Croton gratissimus Burch Herbal Tea Exhibits Anti-Hyperglycemic and Anti-Lipidemic Properties via Inhibition of Glycation and Digestive Enzyme Activities
Abstract
1. Introduction
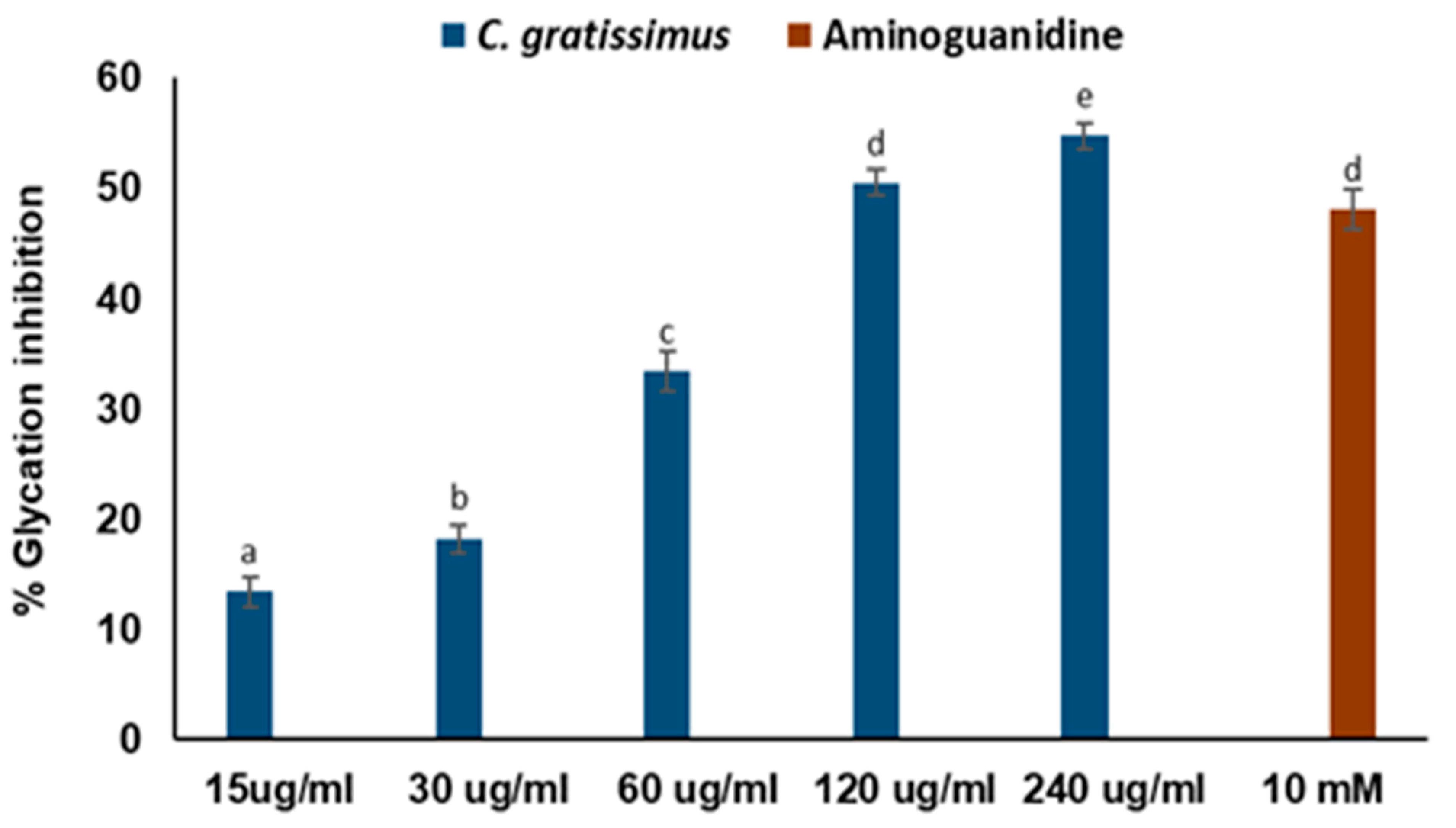
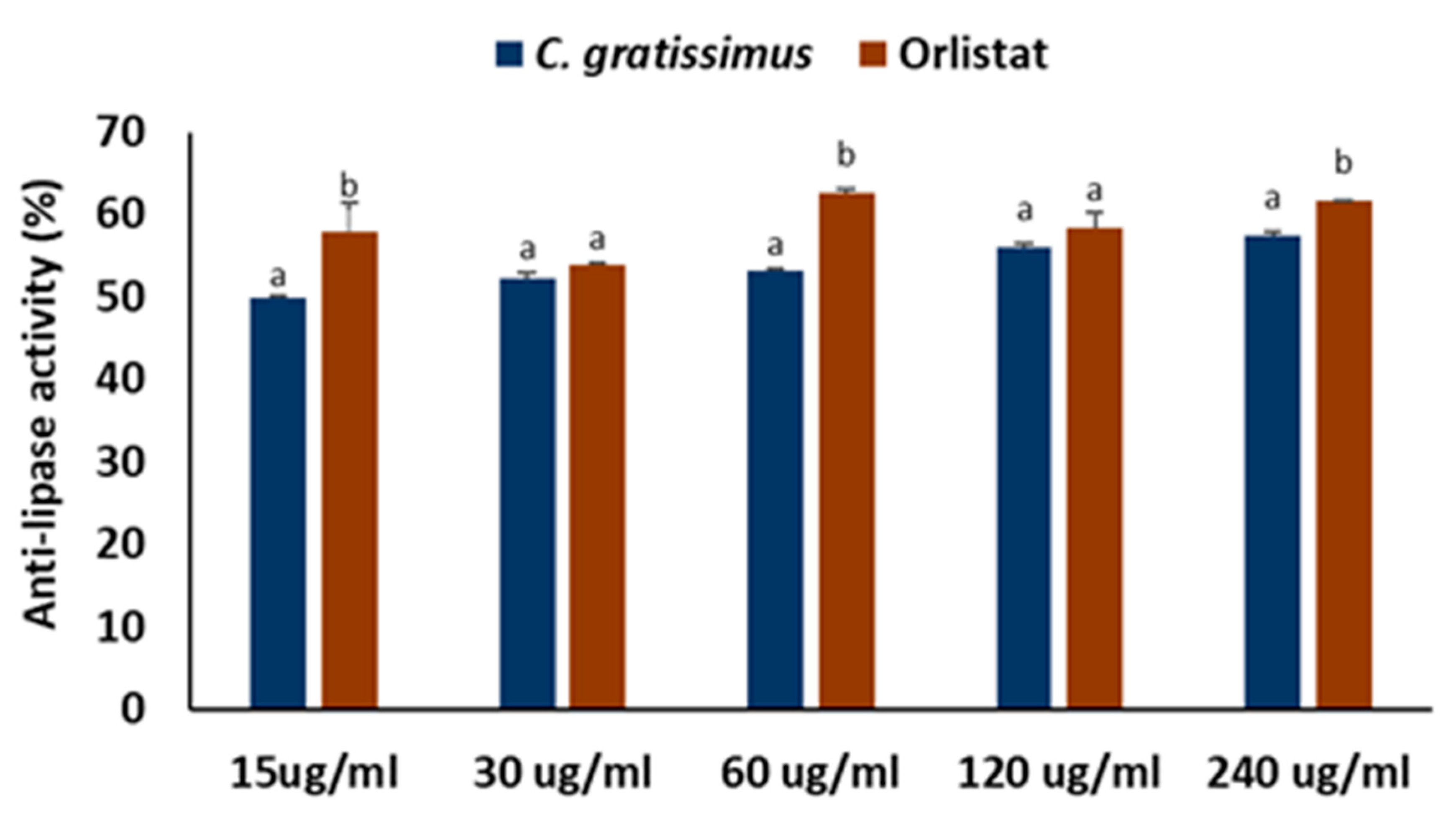
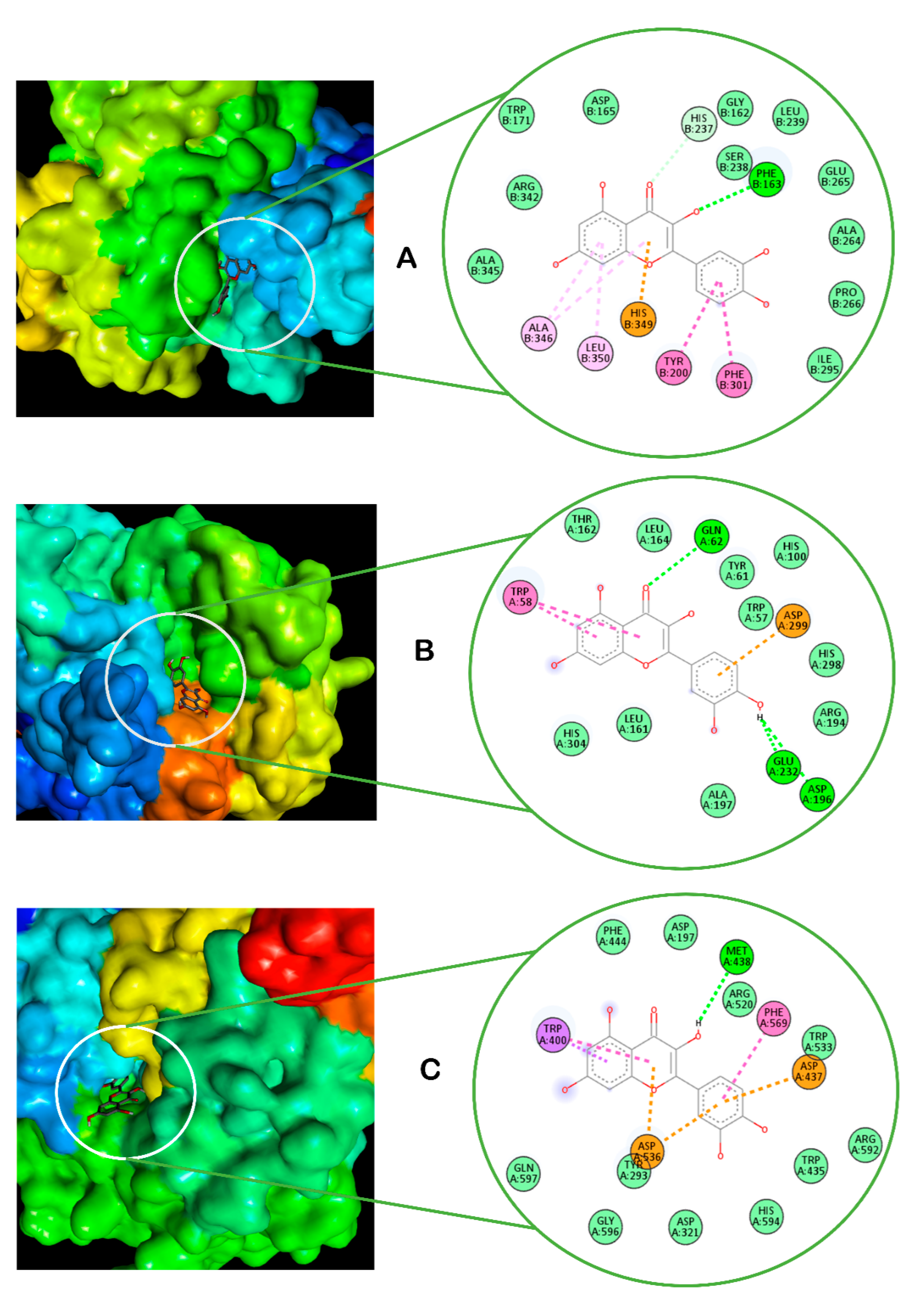
2. Results
3. Discussion
4. Materials and Methods
4.1. Collection of Plants and Verification
4.2. Extraction of Croton gratissimus Herbal Tea
4.3. High-Performance Liquid Chromatography (HPLC) Quantification of C. gratissimus Herbal Infusion Polyphenols
4.4. In Vitro Enzyme Inhibition
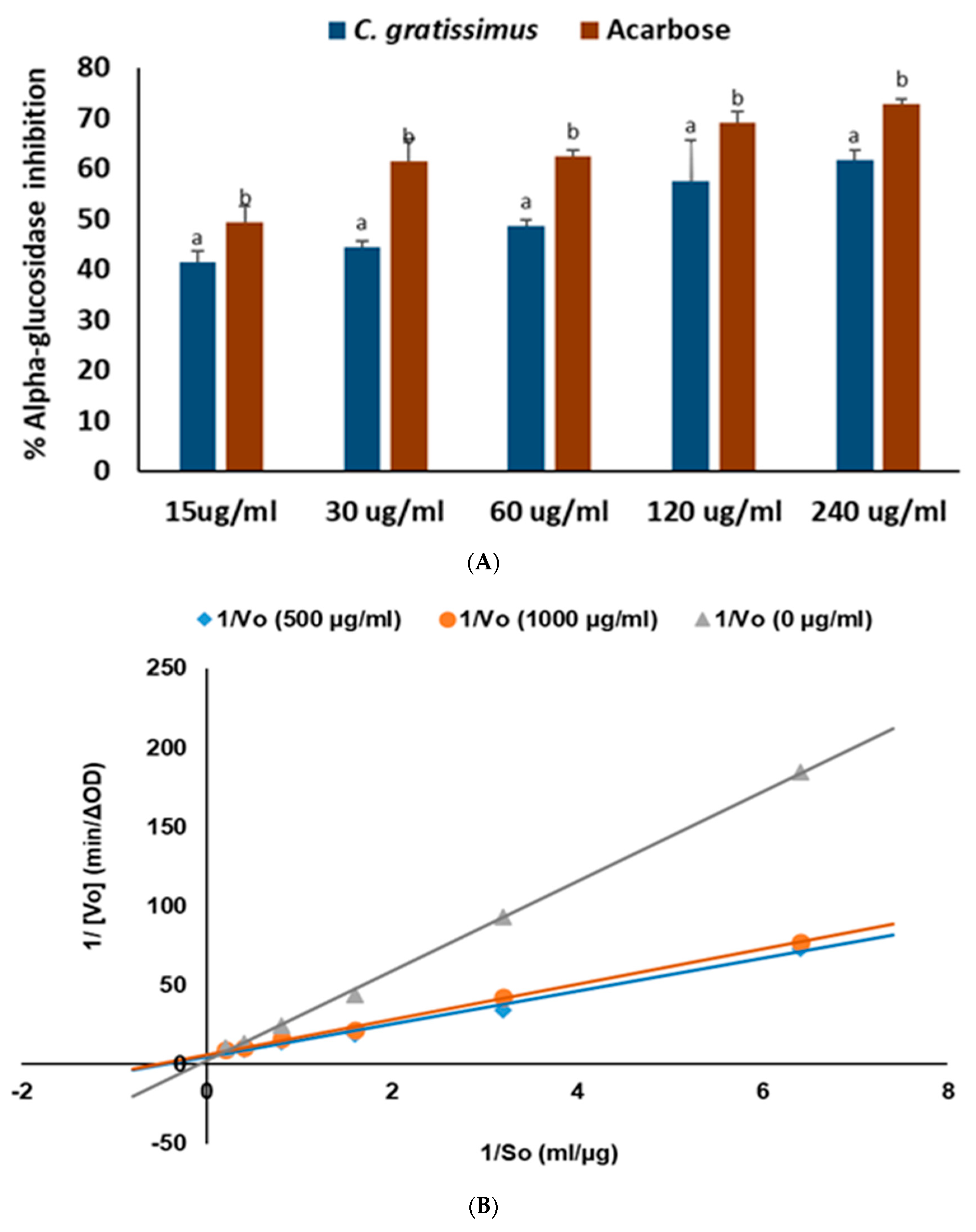
4.4.1. α-Glucosidase Inhibition
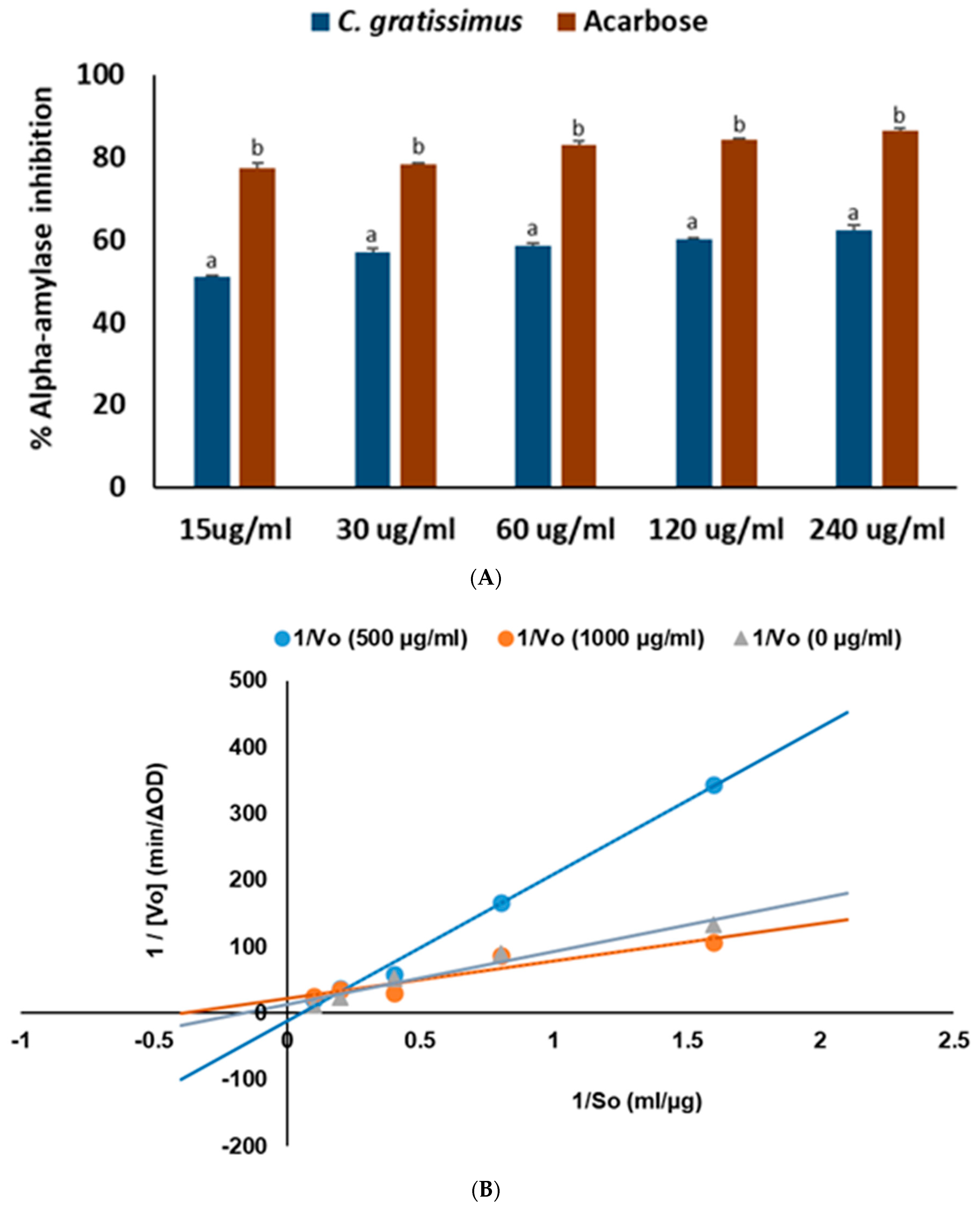
4.4.2. α-Amylase Inhibition
4.4.3. Porcine Pancreatic Lipase Inhibition
4.5. Inhibition Kinetics of Carbohydrate Digestive Enzymes
4.5.1. Mode of α-Glucosidase Inhibition
4.5.2. Mode of α-Amylase Inhibition
4.6. Advanced Glycation End-Products (AGEs) Inhibition
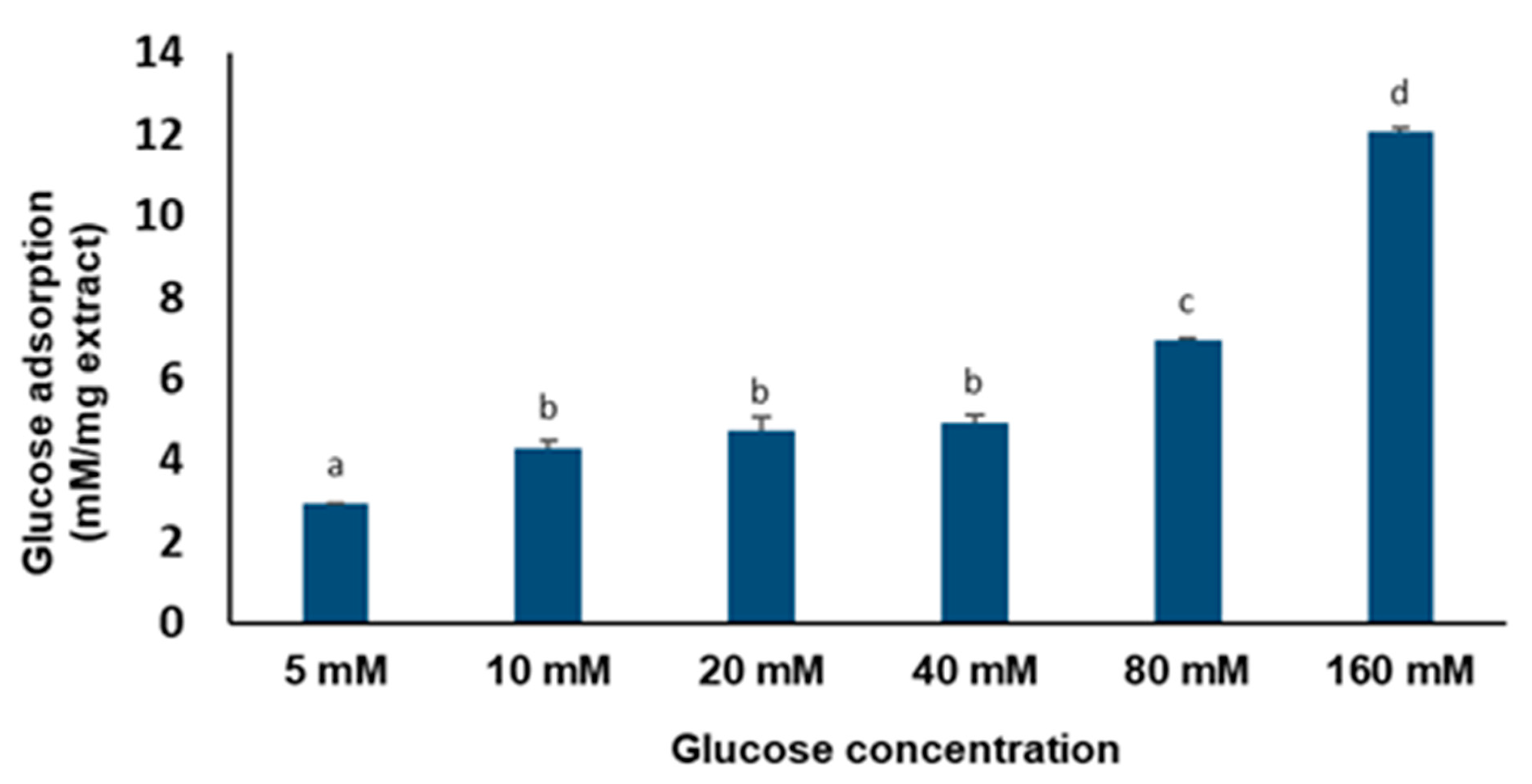
4.7. Glucose Adsorption Capacity of C. gratissimus
4.8. Cell Lines and Cytotoxicity Screening
4.8.1. Cell Maintenance
4.8.2. Cytotoxicity Screening
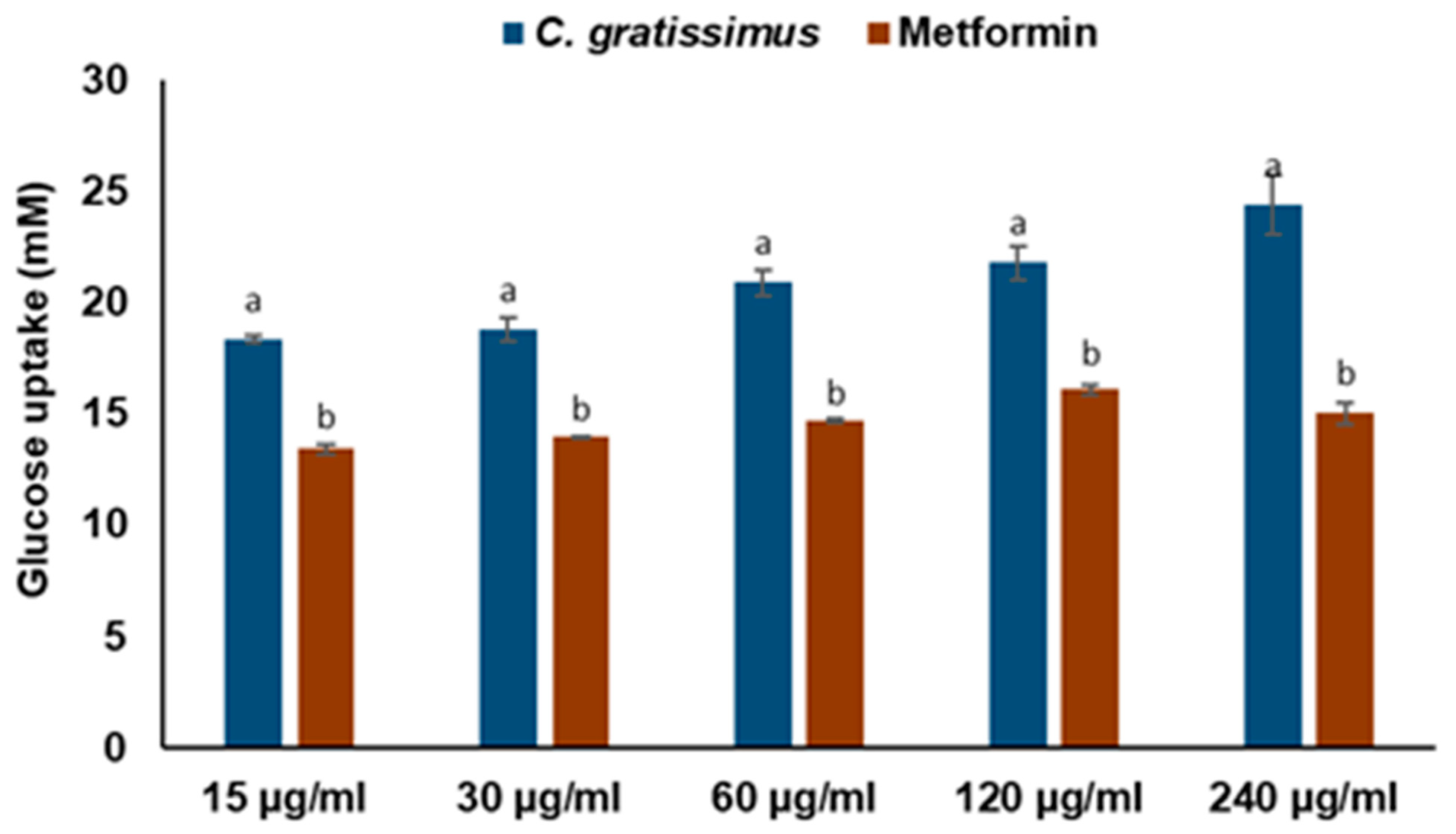
4.9. Glucose Transport/Uptake by Yeast Cells
4.10. Molecular Docking
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes mellitus and its metabolic complications: The role of adipose tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef] [PubMed]
- IDF. International Diabetes Federation (IDF) Diabetes Atlas, 10th ed.; IDF: Brussels, Belgium, 2021. [Google Scholar]
- Cheng, R.; Ma, J.-X. Angiogenesis in diabetes and obesity. Rev. Endocr. Metab. Disord. 2015, 16, 67–75. [Google Scholar] [CrossRef]
- Leitner, D.R.; Frühbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and type 2 diabetes: Two diseases with a need for combined treatment strategies-EASO can lead the way. Obes. Facts 2017, 10, 483–492. [Google Scholar] [CrossRef]
- Beseni, B.K.; Bagla, V.P.; Njanje, I.; Matsebatlela, T.M.; Mampuru, L.; Mokgotho, M.P. Antioxidant, antiglycation, and hypoglycaemic effect of seriphium plumosum crude plant extracts. Evid. Based Complement. Altern. Med. 2017, 2017, 6453567. [Google Scholar] [CrossRef]
- Oyedemi, S.; Koekemoer, T.; Bradley, G.; van de Venter, M.; Afolayan, A. In vitro anti-hyperglycemia properties of the aqueous stem bark extract from Strychnos henningsii (Gilg). Int. J. Diabetes Dev. Ctries. 2013, 33, 120–127. [Google Scholar] [CrossRef]
- Moremi, M.P.; Kamatou, G.P.; Viljoen, A.M.; Tankeu, S.Y. Croton gratissimus-essential oil composition and chemometric analysis of an ethnomedicinally important tree from South Africa. S. Afr. J. Bot. 2021, 138, 141–147. [Google Scholar] [CrossRef]
- Okonkon, J.; Bassey, A.; Obot, J. Antidiabetic activity of ethanolic leaf extract of Croton zambesicus Muell.(thunder plant) in alloxan diabetic rats. Afr. J. Trad. Complement. Altern. Med. 2006, 3, 21–26. [Google Scholar]
- Erhabor, J.O.; Oyenihi, O.R.; Erukainure, O.L.; Matsabisa, M.G. Croton gratissimus Burch.(Lavender croton): A Review of the Traditional Uses, Phytochemistry, Nutritional Constituents and Pharmacological Activities. Trop. J. Nat. Prod. Res. 2022, 6, 842–855. [Google Scholar]
- Mulholland, D.A.; Langat, M.K.; Crouch, N.R.; Coley, H.M.; Mutambi, E.M.; Nuzillard, J.-M. Cembranolides from the stem bark of the southern African medicinal plant, Croton gratissimus (Euphorbiaceae). Phytochemistry 2010, 71, 1381–1386. [Google Scholar] [CrossRef]
- Mthethwa, N.S.; Oyedeji, B.A.; Obi, L.C.; Aiyegoro, O.A. Anti-staphylococcal, anti-HIV and cytotoxicity studies of four South African medicinal plants and isolation of bioactive compounds from Cassine transvaalensis (Burtt. Davy) codd. BMC Complement. Altern. Med. 2014, 14, 1–9. [Google Scholar] [CrossRef]
- Ndhlala, A.R.; Aderogba, M.A.; Ncube, B.; Van Staden, J. Anti-oxidative and cholinesterase inhibitory effects of leaf extracts and their isolated compounds from two closely related Croton species. Molecules 2013, 18, 1916–1932. [Google Scholar] [CrossRef] [PubMed]
- Ncume, P.V.; Salau, V.F.; Mtshali, S.; Olofinsan, K.A.; Erukainure, O.L.; Matsabisa, M.G. Phytochemical Properties of Croton gratissimus Burch (Lavender Croton) Herbal Tea and Its Protective Effect against Iron-Induced Oxidative Hepatic Injury. Plants 2023, 12, 2915. [Google Scholar] [CrossRef] [PubMed]
- Ofusori, D.A.; Komolafe, O.A.; Adewole, O.S.; Obuotor, E.M.; Fakunle, J.B.; Ayoka, A.O. Effect of ethanolic leaf extract of Croton zambesicus (Müll. Arg.) on lipid profile in streptozotocin-induced diabetic rats. Diabetol. Croat. 2012, 41, 69–76. [Google Scholar]
- Magwilu, K.D.; Nguta, J.M.; Mapenay, I.; Matara, D. Phylogeny, phytomedicines, phytochemistry, pharmacological properties, and toxicity of Croton gratissimus Burch (Euphorbiaceae). Adv.Pharm. Pharm. Sci. 2022, 2022, 1238270. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.I.; Beseni, B.K.; Msomi, N.Z.; Salau, V.F.; Erukainure, O.L.; Aljoundi, A.; Islam, M.S. The antioxidant and antidiabetic potentials of polyphenolic-rich extracts of Cyperus rotundus (Linn.). J. Biomol. Struct. Dyn. 2022, 40, 12075–12087. [Google Scholar] [CrossRef] [PubMed]
- Matsabisa, M.; Chukwuma, C.; Chaudhary, S. South African traditional herbal formulation inhibits α-glucosidase, DPP-IV and glycation activities, and modulates glucose utilisation in Chang liver cells and 3T3-L1 adipocytes. S. Afr. J.Bot. 2019, 121, 121–127. [Google Scholar] [CrossRef]
- Mahmoud, A.B.; Danton, O.; Kaiser, M.; Khalid, S.; Hamburger, M.; Mäser, P. HPLC-based activity profiling for antiprotozoal compounds in Croton gratissimus and Cuscuta hyalina. Front. Pharmacol. 2020, 11, 557599. [Google Scholar] [CrossRef] [PubMed]
- Salau, V.F.; Erukainure, O.L.; Islam, M.S. Phenolics: Therapeutic applications against oxidative injury in obesity and type 2 diabetes pathology. In Pathology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 297–307. [Google Scholar]
- Wang, Y.; Alkhalidy, H.; Liu, D. The emerging role of polyphenols in the management of type 2 diabetes. Molecules 2021, 26, 703. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.S.; Hidayathulla, S.; Rehman, M.T.; ElGamal, A.A.; Al-Massarani, S.; Razmovski-Naumovski, V.; Alqahtani, M.S.; El Dib, R.A.; AlAjmi, M.F. Alpha-amylase and alpha-glucosidase enzyme inhibition and antioxidant potential of 3-oxolupenal and katononic acid isolated from Nuxia oppositifolia. Biomolecules 2019, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Ghadyale, V.; Takalikar, S.; Haldavnekar, V.; Arvindekar, A. Effective control of postprandial glucose level through inhibition of intestinal alpha glucosidase by Cymbopogon martinii (Roxb.). Evid-Based Complement. Altern. Med. 2012, 2012, 372909. [Google Scholar] [CrossRef]
- Cele, N.; Awolade, P.; Seboletswe, P.; Olofinsan, K.; Islam, M.S.; Singh, P. α-Glucosidase and α-amylase inhibitory potentials of quinoline–1, 3, 4-oxadiazole conjugates bearing 1, 2, 3-triazole with antioxidant activity, kinetic studies, and computational validation. Pharmaceuticals 2022, 15, 1035. [Google Scholar] [CrossRef]
- Creutzfeldt, W. Effects of the α-glucosidase inhibitor acarbose on the development of long-term complications in diabetic animals: Pathophysiological and therapeutic implications. Diabetes Metab. Res. Rev. 1999, 15, 289–296. [Google Scholar] [CrossRef]
- Okokon, J.E.; Bassey, A.I.; Udom, G.J.; Edem, U.A.; Attah, G. Alpha Amylase and Alpha Glucosidase Inhibitory Activities of Croton zambesicus Leaf Fractions in Wistar Rats. J. Curr. Biomed. Res. 2022, 2, 145–157. [Google Scholar] [CrossRef]
- Bhutkar, M.; Bhinge, S.; Randive, D.; Wadkar, G.; Todkar, S. Studies on glucose adsorption capacity of some indigenous plants. Glob. J. Pharm. Pharm. Sci. 2018, 5, 1–4. [Google Scholar] [CrossRef]
- Ninomiya, K.; Ina, S.; Nakamura, H.; Yamaguchi, Y.; Kumagai, H.; Kumagai, H. Evaluation of the amount of glucose adsorbed on water-soluble dietary fibres by the analysis of its diffusion rate through a dialysis membrane. Food Hydrocoll. 2022, 129, 107626. [Google Scholar] [CrossRef]
- Das, M.; Devi, G. In vitro glucose binding activity of Terminalia bellirica. Asian J. Pharm. Clin. Res. 2015, 8, 320–323. [Google Scholar]
- Pitchaipillai, R.; Ponniah, T. In vitro antidiabetic activity of ethanolic leaf extract of bruguiera Cylindrica L.–glucose uptake by yeast cells method. Int. Biol. Biomed. J. 2016, 2, 171–175. [Google Scholar]
- Bhinge, S.D.; Bhutkar, M.A.; Randive, D.S.; Wadkar, G.H.; Hasabe, T.S. In vitro hypoglycemic effects of unripe and ripe fruits of Musa sapientum. Braz. J. Pharm. Sci. 2017, 53, c00159. [Google Scholar] [CrossRef]
- Rehman, G.; Hamayun, M.; Iqbal, A.; Ul Islam, S.; Arshad, S.; Zaman, K.; Ahmad, A.; Shehzad, A.; Hussain, A.; Lee, I. In vitro antidiabetic effects and antioxidant potential of Cassia nemophila pods. BioMed Res. Int. 2018, 2018, 1824790. [Google Scholar] [CrossRef]
- Mohd Dom, N.S.; Yahaya, N.; Adam, Z.; Hamid, M. Antiglycation and antioxidant properties of Ficus deltoidea varieties. Evid-Based Complemt. Alter, Med. 2020, 2020, 6374632. [Google Scholar]
- Yeh, W.-J.; Hsia, S.-M.; Lee, W.-H.; Wu, C.-H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef]
- Hou, X.-D.; Qin, X.-Y.; Hou, J.; Tang, H.; Ge, G.-B. The potential of natural sources for pancreatic lipase inhibitors: A solution of the obesity crisis? Expert Opin. Drug Discov. 2022, 17, 1295–1298. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Koorbanally, N.A.; Islam, M.S. Ferulic acid promotes muscle glucose uptake and modulate dysregulated redox balance and metabolic pathways in ferric-induced pancreatic oxidative injury. J. Food Biochem. 2022, 46, e13641. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, Y.H.; Lee, J.S.; Jeong, H.I.; Lee, K.W.; Kang, T.H. Anti-obesity effect of DKB-117 through the inhibition of pancreatic lipase and α-amylase activity. Nutrients 2020, 12, 3053. [Google Scholar] [CrossRef]
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflüg. Arch. Eur. J. Physiol. 2020, 472, 1273–1298. [Google Scholar] [CrossRef] [PubMed]
- Nour, V.; Trandafir, I.; Cosmulescu, S. HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. J. Chromatogr. Sci. 2013, 51, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Stoenescu, A.-M.; Trandafir, I.; Cosmulescu, S. Determination of phenolic compounds using HPLC-UV method in wild fruit species. Horticulturae 2022, 8, 84. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G. Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. Exp. Toxicol. Pathol. 2013, 65, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, Y.M.; Kim, H.; Kim, J.; Jang, D.S.; Kim, J.H.; Kim, J.S. Anti-obesity effect of Morus bombycis root extract: Anti-lipase activity and lipolytic effect. J. Ethnopharmacol. 2010, 130, 621–624. [Google Scholar] [CrossRef]
- Kazeem, M.; Adamson, J.; Ogunwande, I. Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of Morinda lucida Benth leaf. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Gołąb, K.; Gburek, J.; Wysokińska, H.; Matkowski, A. Inhibition of advanced glycation end-product formation and antioxidant activity by extracts and polyphenols from Scutellaria alpina L. and S. altissima L. Molecules 2016, 21, 739. [Google Scholar] [CrossRef] [PubMed]
- Nirupama, R.; Devaki, M.; Nirupama, M.; Yajurvedi, H. In vitro and in vivo studies on the hypoglycaemic potential of Ashwagandha (Withania somnifera) root. Pharm. Sci. Monit. 2014, 5, 45–58. [Google Scholar]

| Polyphenols | Parameters | |||||
|---|---|---|---|---|---|---|
| Retention Time (min) | RT (min) | λ Max | % Peak Area | |||
| Standard | Sample | DFS | ||||
| Gallic acid | 4.753 | 4.691 | +0.062 | 35 | 254 | 13.5152 |
| Catechin | 4.548 | 4.567 | −0.019 | 35 | 254 | 15.6037 |
| Caffeic acid | 3.395 | 3.403 | −0.008 | 70 | 254 | 16.3901 |
| Quercetin | 3.594 | 3.522 | +0.072 | 70 | 300 | 15.8977 |
| Activities | C. gratissimus | Acarbose | Orlistat |
|---|---|---|---|
| α-Glucosidase | 60.56 ± 2.78 | 25.21 ± 1.19 | - |
| α-Amylase | 35.67 ± 0.07 | 9.53 ± 0.18 | - |
| Pancreatic lipase | 50.27 ± 1.51 | - | 33.05 ± 2.81 |
| α-Amylase | α-Glucosidase | |||
|---|---|---|---|---|
| Vmax (ΔOD/Min) | Km (µg/mL) | Vmax (ΔOD/Min) | Km (µg/mL) | |
| 0 µg/mL Infusion | 0.079 | 6.311 | 0.426 | 12.06 |
| 500 µg/mL Infusion | 0.087 | 19.075 | 0.218 | 2.266 |
| 1000 µg/mL Infusion | 0.046 | 2.614 | 0.158 | 1.753 |
| Polyphenols | Alpha-Glucosidase | Alpha-Amylase | Lipase |
|---|---|---|---|
| Caffeic acid | −6.2 | −6.5 | −6.7 |
| Gallic acid | −6.0 | −6.2 | −6.0 |
| Catechin | −5.4 | −8.9 | −9.2 |
| Quercetin | −6.3 | −9.0 | −9.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salau, V.F.; Olofinsan, K.A.; Mishra, A.P.; Odewole, O.A.; Ngnameko, C.R.; Matsabisa, M.G. Croton gratissimus Burch Herbal Tea Exhibits Anti-Hyperglycemic and Anti-Lipidemic Properties via Inhibition of Glycation and Digestive Enzyme Activities. Plants 2024, 13, 1952. https://doi.org/10.3390/plants13141952
Salau VF, Olofinsan KA, Mishra AP, Odewole OA, Ngnameko CR, Matsabisa MG. Croton gratissimus Burch Herbal Tea Exhibits Anti-Hyperglycemic and Anti-Lipidemic Properties via Inhibition of Glycation and Digestive Enzyme Activities. Plants. 2024; 13(14):1952. https://doi.org/10.3390/plants13141952
Chicago/Turabian StyleSalau, Veronica F., Kolawole A. Olofinsan, Abhay P. Mishra, Olufemi A. Odewole, Corinne R. Ngnameko, and Motlalepula G. Matsabisa. 2024. "Croton gratissimus Burch Herbal Tea Exhibits Anti-Hyperglycemic and Anti-Lipidemic Properties via Inhibition of Glycation and Digestive Enzyme Activities" Plants 13, no. 14: 1952. https://doi.org/10.3390/plants13141952
APA StyleSalau, V. F., Olofinsan, K. A., Mishra, A. P., Odewole, O. A., Ngnameko, C. R., & Matsabisa, M. G. (2024). Croton gratissimus Burch Herbal Tea Exhibits Anti-Hyperglycemic and Anti-Lipidemic Properties via Inhibition of Glycation and Digestive Enzyme Activities. Plants, 13(14), 1952. https://doi.org/10.3390/plants13141952






