Does the Harvest Type Affect Olive Health? Influence of the Harvesting System and Storage Time on the Chemical, Volatile and Sensory Qualities of Extra Virgin Olive Oils
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material and Climatic Conditions
2.2. Fruit Sampling, Harvesting, Storage and Oil Extraction
2.2.1. Fruit Sampling and Harvesting
- (1)
- Manual Harvesting: This was carried out by gently detaching the fruits from the plant manually and collecting them in woody baskets to avoid shocks and trauma with the fall; then, the olives were transferred to perforated plastic boxes, each containing about 20 kg of fruits.
- (2)
- Mechanical Harvesting: Hand-held olive harvesting by electric vibrating combs (Alice Top, by Campagnola) allows for the drupes to fall on nets around the tree. The fruits were subsequently transferred to perforated plastic boxes, each containing about 20 kg of fruits. As reported by Pegna et al. (2021) [18], the “Alice Top” has two opposed combs moving toward the other with 11 teeth each (6 long and 5 short, alternated), following an elliptical trace movement with an oscillation frequency of 19 Hz.
2.2.2. Fruit Storage Treatments
2.2.3. Oil Extraction and Storage Treatments
2.3. Extraction of Phenolic Compounds from Olives
2.4. Visual Bruising Assessment
2.5. Sensory Evaluation (SE) by Trained Panel
2.6. Instrumental Setup: VOC Detection from Olive Fruits and Olive Oils
2.6.1. Olive Fruits Sample Preparation and Analysis
2.6.2. Olive Oils Sample Preparation and Analysis
2.7. Olive Oil Quality Parameter Analysis
2.8. Statistical Data Analyses
3. Results and Discussion
3.1. Climatic Data and Fruits Characteristics
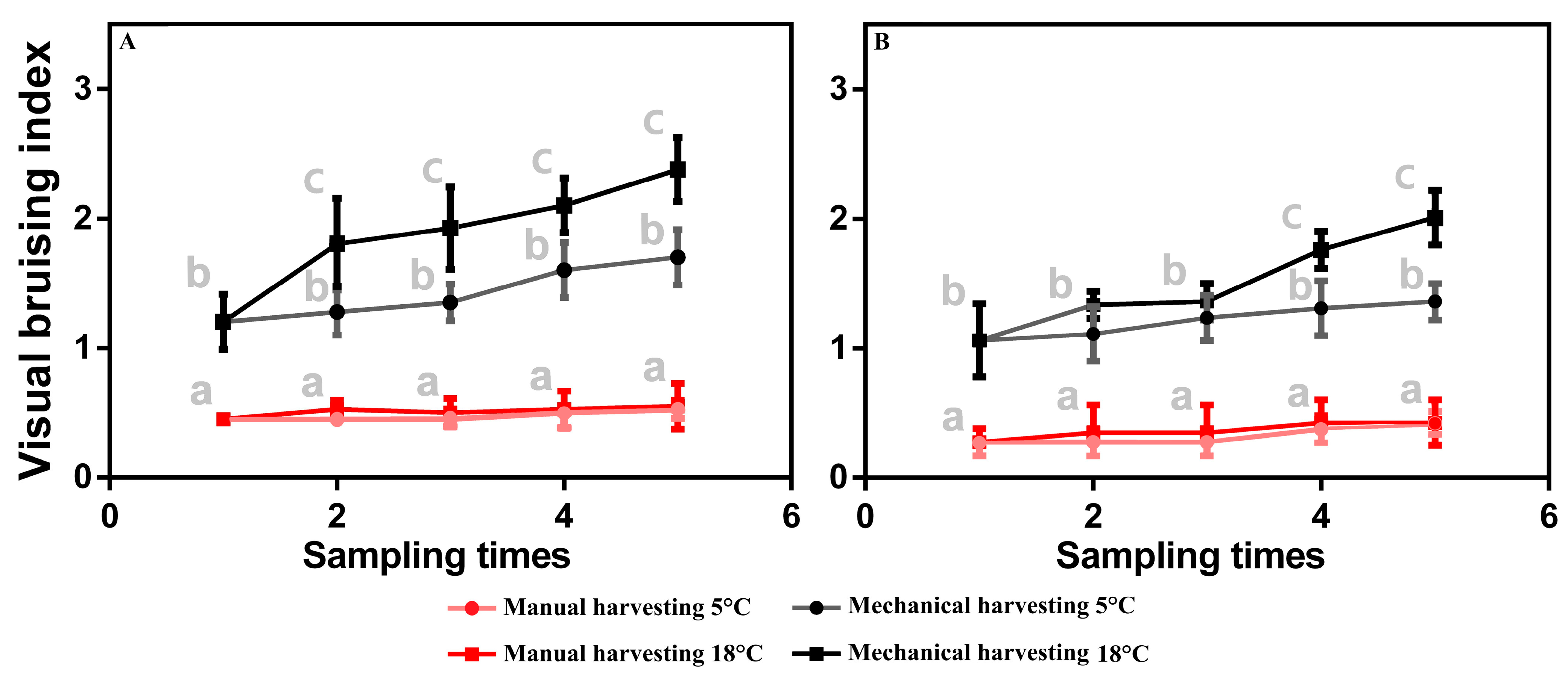
3.2. Visual Bruising Observations in Olive Fruit
3.3. Influence of Olive Cultivar, Harvesting System and Storage Temperature on Phenolics Content in Olive Fruits
3.4. VOCs Results
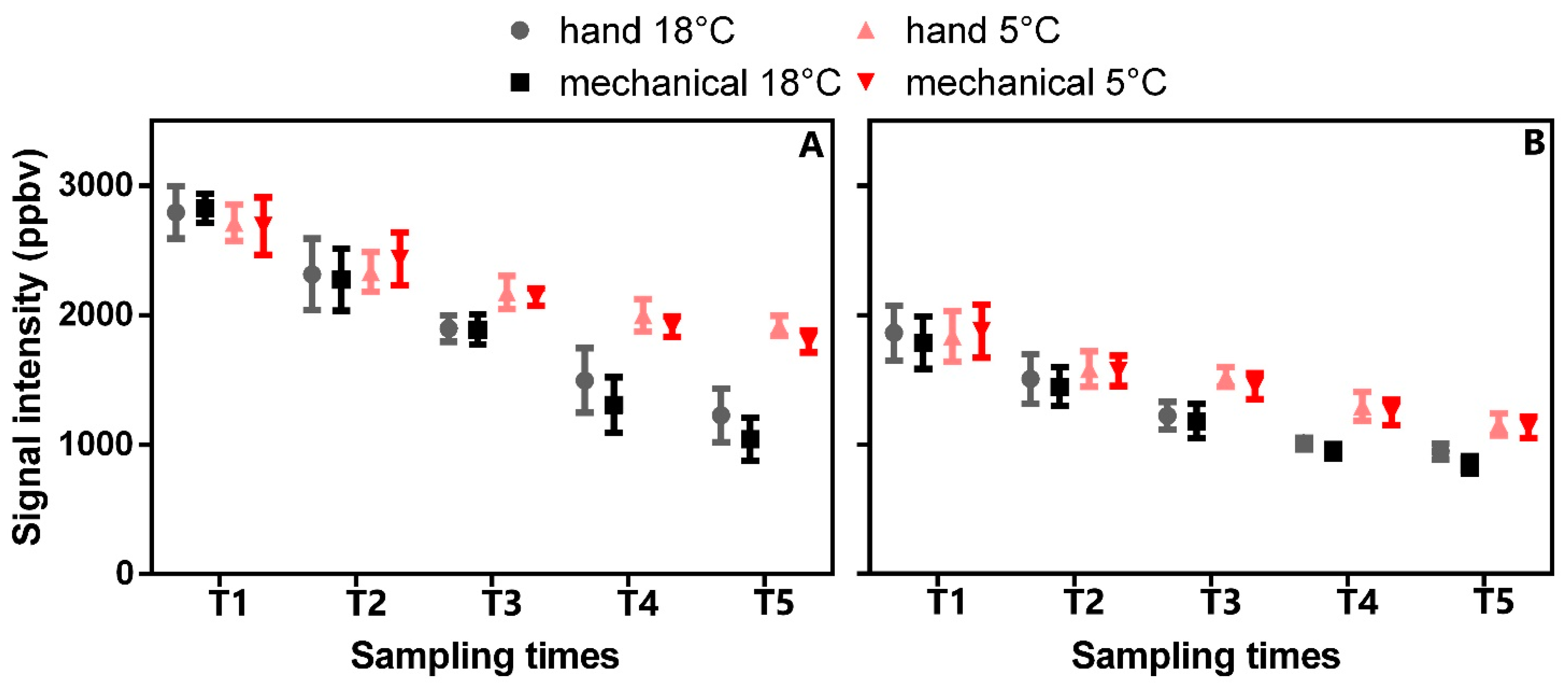
3.4.1. Influence of Olive Cultivar and Time Storage on VOC Emissions in Olive Fruits
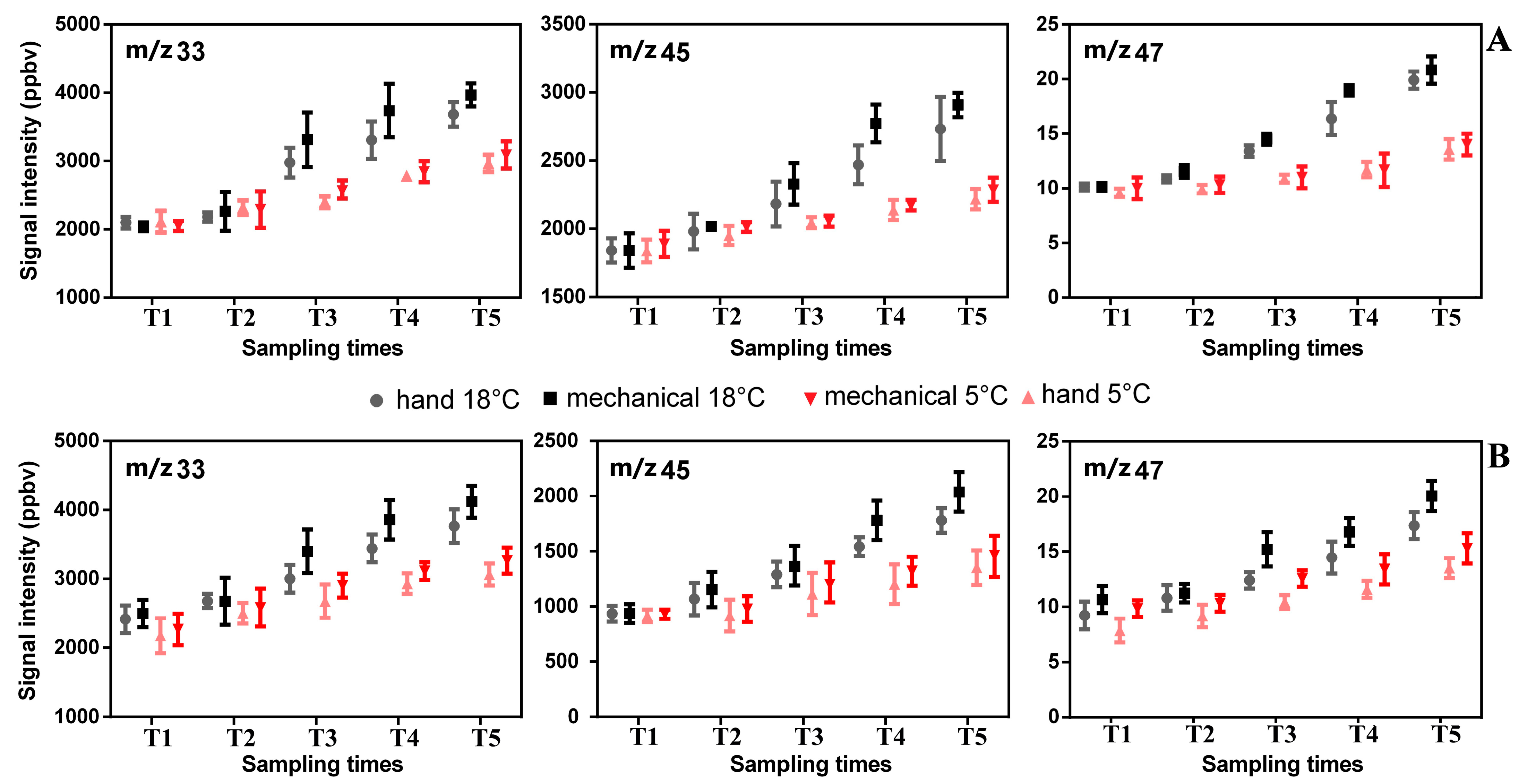
3.4.2. Influence of Olive Cultivar and Time Storage on VOC Emissions in Olive Oil
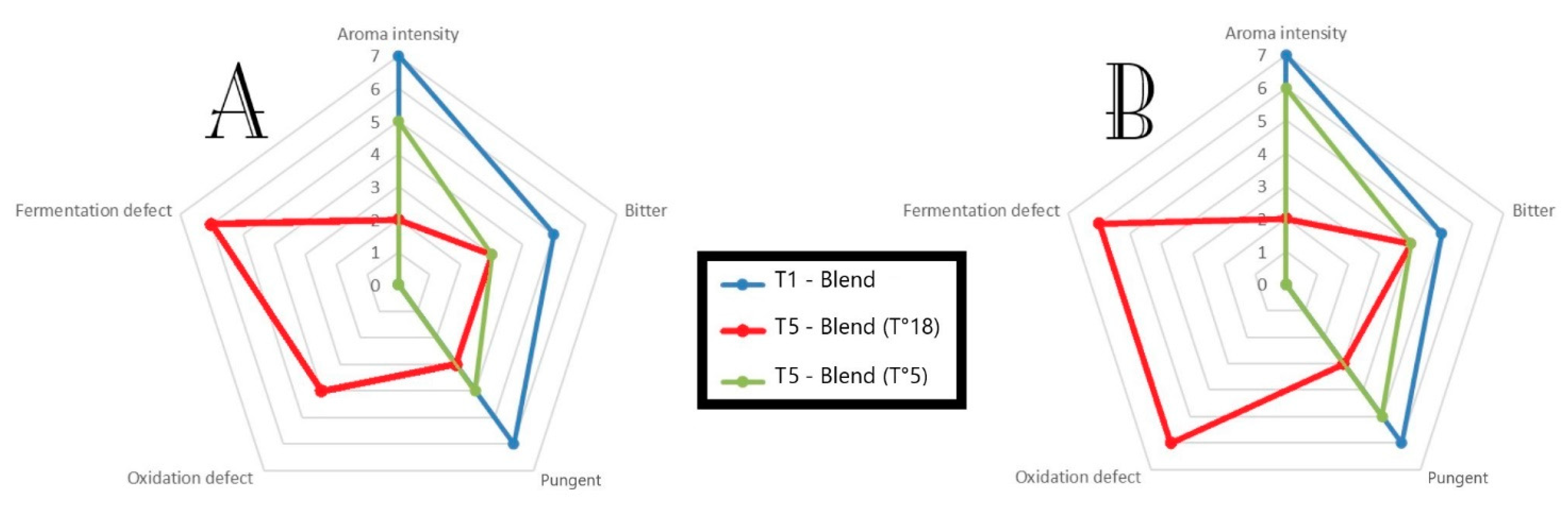
3.5. Sensorial Analysis
3.6. Influence of Olive Cultivar and Time Storage on Olive Oil Quality Parameter
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marone, E.; Fiorino, P. Oleiculture in progress. Adv. Hortic. Sci. 2012, 26, 163–175. [Google Scholar]
- Ferguson, L. Trends in olive fruit handling previous to its industrial transformation. Grasas Y Aceites 2006, 57, 9–15. [Google Scholar] [CrossRef]
- Dag, A.; Boim, S.; Sobotin, Y.; Zipori, I. Effect of mechanically harvested olive storage temperature and duration on oil quality. HortTechnology 2012, 22, 528–533. [Google Scholar] [CrossRef]
- Bernardi, B.; Falcone, G.; Stillitano, T.; Benalia, S.; Strano, A.; Bacenetti, J.; De Luca, A.I. Harvesting system sustainability in Mediterranean olive cultivation. Sci. Total Environ. 2018, 625, 1446–1458. [Google Scholar] [CrossRef]
- Sarri, D.; Vieri, M. Criteria for introducing mechanical harvesting of oil olives: Results of a five-year project in Central Italy. Adv. Hortic. Sci. 2010, 24, 1000–1013. [Google Scholar]
- Famiani, F.; Farinelli, D.; Rollo, S.; Camposeo, S.; Di Vaio, C.; Inglese, P. Evaluation of different mechanical fruit harvesting systems and oil quality in very large size olive trees. Span. J. Agric. Res. 2014, 12, 960–972. [Google Scholar] [CrossRef]
- Sola-Guirado, R.R.; Castro-García, S.; Blanco-Roldán, G.L.; Jiménez-Jiménez, F.; Castillo-Ruiz, F.J.; Gil-Ribes, J.A. Traditional olive tree response to oil olive harvesting technologies. Biosyst. Eng. 2014, 118, 186–193. [Google Scholar] [CrossRef]
- Hussein, Z.; Fawole, O.A.; Opara, U.L. Harvest and postharvest factors affecting bruise damage of fresh fruits. Hortic. Plant J. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- García, J.M.; Gutiérrez, F.; Castellano, J.M.; Perdiguero, S.; Morilla, A.; Albi, M.A. Influence of storage temperature on fruit ripening and olive oil quality. J. Agric. Food Chem. 1996, 44, 264–267. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.; Castro-García, S.; Blanco-Roldán, G.L.; Ferguson, L.; Rosa, U.A.; Gil-Ribes, J.A. Table olive cultivar susceptibility to impact bruising. Postharvest Biol. Technol. 2013, 86, 100–106. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.; Castro-Garcia, S.; Blanco-Roldán, G.L.; González-Sánchez, E.J.; Gil-Ribes, J.A. Isolation of table olive damage causes and bruise time evolution during fruit detachment with trunk shaker. Span. J. Agric. Res. 2013, 11, 65–71. [Google Scholar] [CrossRef]
- Sola-Guirado, R.R.; Bayano-Tejero, S.; Aragon-Rodriguez, F.; Peña, A.; Blanco-Roldan, G. Bruising pattern of table olives (‘Manzanilla’ and ‘Hojiblanca’ cultivars) caused by hand-held machine harvesting methods. Biosyst. Eng. 2022, 215, 188–202. [Google Scholar] [CrossRef]
- Morales-Sillero, A.; Jiménez, M.R.; Suárez, M.P.; Rallo, P.; Casanova, L. Mechanical harvesting at dawn in a super-high-density table olive orchard: Effect on the quality of fruits. J. Sci. Food Agric. 2023, 103, 2989–2996. [Google Scholar] [CrossRef] [PubMed]
- Plasquy, E.; García Martos, J.M.; Florido, M.C.; Sola-Guirado, R.R.; García Martín, J.F. Cold storage and temperature management of olive fruit: The impact on fruit physiology and olive oil quality—A review. Processes 2021, 9, 1543. [Google Scholar] [CrossRef]
- Farinelli, D.; Tombesi, S. Performance and oil quality of ‘Arbequina’and four Italian olive cultivars under super high density hedgerow planting system cultivated in central Italy. Sci. Hortic. 2015, 192, 97–107. [Google Scholar] [CrossRef]
- Mafrica, R.; Piscopo, A.; De Bruno, A.; Poiana, M. Effects of climate on fruit growth and development on olive oil quality in cultivar carolea. Agriculture 2021, 11, 147. [Google Scholar] [CrossRef]
- Ferreira, J. Explotaciones Olivareras Colaboradoras; n. 5; Ministerio de Agricultura: Madrid, Spain, 1979. [Google Scholar]
- Pegna, F.G.; Nourani, A.; Romano, A. Mechanically assisted harvesting of dry and semi-dry dates of average to low quality. J. Agric. Environ. Int. Dev. (JAEID) 2021, 115, 85–96. [Google Scholar]
- Rabiei, V.; Ghorbani, S.; Hajnajari, H. Effect of temperature and storage period of olive (Olea europaea cv. Zard) fruit on olive oil quality. J. Food Agric. Environ. 2011, 9, 74–77. [Google Scholar]
- Saffar Taluri, S.; Jafari, S.M.; Bahrami, A. Evaluation of changes in the quality of extracted oil from olive fruits stored under different temperatures and time intervals. Sci. Rep. 2019, 9, 19688. [Google Scholar] [CrossRef]
- Flamminii, F.; Paciulli, M.; Di Michele, A.; Littardi, P.; Carini, E.; Chiavaro, E.; Pittia, P.; Di Mattia, C.D. Alginate-based microparticles structured with different biopolymers and enriched with a phenolic-rich olive leaves extract: A physico-chemical characterization. Curr. Res. Food Sci. 2021, 4, 698–706. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- IOC/T.20/Doc. No 15/Rev. 10/2018; Sensory Analysis of Olive Oil-Method for the Organoleptic Assessment of Virgin Olive Oil. International Olive Council: Madrid, Spain, 2018.
- IOC/T.20/Doc. No 14/Rev. 4/2013; Sensory Analysis of Olive Oil, Standard, Guide for the Selection, Training and Monitoring of Skilled Olive Oil Tasters. International Olive Council: Madrid, Spain, 2013.
- Masi, E.; Romani, A.; Pandolfi, C.; Heimler, D.; Mancuso, S. PTR-TOF-MS analysis of volatile compounds in olive fruits. J. Sci. Food Agric. 2015, 95, 1428–1434. [Google Scholar] [CrossRef]
- Taiti, C.; Marone, E.; Fiorino, P.; Mancuso, S. The olive oil dilemma: To be or not to be EVOO? chemometric analysis to grade virgin olive oils using 792 fingerprints from, P.T.R.-T.o.F.-M.S. Food Control 2022, 135, 108817. [Google Scholar] [CrossRef]
- Commission Regulation (EEC) No 2568/91 of 11 July 1991 (and later modifications) on the characteristics of olive oil and olive-residue oil and the relevant methods of analysis. Off. J. Eur. Comm. 1991, L248, 1–83.
- Montedoro, G.; Servili, M.; Baldioli, M.; Miniati, E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. J. Agric. Food Chem. 1992, 40, 1571–1576. [Google Scholar] [CrossRef]
- Jiménez, M.R.; Rallo, P.; Rapoport, H.F.; Suárez, M.P. Distribution and timing of cell damage associated with olive fruit bruising and its use in analyzing susceptibility. Postharvest Biol. Technol. 2016, 111, 117–125. [Google Scholar] [CrossRef]
- Segovia-Bravo, K.A.; Jarén-Galán, M.; García-García, P.; Garrido-Fernández, A. Treatments to inhibit the browning reactions in model solutions of olive fruit extracts. Food Chem. 2010, 123, 741–746. [Google Scholar] [CrossRef]
- Segovia-Bravo, K.A.; López, F.A.; García, P.G.; Quintana, M.D.; Fernández, A.G. Treatment of green table olive solutions with ozone. Effect on their polyphenol content and on Lactobacillus pentosus and Saccharomyces cerevisiae growth. Int. J. Food Microbiol. 2007, 114, 60–68. [Google Scholar] [CrossRef]
- Segovia-Bravo, K.A.; Jarén-Galán, M.; García-García, P.; Garrido-Fernández, A. Browning reactions in olives: Mechanism and polyphenols involved. Food Chem. 2009, 114, 1380–1385. [Google Scholar] [CrossRef]
- Morales-Silero, A. Influencia de la recolección en la composición fenólica. Actas Hortic. 2019, 84, 22–25. [Google Scholar]
- Cecchi, L.; Migliorini, M.; Cherubini, C.; Giusti, M.; Zanoni, B.; Innocenti, M.; Mulinacci, N. Phenolic profiles, oil amount and sugar content during olive ripening of three typical Tuscan cultivars to detect the best harvesting time for oil production. Food Res. Int. 2013, 54, 1876–1884. [Google Scholar] [CrossRef]
- Hornero-Mendez, D.; Gallardo-Guerrero, L.; Jaren-Galan, M.; Minguez-Mosquera, M.I. Differences in the activity of superoxidase dismutase, polyphenol oxidase and Cu–Zn content in the fruits of Gordal and Manzanilla olive varieties. Z. Für Naturforschung 2002, 57c, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hbaieb, R.H.; Kotti, F.; García-Rodríguez, R.; Gargouri, M.; Sanz, C.; Pérez, A.G. Monitoring endogenous enzymes during olive fruit ripening and storage: Correlation with virgin olive oil phenolic profiles. Food Chem. 2015, 174, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Yousfi, K.; Weiland, C.M.; García, J.M. Effect of harvesting system and fruit cold storage on virgin olive oil chemical composition and quality of superintensive cultivated ‘Arbequina’ olives. J. Agric. Food Chem. 2012, 60, 4743–4750. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Cunha, S.C.; Baptista, P.; Pereira, J.A. Olive volatiles from Portuguese cultivars Cobrançosa, Madural and Verdeal Transmontana: Role in oviposition preference of Bactrocera oleae (Rossi)(Diptera: Tephritidae). PLoS ONE 2015, 10, e0125070. [Google Scholar] [CrossRef]
- Colzi, I.; Marone, E.; Luti, S.; Pazzagli, L.; Mancuso, S.; Taiti, C. Metabolic Responses in Leaves of 15 Italian Olive Cultivars in Correspondence to Variable Climatic Elements. Plants 2023, 12, 1953. [Google Scholar] [CrossRef]
- Ueda, Y.; Yamanaka, H.; Ose, K.; Imahori, Y.; Wendakoon, S. Production of Ethanol and Methanol in Fresh-Cut Fruits at Different Maturity Stages during Storage. Food Preserv. Sci. 2019, 45, 73–84. [Google Scholar] [CrossRef]
- Beltran, G.; Hueso, A.; Bejaoui, M.A.; Gila, A.M.; Costales, R.; Sánchez-Ortiz, A.; Aguilera, M.P.; Jimenez, A. How olive washing and storage affect fruit ethanol and virgin olive oil ethanol, ethyl esters and composition. J. Sci. Food Agric. 2021, 101, 3714–3722. [Google Scholar] [CrossRef]
- Beltrán, G.; Bejaoui, M.A.; Jimenez, A.; Sanchez-Ortiz, A. Ethanol in olive fruit. Changes during ripening. J. Agric. Food Chem. 2015, 63, 5309–5312. [Google Scholar] [CrossRef]
- Geana, E.I.; Ciucure, C.T.; Apetrei, I.M.; Clodoveo, M.L.; Apetrei, C. Discrimination of Olive Oil and Extra-Virgin Olive Oil from Other Vegetable Oils by Targeted and Untargeted HRMS Profiling of Phenolic and Triterpenic Compounds Combined with Chemometrics. Int. J. Mol. Sci. 2023, 24, 5292. [Google Scholar] [CrossRef]
- Brkić Bubola, K.; Lukić, M.; Novoselić, A.; Krapac, M.; Lukić, I. Olive fruit refrigeration during prolonged storage preserves the quality of virgin olive oil extracted there from. Foods 2020, 9, 1445. [Google Scholar] [CrossRef] [PubMed]
- Famiani, F.; Farinelli, D.; Urbani, S.; Al Hariri, R.; Paoletti, A.; Rosati, A.; Selvaggini, R.; Taticchi, A.; Servili, M. Harvesting system and fruit storage affect basic quality parameters and phenolic and volatile compounds of oils from intensive and super-intensive olive orchards. Sci. Hortic. 2020, 263, 109045. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Conte, L.; Totis, N. Influence of olive fruit storage in bags on oil quality and composition of volatile compounds. Food Technol. Biotechnol. 2002, 40, 129–134. [Google Scholar]
- Yousfi, K.; Weiland, C.M.; García, J.M.; de La Rábida, C.D.P.; Tejero, A.P.G. Responses of fruit physiology and virgin oil quality to cold storage of mechanically harvested ‘Arbequina’ olives cultivated in hedgerow. Grasas Y Aceites 2013, 64, 5. [Google Scholar]
- Serreli, G.; Deiana, M. Biological relevance of extra virgin olive oil polyphenols metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef]

| Number of Italian PGI and DOP | EVOO Quality Label | District | Allowed Time for Olive Storage (h) | Deadline for Olive Harvesting |
|---|---|---|---|---|
| 1 | DOP “Petruziano colline Teramane” | Abruzzo | 48 | 10 December |
| 2 | DOP “Aprutino pescarese” | Abruzzo | 72 | 10 December |
| 3 | DOP “Colline Teatine” | Abruzzo | * | 20 December |
| 4 | IGP “Olio Lucano” | Basilicata | 48 | 31 January |
| 5 | DOP “Vulture” | Basilicata | 24 | 31 December |
| 6 | IGP “Olio di Calabria” | Calabria | 24 | 15 January |
| 7 | DOP “Alto Crotonese” | Calabria | 48 | 31 December |
| 8 | DOP “Bruzio” | Calabria | 48 | 31 December |
| 9 | DOP “Lametia” | Calabria | * | 15 January |
| 10 | DOP “Cilento” | Campania | 48 | 31 December |
| 11 | DOP “Colline salernitane” | Campania | 48 | 31 December |
| 12 | Dop “Irpinia Colline dell’Ufita” | Campania | 48 | 31 December |
| 13 | DOP “Penisola Sorrentina” | Campania | 48 | 31 December |
| 14 | DOP “Terre Aurunche” | Campania | 48 | 31 December |
| 15 | DOP “Brisighella” | Emilia Romagna | 48 | 20 December |
| 16 | DOP “Colline di Romagna” | Emilia Romagna | 48 | 15 December |
| 17 | DOP “Tergeste” | Friuli | 72 | 31 December |
| 18 | DOP “Canino” | Lazio | 36 | 31 December |
| 19 | DOP “Colline pontine” | Lazio | 48 | 31 January |
| 20 | DOP “Sabina” | Lazio | * | 31 January |
| 21 | DOP “Tuscia” | Lazio | 24 | 15 January |
| 22 | DOP “Riviera ligure” | Liguria | * | 31 March |
| 23 | DOP Garda | Lombardia/Veneto/Trentino | 120 | 15 January |
| 24 | DOP “Laghi Lombardi” | Lombardia | * | 15 January |
| 25 | IGP “Marche” | Marche | * | 15 December |
| 26 | DOP “Cartoceto” | Marche | 48 | 25 December |
| 27 | DOP “Molise” | Molise | 48 | * |
| 28 | IGP “Olio di Puglia” | Apulia | 36 | 31 January |
| 29 | DOP “Dauno Gargano” | Apulia | 72 | 31 January |
| 30 | D.O.P. “Collina di Brindisi” | Apulia | * | 31 January |
| 31 | DOP “Terre di Bari” | Apulia | * | 31 January |
| 32 | DOP “Terre d’Otranto” | Apulia | 48 | 31 January |
| 33 | DOP “Terre Tarentine” | Apulia | 72 | * |
| 34 | DOP “Sardegna” | Sardegna | 48 | 31 January |
| 35 | IGP “Sicilia” | Sicily | 48 | 31 January |
| 36 | DOP “Monti Iblei” | Sicily | 48 | * |
| 37 | DOP “Monte Etna” | Sicily | 48 | * |
| 38 | DOP “Valdemone” | Sicily | 48 | 31 January |
| 39 | DOP “Val di Mazara” | Sicily | 48 | 31 December |
| 40 | DOP “Valle del Belice” | Sicily | 48 | 31 December |
| 41 | DOP “Valli Trapanesi” | Sicily | 48 | 31 December |
| 42 | IGP “Toscana” | Tuscany | * | * |
| 43 | Dop “Chianti Classico” | Tuscany | 72 | * |
| 44 | DOP “Lucca” | Tuscany | 48 | 31 December |
| 45 | DOP “Seggiano” | Tuscany | 48 | 15 January |
| 46 | DOP “Terre di Siena” | Tuscany | 72 | 31 December |
| 47 | DOP “Umbria” | Umbria | * | 15 January |
| 48 | DOP “Veneto Valpolicella” | Veneto | * | 15 January |
| 49 | DOP “Veneto Euganei e Berici” | Veneto | * | 15 January |
| 50 | DOP “Veneto del Grappa” | Veneto | * | 15 January |
| Total Rainfall (mm) | Rainy Days (n°) | Average Temperature (°C) | ||||
|---|---|---|---|---|---|---|
| 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | |
| Jan | 66.8 | 32.4 | 13 | 5 | 9.2 | 5.1 |
| Feb | 112 | 72.6 | 10 | 5 | 6.1 | 9.6 |
| Mar | 201.2 | 7.2 | 16 | 4 | 14.4 | 12 |
| Apr | 58.4 | 88 | 8 | 11 | 16.7 | 13.7 |
| May | 74.2 | 136.4 | 8 | 14 | 19.3 | 15.4 |
| Jun | 39.2 | 18.2 | 4 | 2 | 22.4 | 24.5 |
| Jul | 45.4 | 37.6 | 4 | 4 | 25.9 | 26.3 |
| Aug | 71.6 | 14.2 | 5 | 3 | 25.8 | 26.6 |
| Sep | 9.8 | 105.4 | 1 | 9 | 22.6 | 21.7 |
| Oct | 70.6 | 44.6 | 6 | 5 | 18 | 17.5 |
| Nov | 69.6 | 327.8 | 13 | 22 | 12.2 | 12.5 |
| Dec | 73.6 | 138.8 | 9 | 11 | 7.3 | 9.2 |
| Total | 892.4 | 1023.2 | 97 | 95 | ||
| Average | 16.7 | 16.2 | ||||
| Year | Alternate Bearing | Cultivar | FW (g) ± DS | RD (g) | RI |
|---|---|---|---|---|---|
| 2018/19 | OFF | Frantoio | 2.90 ± 0.66 | 460 | 3.2 |
| Moraiolo | 2.50 ± 0.45 | 480 | 2.9 | ||
| 2019/20 | ON | Frantoio | 2.70 ± 0.35 | 500 | 2.3 |
| Moraiolo | 1.80 ± 0.28 | 580 | 1.9 |
| Source | Sum of Squares | Effect (%) | Df | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|---|
| Main Effects | ||||||
| HS | 15.62 | 42.90 | 1 | 15.62 | 566.43 | 0.000 |
| T | 2.92 | 8.00 | 4 | 0.73 | 26.46 | 0.000 |
| cv | 8.70 | 23.90 | 1 | 8.70 | 315.19 | 0.000 |
| Y | 3.67 | 10.00 | 1 | 3.60 | 131.97 | 0.000 |
| Interactions | ||||||
| HS ×T | 0.07 | - | 4 | 0.02 | 0.66 | 0.620 |
| HS × cv | 0.90 | 2.40 | 1 | 0.90 | 32.05 | 0.000 |
| HS × Y | 1.96 | 5.40 | 1 | 1.95 | 70.72 | 0.000 |
| T × cv | 0.01 | - | 4 | 0.00 | 0.13 | 0.969 |
| T × Y | 0.05 | - | 4 | 0.01 | 0.46 | 0.767 |
| cv × Y | 0.02 | - | 1 | 0.02 | 0.68 | 0.412 |
| HS × T × cv | 0.16 | - | 4 | 0.04 | 1.46 | 0.221 |
| HS × T × Y | 0.01 | - | 4 | 0.00 | 0.12 | 0.975 |
| HS × cv × Y | 0.10 | - | 1 | 0.10 | 3.70 | 0.057 |
| T × cv × Y | 0.02 | - | 4 | 0.01 | 0.21 | 0.931 |
| HS × T × cv × Y | 0.03 | - | 4 | 0.01 | 0.27 | 0.895 |
| Residual | 2.21 | 80 | 0.03 | |||
| Total (Corrected) | 36.41 | 100 | 119 | |||
| Source | Sum of Squares | Effect (%) | Df | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|---|
| Main Effects | ||||||
| HS | 4.96 | 26.40 | 1 | 4.96 | 168.66 | 0.0000 |
| T | 3.15 | 16.70 | 4 | 0.79 | 26.77 | 0.0000 |
| cv | 5.98 | 31.80 | 1 | 5.98 | 203.47 | 0.0000 |
| ST | 0.41 | 2.20 | 1 | 0.41 | 13.88 | 0.0004 |
| Interactions | ||||||
| HS × T | 0.02 | - | 4 | 0.05 | 0.19 | 0.9446 |
| HS × cv | 0.67 | 3.30 | 1 | 0.62 | 20.95 | 0.0000 |
| HS × Y | 0.11 | - | 1 | 0.11 | 3.67 | 0.0589 |
| T × cv | 0.32 | 1.70 | 4 | 0.08 | 2.73 | 0.0347 |
| T × Y | 0.23 | - | 4 | 0.06 | 1.98 | 0.1050 |
| cv × Y | 0.13 | 0.70 | 1 | 0.13 | 4.53 | 0.0363 |
| HS × T × cv | 0.04 | - | 4 | 0.01 | 0.34 | 0.8483 |
| HS × T × Y | 0.02 | - | 4 | 0.00 | 0.14 | 0.9649 |
| HS × cv × Y | 0.03 | - | 1 | 0.03 | 0.92 | 0.3409 |
| T × cv × Y | 0.31 | - | 4 | 0.08 | 2.68 | 0.0376 |
| HS × T × cv × Y | 0.14 | - | 4 | 0.04 | 1.23 | 0.3050 |
| Residual | 2.35 | 12.50 | 80 | 0.03 | ||
| Total (Corrected) | 18.84 | 100 | 119 | |||
| Year | Alternate Bearing | Storage T° | Cultivar | Harvesting System | T1 * | T3 * | T4 * | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | SD | |||||
| 2019/2020 | OFF | 18 °C | Frantoio | manual | 19.8 | 0.6 | 23.1 | 0.8 | 21.3 | 0.5 |
| mechanical | 19.9 | 0.8 | 20.7 | 2.1 | 22.8 | 3.0 | ||||
| Moraiolo | manual | 33.5 | 0.6 | 30.5 | 0.3 | 27.8 | 1.4 | |||
| mechanical | 29.3 | 0.9 | 29.9 | 1.2 | 28.2 | 1.1 | ||||
| 5 °C | Frantoio | manual | 26.5 | 1.3 | 24.2 | 0.8 | 24.4. | 0.8 | ||
| mechanical | 21.2 | 1.3 | 22.7 | 1.6 | 22.0 | 1.0 | ||||
| Moraiolo | manual | 43.8 | 1.8 | 43.3 | 1.2 | 41.2 | 0.6 | |||
| mechanical | 40.0 | 0.5 | 40.6 | 0.7 | 39.6 | 0.8 | ||||
| 18 °C | 5 °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Harvesting System | Sampling Times (*) | OHTyr | Ole | Rutin | Verb | OHTyr | Ole | Rutin | Verb | |
| Frantoio | manual | T1 | 0.13 ± 0.03 | 0.07 ± 0.00 | 0.26 ± 0.03 | 2.09 ± 0.38 | 0.14 ± 0.00 | 0.09 ± 0.02 | 0.58 ± 0.00 | 2.63 ± 0.12 |
| T4 | 0.30 ± 0.04 | 0.26 ± 0.01 | 0.41 ± 0.02 | 1.73 ± 0.23 | 0.11 ± 0.00 | 0.17 ± 0.06 | 0.56 ± 0.18 | 2.23 ± 0.35 | ||
| mechanical | T1 | 0.17 ± 0.02 | 0.11 ± 0.01 | 0.56 ± 0.06 | 2.19 ± 0.20 | 0.09 ± 0.03 | 0.07 ± 0.03 | 0.38 ± 0.05 | 1.14 ± 0.00 | |
| T4 | 0.31 ± 0.01 | 0.33 ± 0.13 | 0.56 ± 0.07 | 0.70 ± 0.02 | 0.08 ± 0.00 | 0.09 ± 0.06 | 0.57 ± 0.21 | 1.61 ± 0.17 | ||
| Moraiolo | manual | T1 | 0.14 ± 0.02 | 0.16 ± 0.09 | 0.75 ± 0.00 | 3.53 ± 0.82 | 0.19 ± 0.02 | 0.78 ± 0.03 | 1.30 ± 0.18 | 9.09 ± 0.97 |
| T4 | 0.30 ± 0.02 | 0.86 ± 0.11 | 0.85 ± 0.16 | 2.25 ± 0.07 | 0.14 ± 0.02 | 1.03 ± 0.54 | 1.54 ± 0.24 | 7.03 ± 1.3 | ||
| mechanical | T1 | 0.20 ± 0.03 | 0.61 ± 0.37 | 0.88 ± 0.38 | 3.61 ± 0.73 | 0.17 ± 0.01 | 0.60 ± 0.15 | 1.31 ± 0.04 | 6.64 ± 0.28 | |
| T4 | 0.33 ± 0.00 | 1.04 ± 0.14 | 0.97 ± 0.11 | 1.27 ± 0.03 | 0.18 ± 0.01 | 1.51 ± 0.01 | 1.50 ± 0.12 | 4.93 ± 0.57 | ||
| Storage T (°C) | 18 | 5 | 18 | |||
|---|---|---|---|---|---|---|
| Sampling Times | T1 | T5 | T5 | |||
| Harvesting system | Manual | Mechanical | Manual | Mechanical | Manual | Mechanical |
| Free acidity (%) | 0.18 ± 0.01 | 0.20 ± 0.02 | 0.24 ± 0.03 | 0.27 ± 0.04 | 0.30 ± 0.03 | 0.45 ± 0.03 |
| Peroxide index (meq O2 kg−1) | 5.70 ± 0.30 | 5.60 ± 0.35 | 6.90 ± 0.60 | 9.20 ± 0.70 | 10.20 ± 0.80 | 13.50 ± 0.90 |
| Total polyphenols (mg kg−1) | 420 ± 14 | 417 ± 16 | 411 ± 12 | 381 ± 18 | 372 ± 15 | 315 ± 12 |
| SE results (defective, not defective) | not defective | not defective | not defective | not defective | defective | defective |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taiti, C.; Masi, E.; Flamminii, F.; Di Mattia, C.; Mancuso, S.; Marone, E. Does the Harvest Type Affect Olive Health? Influence of the Harvesting System and Storage Time on the Chemical, Volatile and Sensory Qualities of Extra Virgin Olive Oils. Plants 2023, 12, 3843. https://doi.org/10.3390/plants12223843
Taiti C, Masi E, Flamminii F, Di Mattia C, Mancuso S, Marone E. Does the Harvest Type Affect Olive Health? Influence of the Harvesting System and Storage Time on the Chemical, Volatile and Sensory Qualities of Extra Virgin Olive Oils. Plants. 2023; 12(22):3843. https://doi.org/10.3390/plants12223843
Chicago/Turabian StyleTaiti, Cosimo, Elisa Masi, Federica Flamminii, Carla Di Mattia, Stefano Mancuso, and Elettra Marone. 2023. "Does the Harvest Type Affect Olive Health? Influence of the Harvesting System and Storage Time on the Chemical, Volatile and Sensory Qualities of Extra Virgin Olive Oils" Plants 12, no. 22: 3843. https://doi.org/10.3390/plants12223843
APA StyleTaiti, C., Masi, E., Flamminii, F., Di Mattia, C., Mancuso, S., & Marone, E. (2023). Does the Harvest Type Affect Olive Health? Influence of the Harvesting System and Storage Time on the Chemical, Volatile and Sensory Qualities of Extra Virgin Olive Oils. Plants, 12(22), 3843. https://doi.org/10.3390/plants12223843








