Applications and Market of Micro-Organism-Based and Plant-Based Inputs in Brazilian Agriculture
Abstract
:1. Introduction
2. Characterization and Application of Micro-Organism-Based Biological Inputs
2.1. Direct Mechanisms for Promoting Plant Growth
2.2. Indirect Mechanisms for Promoting Plant Growth
3. Definition and Regulation of Biological Inputs in Brazil
- (i)
- Inoculants: products containing micro-organisms that have a favorable impact on plant growth [26].
- (ii)
- Biofertilizers: products containing an active ingredient or organic agent, free from agrochemical substances, capable of acting directly or indirectly on all or part of cultivated plants, enhancing their productivity, regardless of their hormonal or stimulant value [74].
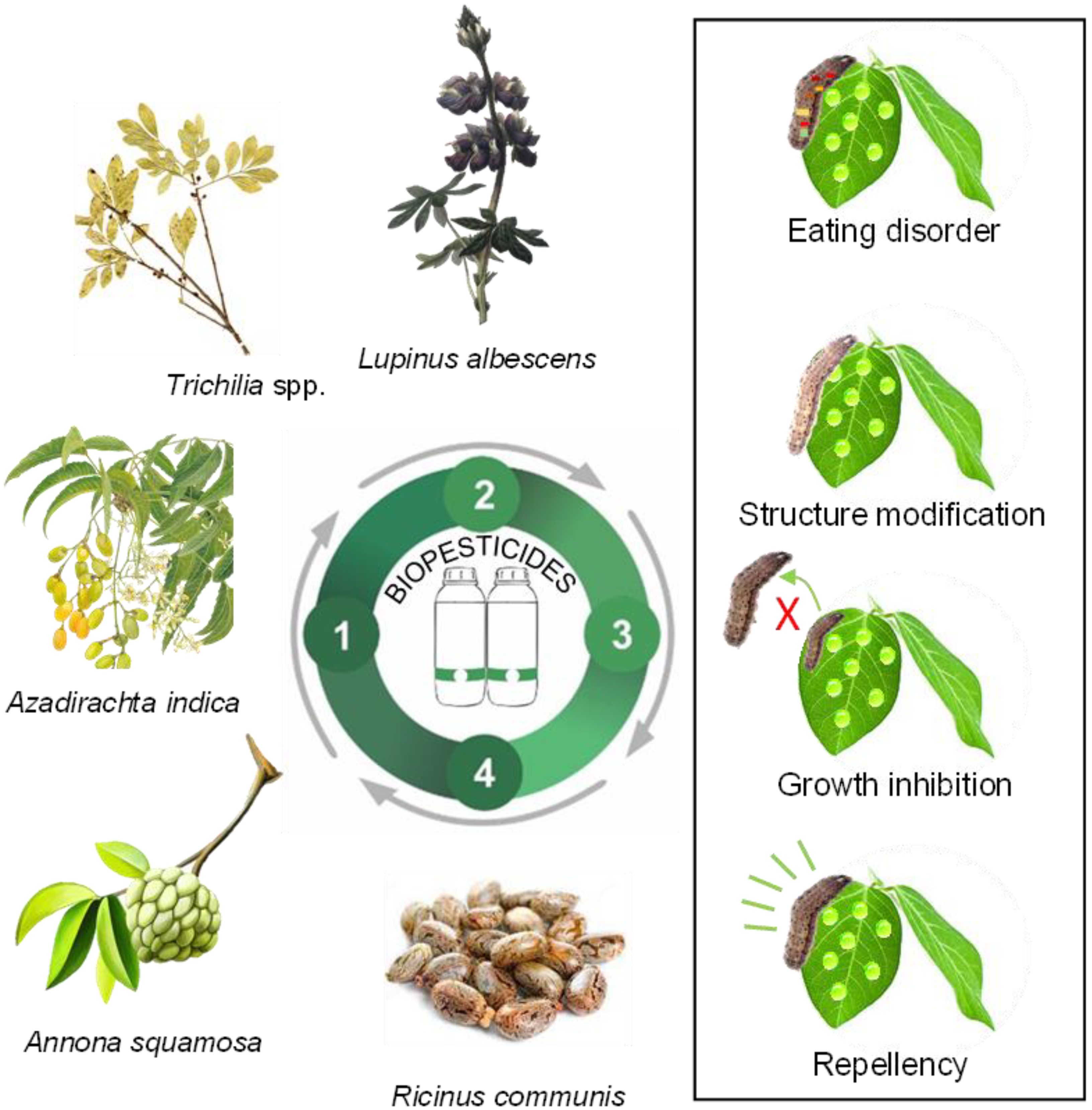
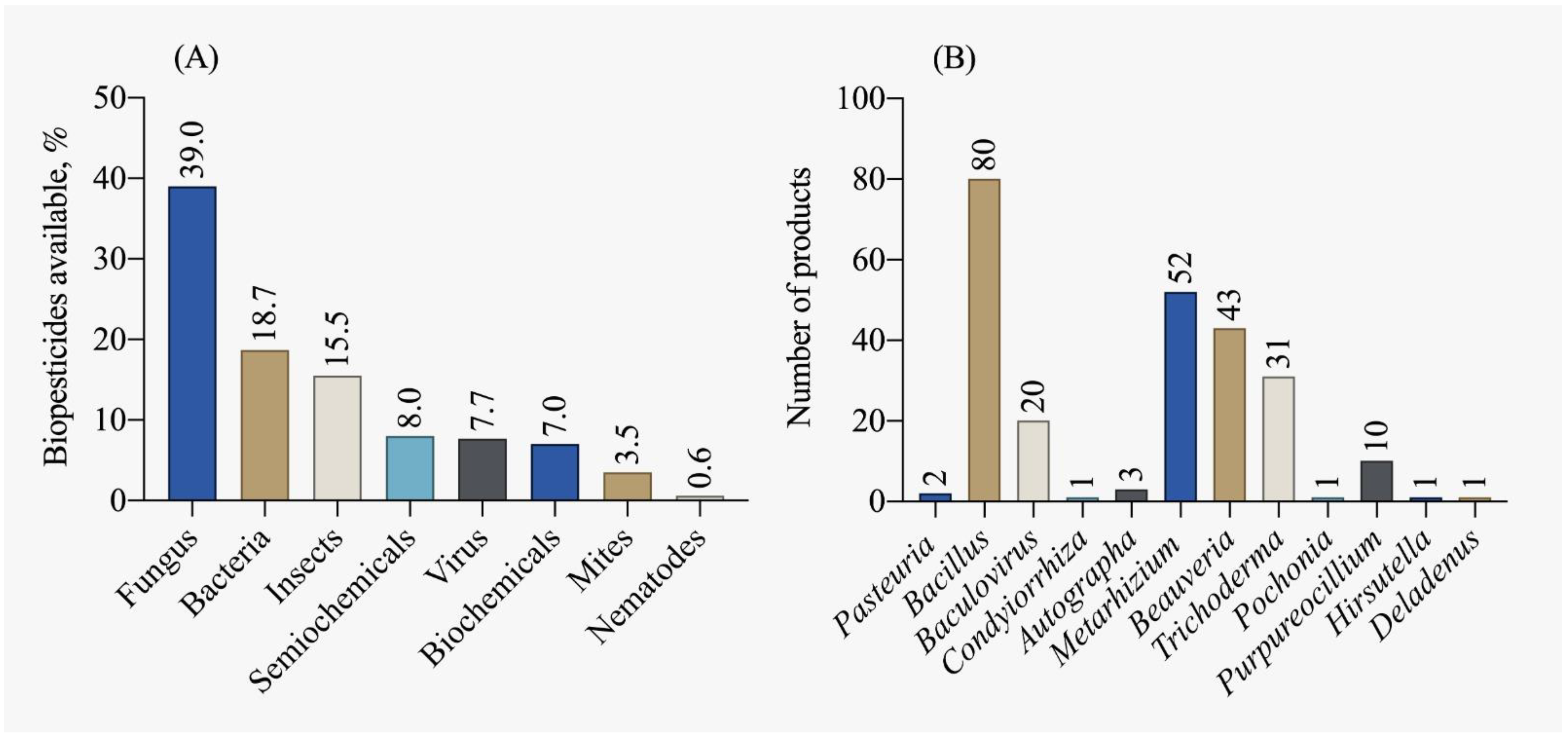
4. Biological and Biochemical Pesticides
4.1. Biological Pesticides
4.2. Biochemical Pesticides
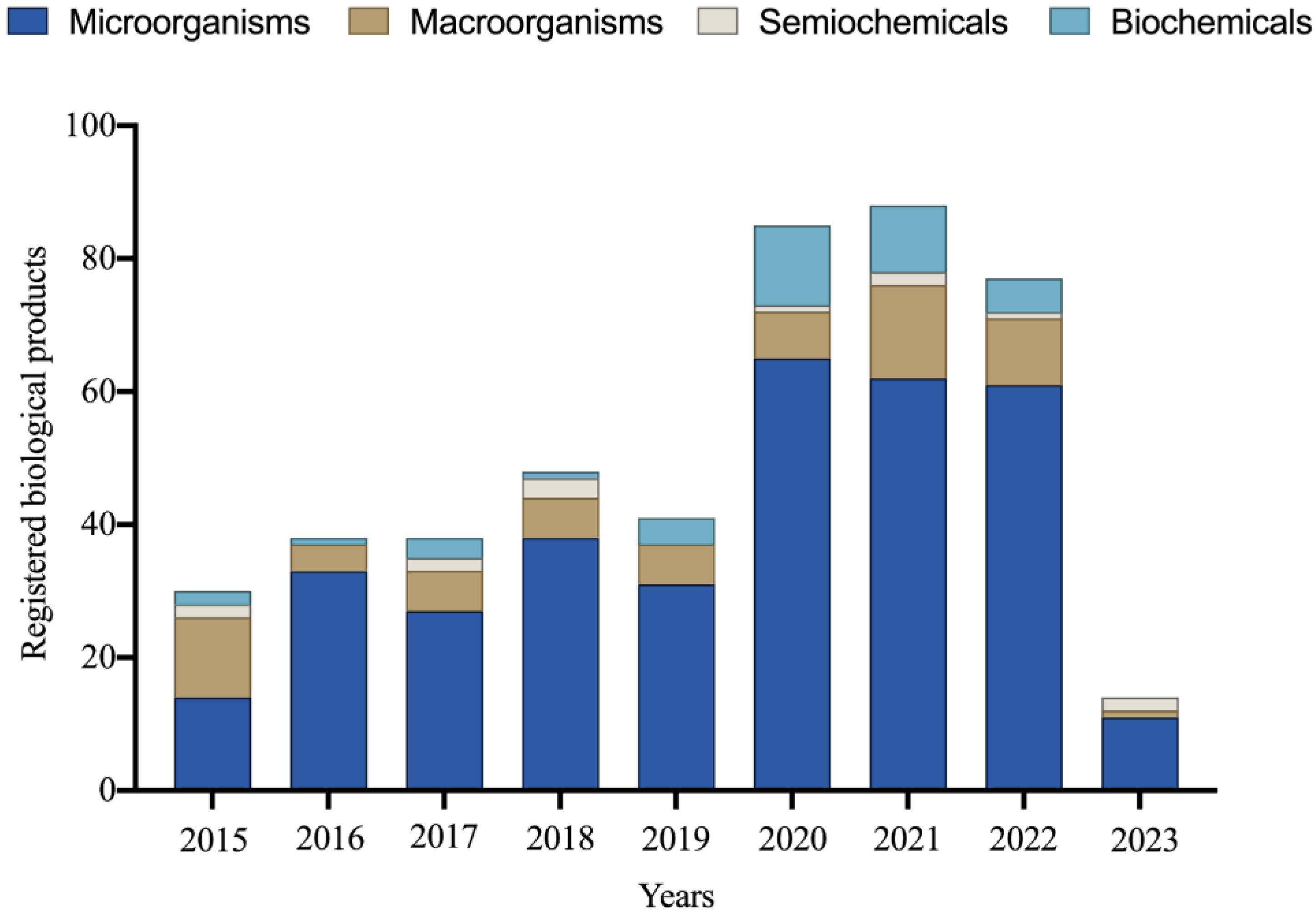
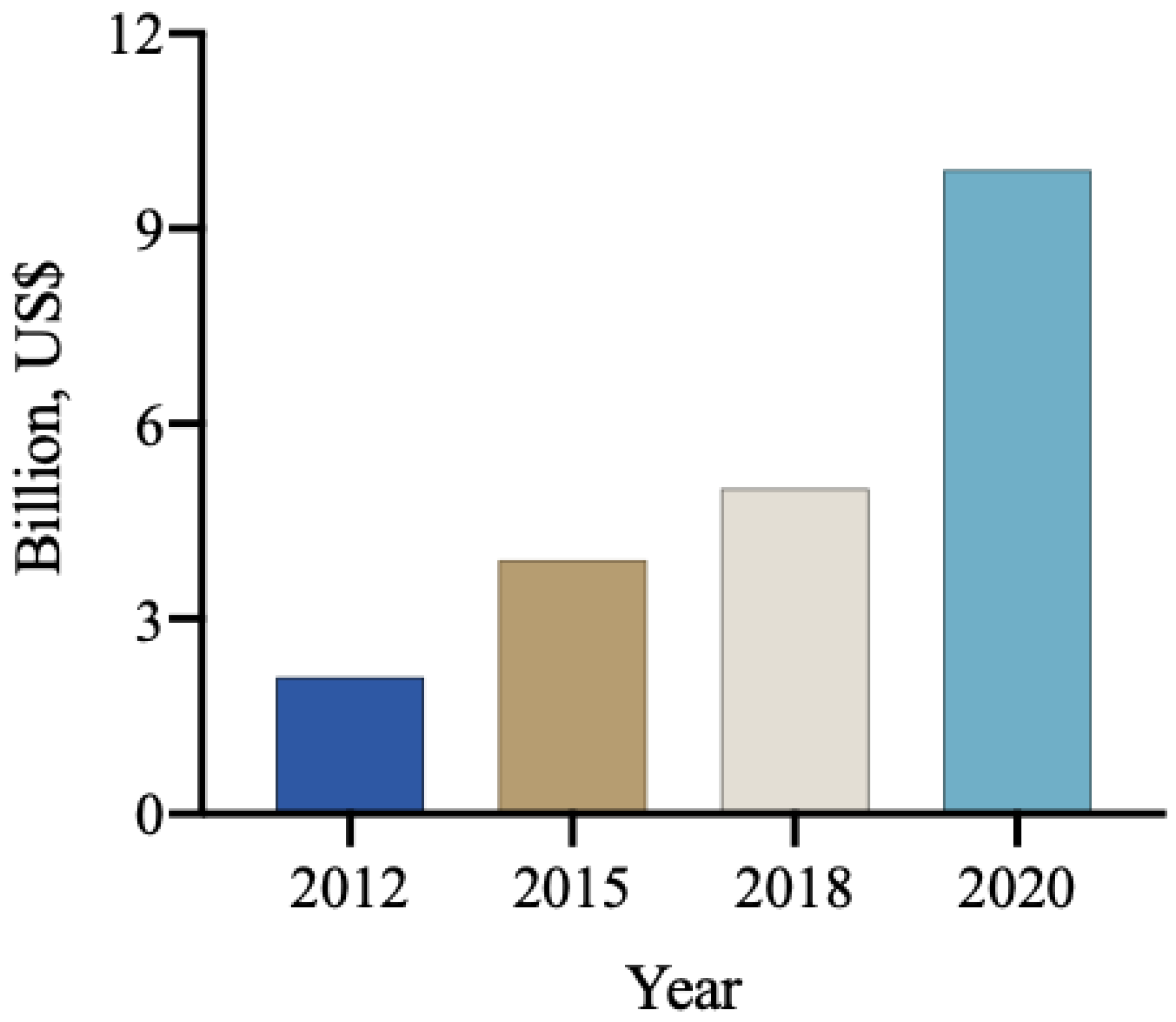
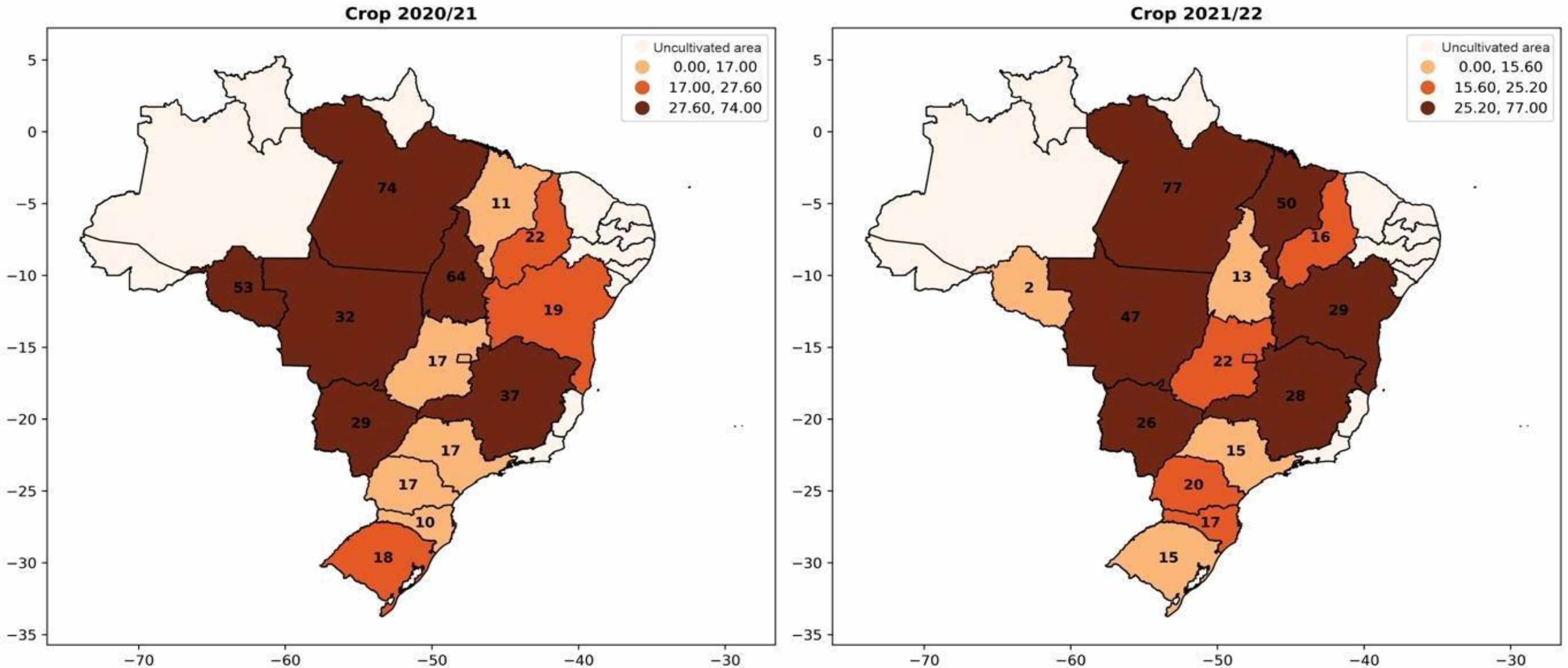
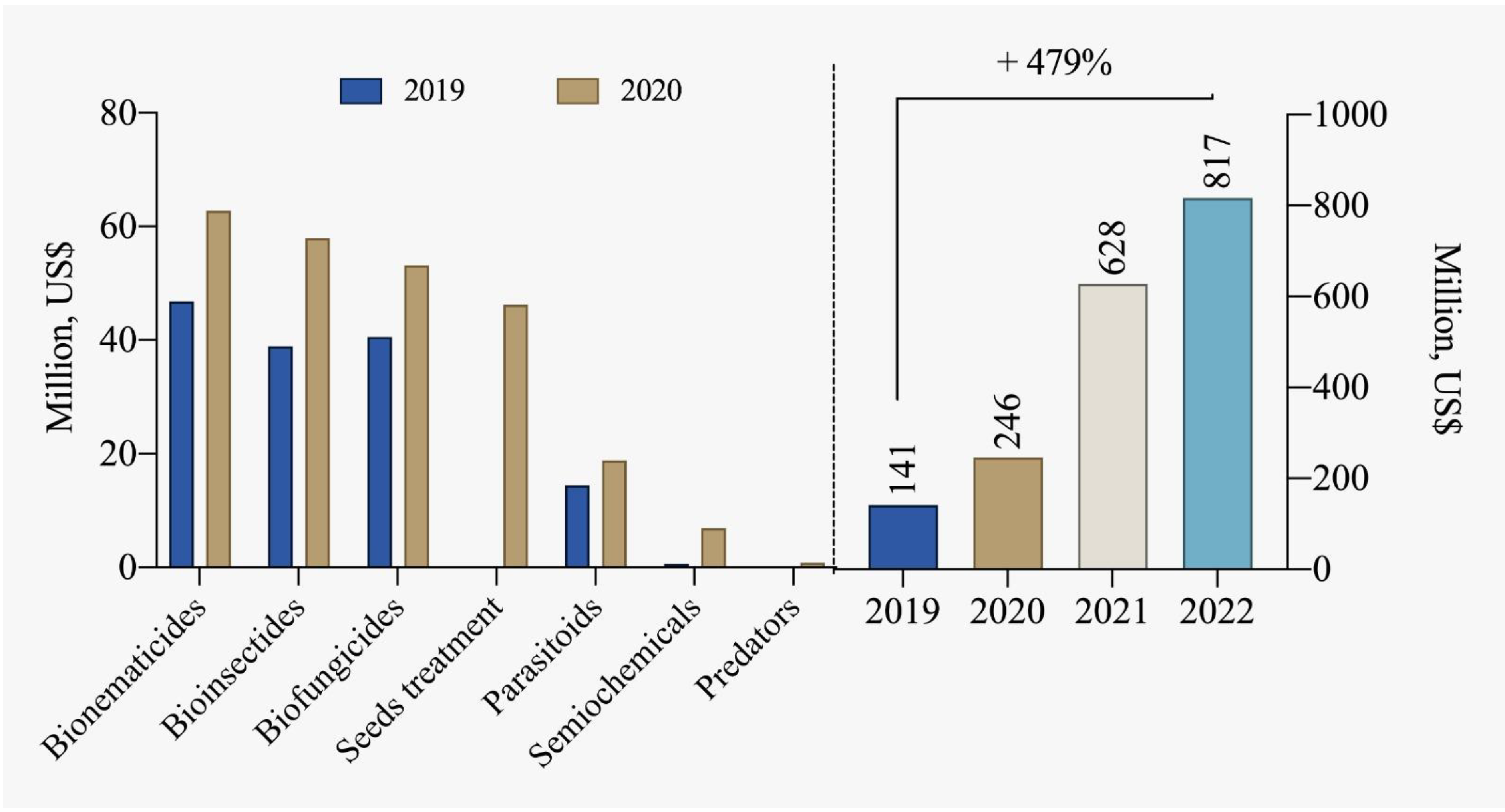
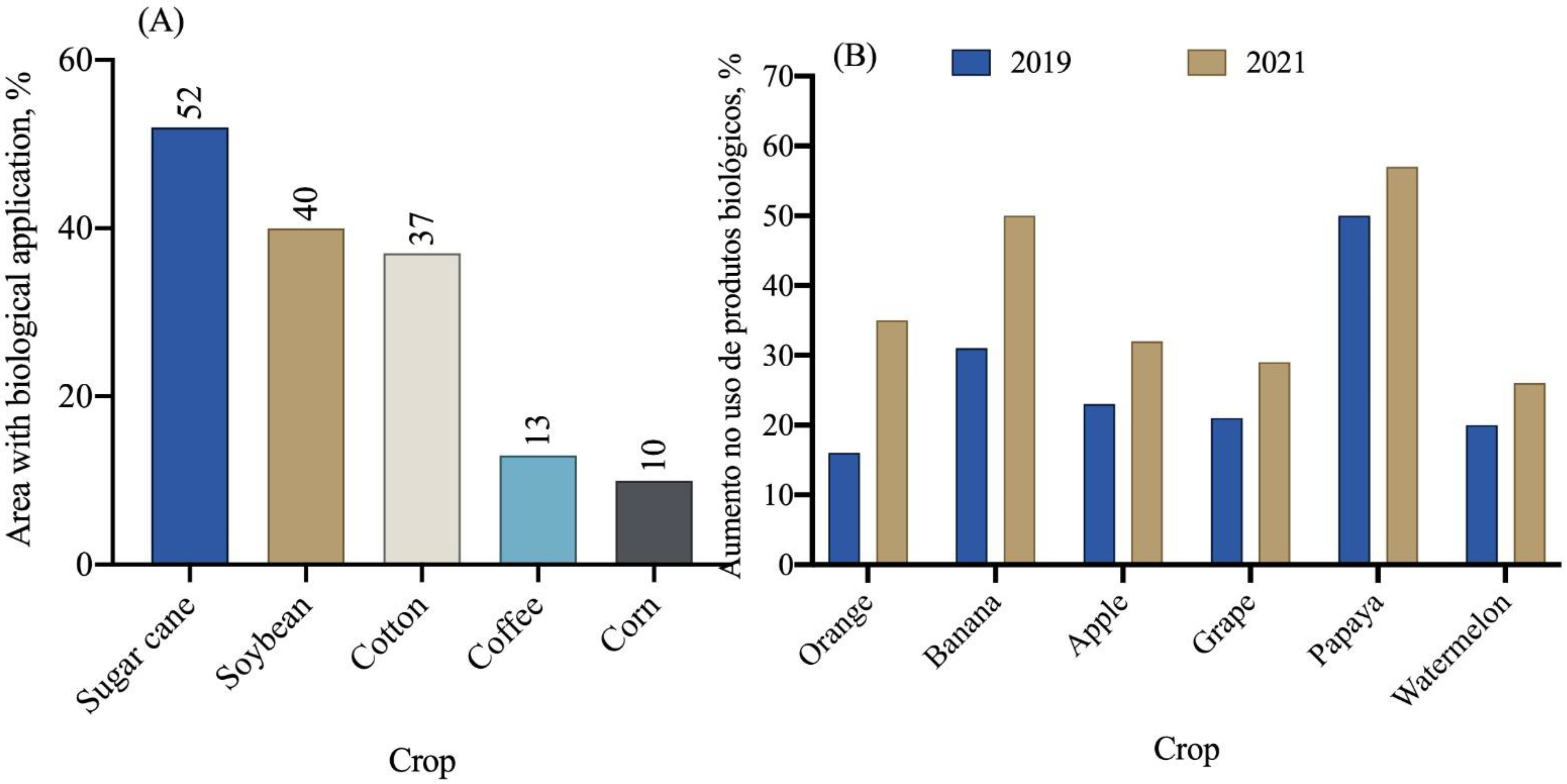
5. Biological Input Market
5.1. Inoculants
5.2. Biodefensives
6. Main Challenges and Opportunities for the Use of Biological Inputs in Brazil
7. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Outstanding Impact of Azospirillum brasilense Strains Ab-V5 and Ab-V6 on the Brazilian Agriculture: Lessons That Farmers Are Receptive to Adopt New Microbial Inoculants. Rev. Bras. Ciênc. Solo 2021, 45, e0200128. [Google Scholar] [CrossRef]
- Milléo, M.V.R.; Pandolfo, M.; Santos, D.S.; Soares, C.R.F.S.; Moscardi, M.L. Agronomic Efficiency of an Inoculant Based on Bacillus amyloliquefaciens FZB45 for Corn and Soybean Crops. Agraria 2023, 18, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Liu, H.; Macdonald, C.A.; Singh, B.K. Application of Microbial Inoculants Significantly Enhances Crop Productivity: A Meta-analysis of Studies from 2010 to 2020. J. Sustain. Agric. Environ. 2022, 1, 216–225. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of Plant Growth Promoting Rhizobacteria (PGPRs) with Multiple Plant Growth Promoting Traits in Stress Agriculture: Action Mechanisms and Future Prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Co-Inoculation of Soybeans and Common Beans with Rhizobia and Azospirilla: Strategies to Improve Sustainability. Biol. Fertil. Soils 2013, 49, 791–801. [Google Scholar] [CrossRef]
- Barbosa, J.Z.; Hungria, M.; Sena, J.V.D.S.; Poggere, G.; Dos Reis, A.R.; Corrêa, R.S. Meta-Analysis Reveals Benefits of Co-Inoculation of Soybean with Azospirillum brasilense and Bradyrhizobium Spp. in Brazil. Appl. Soil Ecol. 2021, 163, 103913. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhiza Symbiosis, 3rd ed.; Academic Press: London, UK, 2008; ISBN 978-0-12-370526-6. [Google Scholar]
- Boulahouat, S.; Cherif-Silini, H.; Silini, A.; Bouket, A.C.; Luptakova, L.; Alenezi, F.N.; Belbahri, L. Biocontrol Efficiency of Rhizospheric Bacillus against the Plant Pathogen Fusarium oxysporum: A Promising Approach for Sustainable Agriculture. Microbiol. Res. 2023, 14, 892–908. [Google Scholar] [CrossRef]
- Vijitrpanth, A.; Jantasorn, A.; Dethoup, T. Potential and Fungicidal Compatibility of Antagonist Endophytic Trichoderma Spp. from Rice Leaves in Controlling Dirty Panicle Disease in Intensive Rice Farming. BioControl 2023, 68, 61–73. [Google Scholar] [CrossRef]
- Seenivasagan, R.; Babalola, O.O. Utilization of Microbial Consortia as Biofertilizers and Biopesticides for the Production of Feasible Agricultural Product. Biology 2021, 10, 1111. [Google Scholar] [CrossRef]
- Ma, M.; Jiang, X.; Wang, Q.; Guan, D.; Li, L.; Ongena, M.; Li, J. Isolation and Identification of PGPR Strain and Its Effect on Soybean Growth and Soil Bacterial Community Composition. Int. J. Agric. Biol. 2018, 20, 1289–1297. [Google Scholar]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Somers, E.; Vanderleyden, J.; Srinivasan, M. Rhizosphere Bacterial Signalling: A Love Parade Beneath Our Feet. Crit. Rev. Microbiol. 2004, 30, 205–240. [Google Scholar] [CrossRef]
- Lehnert, N.; Dong, H.T.; Harland, J.B.; Hunt, A.P.; White, C.J. Reversing Nitrogen Fixation. Nat. Rev. 2008, 2, 278–289. [Google Scholar] [CrossRef]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The Global Nitrogen Cycle in the Twenty-First Century. Philos. Trans. R. Soc. B 2013, 368, 20130164. [Google Scholar] [CrossRef] [PubMed]
- Jordan, D.C. Transfer of Rhizobium Japonicum Buchanan 1980 to Bradyrhizobium Gen. Nov., a Genus of Slow-Growing, Root Nodule Bacteria from Leguminous Plants. Int. J. Syst. Bacteriol. 1982, 32, 136–139. [Google Scholar] [CrossRef]
- Jarvis, B.; Berkum, P.; Chen, W.; Nour, S.; Fernandez, M.P.; Cleyet-Marel, J.-C.; Gillis, M. Transfer of Rhizobium loti, Rhizobium huakuii, Rhizobium ciceri, Rhizobium mediterraneum, Rhizobium tianshanense to Mesorhizobium Gen. Nov. Int. J. Syst. Bacteriol. 1997, 47, 895–898. [Google Scholar] [CrossRef]
- Jordan, D. Taxonomy of the Family Rhizobiaceae Conn 1938 (Approved Lists 1980) Emend. Hördt et al. 2020; NamesforLife, LLC.: East Lansing, MI, USA, 2022. [Google Scholar] [CrossRef]
- Chen, W.X.; Yan, G.H.; Li, J.L. Numerical Taxonomic Study of Fast-Growing Soybean Rhizobia and a Proposal That Rhizobium fredii Be Assigned to Sinorhizobium Gen. Nov. Int. J. Syst. Bacteriol. 1988, 38, 392–397. [Google Scholar] [CrossRef]
- Dreyfus, B.; Garcia, J.L.; Gillis, M. Characterization of Azorhizobium caulinodans Gen. Nov., Sp. Nov., a Stem-Nodulating Nitrogen-Fixing Bacterium Isolated from Sesbania rostrata. Int. J. Syst. Bacteriol. 1988, 38, 89–98. [Google Scholar] [CrossRef]
- Rivas, R.; Velázquez, E.; Willems, A.; Vizcaíno, N.; Subba-Rao, N.S.; Mateos, P.F.; Gillis, M.; Dazzo, F.B.; Martínez-Molina, E. A New Species of Devosia That Forms a Unique Nitrogen-Fixing Root-Nodule Symbiosis with the Aquatic Legume Neptunia natans (L.f.) Druce. Appl. Environ. Microbiol. 2002, 68, 5217–5222. [Google Scholar] [CrossRef]
- Mantelin, S.; Saux, M.F.-L.; Zakhia, F.; Béna, G.; Bonneau, S.; Jeder, H.; De Lajudie, P.; Cleyet-Marel, J.-C. Emended Description of the Genus Phyllobacterium and Description of Four Novel Species Associated with Plant Roots: Phyllobacterium bourgognense Sp. Nov., Phyllobacterium ifriqiyense Sp. Nov., Phyllobacterium leguminum Sp. Nov. and Phyllobacterium brassicacearum Sp. Nov. Int. J. Syst. Evol. Microbiol. 2006, 56, 827–839. [Google Scholar] [CrossRef]
- Teyssier, C.; Marchandin, H.; Jean-Pierre, H.; Diego, I.; Darbas, H.; Jeannot, J.-L.; Gouby, A.; Jumas-Bilak, E. Molecular and Phenotypic Features for Identification of the Opportunistic Pathogens ochrobactrum Spp. J. Med. Microbiol. 2005, 54, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Moulin, L.; Munive, A.; Dreyfus, B.; Boivin-Masson, C. Nodulation of Legumes by Members of the B-Subclass of Proteobacteria. Nature 2001, 411, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-M.; James, E.K.; Prescott, A.R.; Kierans, M.; Sprent, J.I. Nodulation of Mimosa Spp. by the β-Proteobacterium Ralstonia taiwanensis. Mol. Plant Microbe Interact. 2003, 16, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Instrução Normativa SDA No 13, de 24 de Março de 2011. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/fertilizantes/legislacao/in-sda-13-de-24-03-2011-inoculantes.pdf/@@download/file (accessed on 3 October 2023).
- Mehnaz, S. Bioformulations: For Sustainable Agriculture; Arora, N.K., Mehnaz, S., Balestrini, R., Eds.; Springer: New Delhi, India, 2016; Chapter 15; pp. 267–281. [Google Scholar]
- Tiecher, T.; Gomes, M.V.; Ambrosini, V.G.; Amorim, M.B.; Bayer, C. Assessing Linkage between Soil Phosphorus Forms in Contrasting Tillage Systems by Path Analysis. Soil Tillage Res. 2018, 175, 276–280. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant Growth-Promoting Rhizobacteria (PGPR): Emergence in Agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate Solubilizing Microbes: Sustainable Approach for Managing Phosphorus Deficiency in Agricultural Soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.M.S.; Siqueira, J.O. Microbiologia e Bioquímica do Solo, 2nd ed.; Editora UFLA: Lavras, Brazil, 2006; Volume 1, ISBN 85-87692-33-X. [Google Scholar]
- Londoño, D.M.M.; Meyer, E.; González, D.; Hernández, A.G.; Soares, C.R.F.S.; Lovato, P.E. Landrace Maize Varieties Differ from Conventional and Genetically Modified Hybrid Maize in Response to Inoculation with Arbuscular Mycorrhizal Fungi. Mycorrhiza 2019, 29, 237–249. [Google Scholar] [CrossRef]
- Meyer, E.; Betancur-Agudelo, M.; Ventura, B.S.; Dos Anjos, K.G.; Do Scarsanella, J.A.; Vieira, A.S.; Mendes, L.; Stoffel, S.C.G.; Munarini, A.; Soares, C.R.F.S.; et al. Mycorrhizal Root Colonization in Maize Fields Is More Affected by Soil Management and Climate Conditions than by Plant Genotype. Arch. Microbiol. 2021, 203, 4609–4618. [Google Scholar] [CrossRef]
- Hariprasad, P.; Niranjana, S.R. Isolation and Characterization of Phosphate Solubilizing Rhizobacteria to Improve Plant Health of Tomato. Plant Soil 2009, 316, 13–24. [Google Scholar] [CrossRef]
- Rajkumar, M.; Sandhya, S.; Prasad, M.N.V.; Freitas, H. Perspectives of Plant-Associated Microbes in Heavy Metal Phytoremediation. Biotechnol. Adv. 2012, 30, 1562–1574. [Google Scholar] [CrossRef]
- Park, S.-H.; Elhiti, M.; Wang, H.; Xu, A.; Brown, D.; Wang, A. Adventitious Root Formation of in Vitro Peach Shoots Is Regulated by Auxin and Ethylene. Sci. Hortic. 2017, 226, 250–260. [Google Scholar] [CrossRef]
- Park, Y.-G.; Mun, B.-G.; Kang, S.-M.; Hussain, A.; Shahzad, R.; Seo, C.-W.; Kim, A.-Y.; Lee, S.-U.; Oh, K.Y.; Lee, D.Y.; et al. Bacillus aryabhattai SRB02 Tolerates Oxidative and Nitrosative Stress and Promotes the Growth of Soybean by Modulating the Production of Phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef] [PubMed]
- Naz, I.; Bano, A.; Ul Hassan, T. Isolation of Phytohormones Producing Plant Growth Promoting Rhizobacteria from Weeds Growing in Khewra Salt Range, Pakistan and Their Implication in Providing Salt Tolerance to Glycine max L. Afr. J. Biotechnol. 2009, 8, 5762–5768. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Duraivadivel, P.; Hariprasad, P.; Niranjana, S.R. A Novel Split-Pot Bioassay to Screen Indole Acetic Acid Producing Rhizobacteria for the Improvement of Plant Growth in Tomato [Solanum lycopersicum L.]. Sci. Hortic. 2017, 224, 351–357. [Google Scholar] [CrossRef]
- Swarnalakshmi, K.; Yadav, V.; Tyagi, D.; Dhar, D.W.; Kannepalli, A.; Kumar, S. Significance of Plant Growth Promoting Rhizobacteria in Grain Legumes: Growth Promotion and Crop Production. Plants 2020, 9, 1596. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Prasher, I.B. Phosphate Solubilization and Indole-3-Acetic Acid (IAA) Produced by Colletotrichum gloeosporioides and Aspergillus fumigatus strains Isolated from the Rhizosphere of Dillenia indica L. Folia Microbiol. 2023, 68, 219–229. [Google Scholar] [CrossRef]
- Ogugua, U.V.; Kanu, S.A.; Ntushelo, K. Gibberellic Acid Improves Growth and Reduces Heavy Metal Accumulation: A Case Study in Tomato (Solanum lycopersicum L.) Seedlings Exposed to Acid Mine Water. Heliyon 2022, 8, e12399. [Google Scholar] [CrossRef]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E. (Eds.) Ethylene in Plant Biology, 2nd ed.; Academic Press: New York, NY, USA, 1992; ISBN 978-0-08-091628-6. [Google Scholar]
- Stearns, J.C.; Glick, B.R. Transgenic Plants with Altered Ethylene Biosynthesis or Perception. Biotechnol. Adv. 2003, 21, 193–210. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC Deaminase Can Promote Plant Growth and Help to Feed the World. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Yuhashi, K.-I.; Ichikawa, N.; Ezura, H.; Akao, S.; Minakawa, Y.; Nukui, N.; Yasuta, T.; Minamisawa, K. Rhizobitoxine Production by Bradyrhizobium elkanii Enhances Nodulation and Competitiveness on Macroptilium atropurpureum. Appl. Environ. Microbiol. 2000, 66, 2658–2663. [Google Scholar] [CrossRef]
- Ma, W.; Penrose, D.M.; Glick, B.R. Strategies Used by Rhizobia to Lower Plant Ethylene Levels and Increase Nodulation. Can. J. Microbiol. 2002, 48, 947–954. [Google Scholar] [CrossRef]
- Hohma, M.; Shimomura, T. Metabolism of 1-Aminocyclopropane-1-Carboxylic Acid. Agric. Biol. Chem. 1978, 42, 1825–1831. [Google Scholar]
- Glick, B.R. The Enhancement of Plant Growth by Free-Living Bacteria. Can. J. Microbiol. 1995, 41, 109–117. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Development of Biocontrol Products for Postharvest Diseases of Fruit: The Importance of Elucidating the Mechanisms of Action of Yeast Antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C. Plant Responses to Plant Growth-Promoting Rhizobacteria. Eur. J. Plant Pathol. 2007, 119, 243–254. [Google Scholar] [CrossRef]
- Whipps, J.M. Microbial Interactions and Biocontrol in the Rhizosphere. J. Exp. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Srivastava, P.; Sahgal, M.; Sharma, K.; Enshasy, H.A.E.; Gafur, A.; Alfarraj, S.; Ansari, M.J.; Sayyed, R.Z. Optimization and Identification of Siderophores Produced by Pseudomonas Monteilii Strain MN759447 and Its Antagonism toward Fungi Associated with Mortality in Dalbergia sissoo Plantation Forests. Front. Plant Sci. 2022, 13, 984522. [Google Scholar] [CrossRef]
- Singh, P.; Chauhan, P.K.; Upadhyay, S.K.; Singh, R.K.; Dwivedi, P.; Wang, J.; Jain, D.; Jiang, M. Mechanistic Insights and Potential Use of Siderophores Producing Microbes in Rhizosphere for Mitigation of Stress in Plants Grown in Degraded Land. Front. Microbiol. 2022, 13, 898979. [Google Scholar] [CrossRef]
- Segarra, G.; Casanova, E.; Avilés, M.; Trillas, I. Trichoderma asperellum Strain T34 Controls Fusarium Wilt Disease in Tomato Plants in Soilless Culture Through Competition for Iron. Microb. Ecol. 2010, 59, 141–149. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms Underlying the Protective Effects of Beneficial Fungi against Plant Diseases. Biol. Control 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Ahemad, M.; Kibret, M. Mechanisms and Applications of Plant Growth Promoting Rhizobacteria: Current Perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus Lipopeptides: Versatile Weapons for Plant Disease Biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.M.; Mazzola, M. Diversity and Natural Functions of Antibiotics Produced by Beneficial and Plant Pathogenic Bacteria. Annu. Rev. Phytopathol. 2012, 50, 403–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, Z.; Lin, R.; Li, E.; Mao, Z.; Ling, J.; Yang, Y.; Yin, W.-B.; Xie, B. Biosynthesis of Antibiotic Leucinostatins in Bio-Control Fungus Purpureocillium Lilacinum and Their Inhibition on Phytophthora Revealed by Genome Mining. PLoS Pathog. 2016, 12, e1005685. [Google Scholar] [CrossRef]
- Petros Kubheka, B.; Weldegabir Ziena, L. Trichoderma: A Biofertilizer and a Bio-Fungicide for Sustainable Crop Production. In Trichoderma—Technology and Uses; Cezar Juliatti, F., Ed.; IntechOpen: London, UK, 2022; ISBN 978-1-80355-354-2. [Google Scholar]
- El-Tarabily, K.A. An Endophytic Chitinase-Producing Isolate of Actinoplanes Missouriensis, with Potential for Biological Control of Root Rot of Lupin Caused by Plectosporium Tabacinum. Aust. J. Bot. 2003, 51, 257. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant Growth Promoting and Biocontrol Activity of Streptomyces Spp. as Endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef]
- Gogoi, P.; Kakoti, P.; Saikia, J.; Sarma, R.K.; Yadav, A.; Singh, B.P.; Saikia, R. Plant Growth-Promoting Rhizobacteria in Management of Soil-Borne Fungal Pathogens. Management of Fungal Pathogens in Pulses: Current Status and Future Challenges; Springer: Cham, Switzerland, 2020; pp. 1–13. ISBN 978-3-030-35949-2. [Google Scholar]
- Nascimento, F.X.; Hernández, A.G.; Glick, B.R.; Rossi, M.J. Plant Growth-Promoting Activities and Genomic Analysis of the Stress-Resistant Bacillus megaterium STB1, a Bacterium of Agricultural and Biotechnological Interest. Biotechnol. Rep. 2020, 25, e00406. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Recent Advances in Bacterial Amelioration of Plant Drought and Salt Stress. Biology 2022, 11, 437. [Google Scholar] [CrossRef]
- Alotaibi, S.S.; Darwish, H.; Alzahrani, A.K.; Alharthi, S.; Alghamdi, A.S.; Al-Barty, A.M.; Helal, M.; Maghrabi, A.; Baazeem, A.; Alamari, H.A.; et al. Environment-Friendly Control Potential of Two Citrus Essential Oils against Aphis punicae and Aphis illinoisensis (Hemiptera: Aphididae). Agronomy 2022, 12, 2040. [Google Scholar] [CrossRef]
- Dos Santos, M.L.; Soares, C.R.F.S.; Comin, J.J.; Lovato, P.E. The Phytoprotective Effects of Arbuscular Mycorrhizal Fungi on Enterolobium Contorstisiliquum (Vell.) Morong in Soil Containing Coal-Mine Tailings. Int. J. Phytoremediat. 2017, 19, 1100–1108. [Google Scholar] [CrossRef]
- Brunetto, G.; Rosa, D.J.; Ambrosini, V.G.; Heinzen, J.; Ferreira, P.A.A.; Ceretta, C.A.; Soares, C.R.F.S.; Melo, G.W.B.; Soriani, H.H.; Nicoloso, F.T.; et al. Use of Phosphorus Fertilization and Mycorrhization as Strategies for Reducing Copper Toxicity in Young Grapevines. Sci. Hortic. 2019, 248, 176–183. [Google Scholar] [CrossRef]
- Lei No 6.894 de 16 de Dezembro de 1980. Available online: https://www.planalto.gov.br/ccivil_03/leis/1980-1988/l6894.htm (accessed on 3 October 2023).
- Decreto No 4.954 de 14 de Janeiro de 2004. Available online: https://www.planalto.gov.br/ccivil_03/_ato2004-2006/2004/decreto/d4954.htm (accessed on 3 October 2023).
- Instrução Normativa No 61 de 08 de Julho de 2020. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/fertilizantes/legislacao/in-61-de-8-7-2020-organicos-e-biofertilizantes-dou-15-7-20.pdf (accessed on 3 October 2023).
- Lei No 7.802 de 11 de Julho de 1989. Available online: https://www.planalto.gov.br/ccivil_03/leis/l7802.htm (accessed on 3 October 2023).
- De Oliveira, H.N.; Ávila, C.J. Controle biológico de pragas no Centro-Oeste brasileiro. Rev. Controle Biol. 2010, 11–13. [Google Scholar]
- Souza, M.; Vargas, M.M.M.; Ventura, B.S.; Müller Júnior, V.; Soares, C.R.F.S.; Kurtz, C.; Comin, J.J.; Lovato, P.E. Microbial Activity in Soil with Onion Grown in a No-Tillage System with Single or Intercropped Cover Crops. Cienc. Rural 2020, 50, e20190849. [Google Scholar] [CrossRef]
- Biodefensivos, Cada Vez Mais Presentes No Campo. Available online: https://croplifebrasil.org/noticias/biodefensivos-cada-vez-mais-presentes-no-campo/ (accessed on 3 October 2023).
- Neves, T.N.; Foresti, J.; Silva, P.R.; Alves, E.; Rocha, R.; Oliveira, C.; Picanço, M.C.; Pereira, E.J. Insecticide Seed Treatment against Corn Leafhopper: Helping Protect Grain Yield in Critical Plant Growth Stages. Pest Manag. Sci. 2022, 78, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Deshwal, B.; Thalluri, R.; Meena, S. Biopesticides as Promising Alternatives to Traditional Approaches. Vigyan Varta 2022, 3, 73–76. [Google Scholar]
- Reddy, D.S.; Chowdary, N.M. Botanical Biopesticide Combination Concept—A Viable Option for Pest Management in Organic Farming. Egypt. J. Biol. Pest Control 2021, 31, 23. [Google Scholar] [CrossRef]
- Ruiu, L. Microbial Biopesticides in Agroecosystems. Agronomy 2018, 8, 235. [Google Scholar] [CrossRef]
- El Aimani, A.; Houari, A.; Laasli, S.-E.; Mentag, R.; Iraqi, D.; Diria, G.; Khayi, S.; Lahlali, R.; Dababat, A.A.; Mokrini, F. Antagonistic Potential of Moroccan Entomopathogenic Nematodes against Root-Knot Nematodes, Meloidogyne Javanica on Tomato under Greenhouse Conditions. Sci. Rep. 2022, 12, 2915. [Google Scholar] [CrossRef]
- Subedi, S.; Thapa, B.; Shrestha, J. Root-Knot Nematode (Meloidogyne Incognita) and Its Management: A Review. J. Agric. Nat. Res. 2020, 3, 21–31. [Google Scholar] [CrossRef]
- Produtos Fitossanitários Com Uso Aprovado Para a Agricultura Orgânica Registrados. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/agrotoxicos/produtos-fitossanitarios/ProdutosFitossanitarioscomusoAprovadoparaaAgriculturaOrganicaRegistradosatualizadoem151221.pdf (accessed on 3 October 2023).
- Belal, E.B.; Shalaby, M.E.; El-Said, R.A.R.; Abdelrazek, M.A.S.; Ebrahim, A.E.E.; Gad, W.A. Utilization of Paper Wastes for Cellulolytic Enzyme Production by Aspergillus niger Strain 13A and Using the Bioorganic Materials in the Biocontrol of Fusarium Wilt of Cucumber (Cucumis sativus L.). Appl. Ecol. Environ. Res. 2021, 19, 1233–1246. [Google Scholar] [CrossRef]
- Brun, T.; Rabuske, J.E.; Confortin, T.C.; Luft, L.; Todero, I.; Fischer, M.; Zabot, G.L.; Mazutti, M.A. Weed Control by Metabolites Produced from Diaporthe schini. Environ. Technol. 2022, 43, 139–148. [Google Scholar] [CrossRef]
- Chaves Neto, J.R.; Nascimento Dos Santos, M.S.; Mazutti, M.A.; Zabot, G.L.; Tres, M.V. Phoma dimorpha Phytotoxic Activity Potentialization for Bioherbicide Production. Biocatal. Agric. Biotechnol. 2021, 33, 101986. [Google Scholar] [CrossRef]
- Sayyad, I.G.; Mogle, U. In Vitro Antifungal Activity of Plant Extracts on Pathogenic Fungi of Chilli (Capsicum annum L.). Int. J. Curr. Sci. 2023, 13, 673–681. [Google Scholar]
- Confortin, T.C.; Todero, I.; Luft, L.; Schmaltz, S.; Ferreira, D.F.; Barin, J.S.; Mazutti, M.A.; Zabot, G.L.; Tres, M.V. Extraction of Bioactive Compounds from Senecio Brasiliensis Using Emergent Technologies. 3 Biotech 2021, 11, 284. [Google Scholar] [CrossRef]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M.; et al. Status and Prospects of Botanical Biopesticides in Europe and Mediterranean Countries. Biomolecules 2022, 12, 311. [Google Scholar] [CrossRef]
- García-Gómez, A.; Figueroa-Brito, R.; Serrano, L.A.G.; Jiménez-Pérez, A. Trichilia (Meliaceae) Plants: An Important Source of Biomolecules with Insecticidal Properties. Fla. Entomol. 2018, 101, 470–479. [Google Scholar] [CrossRef]
- De La Torre-Anzures, J.; Figueroa-Brito, R.; Carrazco-Aquino, R.J.; Garcia-Serrano, L.A.; Ramos-Lopez, M.A.; Sotelo-Leyva, C.; Salinas-Sanchez, D.O.; Lopez-Olguin, J.F. Chemical Compounds of Ethanolic Extract from Trichilia havanensis Seeds and Its Insecticidal Activity. Chem. Nat. Compd. 2023, 59, 124–127. [Google Scholar] [CrossRef]
- Nuryanti, N.; Yuriansyah; Budiarti, L. Toxicity and Compatibility of Botanical Insecticide From Clove (Syzygium aromaticum), Lime (Citrus aurantifolia) and Garlic (Allium sativum) Essential Oil Against Callasobruchus chinensis L. IOP Conf. Ser. Earth Environ. Sci. 2022, 1012, 012036. [Google Scholar] [CrossRef]
- Rodrigues, F.S. Inseticida de Extrato de Sementes de Annona Squamosa Sobre Chrysodeixis Includens. Master’s Thesis, Universidade Federal de Santa Maria, Santa Maria, Brazil, 2022. [Google Scholar]
- Fite, T.; Tefera, T.; Negeri, M.; Damte, T. Effect of Azadirachta indica and Milletia ferruginea Extracts against Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) Infestation Management in Chickpea. Cogent Food Agric. 2020, 6, 1712145. [Google Scholar] [CrossRef]
- Anchieta, M.G.; Pigatto, G.; Baisch, J.S.; Dolianitis, B.M.; Coradi, P.C.; Guedes, J.V.C.; Mazutti, M.A.; Tres, M.V.; Zabot, G.L. Pre- and Post-Emergence Control of Hovenia Dulcis with Extracts Obtained from Pepper (Capsicum baccatum). Rev. Eng. Agric. REVENG 2022, 30, 371–382. [Google Scholar] [CrossRef]
- Moustafa, M.A.M.; Awad, M.; Amer, A.; Hassan, N.N.; Ibrahim, E.-D.S.; Ali, H.M.; Akrami, M.; Salem, M.Z.M. Insecticidal Activity of Lemongrass Essential Oil as an Eco-Friendly Agent against the Black Cutworm Agrotis ipsilon (Lepidoptera: Noctuidae). Insects 2021, 12, 737, Erratum in Insects 2021, 12, 991. [Google Scholar] [CrossRef] [PubMed]
- Dolianitis, B.M. Alelopatia de Extratos Vegetais Sobre Eragrostis Plana Ness e Hidrólise Da Sua Biomassa Para Prospecção de Compostos Químicos. Master’s Thesis, Universidade Federal de Santa Maria, Santa Maria, Brazil, 2022. [Google Scholar]
- Confortin, T.C.; Todero, I.; Soares, J.F.; Luft, L.; Brun, T.; Rabuske, J.E.; Nogueira, C.U.; Mazutti, M.A.; Zabot, G.L.; Tres, M.V. Extracts from Lupinus Albescens: Antioxidant Power and Antifungal Activity In Vitro against Phytopathogenic Fungi. Environ. Technol. 2019, 40, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Akbar, R.; Khan, I.A.; Alajmi, R.A.; Ali, A.; Faheem, B.; Usman, A.; Ahmed, A.M.; El-Shazly, M.; Farid, A.; Giesy, J.P.; et al. Evaluation of Insecticidal Potentials of Five Plant Extracts against the Stored Grain Pest, Callosobruchus maculatus (Coleoptera: Bruchidae). Insects 2022, 13, 1047. [Google Scholar] [CrossRef] [PubMed]
- Sotelo-Leyva, C.; Salinas-Sánchez, D.O.; Peña-Chora, G.; Trejo-Loyo, A.G.; González-Cortázar, M.; Zamilpa, A. Insecticidal Compounds in Ricinus communis L. (Euphorbiaceae) to Control Melanaphis sacchari Zehntner (Hemiptera: Aphididae). Fla. Entomol. 2020, 103, 91. [Google Scholar] [CrossRef]
- Baldin, E.L.L.; Schlick-Souza, E.C.; Soares, M.C.E.; Lopes, N.P.; Lopes, J.L.C.; Bogorni, P.C.; Vendramim, J.D. Insecticidal and Inhibitory Effects of Meliaceae and Asteraceae Extracts to Silverleaf Whitefly. Hortic. Bras. 2020, 38, 280–287. [Google Scholar] [CrossRef]
- Ugwu, J.A.; Alabi, O.Y.; Aluko, O.J. Insecticidal Activity of Crude Extracts of Three Spices and Commercial Botanical Pesticide on Oriental Fruit Fly under Laboratory Conditions. J. Basic Appl. Zool. 2021, 82, 26. [Google Scholar] [CrossRef]
- Copping, L.; Peregrine, J.; Matthews, G. The 2007 BCPC/IPPC Congress in Glasgow. Outlooks Pest Manag. 2007, 18, 245–253. [Google Scholar] [CrossRef]
- Yoon, M.-Y.; Cha, B.; Kim, J.-C. Recent Trends in Studies on Botanical Fungicides in Agriculture. Plant Pathol. J. 2013, 29, 1–9. [Google Scholar] [CrossRef]
- Adak, T.; Barik, N.; Patil, N.B.; Govindharaj, G.-P.-P.; Gadratagi, B.G.; Annamalai, M.; Mukherjee, A.K.; Rath, P.C. Nanoemulsion of Eucalyptus Oil: An Alternative to Synthetic Pesticides against Two Major Storage Insects (Sitophilus oryzae (L.) and Tribolium castaneum (Herbst)) of Rice. Ind. Crops Prod. 2020, 143, 111849. [Google Scholar] [CrossRef]
- Bharti, A.; Dubey, I.; Chandel, B. Phago-Repellent Biopotency of Cichorium intybus, Inula racemosa, Tagetes minuta and Chrysanthemum cinerariaefolium as Herbal Alternatives to Synthetic Insecticides against Earias vittella Fabricius (Lepidoptera: Noctuidae) on Okra in Kanpur Region. Int. J. Fauna Biol. Stud. 2021, 8, 11–17. [Google Scholar] [CrossRef]
- Annual New Product Introductions: Biological vs. Conventional. Available online: https://www.spglobal.com/commodityinsights/en/ci/research-analysis/biologicals-innovation.html#:~:text=Innovation%20in%20the%20Biologicals%20Segment,600) (accessed on 3 October 2023).
- Meyer, M.C. Bioinsumos na Cultura da Soja; Embrapa: Brasilia, Brazil, 2022; ISBN 9786587380964. [Google Scholar]
- DunhamTrimmer. State of the Biological Products Industry; DunhamTrimmer: Lakewood Ranch, FL, USA, 2021. [Google Scholar]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Inoculação: Uma Prática Essencial—Outlook GlobalFert. Available online: http://www.anpii.org.br/tag/globalfert/ (accessed on 3 October 2023).
- Mercado de Insumos Biológicos No Brasil Poderá Alcançar R$ 6,2 Bilhões Até 2025. Available online: https://ciorganicos.com.br/sustentabilidade/mercado-de-insumos-biologicos-no-brasil-podera-alcancar-r-6-2-milhoes-ate-2025/ (accessed on 3 October 2023).
- Relatório de Comercialização de Agrotóxicos. Available online: http://www.ibama.gov.br/agrotoxicos/relatorios-de-comercializacao-de-agrotoxicos#historicodecomercializacao (accessed on 3 October 2023).
- Acompanhamento Da Safra Brasileira. Available online: https://www.conab.gov.br/info-agro/safras (accessed on 3 October 2023).


| Pesticide Product | Definition | Active Substances |
|---|---|---|
| Biochemicals | Products consisting of naturally occurring chemical substances with a nontoxic mode of action, used in the control of diseases or pests as agents that promote chemical or biological processes |
|
| Semiochemicals | Products consisting of chemical substances that evoke behavioral or physiological responses in recipient organisms and are used to detect, monitor, and control a population or biological activity of living organisms |
|
| Biological Control Agents | Living organisms, naturally occurring or obtained through genetic manipulation, introduced into the environment for the control of a population or biological activities of another organism considered harmful |
|
| Microbiological |
|
| Plant | Family | Part Used | Class of Compounds | Target Pest | Reference |
|---|---|---|---|---|---|
| Allium sativum | Amaryllidaceae | Bulbs | Dimethyl trisulfide, diallyl disulfide, diallyl sulfide, diallyl tetrasulfide, 3-vinyl-[4 H]-1,2-dithiin, diallyl trisulfide, allyl trisulfide, 1,4 -dimethyl tetrasulfide, allyl disulfide, methyl allyl disulfide, and methyl allyl trisulfide | Callosobruchus chinensis | [94] |
| Annona squamosa | Annonaceae | Seeds | Caryophyllene oxide and acetogenins | Chrysodeixis includens | [95] |
| Azadirachta indica | Meliaceae | Seeds | Rotenone, deguelin, and tephrosin | Helicoverpa armigera | [96] |
| Capsicum baccatum | Solanaceae | Fruits | Capsaicinoids, carotenoids, and ascorbic acid | Hovenia dulcis | [97] |
| Cymbopogon flexuosus | Poaceae | Leaves | α-citral and β-citral | Agrotis ipsilon | [98] |
| Eucalyptus camaldulensis | Myrtaceae | Leaves | 1,8-cineole, l-α-terpineol, and α-pinene | Eragrostis plana | [99] |
| Lupinus albescens | Fabaceae | Roots, stalks, leaves, and flowers | Stigmasterol, Ergosterol, Vitamin E, Methyl commate, Eicosanol, Epiergostanol, and Tetracosanol | Fusarium oxysporum; Fusarium verticillioides | [100] |
| Nicotiana tabacum | Solanaceae | Leaves | Alkaloids, Saponins, Diterphenes, Phytosterol, Flavonoids, and Phenols | Callosobruchus maculatus | [101] |
| Ricinus communis | Euphorbiaceae | Fruits | Carotenoid, Tocopherol, Tocotrienol, Phytosterol, and Phospholipid | Melanaphis sacchari | [102] |
| Trichilia spp. | Meliaceae | Fruits | Β-Sitosterol, Β-Amyrin, Stigmasterol, Campesterol, Sitostenone, Lupeol, Lupenone, Cryptomeridiol, and A-Amyrin | Bemisia tabaci | [103] |
| Zingiber officinale | Zingiberaceae | Rhizomes | Gingerol, Paradol, Shogaols, and Zingerone | Bactrocera dorsalis | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, C.R.F.S.; Hernández, A.G.; da Silva, E.P.; de Souza, J.E.A.; Bonfim, D.F.; Zabot, G.L.; Ferreira, P.A.A.; Brunetto, G. Applications and Market of Micro-Organism-Based and Plant-Based Inputs in Brazilian Agriculture. Plants 2023, 12, 3844. https://doi.org/10.3390/plants12223844
Soares CRFS, Hernández AG, da Silva EP, de Souza JEA, Bonfim DF, Zabot GL, Ferreira PAA, Brunetto G. Applications and Market of Micro-Organism-Based and Plant-Based Inputs in Brazilian Agriculture. Plants. 2023; 12(22):3844. https://doi.org/10.3390/plants12223844
Chicago/Turabian StyleSoares, Cláudio Roberto Fonsêca Sousa, Anabel González Hernández, Emanuela Pille da Silva, Julia Emanuela Almeida de Souza, Danyella Fernandes Bonfim, Giovani Leone Zabot, Paulo Ademar Avelar Ferreira, and Gustavo Brunetto. 2023. "Applications and Market of Micro-Organism-Based and Plant-Based Inputs in Brazilian Agriculture" Plants 12, no. 22: 3844. https://doi.org/10.3390/plants12223844
APA StyleSoares, C. R. F. S., Hernández, A. G., da Silva, E. P., de Souza, J. E. A., Bonfim, D. F., Zabot, G. L., Ferreira, P. A. A., & Brunetto, G. (2023). Applications and Market of Micro-Organism-Based and Plant-Based Inputs in Brazilian Agriculture. Plants, 12(22), 3844. https://doi.org/10.3390/plants12223844








