- Pompei, A.; Toniato, E.; Innocenti, P.D.; Alimonte, I.; Cellini, C.; Mattoscio, D.; Cotellese, R.; Bosco, D.; Ciccarelli, R.; Dadorante, V.D.; et al. Cyanidin reduces preadipocyte differentiation and relative ChREBP expression. J. Biol. Regul. Homeost. Agents 2012, 26, 253–264. [Google Scholar]
- Guo, H.; Liu, G.; Zhong, R.; Wang, Y.; Wang, D.; Xia, M. Cyanidin-3-O-β-glucoside regulates fatty acid metabolism via an AMP-activated protein kinase-dependent signaling pathway in human HepG2 cells. Lipids Health Dis. 2012, 11, 10. [Google Scholar] [CrossRef]
- Guo, H.; Guo, J.; Jiang, X.; Li, Z.; Ling, W. Cyanidin-3-O-β-glucoside, a typical anthocyanin, exhibits antilipolytic effects in 3T3-L1 adipocytes during hyperglycemia: Involvement of FoxO1-mediated transcription of adipose triglyceride lipase. Food Chem. Toxicol. 2012, 50, 3040–3047. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, J.N.; Han, S.N.; Nam, J.H.; Na, H.N.; Ha, T.J. Black soybean anthocyanins inhibit adipocyte differentiation in 3T3-L1 cells. Nutr. Res. 2012, 32, 770–777. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Zhang, Y.; Sun, R.; Xia, M. Anthocyanin increases adiponectin secretion and protects against diabetes-related endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E975–E988. [Google Scholar] [CrossRef]
- Park, S.; Kang, S.; Jeong, D.Y.; Jeong, S.Y.; Park, J.J.; Yun, H.S. Cyanidin and malvidin in aqueous extracts of black carrots fermented with Aspergillus oryzae prevent the impairment of energy, lipid and glucose metabolism in estrogen-deficient rats by AMPK activation. Genes Nutr. 2015, 10, 455. [Google Scholar] [CrossRef]
- Matsukawa, T.; Inaguma, T.; Han, J.; Villareal, M.O.; Isoda, H. Cyanidin-3-glucoside derived from black soybeans ameliorate type 2 diabetes through the induction of differentiation of preadipocytes into smaller and insulin-sensitive adipocytes. J. Nutr. Biochem. 2015, 26, 860–867. [Google Scholar] [CrossRef]
- Bjork, C.; Wilhelm, U.; Mandrup, S.; Larsen, B.D.; Bordoni, A.; Heden, P.; Ryden, M.; Arner, P.; Laurencikiene, J. Effects of selected bioactive food compounds on human white adipocyte function. Nutr. Metab. 2016, 13, 4. [Google Scholar] [CrossRef]
- Choi, K.H.; Lee, H.A.; Park, M.H.; Han, J.S. Cyanidin-3-rutinoside increases glucose uptake by activating the PI3K/Akt pathway in 3T3-L1 adipocytes. Environ. Toxicol. Pharmacol. 2017, 54, 1–6. [Google Scholar] [CrossRef]
- Luna-Vital, D.; Weiss, M.; Mejia, E.G.D. Anthocyanins from purple corn ameliorated tumor necrosis factor-α-induced inflammation and insulin resistance in 3T3-L1 adipocytes via activation of insulin signaling and enhanced GLUT4 translocation. Mol. Nutr. Food Res. 2017, 61, 1700362. [Google Scholar] [CrossRef]
- Matsukawa, T.; Villareal, M.O.; Motojima, H.; Isoda, H. Increasing cAMP levels of preadipocytes by cyanidin-3-glucoside treatment induces the formation of beige phenotypes in 3T3-L1 adipocytes. J. Nutr. Biochem. 2017, 40, 77–85. [Google Scholar] [CrossRef]
- Pei, L.; Wan, T.; Wang, S.; Ye, M.; Qiu, Y.; Jiang, R.; Pang, N.; Huang, Y.; Zhou, Y.; Jiang, X.; et al. Cyanidin-3-O-β-glucoside regulates the activation and the secretion of adipokines from brown adipose tissue and alleviates diet induced fatty liver. Biomed. Pharmacother. 2018, 105, 625–632. [Google Scholar] [CrossRef]
- Muscarà, C.; Molonia, M.S.; Speciale, A.; Bashllari, R.; Cimino, F.; Occhiuto, C.; Saija, A.; Cristani, M. Anthocyanins ameliorate palmitate-induced inflammation and insulin resistance in 3T3-L1 adipocytes. Phytother. Res. 2019, 33, 1888–1897. [Google Scholar] [CrossRef]
- Thilavech, T.; Adisakwattana, S. Cyanidin-3-rutinoside acts as a natural inhibitor of intestinal lipid digestion and absorption. BMC Complement. Altern. Med. 2019, 19, 242. [Google Scholar] [CrossRef]
- Vijayaraj, P.; Nakagawa, H.; Yamaki, K. Cyanidin and cyanidin-3-glucoside derived from Vigna unguiculata act as noncompetitive inhibitors of pancreatic lipase. J. Food Biochem. 2019, 43, e12774. [Google Scholar] [CrossRef]
- Saulite, L.; Jekabsons, K.; Klavins, M.; Muceniece, R.; Riekstina, U. Effects of malvidin, cyanidin and delphinidin on human adipose mesenchymal stem cell differentiation into adipocytes, chondrocytes and osteocytes. Phytomedicine 2019, 53, 86–95. [Google Scholar] [CrossRef]
- Zhang, Q.; Mejia, E.G.D.; Vital, D.L.; Tao, T.; Chandrasekaran, S.; Chatham, L.; Juvik, J.; Singh, V.; Kumar, D. Relationship of phenolic composition of selected purple maize (Zea mays L.) genotypes with their anti-inflammatory, anti-adipogenic and anti-diabetic potential. Food Chem. 2019, 289, 739–750. [Google Scholar] [CrossRef]
- Molonia, M.S.; Occhiuto, C.; Muscara, C.; Speciale, A.; Bashllari, R.; Villarroya, F.; Saija, A.; Cimino, F.; Cristani, M. Cyanidin-3-O-glucoside restores insulin signaling and reduces inflammation in hypertrophic adipocytes. Arch. Biochem. Biophys. 2020, 691, 108488. [Google Scholar] [CrossRef]
- Madduma Hewage, S.; Prashar, S.; Debnath, S.C.; Karmin, O.; Siow, Y.L. Inhibition of Inflammatory Cytokine Expression Prevents High-Fat Diet-Induced Kidney Injury: Role of Lingonberry Supplementation. Front. Med. 2020, 7, 80. [Google Scholar] [CrossRef]
- Han, S.; Yang, Y.; Lu, Y.; Guo, J.; Han, X.; Gao, Y.; Huang, W.; You, Y.; Zhan, J. Cyanidin-3-O-glucoside regulates the expression of Ucp1 in brown adipose tissue by activating Prdm16 gene. Antioxidants 2021, 10, 1986. [Google Scholar] [CrossRef] [PubMed]
- Molonia, M.S.; Quesada-Lopez, T.; Speciale, A.; Muscara, C.; Saija, A.; Villarroya, F.; Cimino, F. In vitro effects of Cyanidin-3-O-Glucoside on inflammatory and insulin-sensitizing genes in human adipocytes exposed to palmitic acid. Chem. Biodivers. 2021, 12, e2100607. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Ferdousi, F.; Zheng, Y.-W.; Oda, T.; Isoda, H. Human amniotic epithelial cells as a tool to investigate the effects of cyanidin 3-O-Glucoside on cell differentiation. Int. J. Mol. Sci. 2021, 22, 3768. [Google Scholar] [CrossRef]
- Channuwong, P.; Salae, K.; Chongruchiroj, S.; Cheng, H.; Suantawee, T.; Thilavech, T.; Adisakwattana, S. Dietary anthocyanins inhibit insulin fibril formation and cytotoxicity in 3T3-L1 preadipocytes. Int. J. Biol. Macromol. 2022, 223, 1578–1585. [Google Scholar] [CrossRef]
- Kongthitilerd, P.; Barras, E.; Rong, W.; Thibodeaux, A.; Rigdon, M.; Yao, S.; Adisakwattana, S.; Suantawee, T.; Cheng, H. Cyanidin inhibits adipogenesis in 3T3-L1 preadipocytes by activating the PLC-IP3 pathway. Biomed. Pharmacother. 2023, 162, 114677. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Liu, H.; Xie, J.; Yin, W.; Xu, Z.; Ma, H.; Wu, W.; Zheng, M.; Liu, M.; et al. Characterization of the synergistic inhibitory effect of cyanidin-3-O-glucoside and catechin on pancreatic lipase. Food Chem. 2023, 404, 134672. [Google Scholar] [CrossRef]
- Molonia, M.S.; Salamone, F.L.; Muscarà, C.; Costa, G.; Vento, G.; Saija, A.; Speciale, A.; Cimino, F. Regulation of mitotic clonal expansion and thermogenic pathway are involved in the antiadipogenic effects of cyanidin-3-O-glucoside. Front. Pharmacol. 2023, 14, 1225586. [Google Scholar] [CrossRef]
- Vangoori, Y.; Dakshinamoorthi, A.; Kavimani, S. Prominent Pancreatic Lipase Inhibition and Free Radical Scavenging Activity of a Myristica fragrans Ethanolic Extract in vitro. Potential Role in Obesity Treatment. Maedica 2019, 14, 254–259. [Google Scholar]
- Chen, L.; Chen, W.; Li, D.; Liu, X. Anthocyanin and proanthocyanidin from Aronia melanocarpa (Michx.) Ell.: Purification, fractionation, and enzyme inhibition. Food Sci. Nutr. 2023, 11, 3911–3922. [Google Scholar] [CrossRef]
- Xie, L.; Xie, J.; Xu, Y.; Chen, W. Discovery of anthocyanins from cranberry extract as pancreatic lipase inhibitors using a combined approach of ultrafiltration, molecular simulation and spectroscopy. Food Funct. 2020, 11, 8527–8536. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef]
- Hogan, S.; Canning, C.; Sun, S.; Sun, X.; Zhou, K. Effects of grape pomace antioxidant extract on oxidative stress and inflammation in diet induced obese mice. J. Agric. Food Chem. 2010, 58, 11250–11256. [Google Scholar] [CrossRef] [PubMed]
- Kanamoto, Y.; Yamashita, Y.; Nanba, F.; Yoshida, T.; Tsuda, T.; Fukuda, I.; Nakamura-Tsuruta, S.; Ashida, H. A black soybean seed coat extract prevents obesity and glucose intolerance by up-regulating uncoupling proteins and down-regulating inflammatory cytokines in high-fat diet-fed mice. J. Agric. Food Chem. 2011, 59, 8985–8993. [Google Scholar] [CrossRef]
- Kaume, L.; Gilbert, W.C.; Brownmiller, C.; Howard, L.R.; Devareddy, L. Cyanidin 3-O-β-D-glucoside-rich blackberries modulate hepatic gene expression, and anti-obesity effects in ovariectomized rats. J. Funct. Foods 2012, 4, 480–488. [Google Scholar] [CrossRef]
- Guo, H.; Xia, M.; Zou, T.; Ling, W.; Zhong, R.; Zhang, W. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. J. Nutr. Biochem. 2012, 23, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Qi, X.; Liu, Y.; Guo, J.; Zhu, R.; Chen, W.; Zheng, X.; Yu, T. Dietary supplementation with purified mulberry (Morus australis Poir) anthocyanins suppresses body weight gain in high-fat diet fed C57BL/6 mice. Food Chem. 2013, 141, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Ren-Qiang, Y.; Xiao-You, Q.; Xiang, Z.; Jing, Z.; Lu-Yi, M. Cyanidin-3-glucoside attenuates body weight gain, serum lipid concentrations and insulin resistance in high-fat diet-induced obese rats. Chin. J. Contemp. Pediatr. 2014, 16, 534–538. [Google Scholar]
- Esposito, D.; Damsud, T.; Wilson, M.; Grace, M.H.; Strauch, R.; Li, X.; Lila, M.A.; Komarnytsky, S. Black Currant Anthocyanins Attenuate Weight Gain and Improve Glucose Metabolism in Diet-Induced Obese Mice with Intact, but Not Disrupted, Gut Microbiome. J. Agric. Food Chem. 2015, 63, 6172–6180. [Google Scholar] [CrossRef]
- Farrell, N.J.; Norris, G.H.; Ryan, J.; Porter, C.M.; Jiang, C.; Blesso, C.N. Black elderberry extract attenuates inflammation and metabolic dysfunction in diet-induced obese mice. Br. J. Nutr. 2015, 114, 1123–1131. [Google Scholar] [CrossRef]
- Yamane, T.; Kozuka, M.; Konda, D.; Nakano, Y.; Nakagaki, T.; Ohkubo, I.; Ariga, H. Improvement of blood glucose levels and obesity in mice given aronia juice by inhibition of dipeptidyl peptidase IV and α-glucosidase. J. Nutr. Biochem. 2016, 31, 106–112. [Google Scholar] [CrossRef]
- Wu, T.; Guo, X.; Zhang, M.; Yang, L.; Liu, R.; Yin, J. Anthocyanins in black rice, soybean and purple corn increase fecal butyric acid and prevent liver inflammation in high fat diet-induced obese mice. Food Funct. 2017, 8, 3178–3186. [Google Scholar] [CrossRef]
- Biswas, D.; Sarkar, S.; De Silva, A.B.K.H.; D’Souza, K.; Kienesberger, P.; Rupasinghe, H.P.V.; Pulinilkunnil, T. Cyanidin-3-O-Glucoside rich extract from haskap berry improves glucose homeostasis and insulin sensitivity in diet-induced obese mice. Can. J. Diabetes 2018, 42, S24–S61. [Google Scholar] [CrossRef]
- Shi, M.; O’Keefe, L.; Simcocks, A.C.; Su, X.Q.; McAinch, A.J. The effect of cyanidin-3-O-β-glucoside and peptides extracted from yoghurt on glucose uptake and gene expression in human primary skeletal muscle myotubes from obese and obese diabetic participants. J. Funct. Foods 2018, 51, 55–64. [Google Scholar] [CrossRef]
- Daveri, E.; Cremonini, E.; Mastaloudis, A.; Hester, S.N.; Wood, S.M.; Waterhouse, A.L.; Anderson, M.; Fraga, C.G.; Oteiza, P.I. Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Biol. 2018, 18, 16–24. [Google Scholar] [CrossRef]
- You, Y.; Han, X.; Guo, J.; Guo, Y.; Yin, M.; Liu, G.; Huang, W.; Zhan, J. Cyanidin-3-glucoside attenuates high-fat and high-fructose diet-induced obesity by promoting the thermogenic capacity of brown adipose tissue. J. Funct. Foods 2018, 41, 62–71. [Google Scholar] [CrossRef]
- Shi, M.; Mathai, M.L.; Xu, G.; McAinch, A.J.; Su, X.Q. The effects of supplementation with blueberry, cyanidin-3-O-β-glucoside, yoghurt and its peptides on obesity and related comorbidities in a diet-induced obese mouse model. J. Funct. Foods 2019, 56, 92–101. [Google Scholar] [CrossRef]
- Lim, S.-M.; Lee, H.S.; Jung, J.I.; Kim, S.M.; Kim, N.Y.; Seo, T.S.; Bae, J.-S.; Kim, E.J. Cyanidin-3-O-Galactoside-enriched Aronia melanocarpa extract attenuates weight gain and adipogenic pathways in high-fat diet-induced obese C57BL/6 mice. Nutrients 2019, 11, 1190. [Google Scholar] [CrossRef]
- Zhao, R.; Xiang, B.; Dolinsky, V.W.; Xia, M.; Shen, G.X. Saskatoon berry powder reduces hepatic steatosis and insulin resistance in high fat-high sucrose diet-induced obese mice. J. Nutr. Biochem. 2021, 95, 108778. [Google Scholar] [CrossRef]
- Jiao, X.; Shen, Y.; Deng, H.; Zhang, Q.; Zhao, J. Cyanidin-3-O-galactoside from Aronia melanocarpa attenuates high-fat diet-induced obesity and inflammation via AMPK, STAT3, and NF-κB p65 signaling pathways in Sprague-Dawley rats. J. Funct. Foods 2021, 85, 104616. [Google Scholar] [CrossRef]
- Shi, M.; Mathai, M.L.; Xu, G.; Su, X.Q.; McAinch, A.J. The effect of dietary supplementation with blueberry, cyanidin-3-O-β-glucoside, yoghurt and its peptides on gene expression associated with glucose metabolism in skeletal muscle obtained from a high-fat-high-carbohydrate diet induced obesity model. PLoS ONE 2022, 17, e0270306. [Google Scholar] [CrossRef]
- Cremonini, E.; Daveri, E.; Iglesias, D.E.; Kang, J.; Wang, Z.; Gray, R.; Mastaloudis, A.; Kay, C.D.; Hester, S.N.; Wood, S.M.; et al. A randomized placebo-controlled cross-over study on the effects of anthocyanins on inflammatory and metabolic responses to a high-fat meal in healthy subjects. Redox Biol. 2022, 51, 102273. [Google Scholar] [CrossRef]
- Lyu, Q.; Deng, H.; Wang, S.; El-Seedi, H.; Cao, H.; Chen, L.; Teng, H. Dietary supplementation with casein/cyanidin-3-O-glucoside nanoparticles alters the gut microbiota in high-fat fed C57BL/6 mice. Food Chem. 2023, 412, 135494. [Google Scholar] [CrossRef]
- Tomay, F.; Marinelli, A.; Leoni, V.; Caccia, C.; Matros, A.; Mock, H.P.; Tonelli, C.; Petroni, K. Purple corn extract induces long-lasting reprogramming and M2 phenotypic switch of adipose tissue macrophages in obese mice. J. Transl. Med. 2019, 17, 237. [Google Scholar] [CrossRef]
- Takahashi, A.; Shimizu, H.; Okazaki, Y.; Sakaguchi, H.; Taira, T.; Suzuki, T.; Chiji, H. Anthocyanin-rich Phytochemicals from Aronia Fruits Inhibit Visceral Fat Accumulation and Hyperglycemia in High-fat Diet-induced Dietary Obese Rats. J. Oleo. Sci. 2015, 64, 1243–1250. [Google Scholar] [CrossRef]
- Meleleo, D.; Avato, P.; Conforti, F.; Argentieri, M.P.; Messina, G.; Cibelli, G.; Mallamaci, R. Interaction of Quercetin, Cyanidin, and Their O-Glucosides with Planar Lipid Models: Implications for Their Biological Effects. Membranes 2023, 13, 600. [Google Scholar] [CrossRef]
| Model | Concentration | Mechanism(s) | Year of Publication | References |
|---|---|---|---|---|
| Rat adipocytes | 100 µM |
| 2004 | [35] |
| Human preadipocytes | 100 µM |
| 2006 | [36] |
| 3T3-L1 adipocytes and RAW 264.7 cells | 10, 50, and 100 μM |
| 2007 | [37] |
| H2O2- and TNF-α-induced insulin resistance in 3T3-L1 adipocytes | 10, 20, and 40 µM |
| 2008 | [38] |
| Primary brown preadipocytes | 100 µM |
| 2010 | [39] |
| 3T3-L1 adipocytes | 20 and 100 μM |
| 2011 | [40] |
| Skeletal muscle cells and adipocytes from female KK-Ay mice | 10, 50, and 100 μmol/L |
| 2011 | [41] |
| Human omental adipocytes and 3T3-L1 cells. | Human omental adipocytes—50 and 100 µmol/L; 3T3-L1 cells—10 and 100 µmol/L |
| 2011 | [42] |
| Preadipocytes from human adipose explant tissue | 50 μM |
| 2012 | [43] |
| Human HepG2 cells | 100 μM |
| 2012 | [44] |
| 3T3-L1 adipocytes | 50 µM |
| 2012 | [45] |
| Preadipocyte 3T3-L1 cells | Black soybean anthocyanins; 12.5 and 50 µg/mL |
| 2012 | [46] |
| 3T3 adipocytes | 12.5, 25, and 50 µM |
| 2014 | [47] |
| 3T3-L1 fibroblasts | 5 or 20 µM |
| 2015 | [48] |
| 3T3-L1 adipocytes | 20 and 100 μM |
| 2015 | [49] |
| Primary human preadipocytes | 100 μM |
| 2016 | [50] |
| 3T3-L1 adipocytes | 10 and 50 μM |
| 2017 | [51] |
| 3T3-L1 adipocytes | 50 μM |
| 2017 | [52] |
| 3T3-L1 adipocytes | 50 and 100 μM |
| 2017 | [53] |
| Brown adipose tissue C3H10T1/2 clone 8 cells induced by palmitate | Isolated from mulberry; 100 and 200 μg/mL |
| 2018 | [54] |
| Palmitic acid-induced 3T3-L1 adipocytes | Anthocyanin-rich extract; 10 and 20 μg/mL |
| 2019 | [55] |
| Macrophage–adipocyte interaction, using mono- and co-culture | 1.0 mg/mL of the anthocyanin-rich extracts of purple and red maize or 50 μM of pure anthocyanins |
| 2019 | [2] |
| Pancreatic lipase and cholesterol esterase Caco-2 cells | 12.5–100 μM |
| 2019 | [56] |
| Pancreatic lipase activity | 50 to 350 µM |
| 2019 | [57] |
| Human adipose tissue | 25 μM |
| 2019 | [58] |
| RAW 264.7 macrophages and 3T3-L1 adipocytes | Anthocyanin-rich water extracts (PMWs) from purple maize; 1 mg/mL |
| 2019 | [59] |
| 3T3-L1 hypertrophic adipocytes exposed to palmitic acid | 5–10 μM |
| 2020 | [60] |
| Palmitic acid-induced proximal tubular cells | 2, 10, and 20 μM |
| 2020 | [61] |
| HepG2 cells and C2C12 myotubes | 10 and 50 μM |
| 2020 | [19] |
| C3H/10T1/2 brown adipose cells | 10–40 µM |
| 2021 | [62] |
| Human SGBS adipocytes and murine 3T3-L1 cells. | 1–20 μM |
| 2021 | [63] |
| Human amniotic epithelial cells (hAECs) | 20 µM |
| 2021 | [64] |
| 3T3-L1 preadipocytes | 50–200 μM |
| 2022 | [65] |
| 3T3-L1 preadipocytes | 30–100 μM |
| 2023 | [66] |
| Pancreatic lipase inhibitory assay | 0.05 to 0.35 mg/mL |
| 2023 | [67] |
| 3T3-L1 preadipocytes | 5 and 10 μM |
| 2023 | [68] |
| Model | Dose | Mechanism(s) | Year of Publication | References |
|---|---|---|---|---|
| HFD-induced C57BL/6J mice | Purple corn extract; 2 g/kg diet |
| 2003 | [72] |
| HFD-induced C57/Bl6 mice | 90 mg/kg/day |
| 2010 | [39] |
| HFD-induced C57BLK/6J mice | C3G-rich grape pomace extract; 250 mg/kg b.w. per day |
| 2010 | [73] |
| KK-Ay mice | 10, 50, and 100 μmol/L; 1 g/kg |
| 2011 | [41] |
| HFD-induced C57BL/6 mice | 3G-rich black soybean seed coat extract for 14 weeks |
| 2011 | [74] |
| Sprague-Dawley rats—ovariectomized (OVX) | 5% and 10% (w/w) black berries |
| 2012 | [75] |
| HFD-fed C57BL/6J mice and db/db and db/+ mice | 0.2% |
| 2012 | [76] |
| HFD-fed C57BL/6 mice | 40 and 200 mg/kg food for 12 weeks |
| 2013 | [77] |
| HFD-induced rats | 100 mg/kg |
| 2014 | [78] |
| db/db mice | 2 g/kg diet |
| 2014 | [47] |
| Estrogen-deficient animals with diet-induced obesity in OVX rats | Black carrot extract; 2% for 12 weeks |
| 2015 | [48] |
| HFD-induced C57BL/6J mice | Cyanidin-based anthocyanin-rich blackcurrant extract; 1% for 8 weeks |
| 2015 | [79] |
| HFD-induced C57BL/6J mice | Anthocyanin-rich black elderberry extract; 20–40 mg and 100–200 mg/kg b.w. for 16 weeks |
| 2015 | [80] |
| Diabetes model in KK-Ay mice | C3G-rich aronia juice; free intake |
| 2016 | [81] |
| HFD-induced mice | Purple corn anthocyanin; 200 mg/kg |
| 2017 | [82] |
| db/db mice | 1 mg/mL for 16 weeks |
| 2017 | [17] |
| C57BL/6 J mice fed with a high-fat high-cholesterol diet | 200 mg/kg |
| 2018 | [54] |
| HFD-induced obese mice | Haskap Berry; 0.192% C3G-rich extract |
| 2018 | [83] |
| Human primary myotubes derived from obese and obese T2DM participants | 10 and 100 μM |
| 2018 | [84] |
| HFD-fed C57BL/6J mice | AC-rich blend; 2, 20, or 40 mg/kg body weight |
| 2018 | [85] |
| HF and high-fructose diet-induced mice | 1 mg/mL |
| 2018 | [86] |
| Diet-induced mouse model | 0.02 g/kg BW/ day |
| 2019 | [87] |
| HFD-induced C57BL/6N mice | Aronia melanocarpa extract (70% ethanol extract); 50, 100, and 200 mg/kg body weight/day |
| 2019 | [88] |
| C57BL/6 J mice | 50 mg/day/body weight for 8 weeks |
| 2020 | [19] |
| HFD-induced C57BL/6J mice | Lingonberry (5% w/w) and its anthocyanin; C3G for 12 weeks |
| 2020 | [61] |
| HF and high-fructose diet-induced db/db mice | 1 mg/mL |
| 2021 | [62] |
| HFD-induced mice | Purple corn anthocyanin; 400 mg/kg |
| 2021 | [4] |
| High-fat high-sucrose diet-indued mice | 45.2 mg/kg for 10 weeks |
| 2021 | [89] |
| HFD Sprague-Dawley rats | Isolated from Aronia melanocarpa, Haicheng, China; 100 and 200 mg/kg bw/day) for 8 weeks. |
| 2021 | [90] |
| HF-high-carbohydrate diet-induced obesity model | 0.02 g/kg BW/d |
| 2022 | [91] |
| High-fat meal in healthy humans | Cyanidin- and delphinidin-rich extract (1 g consisted of 150 mg bilberry extract, 230 mg black currant extract, and 620 mg black rice extract) |
| 2022 | [92] |
| HFD-fed C57BL/6 mice | Casein/C3G nanoparticles |
| 2023 | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deepa, P.; Hong, M.; Sowndhararajan, K.; Kim, S. A Review of the Role of an Anthocyanin, Cyanidin-3-O-β-glucoside in Obesity-Related Complications. Plants 2023, 12, 3889. https://doi.org/10.3390/plants12223889
Deepa P, Hong M, Sowndhararajan K, Kim S. A Review of the Role of an Anthocyanin, Cyanidin-3-O-β-glucoside in Obesity-Related Complications. Plants. 2023; 12(22):3889. https://doi.org/10.3390/plants12223889
Chicago/Turabian StyleDeepa, Ponnuvel, Minji Hong, Kandhasamy Sowndhararajan, and Songmun Kim. 2023. "A Review of the Role of an Anthocyanin, Cyanidin-3-O-β-glucoside in Obesity-Related Complications" Plants 12, no. 22: 3889. https://doi.org/10.3390/plants12223889
APA StyleDeepa, P., Hong, M., Sowndhararajan, K., & Kim, S. (2023). A Review of the Role of an Anthocyanin, Cyanidin-3-O-β-glucoside in Obesity-Related Complications. Plants, 12(22), 3889. https://doi.org/10.3390/plants12223889







