Using Mediterranean Native Plants for the Phytoremediation of Mining Sites: An Overview of the Past and Present, and Perspectives for the Future
Abstract
1. Introduction
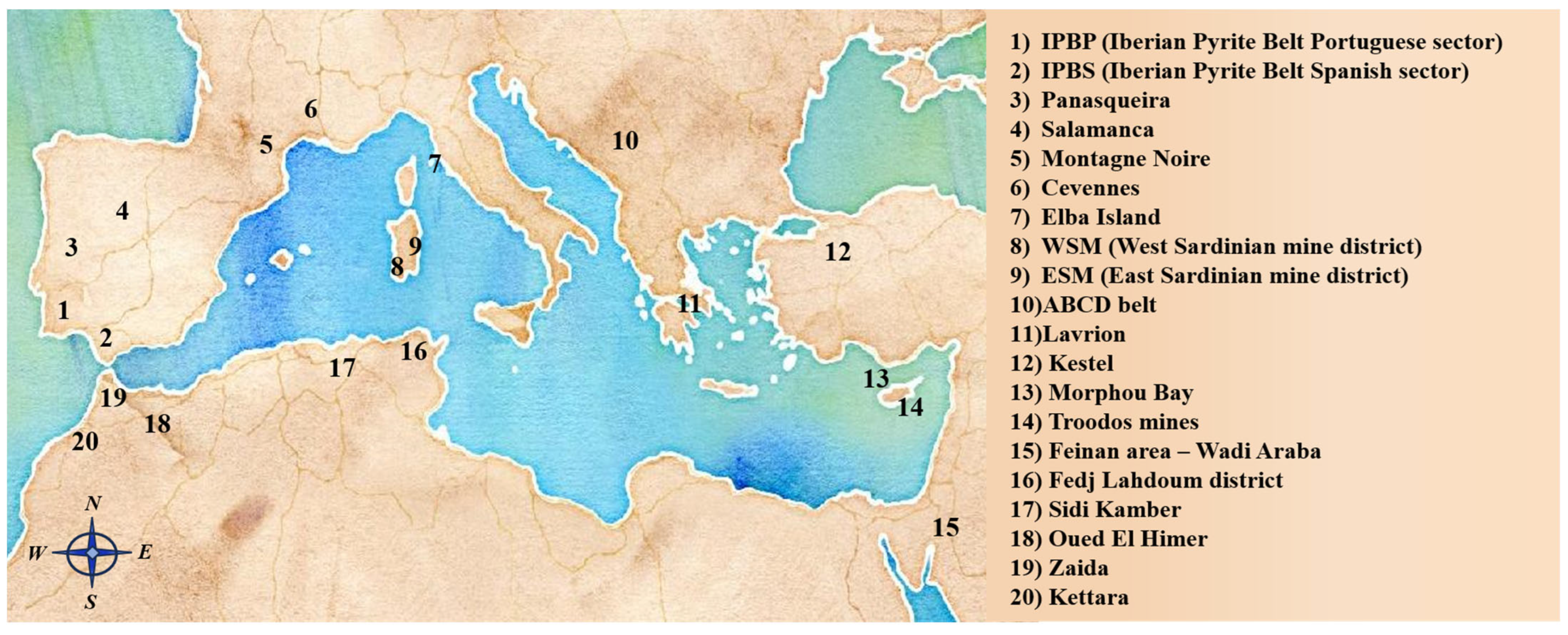
2. Main Mediterranean Ores and Mine Pollution Hotspots
3. Mine Wastes: Main Issues in the Mediterranean Climate Frame and Consequences on Human Health
4. Phytoremediation of Mine Areas in the Mediterranean Biogeographic Region
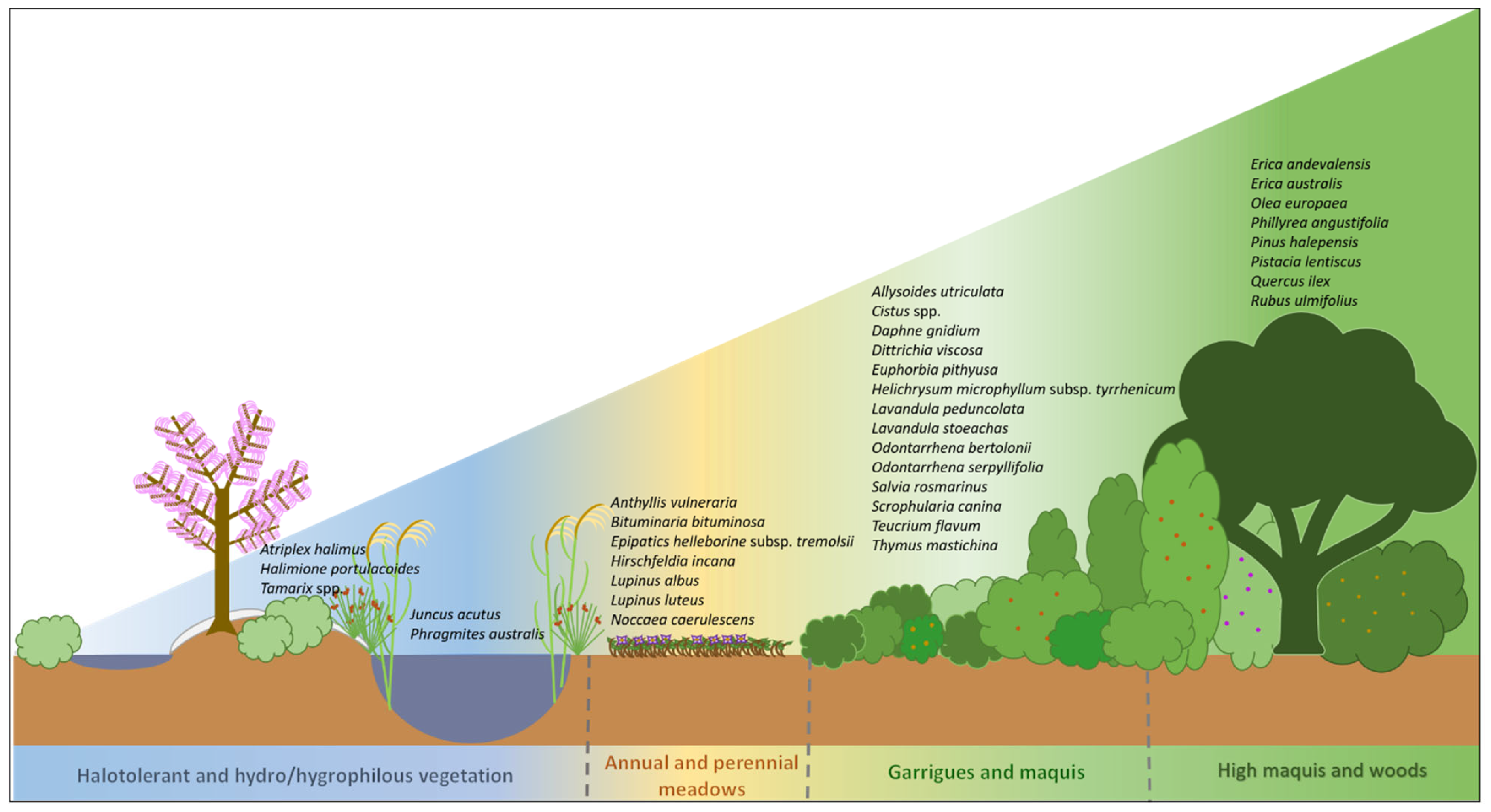
5. Mediterranean Plant Species for Phytoremediation
5.1. Halotolerant and Hydro/Hygrophilous Vegetation
5.2. Annual and Perennial Meadows
5.3. Garrigues and Maquis
5.4. High Maquis and Woods
6. Discussion and Perspectives for the Future
6.1. The Role of Native and Endemic Flora of Mine Areas
6.2. Local Policies and Guidelines for Environmental Restoration
6.3. The Importance of the Multidisciplinary Approach and Its Future Implementation
6.4. Phytostabilisation or Phytoextraction?
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Plant Species | Metal(Loid)s | Metal(Loid)s in Plant | BCF | BAC | TF | References |
|---|---|---|---|---|---|---|
| Alyssoides utriculata * | Ni | 5000 mg/kg R >1000 mg/kg L | - | >1 | >1 | [133] |
| Alyssum serpyllifolium * | Ni | 3405 mg/kg (entire plant) | 2.98 | 4.35 | [161] | |
| Atriplex halimus | As | 6–8 mg/kg L | - | - | - | [138] |
| Pb | 8–12 mg/kg L | - | - | - | ||
| Zn | 6 mg/kg L | - | - | - | ||
| Bituminaria bituminosa | Pb | 4.7 mg/kg R 16.7 mg/kg S 4 mg/kg L | 0.27 | - | 0.85 | [53] |
| Zn | 62.9 mg/kg R 107 mg/kg S 70 mg/kg L | 0.96 | - | 1.10 | ||
| Cistus albidus | As | 10.6 mg/kg EO | - | 0.13 | - | [133] |
| Mn | 115 mg/kg EO | - | 0.09 | - | ||
| Pb | 6.74 mg/kg EO | - | 0.03 | - | ||
| Zn | 124 mg/kg EO | - | 0.86 | - | ||
| C. ladanifer * | Pb | 5–69 mg/kg R 5–19 mg/kg S 5–69 mg/kg L | - | 1.03 | 3.87 | [176] |
| Zn | 29–203 mg/kg R 57–214 mg/kg S 124–703 mg/kg L | - | 0.05 | 3.61 | ||
| As | 12 mg/kg R 1.7 mg/kg L | - | <1 | 0.7–0.14 | [137] | |
| Pb | 53.88 mg/kg R 55.79 mg/kg L | - | <1 | 0.67–1.46 | ||
| Zn | 41 mg/kg R 147 mg/kg L | - | >1 | 0.53–4.82 | ||
| C. libanotis | Pb | 823–1820 mg/kg EO | - | - | - | [181] |
| Zn | 89–1297 mg/kg EO | - | - | - | ||
| C. monspeliensis * | As | 11.4 mg/kg R 29.7 mg/kg S | - | 0.02 | 2.13 | [35] |
| Mn | 764 mg/kg R 1165 mg/kg EO | 3.29 | 2.18 | |||
| Pb | 35 mg/kg R 15.6 mg/kg S | - | 0.01 | 0.60 | ||
| Zn | 91 mg/kg R 217 mg/kg S | - | 1.60 | 2.61 | ||
| C. populifolius | As | 0.8–4.8 mg/kg R 0.5–1.1 mg/kg EO | - | <1 | 0.2–0.7 | [175] |
| Sb | 0.01–0.2 mg/kg R 0.03–0.07 mg/kg EO | - | <1 | 0.1–0.8 | ||
| Pb | 8.9–333 mg/kg R 0.7–20.3 mg/kg EO | - | <1 | 0.1–0.4 | ||
| As | 5.40 mg/kg EO | - | 0.053 | - | [133] | |
| Mn | 747 mg/kg EO | 12 | - | |||
| Pb | 5.51 mg/kg EO | - | 0.036 | - | ||
| Zn | 154 mg/kg EO | - | 3.27 | - | ||
| C. salviifolius * | Pb | 185 mg/kg EO | - | - | - | [170] |
| 1560 mg/kg EO | - | - | - | |||
| Pb | 1.7 mg/kg R 2.1 mg/kg S 3.1 mg/kg L | 0.21 | - | 1.82 | [53] | |
| Zn | 26 mg/kg R 58.6 mg/kg S 113.4 mg/kg L | 1.55 | - | 4.36 | ||
| As | 0.6–7.2 mg/kg R 0.1–10 mg/kg EO | - | <1 | 0.2–0.7 | [175] | |
| Sb | 0.01–0.9 mg/kg R 0.02–0.2 mg/kg EO | - | <1 | 0.1–0.8 | ||
| Pb | 23.9–312 mg/kg R 1.3–39.6 mg/kg EO | - | <1 | 0.1–0.4 | ||
| As | 5.74 mg/kg EO | - | 0.02 | - | [133] | |
| Pb | 8.03 mg/kg EO | - | 0.04 | - | ||
| Zn | 286 mg/kg EO | - | 2.74 | - | ||
| Daphne gnidium | As | 4.35 mg/kg EO | - | - | - | [183] |
| Zn | 104 mg/kg EO | - | - | - | ||
| Pb | 11.5 mg/kg R 2.9 mg/kg S | - | 0.002 | 0.25 | [132] | |
| Zn | 243.7 mg/kg R 77.4 mg/kg S | - | 0.04 | 0.32 | ||
| Dittrichia viscosa subsp. viscosa * | Zn | 2000 mg/kg L | - | - | - | [166] |
| Pb | 250 mg/kg L | - | - | - | ||
| As | 12.7 mg/kg R 18.5 mg/kg S 26.2 mg/kg L | 12.48 | - | 2.06 | [53] | |
| Pb | 4.5 mg/kg R 7.3 mg/kg S 25 mg/kg L | 1.69 | - | 5.56 | ||
| Zn | 20.3 mg/kg R 27.4 mg/kg S 133.2 mg/kg L | 1.82 | - | 6.56 | ||
| As | 23 mg/kg EO | [167] | ||||
| Pb | 270 mg/kg EO | |||||
| Zn | 640 mg/kg EO | |||||
| Erica andevalensis | As | 2.5–4.4 mg/kg L | - | 0.007–0.02 | - | [112] |
| Mn | 790–1100 mg/kg | - | 0.8–15 | - | ||
| Pb | 2.6–6.31 mg/kg L | - | 0.01–0.03 | - | ||
| Zn | 15.64–146 mg/kg L | - | 0.1–0.3 | - | ||
| As | 7.5 mg/kg R 7.5 mg/kg EO | - | 0.4 bf | 1.4 | [88] | |
| Mn | 500 mg/kg R 1400 mg/kg EO | - | 173 bf | 3.1 | ||
| Pb | 100 mg/kg R 100 mg/kg EO | - | 1 bf | 0.9 | ||
| Zn | 60 mg/kg R 40 mg/kg EO | - | 3.1 bf | 0.8 | ||
| E. australis * | Mn | 500 mg/kg R 325 mg/kg L | - | >1 | >1 | [37] |
| Pb | 5 mg/kg R 3 mg/kg L | - | <1 | >1 | ||
| Zn | 5 mg/kg R 22.5 mg/kg L | - | <1 | <1 | ||
| As | 26 mg/kg R 3.7 mg/kg EO | - | 0.42 bf | 1.08 | [88] | |
| Mn | 300 mg/kg R 250 mg/kg EO | - | 189 bf | 1.1 | ||
| Pb | 125 mg/kg R | - | 0.76 bf | 1.2 | ||
| Zn | 7.5 mg/kg R 17.5 mg/kg EO | - | 8.5 bf | 2.4 | ||
| Euphorbia pithyusa subsp. cupanii | Zn | 300 mg/kg L | - | - | - | [170] |
| Halimione portulacoides | As | 9.8 mg/kg R 0.6 mg/kg S | 0.04–0.1 | [136] | ||
| Pb | 312.5 mg/kg R 6.9 mg/kg S | 0.02–0.03 | ||||
| Zn | 2.1 mg/kg R 67.9 mg/kg S | 0.03–0.04 | ||||
| Helichrysum microphyllum subsp. tyrrhenicum | Zn | 2600 mg/kg R 3400 mg/kg EO | 0.1 | 0.12 | 1.7 | [110,164] |
| Pb | 500 mg/kg R 1000 mg/kg EO | 0.1 | 0.20 | 2.45 | ||
| Cd | 20 mg/kg R 19 mg/kg EO | 0.2 | 0.16 | 1.1 | ||
| Hirschfeldia incana * | Pb | 11.7–204.4 mg/kg R 25.4–222.6 mg/kg EO | - | 0.009–0.036 | 1.09–2.16 | [132] |
| Cd | 33.5 mg/kg R | 0.07 | 2.43 | |||
| 81.2 mg/kg EO | ||||||
| Zn | 46.9–528.9 mg/kg R 144.1–911.3 mg/kg EO | - | 0.09–0.21 | 1.72–3.07 | ||
| Juncus acutus | As | 0.0094 mg/kg external R 0.0046 mg/kg inner R 0.00013 mg/kg S | - | - | - | [16] |
| Pb | 2100 mg/kg external R 580 mg/kg inner R 15 mg/kg S | 0.09 | 0.002 | 0.03 | ||
| Zn | 16,000 mg/kg external R 3500 mg/kg inner R 380 mg/kg S | 0.04 | 0.005 | 0.1 | ||
| Pb | 30 mg/kg R 2.2 mg/kg S | - | 0.001 | 0.07 | [132] | |
| Zn | 142.3 mg/kg R 25.8 mg/kg S | - | 0.01 | 0.18 | ||
| As | 752 mg/kg R 3.6 mg/kg EO 4 mg/kg F | - | - | - | [115] | |
| Zn | 234 mg/kg R 19 mg/kg EO 38 mg/kg L | 2.25 | - | - | ||
| Lavandula stoechas * | Pb | 1.2 mg/kg R 2 mg/kg S 4.6 mg/kg L | 0.31 | - | 3.83 | [53] |
| Zn | 25.8 mg/kg R 29.1 mg/kg S 82.7 mg/kg L | 1.13 | - | 3.21 | ||
| L. stoechas subsp. luisieiri * | Mn | 577 mg/kg R 890 mg/kg S | - | <1 | >1 | [171] |
| Zn | 47 mg/kg R 131 mg/kg S | - | >1 | >1 | ||
| As | 1.7 mg/kg EO | - | - | - | [176] | |
| Pb | 6.96 mg/kg EO | - | - | - | ||
| Zn | 139 mg/kg EO | - | - | - | ||
| L. pedunculata | As | 12.3 mg/kg R 14.6 mg/kg S | <1 | >1 | [213] | |
| Pb | 26.3 mg/kg R 21.7 mg/kg S | <1 | <1 | |||
| Zn | 106 mg/kg R 229 mg/kg S | <1 | - | |||
| Noccaea caerulescens | Zn | 53,450 mg/kg L | - | - | - | [143] |
| Cd | 2908 mg/kg L | - | - | - | ||
| Olea europaea var. sylvestris | As | 0.32 mg/kg L | - | <1 | - | [34] |
| Cd | 0.07 mg/kg L | - | <1 | - | ||
| Pb | 0.89 mg/kg L | - | <1 | - | ||
| Zn | 42.2 mg/kg L | - | <1 | - | ||
| Phillyrea angustifolia | As | 3.28 mg/kg EO | - | 0.06 | - | [133] |
| Pb | 3.94 mg/kg EO | - | 0.05 | - | ||
| Zn | 73.1 mg/kg EO | - | 0.7 | - | ||
| Phragmites australis | Cd | 55 mg/kg R 19 mg/kg EO | - | - | < 0.1 | [16] |
| Pb | 3000 mg/kg R 750 mg/kg EO | 3 | - | < 0.1 | ||
| Zn | 10,000 mg/kg R 4000 mg/kg EO | - | - | < 0.1 | ||
| As | 5.18 mg/kg EO | - | 0.0014 | - | [133] | |
| Pb | 1.17 mg/kg EO | - | 0.0001 | - | ||
| Zn | 43.1 mg/kg EO | - | 0.03 | - | ||
| Pinus halepensis | Zn | 664.65–2710 mg/kg R | 0.18 | - | 0.03–0.19 | [189] |
| Pb | 58.39–735.88 mg/kg R | 0.17 | - | 0.03–0.32 | ||
| Cd | 4.86–11.02 mg/kg R | 0.19 | - | 0.04–0.14 | ||
| Pistacia lentiscus | Zn | 450 mg/kg R 350 mg/kg S 150 mg/kg L | <1 | <1 | <1 | [18] |
| Pb | 30 mg/kg R 25 mg/kg S 10 mg/kg L | <1 | <1 | <1 | ||
| Hg | 0.09 mg/kg R 0.04 mg/kg S 0.250 mg/kg L | <1 | <1 | 2 | ||
| Pb | 9.93 mg/kg EO | - | 0.13 | - | [133] | |
| Zn | 23 mg/kg EO | - | 0.25 | - | ||
| Quercus ilex | As | 0.56 mg/kg L | - | <0.2 | - | [34] |
| Cd | 0.21 mg/kg L | - | <0.5 | - | ||
| Pb | 2.48 mg/kg L | - | <0.2 | - | ||
| Zn | 80 mg/kg L | - | <0.5 | - | ||
| Rubus ulmifolius | As | <1000 mg/kg R, S, L | 0.02–0.16 | [186] | ||
| Ni | <1000 mg/kg R | |||||
| Pb | <1000 mg/kg R, S, L | 0.08–0.13 | ||||
| Salvia rosmarinus | As | 0.79 mg/kg L | - | <1 | - | [34] |
| Cd | 0.04 mg/kg L | - | <1 | - | ||
| Pb | 2.01 mg/kg L | - | <1 | - | ||
| Zn | 51.2 mg/kg L | - | <1 | - | ||
| As | 8.54 mg/kg EO | - | 0.01 | - | [133] | |
| Pb | 4.89 mg/kg EO | - | 0.002 | - | ||
| Zn | 95 mg/kg EO | - | 0.3 | - | ||
| Cd | 1.7 mg/kg R 0.3 mg/kg S | - | 0.12 | 0.18 | [132] | |
| Pb | 9.6 mg/kg R 3.2 mg/kg S | - | 0.001 | 0.33 | ||
| Zn | 30 mg/kg R 19.5 mg/kg S | - | 0.01 | 0.65 | ||
| Scrophularia canina subsp. bicolor * | Pb | 120 mg/kg EO | - | - | >1 | [15,170] |
| Zn | 1200 mg/kg EO | - | - | >1 | ||
| S. canina | Pb | 3.8 mg/kg R 5.4 mg/kg S | - | 0.001 | 1.44 | [132] |
| Zn | 20.5 mg/kg R 24.1 mg/kg S | - | 0.01 | 1.17 | ||
| Tamarix africana * | As | 0.10 mg/kg R 0.007 mg/kg S | - | - | 0.8 | [136] |
| Pb | 0.6 mg/kg R 0.7 mg/kg S | - | - | 1.1 | ||
| Zn | 27.8 mg/kg R 56.6 mg/kg S | - | - | 2 | ||
| T. gallica | As | 520 mg/kg R 68 mg/kg S | - | - | - | [121] |
| Hg | 1536 mg/kg R 22 mg/kg S | - | - | - | ||
| Teucrium flavum subsp. glaucum | Pb | 346 mg/kg EO | - | - | - | [170] |
| Zn | 1130 mg/kg EO | - | - | - | ||
| Thymus mastichina * | Zn | 4.0–240 mg/kg R 8.0–65.0 mg/kg S 17.5–145 mg/kg L | - | - | 1.7–5.8 | [173] |
| Ni | 2.5–100 mg/kg R 2.5–35.0 mg/kg S 4.0–180 mg/kg L | - | - | 1.4–3.0 |
References
- Biggeri, A.; Lagazio, C.; Catelan, D.; Pirastu, R.; Casson, F.; Terracini, B. Report on Health Status of Residents in Areas with Industrial, Mining or Military Sites in Sardinia, Italy. Epidemiol. Prev. 2006, 30, 5–95. [Google Scholar] [PubMed]
- Sanna, E.; Floris, G.; Vallascas, E. Town and Gender Effects on Hair Lead Levels in Children from Three Sardinian Towns (Italy) with Different Environmental Backgrounds. Biol. Trace Elem. Res. 2008, 124, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Varrica, D.; Tamburo, E.; Milia, N.; Vallascas, E.; Cortimiglia, V.; Giudici, G.; Dongarrà, D.; Sanna, E.; Monna, F.; Losno, R. Metals and Metalloids in Hair Samples of Children Living near the Abandoned Mine Sites of Sulcis-Iglesiente (Sardinia, Italy). Environ. Res. 2014, 134, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.; Costa, S.; Costa, C.; Silva, S.; Walter, A.; Ranville, J.; Pastorinho, M.R.; Harrington, C.; Taylor, A.; Dall’Armi, V.; et al. Biomonitoring of Several Toxic Metal(loid)s in Different Biological Matrices from Environmentally and Occupationally Exposed Populations from Panasqueira Mine Area, Portugal. Environ. Geochem. Health 2014, 36, 255–269. [Google Scholar] [CrossRef]
- Medail, F.; Quezel, P. Biodiversity Hotspots in the Mediterranean Basin: Setting Global Conservation Priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Frau, F.; Medas, D.; Pelo, S.; Wanty, R.B.; Cidu, R. Environmental Effects on the Aquatic System and Metal Discharge to the Mediterranean Sea from a Near-Neutral Zinc-Ferrous Sulfate Mine Drainage. Water Air Soil Pollut. 2015, 226, 55. [Google Scholar] [CrossRef]
- Doumas, P.; Munoz, M.; Banni, M.; Becerra, S.; Bruneel, O.; Casiot, C.; Cleyet-Marel, J.C.; Gardon, J.; Noack, Y.; Sappin-Didier, V. Polymetallic Pollution from Abandoned Mines in Mediterranean Regions: A Multidisciplinary Approach to Environmental Risks. Reg. Environ. Chang. 2018, 18, 677–692. [Google Scholar] [CrossRef]
- Rodríguez-Seijo, A.; Lourenço, J.; Arenas-Lago, D.; Mendo, S.; Vega, F.A.; Pereira, R. Chemical availability versus bioavailability of potentially toxic elements in mining and quarry soils. Chemosphere 2020, 251, 126421. [Google Scholar] [CrossRef]
- Iyyappan, J.; Baskar, G.; Deepanraj, B.; Vivek Anand, A.; Saravanan, R.; Awasthi, M.K. Promising Strategies of Circular Bioeconomy Using Heavy Metal Phytoremediated Plants—A Critical Review. Chemosphere 2023, 313, 137097. [Google Scholar] [CrossRef]
- European Commission. EU Biodiversity Strategy for 2030 Bringing Nature Back into Our Lives; Communication from the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; EC: Brussels, Belgium, 2020. [Google Scholar]
- European Commission. Guidelines on Biodiversity-Friendly Afforestation, Reforestation and Tree Planting; Commission Staff Working Document; EC: Brussels, Belgium, 2023. [Google Scholar]
- Shah, V.; Daverey, A. Phytoremediation: A Multidisciplinary Approach to Clean up Heavy Metal Contaminated Soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Tamburini, E.; Cappai, G.; Sergi, S.; Ruggeri, C.; Bacchetta, G.; Giudici, G.; Carucci, A. Bioaugmentation-Assisted Phytostabilisation of Abandoned Mines by Plant-Growth Promoter Serratia sp. Phytoremediation Bioremediation Technologies for Removal of Heavy Metals. In Proceedings of the e-Proceedings of the 6th European Bioremediation Conference, Chania, Greece, 29 June–2 July 2015; pp. 171–174. [Google Scholar]
- Tamburini, E.; Sergi, S.; Serreli, L.; Bacchetta, G.; Milia, S.; Cappai, G.; Carucci, A. Bioaugmentation-assisted phytostabilization of abandoned mine sites in south west Sardinia. Bull. Environ. Contam. Toxicol. 2016, 98, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, G.; Cao, A.; Cappai, G.; Carucci, A.; Casti, M.; Fercia, M.L.; Lonis, R.; Mola, F. A Field Experiment on the Use of Pistacia lentiscus L. and Scrophularia canina L. subsp. bicolor (Sibth. et Sm.) Greuter for the Phytoremediation of Abandoned Mining Areas. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2012, 146, 1054–1063. [Google Scholar] [CrossRef]
- Bacchetta, G.; Cappai, G.; Carucci, A.; Tamburini, E. Use of Native Plants for the Remediation of Abandoned Mine Sites in Mediterranean Semiarid Environments. Bull. Environ. Contam. Toxicol. 2015, 94, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Boi, M.E.; Cappai, G.; Giudici, G.; Medas, D.; Piredda, M.; Porceddu, M.; Bacchetta, G. Ex Situ Phytoremediation Trial of Sardinian Mine Waste Using a Pioneer Plant Species. Environ. Sci. Pollut. Res. 2021, 28, 55736–55753. [Google Scholar] [CrossRef]
- Concas, S.; Lattanzi, P.; Bacchetta, G.; Barbafieri, M.; Vacca, A. Zn, Pb and Hg Contents of Pistacia lentiscus L. Grown on Heavy Metal Rich Soils: Implications for Phytostabilization. Water Air Soil Pollut. 2015, 226, 340–355. [Google Scholar] [CrossRef]
- Fois, M.; Murgia, L.; Bacchetta, G. Plant Diversity and Species Composition of the Abandoned Mines of the Iglesiente Mining District (Sardinia, Italy): A Restoration Perspective. Ecol. Eng. 2023, 188, 106879. [Google Scholar] [CrossRef]
- Su, R.; Ou, Q.; Wang, H.; Dai, X.; Chen, Y.; Luo, Y.; Yao, H.; Ouyang, D.; Li, Z.; Wang, Z. Organic—Inorganic Composite Modifiers Enhance Restoration Potential of Nerium oleander L. to Lead–Zinc Tailing: Application of Phytoremediation. Environ. Sci. Pollut. Res. 2023, 30, 56569–56579. [Google Scholar] [CrossRef]
- Mandaresu, M.; Dessì, L.; Lallai, A.; Porceddu, M.; Boi, M.E.; Bacchetta, G.; Pivetta, T.; Lussu, R.; Ardu, R.; Pinna, M.; et al. Helichrysum microphyllum subsp. tyrrhenicum, Its Root-Associated Microorganisms, and Wood Chips Represent an Integrated Green Technology for the Remediation of Petroleum Hydrocarbon-Contaminated Soils. Agronomy 2023, 13, 812. [Google Scholar]
- Arvanitoyannis, I.S.; Ladas, D.; Mavromatis, A. Potential Uses and Applications of Treated Wine Waste: A Review. Int. J. Food Sci. Technol. 2006, 41, 475–487. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Said-Pullicino, D.; Paredes, C.; Cecilia, J.A.; Moral, R. Influences of Winery—Distillery Waste Compost Stability and Soil Type on Soil Carbon Dynamics in Amended Soils. Waste Manag. 2010, 30, 1966–1975. [Google Scholar] [CrossRef]
- Perra, M.; Leyva-Jiménez, F.J.; Manca, M.L.; Manconi, M.; Rajha, H.N.; Borrás-Linares, I.; Segura-Carretero, A.; Lozano-Sánchez, J. Application of Pressurized Liquid Extraction to Grape By-Products as a Circular Economy Model to Provide Phenolic Compounds Enriched Ingredient. J. Clean. Prod. 2023, 402, 136712. [Google Scholar] [CrossRef]
- Perra, M.; Bacchetta, G.; Muntoni, A.; Gioannis, G.; Castangia, I.; Rajha, H.N.; Manca, M.L.; Manconi, M. An Outlook on Modern and Sustainable Approaches to the Management of Grape Pomace by Integrating Green Processes, Biotechnologies and Advanced Biomedical Approaches. J. Funct. Foods 2022, 98, 105276. [Google Scholar] [CrossRef]
- Hidalgo, J.; Anza, M.; Epelde, L.; Becerril, J.M.; Garbisu, C. Zero-Valent Iron Nanoparticles and Organic Amendment Assisted Rhizoremediation of Mixed Contaminated Soil Using Brassica napus. Environ. Technol. Innov. 2022, 28, 102621. [Google Scholar] [CrossRef]
- Hong, Y.; Li, D.; Xie, C.; Zheng, X.; Yin, J.; Li, Z.; Zhang, K.; Jiao, Y.; Wang, B.; Hu, Y.; et al. Combined Apatite, Biochar, and Organic Fertilizer Application for Heavy Metal Co-Contaminated Soil Remediation Reduces Heavy Metal Transport and Alters Soil Microbial Community Structure. Sci. Total Environ. 2022, 851, 158033. [Google Scholar] [CrossRef] [PubMed]
- Sprocati, A.R.; Alisi, C.; Pinto, V.; Montereali, M.R.; Marconi, P.; Tasso, F.; Turnau, K.; De Giudici, G.; Goralska, K.; Bevilacqua, M.; et al. Assessment of the Applicability of a “Toolbox” Designed for Microbially Assisted Phytoremediation: The Case Study at Ingurtosu Mining Site (Italy). Environ. Sci. Pollut. Res. 2014, 21, 6939–6951. [Google Scholar] [CrossRef]
- Majumdar, S.; Peralta-Videa, J.R.; Castillo-Michel, H.; Hong, J.; Rico, C.M.; Gardea-Torresdey, J.L. Applications of Synchrotron μ-XRF to Study the Distribution of Biologically Important Elements in Different Environmental Matrices: A Review. Anal. Chim. Acta 2012, 755, 1–16. [Google Scholar] [CrossRef]
- De Giudici, G.; Medas, D.; Meneghini, C.; Casu, M.A.; Gianoncelli, A.; Iadecola, A.; Podda, S.; Lattanzi, P. Microscopic Biomineralization Processes and Zn Bioavailability: A Synchrotron-Based Investigation of Pistacia lentiscus L. Roots. Environ. Sci. Pollut. Res. 2015, 22, 19352–19361. [Google Scholar] [CrossRef]
- Medas, D.; Giudici, G.; Casu, M.A.; Musu, E.; Giannoncelli, A.; Iadecola, A.; Meneghini, C.; Tamburini, E.; Sprocati, A.R.; Turnau, K.; et al. Microscopic Processes Ruling the Bioavailability of Zn to Roots of Euphorbia pithyusa L. Pioneer Plant. Environ. Sci. Technol. 2015, 49, 1400–1408. [Google Scholar] [CrossRef]
- He, L.; Su, R.; Chen, Y.; Zeng, P.; Du, L.; Cai, B.; Zhang, A.; Zhu, H. Integration of Manganese Accumulation, Subcellular Distribution, Chemical Forms, and Physiological Responses to Understand Manganese Tolerance in Macleaya cordata. Environ. Sci. Pollut. Res. 2022, 29, 39017–39026. [Google Scholar] [CrossRef]
- Garbisu, C.; Alkorta, I. Phytoextraction: A Cost-Effective Plant-Based Technology for the Removal of Metals from the Environment. Bioresour. Technol. 2001, 77, 229–236. [Google Scholar] [CrossRef]
- Domínguez, M.T.; Maranón, T.; Murillo, J.M.; Schulin, R.; Robinson, B.H. Trace element accumulation in woody plants of the Guadiamar Valley, SW Spain: A large scale phytomanagement case study. Environ. Pollut. 2008, 152, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Lago, D.; Santos, E.S.; Carvalho, L.C.; Abreu, M.M.; Andrade, M.L. Cistus monspeliensis L. as a potential species for rehabilitation of soils with multielemental contamination under Mediterranean conditions. Environ. Sci. Pollut. Res. 2018, 25, 6443–6455. [Google Scholar] [CrossRef]
- Álvarez-Rogel, J.; Peñalver-Alcalá, A.; Nazaret González-Alcaraz, M. Spontaneous Vegetation Colonizing Abandoned Metal(loid) Mine Tailings Consistently Modulates Climatic, Chemical and Biological Soil Conditions throughout Seasons. Sci. Total Environ. 2022, 838, 155945. [Google Scholar] [CrossRef] [PubMed]
- Monaci, F.; Trigueros, D.; Dingorance, M.D.; Rossini-Oliva, S. Phytostabilization Potential of Erica australis L. and Nerium oleander L.: A Comparative Study in the Riotinto Mining Area (SW Spain). Environ. Geochem. Health 2019, 42, 2345–2360. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, F.; Clemente, L.; Dìaz Barrientos, E.; López, R.; Murillo, J.M. Heavy Metal Pollution of Soils Affected by the Guadiamar Toxic Flood. Sci. Total Environ. 1999, 242, 117–129. [Google Scholar] [CrossRef]
- Garralón, A.; Gómez, P.; Turrero, M.J.; Sánchez, M.; Melón, A.M. The Geochemical Aspects of Toxic Waters Retained in the Entremuros Area (Spain). Sci. Total Environ. 1999, 242, 27–40. [Google Scholar] [CrossRef]
- Grimalt, J.O.; Ferrer, M.; Macpherson, E. The Mine Tailing Accident in Aznalcóllar. Sci. Total Environ. 1999, 242, 3–11. [Google Scholar] [CrossRef]
- Calvo, G.; Valero, A.; Valero, A. How Can Strategic Metals Drive the Economy? Tungsten and Tin Production in Spain during Periods of War. Extr. Ind. Soc. 2019, 6, 8–14. [Google Scholar] [CrossRef]
- Castro-Gomes, J.P.; Silva, A.P.; Cano, R.P.; Durán Suarez, J.; Albuquerque, A. Potential for Reuse of Tungsten Mining Waste-Rock in Technical-Artistic Value Added Products. J. Clean. Prod. 2012, 25, 34–41. [Google Scholar] [CrossRef]
- Huertas, F.J.; Gervilla, F.; Gwatkin, G. Uranium Mineralization in the Retortillo-Santidad Area (Salamanca, Spain): Role of Late Alteration. In Mineral Deposit Research for a High-Tech World, Proceedings of the 12th Biennial SGA Meeting, Uppsala, Sweden, 12–15 August 2013; Jonsson, E., Ed.; Elanders Sverige AB: Mölnlycke, Sweden, 2013; Volume 4, pp. 1594–1597. [Google Scholar]
- Ávila, P.F.; Silva, E.F.d.; Salgueiro, A.R.; Farinha, J.A. Geochemistry and Mineralogy of Mill Tailings Impoundments from the Panasqueira Mine (Portugal): Implications for the Surrounding Environment. Mine Water Environ. 2008, 27, 210–224. [Google Scholar] [CrossRef]
- Vance, R.E.; Cameron, R.; Hinton, N.; Huffman, D.; Harris, F.; Arnold, N.; Ruokonen, E.; Jakubick, A.; Tyulyubayev, Z.; Till, W.V.; et al. Managing Environmental and Health Impacts of Uranium Mining; NEA.7062; Organisation for Economic Co-Operation and Development (OECD): Paris, France, 2014. [Google Scholar]
- Gil-Pacheco, E.; Suárez-Navarro, J.A.; Sánchez-González, S.M.; Suarez-Navarro, M.J.; Hernáiz, G.; García-Sánchez, A. A Radiological Index for Evaluating the Impact of an Abandoned Uranium Mining Area in Salamanca, Western Spain. Environ. Pollut. 2020, 258, 113825. [Google Scholar] [CrossRef] [PubMed]
- Quindós Poncela, L.S.; Fernández Navarro, P.L.; Gómez Arozamena, J.; Ródenas Palomino, C.; Sainz, C.; Martin Matarranz, J.L.; Arteche, J. Population Dose in the Vicinity of Old Spanish Uranium Mines. Sci. Total Environ. 2004, 329, 283–288. [Google Scholar] [CrossRef]
- Boni, M.; Costabile, S.; Vivo, B.; Gasparrini, M. Potential Environmental Hazard in the Mining District of Southern Iglesiente (SW Sardinia, Italy. J. Geochem. Explor. 1999, 67, 417–430. [Google Scholar] [CrossRef]
- Frau, F.; Ardau, C.; Fanfani, L. Environmental Geochemistry and Mineralogy of Lead at the Old Mine Area of Baccu Locci (South-East Sardinia, Italy. J. Geochem. Explor. 2008, 100, 105–115. [Google Scholar] [CrossRef]
- Cidu, R.; Fanfani, L. Overview of the Environmental Geochemistry of Mining Districts in Southwestern Sardinia, Italy. Geochem. Explor. Environ. Anal. 2002, 2, 243–251. [Google Scholar] [CrossRef]
- Jiménez, M.N.; Bacchetta, G.; Casti, M.; Navarro, F.B.; Lallena, A.M.; Fernandèz-Ondono, E. Potential Use in Phytoremediation of Three Plant Species Growing on Contaminated Mine-Tailing Soils in Sardinia. Ecol. Eng. 2011, 37, 392–398. [Google Scholar] [CrossRef]
- Tanelli, G.; Benvenuti, M.; Costagliola, P.; Dini, A.; Lattanzi, P.; Maineri, C.; Mascaro, I.; Ruggieri, G. The iron mineral deposits of Elba Island: State of the art. Ofioliti 2001, 26, 239–248. [Google Scholar]
- Pistelli, L.; D’Angiolillo, F.; Morelli, E.; Basso, B.; Rosellini, I.; Posarelli, M.; Barbafieri, M. Response of Spontaneous Plants from an Ex-Mining Site of Elba Island (Tuscany, Italy) to Metal(loid) Contamination. Environ. Sci. Pollut. Res. 2017, 24, 7809–7820. [Google Scholar] [CrossRef]
- ARPAT-Agenzia Regionale per la Protezione Ambientale della Toscana. Indagine Ambientale Sulle Aree Ex-Minerarie Dell’isola D’elba; ARPAT: San Giovanni Valdarno, Italy, 2002; 133p. [Google Scholar]
- Pagès, G.; Dillmann, P.; Vega, E.; Berranger, M.; Bauvais, S.; Long, L.; Fluzin, P. Vice-Versa: The Iron Trade in the Western Roman Empire between Gaul and the Mediterranean. PLoS ONE 2022, 17, e0268209. [Google Scholar] [CrossRef]
- Baron, S.; Carignan, J.; Laurent, S.; Ploquin, A. Medieval Lead Making on Mont-Lozère Massif (Cévennes-France): Tracing Ore Sources Using Pb Isotopes. Appl. Geochem. 2006, 21, 241–252. [Google Scholar] [CrossRef]
- Atega, P.L.E.; Vinchesa, M.; Casiot, C.; Pistre, S. Development and Implementation of Amulti-Criteria Aggregation Operator to Estimate the Contributions of the Natural Geochemical Background and Anthropogenic Inputs in Groundwater in Former Mining Regions: An Application to Arsenic and Antimony in the Gardon River Watershed (Southern France). Sci. Total Environ. 2022, 814, 151936. [Google Scholar]
- Brunel, C.; Munoz, M.; Probst, A. Remobilisation of Zn and Pb in a Mountain Stream Contaminated by Mining Wastes during a Moderate Flood Event (Ariège, France). J. Phys. 2003, 107, 233–236. [Google Scholar] [CrossRef][Green Version]
- Westner, K.J.; Vaxevanopoulos, M.; Blichert-Toft, J.; Davis, G.; Albarède, F. Isotope and trace element compositions of silver-bearing ores in the Balkans as possible metal sources in antiquity. J. Archaeol. Sci. 2023, 155, 105791. [Google Scholar] [CrossRef]
- Heinrich, C.A.; Franz Neubauer, F. Cu-Au-Pb-Zn-Ag Metallogeny of the Alpine-Balkan-Carpathian-Dinaride Geodynamic Province. Miner. Depos. 2002, 37, 533–540. [Google Scholar] [CrossRef]
- Kypritidou, Z.; Kourgia, P.-M.; Argyraki, A.; Demetriades, A. Do Humans Take Good Care of Their Offspring as Animals Do…! The Lavreotiki and Lavrion “Sagas”, Hellenic Republic—Part 1: Historical Outline and Mapping of Lead Contamination. Environ. Geochem. Health 2023, 45, 1107–1116. [Google Scholar] [CrossRef]
- Sözen, S.; Orhon, D.; Dinçer, H.; Ateşok, G.; Baştürkçü, H.; Yalçın, T.; Öznesil, H.; Karaca, C.; Allı, B.; Dulkadiroğlu, H.; et al. Resource Recovery as a Sustainable Perspective for the Remediation of Mining Wastes: Rehabilitation of the CMC Mining Waste Site in Northern Cyprus. Bull. Eng. Geol. Environ. 2017, 76, 1535–1547. [Google Scholar] [CrossRef]
- Powell, W.; Yazgan, E.; Johnson, M.; Yener, K.A.; Mathur, R. Mineralogical Analysis of the Kestel Mine: An Early Bronze Age Source of Tin Ore in the Taurus Mountains, Turkey. Minerals 2021, 11, 91. [Google Scholar] [CrossRef]
- Hunt, C.O.; Gilbertson, D.D.; El-Rishi, H.A. An 8000-Year History of Landscape, Climate, and Copper Exploitation in the Middle East: The Wadi Faynan and the Wadi Dana National Reserve in Southern Jordan. J. Archaeol. Sci. 2007, 34, 1306–1338. [Google Scholar] [CrossRef]
- Grattan, J.P.; Gilbertson, D.D.; Hunt, C.O. The Local and Global Dimensions of Metalliferous Pollution Derived from a Reconstruction of an Eight Thousand Year Record of Copper Smelting and Mining at a Desert-Mountain Frontier in Southern Jordan. J. Archaeol. Sci. 2007, 34, 83–110. [Google Scholar] [CrossRef]
- Al Tarawneh, K. A Comprehensive Outlook of Mining Industry in Jordan, Opportunities and Threats. Open J. Geol. 2016, 6, 1137–1148. [Google Scholar] [CrossRef]
- Witten, A.J.; Levy, T.E.; Adams, R.B.; Won, I.J. Geophysical Surveys in the Jebel Hamrat Fidan, Jordan. Geoarchaeology 2000, 15, 135–150. [Google Scholar] [CrossRef]
- Hakkou, R.; Benzaazoua, M.; Bussière, B. Acid Mine Drainage at the Abandoned Kettara Mine (Morocco): 1. Environ. Charact. Mine Water Environ. 2008, 27, 145–159. [Google Scholar] [CrossRef]
- El Hachimi, M.L.; El Founti, L.; Bouabdli, A.; Saïdi, N.; Fekhoui, M.; Tassé, N. Pb et As dans des eaux alcalines minières: Contamination, comportement et risques (mine abandonnée de Zeïda, Maroc). Rev. Sci. L’eau J. Water Sci. 2007, 20, 1–13. [Google Scholar] [CrossRef]
- Mendez, M.O.; Maier, R.M. Phytostabilization of mine tailings in arid and semiarid environments—An emerging remediation technology. Environ. Health Perspect. 2008, 116, 278–283. [Google Scholar] [CrossRef]
- Garcıá-Rizo, C.; Martínez-Sánchez, J.; Pérez-Sirvent, C. Environmental Transfer of Zinc in Calcareous Soils in Zones near Old Mining Sites with Semi-Aridic Climate. Chemosphere 1999, 39, 209–227. [Google Scholar] [CrossRef]
- Cidu, R.; Frau, F.; Pelo, S. Drainage at abandoned mine sites: Natural attenuation of contaminants in different seasons. Mine Water Environ. 2011, 30, 113–126. [Google Scholar] [CrossRef]
- Angiolini, C.; Bacchetta, G.; Brullo, S.; Casti, M.; Galdo, G.; Guarino, R. The Vegetation of Mining Dumps in SW-Sardinia. Feddes Repert. 2005, 116, 243–276. [Google Scholar] [CrossRef]
- Bacchetta, G.; Casti, M.; Zavattero, L. Integration of Vegetational and Multitemporal Analysis: A Case Study in the Abandoned Mine District of Montevecchio (South-Western Sardinia). Ann. Bot. Nuova Ser. 2007, 7, 163–173. [Google Scholar]
- Munshower, F.F. Practical Handbook of Disturbed Land Revegetation; Lewis Publishing: Boca Raton, FL, USA, 1994. [Google Scholar]
- Loredo-Jasso, A.U.; Villalobos, M.; Ponce-Pérez, D.B.; Pi-Puig, T.; Meza-Figueroa, D.; Rio-Salas, R.; Ochoa-Landín, L. Characterization and pH Neutralization Products of Efflorescent Salts from Mine Tailings of (Semi-)Arid Zones. Chem. Geol. 2021, 580, 120370. [Google Scholar] [CrossRef]
- Cala-Rivero, V.; Arranz-González, J.C.; Rodríguez-Gómez, V.; Fernández-Naranjo, F.J.; Vadillo-Fernández, L. A Preliminary Study of the Formation of Efflorescent Sulfate Salts in Abandoned Mining Areas with a View to Their Harvesting and Subsequent. Miner. Eng. 2018, 129, 37–40. [Google Scholar] [CrossRef]
- Grande, J.A.; Valente, T.; Torre, M.L.; Santisteban, M.; Ceron, J.C.; Perez-Ostale, E. Characterization of Acid Mine Drainage Sources in the Iberian Pyrite Belt: Base Methodology for Quantifying Affected Areas and for Environmental Management. Environ. Earth Sci. 2014, 71, 2729–2738. [Google Scholar] [CrossRef]
- Alcolea, A.; Vázquez, M.; Caparrós, A.; Ibarra, I.; García, C.; Linares, R.; Rodríguez, R. Heavy Metal Removal of Intermittent Acid Mine Drainage with an Open Limestone Channel. Miner. Eng. 2012, 26, 86–98. [Google Scholar] [CrossRef]
- Gökçekus, H.; Kabdasli, S.; Kabdasli, I.; Turker, U.; Tunay, O.; Olmez, T. Pollution of Coastal Region Impacted by Acid Mine Drainage in Morphou Bay, Northern Cyprus. J. Environ. Sci. Health A 2003, 38, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Egal, M.; Casiot, C.; Morin, G.; Elbaz-Poulichet, F.; Cordier, M.A.; Bruneel, O. An Updated Insight into the Natural Attenuation of As Concentrations in Reigous Creek (Southern France. Appl. Geochem. 2010, 25, 1949–1957. [Google Scholar] [CrossRef]
- Pereira, R.; Ribeir, R.; Goncálves, F. Scalp Hair Analysis as a Tool in Assessing Human Exposure to Heavy Metals (S. Domingos Mine, Portugal). Sci. Total Environ. 2004, 327, 81–92. [Google Scholar] [CrossRef]
- Angius, R.; Bacchetta, G.; Pontecorvo, C. Floristic and vegetational features of Monte Marganai (SW Sardinia). Conserv. Habitat Invertebr. 2011, 5, 57–132. [Google Scholar]
- Abreu, M.M.; Tavares, M.T.; Batista, M.J. Potential Use of Erica andevalensis and Erica australis in Phytoremediation of Sulphide Mine Environments: São Domingos, Portugal. J. Geochem. Explor. 2008, 96, 210–222. [Google Scholar] [CrossRef]
- Rodríguez, N.; Amils, R.; Jiménez-Ballesta, R.; Rufo, L.; Fuente, V. Heavy Metal Content in Erica andevalensis: An Endemic Plant from the Extreme Acidic Environment of Tinto River and Its Soils. Arid Land Res. Manag. 2007, 21, 51–65. [Google Scholar] [CrossRef]
- Monaci, F.; Leidi, E.O.; Mingorance, M.D.; Valdés, B.; Rossini Oliva, S.; Bargagli, R. Selective Uptake of Major and Trace Elements in Erica andevalensis, an Endemic Species to Extreme Habitats in the Iberian Pyrite Belt. J. Environ. Sci. 2011, 23, 444–452. [Google Scholar] [CrossRef]
- Márquez-García, B.; Márquez, C.; Sanjosé, I.; Nieva, F.J.J.; Rodríguez-Rubio, P.; Muñoz-Rodríguez, A.F. The Effects of Heavy Metals on Germination and Seedling Characteristics in Two Halophyte Species in Mediterranean Marshes. Mar. Pollut. Bull. 2013, 70, 119–124. [Google Scholar] [CrossRef]
- Pérez-López, R.; Márquez-García, B.; Abreu, M.M.; Nieto, J.M.; Córdoba, F. Erica andevalensis and Erica australis Growing in the Same Extreme Environments: Phytostabilization Potential of Mining Areas. Geoderma 2014, 230–231, 194–203. [Google Scholar] [CrossRef]
- Fois, M.; Farris, E.; Calvia, G.; Campus, G.; Fenu, G.; Porceddu, M.; Bacchetta, G. The Endemic Vascular Flora of Sardinia: A Dynamic Checklist with an Overview of Biogeography and Conservation Status. Plants 2022, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Whiting, S.N.; Reeves, R.D.; Richards, D.; Johnson, M.S.; Cooke, J.A.; Malaisse, F.; Paton, A.; Smith, J.A.C.; Angle, J.S.; Chaney, R.L.; et al. Research Priorities for Conservation of Metallophyte Biodiversity and Their Potential for Restoration and Site Remediation. Restor. Ecol. 2004, 12, 106–116. [Google Scholar] [CrossRef]
- Pollard, A.J.; Powell, K.D.; Harper, F.A.; Smith, J.A.C. The Genetic Basis of Metal Hyperaccumulation in Plants. Crit. Rev. Plant Sci. 2002, 21, 539–566. [Google Scholar] [CrossRef]
- Paul, A.L.D.; Erskine, P.D.; Ent, A. Metallophytes on Zn-Pb Mineralised Soils and Mining Wastes in Broken Hill, NSW, Australia. Aust. J. Bot. 2018, 66, 124–133. [Google Scholar] [CrossRef]
- Navarro-Cano, J.A.; Verdú, M.; Goberna, M. Trait-Based Selection of Nurse Plants to Restore Ecosystem Functions in Mine Tailings. J. Appl. Ecol. 2018, 55, 1041–1565. [Google Scholar] [CrossRef]
- Santa-Cruz, J.; Robinson, B.; Krutyakov, Y.A.; Shapoval, O.A.; Peñaloza, P.; Yáñez, C.; Neaman, A. An Assessment of the Feasibility of Phytoextraction for the Stripping of Bioavailable Metals from Contaminated Soils. Environ. Toxicol. Chem. 2023, 42, 558–565. [Google Scholar] [CrossRef]
- Kanso, A.; Benizri, E.; Azoury, S.; Echevarria, G.; Sirguey, C. Maximizing Trace Metal Phytoextraction through Planting Methods: Role of Rhizosphere Fertility and Microbial Activities. Chemosphere 2023, 340, 139833. [Google Scholar] [CrossRef]
- Tapia, Y.; Cala, V.; Eymar, E.; Frutos, I.; Gárate, A.; Masaguer, A. Phytoextraction of Cadmium by Four Mediterranean Shrub Species. Int. J. Phytoremediat. 2011, 13, 567–579. [Google Scholar] [CrossRef]
- Marchiol, L.; Sacco, P.; Assolari, S.; Zerbi, G. Reclamation of Polluted Soil: Phytoremediation Potential of Crop-Related Brassica Species. Water Air Soil Pollut. 2004, 158, 345–356. [Google Scholar] [CrossRef]
- Petruzzelli, G.; Pedron, F.; Rosellini, I.; Barbafieri, M. The Bioavailability Processes as a Key to Evaluate Phytoremediation Efficiency. In Phytoremediation: Management of Environmental Contaminants; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 1, pp. 31–43. ISBN 978-3-319-10395-2. [Google Scholar]
- Mang, K.C.; Ntushelo, K. Phytoextraction and Phytostabilisation Approaches of Heavy Metal Remediation in Acid Mine Drainage with Case Studies: A Review. Appl. Ecol. Environ. Res. 2019, 17, 6129–6149. [Google Scholar] [CrossRef]
- Gao, S.; Guo, Y.; Cao, X.; Qiu, C.; Qiu, H.; Zhao, X. Enhanced Phytoremediation for Trace-Metal-Polluted Farmland with Hibiscus cannabinus—Sedum plumbizincicola Rotation: A Case Study in Hunan, China. Agronomy 2023, 13, 1231. [Google Scholar] [CrossRef]
- Brooks, R.R. Plants That Hyperaccumulate Heavy Metals: Their Role in Phytoremediation, Microbiology, Archaeology, Mineral Exploration and Phyto-Mining; CAB International: Oxford, UK, 1998. [Google Scholar]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in Native Plants Growing on a Contaminated Floridasite. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Solomou, A.D.; Germani, R.; Proutsos, N.; Petropoulou, M.; Koutroumpilas, P.; Galanis, C.; Maroulis, G.; Kolimenakis, A. Utilizing Mediterranean plants to remove contaminants from the soil environment: A short review. Agriculture 2022, 12, 238. [Google Scholar] [CrossRef]
- Tosini, L.; Folzerb, H.; Heckenroth, A.; Prudentd, P.; Santonja, M.; Farnet, A.M.; Salducci, M.D.; Vassalo, L.; Labroussee, Y.; Ourself, B.; et al. Gain in Biodiversity but Not in Phytostabilization after 3 Years of Ecological Restoration of Contaminated Mediterranean Soils. Ecol. Eng. 2020, 157, 105998. [Google Scholar] [CrossRef]
- Navarro-Cano, J.A.; Goberna, M.; Valiente-Banuet, A.; Montesinos-Navarro, A.; Garcia, C.; Verdú, M. Plant Phylodiversity Enhances Soil Microbial Productivity in Facilitation-Driven Communities. Oecologia 2014, 174, 909–920. [Google Scholar] [CrossRef]
- Zine, H.; Midhat, L.; Hakkou, R.; El Adnani, M.; Ouhammou, A. Guidelines for a Phytomanagement Plan by the Phytostabilization of Mining Wastes. Sci. Afr. 2020, 10, e00654. [Google Scholar] [CrossRef]
- Mukherjee, I.; Campbell, N.H.; Ash, J.S.; Connolly, E.L. Expression Profiling of the Arabidopsis Ferric Chelate Reductase (FRO) Gene Family Reveals Differential Regulation by Iron and Copper. Planta 2006, 223, 1178–1190. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Kakei, Y.; Shimo, H.; Bashir, K.; Sato, Y.; Sato, Y.; Uozumi, N.; Nakanishi, H.; Nishizawa, N.K. A Rice Phenolic Efflux Transporter Is Essential for Solubilizing Precipitated Apoplasmic Iron in the Plant Stele. J. Biol. Chem. 2011, 286, 24649–24655. [Google Scholar] [CrossRef]
- Boi, M.E.; Medas, D.; Aquilanti, G.; Bacchetta, G.; Birarda, G.; Cappai, G.; Carlomagno, I.; Casu, M.A.; Gianoncelli, A.; Meneghini, C.; et al. Mineralogy and Zn Chemical Speciation in a Soil-Plant System from a Metal-Extreme Environment: A Study on Helichrysum microphyllum subsp. tyrrhenicum. Minerals 2020, 10, 259. [Google Scholar]
- Boi, M.E.; Porceddu, M.; Cappai, G.; DeGiudici, G.; Bacchetta, G. Effects of Zinc and Lead on Seed Germination of Helichrysum microphyllum subsp. tyrrhenicum, a Metal-Tolerant Plant. Int. J. Environ. Sci. Technol. 2020, 17, 1917–1928. [Google Scholar] [CrossRef]
- Boi, M.E.; Sanna Angotzi, M.; Porceddu, M.; Musu, E.; Mameli, V.; Bacchetta, G.; Cannas, C. Germination and Early Seedling Development of Helichrysum microphyllum Cambess. subsp. tyrrhenicum Bacch., Brullo & Giusso in the Presence of Arsenates and Arsenites. Heliyon 2022, 8, 10693. [Google Scholar]
- Márquez-García, B.; Córdoba, F. Antioxidative System in Wild Populations of Erica andevalensis. Environ. Exp. Bot. 2010, 68, 58–65. [Google Scholar] [CrossRef]
- Mirete, S.; de Figueras, C.G.; González-Pastor, J.E. Novel Nickel Resistance Genes from the Rhizosphere Metagenome of Plants Adapted to Acid Mine Drainage. Appl. Environ. Microbiol. 2007, 73, 6001–6011. [Google Scholar] [CrossRef]
- Soldevilla, M.; Maranón, T.; Cabrera, F. Heavy Metal Content in Soil and Plants from a Pyrite Mining Area in Southwest Spain. Commun. Soil Sci. Plant Anal. 1992, 23, 1301–1319. [Google Scholar] [CrossRef]
- Freitas, M.C.; Pacheco, A.M.G.; Anawar, H.M.; Dionísio, I.; Dung, H.M.; Canha, N.; Bettencourt, A.; Henriques, F.; Pinto-Gomes, C.J.; Capelo, S. Determination of Phytoextraction Potential of Plant Species for Toxic Elements in Soils of Abandoned Sulphide-Mining Areas. J. Radioanal. Nucl. Chem. 2009, 282, 21–27. [Google Scholar] [CrossRef]
- Martínez-Fernández, D.; Walker, D.J.; Romero-Espinar, P.; Flores, P.; Río, J.A. Physiological responses of Bituminaria bituminosa to heavy metals. J. Plant Physiol. 2011, 168, 2206–2211. [Google Scholar] [CrossRef]
- Fernandez, R.; Bertrand, A.; Reis, R.; Mourato, M.P.; Martins, L.L.; Gonzales, A. Growth and Physiological Responses to Cadmium, Stress of Two Population of Dittrichia viscosa (L) Greuter. J. Hazard. Mater. 2013, 244–245, 555–562. [Google Scholar] [CrossRef]
- Santos, E.S.; Abreu, M.M.; Magalhães, M.C.F. Cistus ladanifer phytostabilizing soils contaminated with non-essential chemical elements. Ecol. Eng. 2016, 94, 107–116. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Vieira, C.; Abreu, M.M.; Magalhães, M.C.F. Physiological Response of Cistus salviifolius L. to High Arsenic Concentrations. Environ. Geochem. Health 2019, 42, 2305–2319. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Santos, E.S.; Abreu, M.M. Unraveling the Crucial Role of the Ascorbate-Glutathione Cycle in the Resilience of Cistus monspeliensis L. to Withstand High As Concentrations. Ecotoxicol. Environ. Saf. 2019, 171, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.; Peñalosa, J.M.; Esteban, E.; Pilar Bernal, M. Feasibility of Arsenic Phytostabilisation Using Mediterranean Shrubs: Impact of Root Mineralisation on As Availability in Soils. J. Environ. Monit. 2009, 11, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Medas, D.; Meneghini, C.; Pusceddu, C.; Carlomagno, I.; Aquilanti, G.; Dore, E.; Murgia, V.; Podda, F.; Rimondi, V.; Vacca, S.; et al. Plant-Minerals-Water Interactions: An Investigation on Juncus acutus Exposed to Different Zn Sources. Sci. Total Environ. 2023, 870, 161931. [Google Scholar] [CrossRef] [PubMed]
- Medas, D.; Giudici, G.; Pusceddu, C.; Casu, M.A.; Birarda, G.; Vaccari, L.; Giannoncelli, A.; Meneghini, C. Impact of Zn Excess on Biomineralization Processes in Juncus acutus Grown in Mine Polluted Sites. J. Hazard. Mater. 2017, 370, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Sandhya, S.; Prasad, M.N.V.; Freitas, H. Perspectives of Plant-Associated Microbes in Heavy Metal Phytoremediation. Biotechnol. Adv. 2012, 30, 1562–1574. [Google Scholar] [CrossRef]
- Jin, Z.; Deng, S.; Wen, Y.; Jin, Y.; Pan, L.; Zhang, Y.; Zhang, D. Application of Simplicillium chinense for Cd and Pb Biosorption and Enhancing Heavy Metal Phytoremediation of Soils. Sci. Total Environ. 2019, 697, 134148. [Google Scholar] [CrossRef]
- Baumann, A. Das Verhalten von Zinksalzen gegen Pflanzen und in Boden. Die landwirtsch. Versuchsstat 1885, 31, 53. [Google Scholar]
- Bazan, G.; Galizia, G. Geographical and Ecological Outline of Metal(loid) Accumulating Plants in Italian Vascular Flora. Ecocycles 2018, 4, 47–64. [Google Scholar] [CrossRef]
- Thompson, J.D. Plant Evolution in the Mediterranean: Insights for Conservation; Oxford University Press: Cary, NC, USA, 2020. [Google Scholar]
- González-Orenga, S.; Grigore, M.N.; Boscaiu, M.; Vicente, O. Constitutive and Induced Salt Tolerance Mechanisms and Potential Uses of Limonium Mill. Species. Agronomy 2021, 11, 413. [Google Scholar] [CrossRef]
- Dar, M.I.; Khan, F.A.; Rehman, F.; Masoodi, A.; Ansari, A.A.; Varshney, D.; Naushin, F.; Naikoo, M.I. Roles of Brassicaceae in Phytoremediation of Metals and Metalloids in A.A. In Phytoremediation: Management of Environmental Contaminants; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Manousaki, E.; Kalogerakis, N. Halophytes—An Emerging Trend in Phytoremediation. Int. J. Phytoremediat. 2011, 13, 959–969. [Google Scholar] [CrossRef]
- Chaabani, S.; Abdelmalek-Babbou, C.; Ben Ahmed, H.; Chaabani, A.; Sebei, A. Phytoremediation Assessment of Native Plants Growing on Pb-Zn Mine Site in Northern Tunisia. Environ. Earth Sci. 2017, 76, 585–600. [Google Scholar] [CrossRef]
- De la Fuente, V.; Rufo, L.; Rodríguez, N.; Amils, R.; Zuluaga, J. Metal Accumulation Screening of the Río Tinto Flora (Huelva, Spain). Biol. Trace Element. Res. 2010, 134, 318–341. [Google Scholar] [CrossRef] [PubMed]
- De Giudici, G.; Pusceddu, C.; Medas, D.; Meneghini, C.; Gianoncelli, A.; Rimondi, V.; Podda, F.; Cidu, R.; Lattanzi, P.; Wanty, R.B.; et al. The Role of Natural Biogeochemical Barriers in Limiting Metal Loading to a Stream Affected by Mine Drainage. Appl. Geochem. 2017, 76, 124–135. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Esteban, E.; Carpena-Ruiz, R.O.; Lobo, M.C.; Peñalosa, J.M. Phytostabilisation with Mediterranean Shrubs and Liming Improved Soil Quality in a Pot Experiment with a Pyrite Mine Soil. J. Hazard. Mater. 2012, 201–202, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.S.; Abreu, M.M.; Peres, S.; Magalhães, M.C.F.; Leitão, S.; Santos Pereira, A.; Cerejeira, M.J. Potential of Tamarix africana and Other Halophyte Species for Phytostabilisation of Contaminated Salt Marsh Soils. J. Soils Sediments 2017, 17, 1459–1473. [Google Scholar] [CrossRef]
- Santos, E.S.; Abreu, M.M.; Nabais, C.; Magalhães, M.C.F. Trace elements distribution in soils developed on gossan mine wastes and Cistus ladanifer L. tolerance and bioaccumulation. J. Geochem. Explor. 2012, 123, 45–51. [Google Scholar] [CrossRef]
- Clemente, R.; Walker, D.J.; Pardo, T.; Martínez-Fernández, D.; Bernal, M.P. The Use of a Halophytic Plant Species and Organic Amendments for the Remediation of a Trace Elements-Contaminated Soil under Semi-Arid Conditions. J. Hazard. Mater. 2012, 223–224, 63–71. [Google Scholar] [CrossRef]
- Broadley, M.; Willey, M.J.; Wilkins, J.C.; Baker, A.J.M.; Mead, A.; White, P.J. Phylogenetic Variation in Heavy Metal Accumulation in Angiosperms. New Phytol. 2001, 152, 9–27. [Google Scholar] [CrossRef]
- Cecchi, L.; Gabbrielli, R.; Arnetoli, M.; Gonnelli, C.; Hasko, A.; Selvi, F. Evolutionary Lineages of Nickel Hyperaccumulation and Systematics in European Alysseae Brassicaceae: Evidence from nrDNA Sequence Data. Ann. Bot. 2010, 106, 751–767. [Google Scholar] [CrossRef]
- Cecchi, L.; Coppi, A.; Selvi, F. Evolutionary Dynamics of Serpentine Adaptation in Onosma (Boraginaceae) as Revealed by ITS Sequence Data. Plant Syst. Evol. 2011, 297, 185–199. [Google Scholar] [CrossRef]
- Cecchi, L.; Španiel, S.; Bianchi, E.; Coppi, A.; Gonnelli, C.; Federico, S. Odontarrhena stridii (Brassicaceae), a New Nickel-hyperaccumulating Species from Mainland Greece. Plant Syst. Evol. 2020, 306, 69. [Google Scholar] [CrossRef]
- Krämer, U. Metal Hyperaccumulation in Plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Roccotiello, E.; Serrano, H.C.; Mariotti, M.G.; Branquinho, C. Nickel Phytoremediation Potential of the Mediterranean Alyssoides utriculata (L.) Medik. Chemosphere 2015, 119, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.D.; Brooks, R.R. Hyperaccumulation of Lead and Zinc by Two Metallophytes from Mining Areas of Central Europe. Environ. Pollut. 1983, 31, 277–285. [Google Scholar] [CrossRef]
- Brown, S.L.; Chaney, R.L.; Angle, J.S.; Baker, A.J.M. Zinc and cadmium uptake by hyperaccumulator Thlaspi caerulescens and metal tolerant Silene vulgaris grown on sludge amended soils. Environ. Sci. Technol. 1995, 29, 1581–1585. [Google Scholar] [CrossRef]
- Madejón, P.; Murillo, J.M.; Marãnón, T.; Leppa, N.W. Factors Affecting Accumulation of Thallium and Other Trace Elements in Two Wild Brassicaceae Spontaneously Growing on Soils Contaminated by Tailings Dam Waste. Chemosphere 2007, 67, 20–28. [Google Scholar] [CrossRef]
- Poschenrieder, P.; Bech, J.; Llugany, M.; Pace, A.; Fenés, E. Copper in Plant Species in a Copper Gradient in Catalonia (North East Spain) and Their Potential for Phytoremediation. Plant Soil 2001, 2, 247–256. [Google Scholar] [CrossRef]
- Gisbert, C.; Clemente, R.; Navarro-Aviñó, J.; Baixauli, C.; Ginér, A.; Serrano, R.; Walker, D.J.; Bernal, M.P. Tolerance and Accumulation of Heavy Metals by Brassicaceae Species Grown in Contaminated Soils from Mediterranean Regions of Spain. Environ. Exp. Bot. 2006, 56, 19–27. [Google Scholar] [CrossRef]
- El Aafi, N.; Saidi, N.; Maltouf, A.F.; Perez-Palacios, P.; Dary, M.; Brhada, F.; Pajuelo, E. Prospecting Metal-Tolerant Rhizobia for Phytoremediation of Mining Soils from Morocco Using Anthyllis vulneraria L. Environ. Sci. Pollut. Res. 2015, 22, 4500–4512. [Google Scholar] [CrossRef]
- Prasad, M.N.V.; Freitas, H.M.O. Metal Hyperaccumulation in Plants—Biodiversity Prospecting for Phytoremediation Technology. Electron. J. Biotechnol. 2003, 6, 285. [Google Scholar] [CrossRef]
- Vázquez, S.; Agha, R.; Granado, A.; Sarro, M.; Esteban, E.; Peñalosa, J.; Carpena, R. Use of White Lupin Plant for Phytostabilization of Cd and As Polluted Acid Soil. Water Air Soil Pollut. 2006, 177, 349–365. [Google Scholar] [CrossRef]
- Hao, X.; Taghavi, S.; Xie, P.; Orbach, M.J.; Alwathnani, H.A.; Rensing, C.; Wei, G. Phytoremediation of Heavy and Transition Metals Aided by Legume-Rhizobia Symbiosis. Int. J. Phytoremediat. 2014, 16, 179–202. [Google Scholar] [CrossRef] [PubMed]
- De Agostini, A.; Caltagirone, C.; Caredda, A.; Cicatelli, A.; Cogoni, A.; Farci, D.; Guarino, F.; Garau, A.; Labra, M.; Lussu, M.; et al. Heavy Metal Tolerance of Orchid Populations Growing on Abandoned Mine Tailings: A Case Study in Sardinia Island (Italy). Ecotoxicol. Environ. Saf. 2020, 189, 110018. [Google Scholar] [CrossRef] [PubMed]
- Shefferson, R.P.; Kull, T.; Tali, K. Mycorrhizal Interactions of Orchids Colonizing Estonian Mine Tailings Hills. Am. J. Bot. 2008, 95, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Heckenroth, A.; Rabier, J.; Dutoit, T.; Torre, F.; Prudent, P.; Laffont-Schwob, I. Selection of Native Plants with Phytoremediation Potential for Highly Contaminated Mediterranean Soil Restoration: Tools for a Non-Destructive and Integrative Approach. J. Environ. Manag. 2016, 183, 850–863. [Google Scholar] [CrossRef]
- Cecchi, L.; Colzi, I.; Coppi, A.; Gonnelli, C.; Selvi, F. Diversity and Biogeography of Ni-Hyperaccumulators of Alyssum Section Odontarrhena (Brassicaceae) in the Central Western Mediterranean: Evidence from Karyology, Morphology and DNA Sequence Data. Bot. J. Linn. Soc. 2013, 173, 269–289. [Google Scholar] [CrossRef]
- Cecchi, L.; Bettarini, I.; Colzi, I.; Coppi, A.; Echevarria, G.; Pazzagli, L.; Bani, A.; Gonnelli, C.; Selvi, F. The Genus Odontarrhena (Brassicaceae) in Albania: Taxonomy and Nickel Accumulation in a Critical Group of Metallophytes from a Major Serpentine Hot-Spot. Phytotaxa 2018, 35, 1–28. [Google Scholar] [CrossRef]
- Mengoni, A.; Cecchi, L.; Gonnelli, C. Nickel Hyperaccumulating Plants and Alyssum bertolonii: Model Systems for Studying Biogeochemical Interactions in Serpentine Soils. In Bio-Geo Interactions in Metal-Contaminated Soils; Springer: Berlin/Heidelberg, Germany, 2011; pp. 279–296. [Google Scholar]
- Minguzzi, C.; Vergnano Gambi, O. Il contenuto di nichel nelle ceneri di Alyssum bertolonii Desv. Mem. Soc. Sci. Nat. 1948, 55, 49–74. [Google Scholar]
- Morais, I.; Campos, J.S.; Favas, P.J.C.; Pratas, J.; Pita, F.; Prasad, M.N.V. Nickel Accumulation by Alyssum serpyllifolium subsp. lusitanicum (Brassicaceae) from Serpentine Soils of Bragança and Morais (Portugal) Ultramafic Massifs: Plant-Soil Relationships and Prospects for Phytomining. Aust. J. Bot. 2015, 63, 17–30. [Google Scholar] [CrossRef]
- Bacchetta, G.; Brullo, S.; Cusma Velari, T.; Feoli Campiella, L.; Kosovel, V. Taxonomic Notes on the Genista ephedroides Group (Fabaceae) from the Mediterranean Area. Novon 2011, 21, 4–19. [Google Scholar] [CrossRef]
- Cao, A.; Cappai, G.; Carucci, A.; Muntoni, A. Selection of Plants for Zinc and Lead Phytoremediation. J. Environ. Sci. Health 2004, 39, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, G.; Boi, M.E.; Cappai, G.; Giudici, G.; Piredda, M.; Porceddu, M. Metal tolerance capability of Helichrysum microphyllum Cambess. subsp. tyrrhenicum Bacch., Brullo & Giusso: A candidate for phytostabilization in abandoned mine sites. Bull. Environ. Contam. Toxicol. 2018, 101, 758–765. [Google Scholar] [PubMed]
- Barbafieri, M.; Dadea, C.; Tassi, E.; Bretzel, F.; Fanfani, L. Uptake of Heavy Metals by Native Species Growing in a Mining Area in Sardinia, Italy: Discovering Native Flora for Phytoremediation. Int. J. Phytoremediat. 2011, 13, 985–997. [Google Scholar] [CrossRef]
- Jiménez, M.N.; Fernandez, E.; Navarro, E.B.; Contini, E.; Casti, M.; Bacchetta, G. Livelli di metalli pesanti in Dittrichia viscosa (L.) Greuter, Cistus salviifolius L. e Euphorbia cupanii Bertol. ex Moris su suoli contaminati e non contaminati dalle attività estrattive nell’Iglesiente (Sardegna sudoccidentale). Inf. Bot. Ital. 2005, 37, 794–795. [Google Scholar]
- Conesa, H.M.; María-Cervantes, A.; Álvarez-Rogel, J.; González-Alcaraz, M.N. Influence of Soil Properties on Trace Element Availability and Plant Accumulation in a Mediterranean Salt Marsh Polluted by Mining Wastes: Implications for Phytomanagement. Sci. Total Environ. 2011, 409, 4470–4479. [Google Scholar] [CrossRef] [PubMed]
- Freitas, H.; Prasad, M.N.V.; Pratas, J. Analysis of Serpentinophytes from North–East of Portugal for Trace Metal Accumulation—Relevance to the Management of Mine Environment. Chemosphere 2004, 54, 1625–1642. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Zornoza, R.; Conesa, E.; Gómez-López, M.D.; Faza, A. Seedling Emergence, Growth and Trace Elements Tolerance and Accumulation by Lamiaceae Species in a Mine Soil. Chemosphere 2014, 113, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Carucci, A.; Lai, T.; Bacchetta, G.; Casti, M. Use of Native Species and Biodegradable Chelating Agent in Phytoremediation of Abandoned Mining Area. J. Chem. Technol. Biotechnol. 2009, 84, 884–889. [Google Scholar] [CrossRef]
- Rossini-Oliva, S.; Santos, E.S.; Abreu, M.M. Accumulation of Mn and Fe in Aromatic Plant Species from the Abandoned Rosalgar Mine and Their Potential Risk to Human Health. Appl. Geochem. 2019, 104, 42–50. [Google Scholar]
- Moreno-Jiménez, E.; Rebeca Manzano, R.; Esteban, E.; Peñalosa, J. The Fate of Arsenic in Soils Adjacent to an Old Mine Site (Bustarviejo, Spain): Mobility and Transfer to Native Flora. J. Soils Sediments 2010, 10, 301–312. [Google Scholar] [CrossRef]
- Díez-Lázaro, J.; Kidd, P.S.; Monterroso, C. A phytogeochemical study of the Trásos Montes region (NE Portugal): Possible species for plant-based soil remediation technologies. Sci. Total Environ. 2006, 354, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.N.; Bacchetta, G.; Navarro, F.B.; Casti, M.; Fernández-Ondoño, E. Native Plant Capacity for Gentle Remediation in Heavily Polluted Mines. Appl. Sci. 2021, 11, 1769. [Google Scholar] [CrossRef]
- Abreu, M.M.; Santos, E.S.; Magalhães, M.C.F.; Fernandes, E. Trace Elements Tolerance, Accumulation and Translocation in Cistus populifolius, Cistus salviifolius and Their Hybrid Growing in Polymetallic Contaminated Mine Areas. J. Geochem. Explor. 2012, 123, 52–60. [Google Scholar] [CrossRef]
- Durães, N.; Bobos, I.; Silva, E.; Dekayir, A. Copper, Zinc and Lead Biogeochemistry in Aquatic and Land Plants from the Iberian Pyrite Belt (Portugal) and North of Morocco Mining Areas. Environ. Sci. Pollut. Res. 2015, 22, 2086–2105. [Google Scholar] [CrossRef]
- Rossini-Oliva, S.; Mingorance, M.D.; Monaci, F.; Valdés, B. Ecophysiological Indicators of Native Cistus ladanifer L. at Riotinto Mine Tailings (SW Spain) for Assessing Its Potential Use for Rehabilitation. Ecol. Eng. 2016, 91, 93–100. [Google Scholar] [CrossRef]
- Batista, M.J.; Gonzalez-Fernandez, O.; Abreu, M.M.; Queralt, I.; Carvalho, M.L. Pioneer Mediterranean shrub species revegetating soils developed on mining soils/spoils. Land Degrad. Dev. 2017, 28, 718–730. [Google Scholar] [CrossRef]
- El Mamoun, I.; Mouna, F.; Mohammed, A.; Najib, B.; Zine-El Abidine, T.; Abdelkarim, G.; Didier, B.; Laurent, L.; Abdelaziz, S. Zinc, Lead, and Cadmium Tolerance and Accumulation in Cistus libanotis, Cistus albidus, and Cistus salviifolius: Perspectives on Phytoremediation. Remediation 2020, 30, 73–80. [Google Scholar] [CrossRef]
- Santos, E.S.; Abreu, M.M.; Macías, F.; Batista, M.J.; Magalhães, M.C.F.; Fernandes, E. Inter-Population Variation on the Accumulation and Translocation of Potentially Harmful Chemical Elements in Cistus ladanifer L. from Brancanes, Caveira, Chança, Lousal, Neves Corvo and São Domingos Mines in the Portuguese Iberian Pyrite Belt. J. Soil Sediments 2014, 14, 758–772. [Google Scholar] [CrossRef]
- Laplaze, L.; Doumas, P.; Smouni, A.; Brhada, F.; Ater, M. Phytoremédiation du Plomb par Cistus libanotis: Demande de Brevet Prioritaire. Patentscope, 2009. Available online: https://patentscope.wipo.int/search/fr/detail.jsf?docId=WO2010130730 (accessed on 6 November 2023).
- Lai, T.; Cappai, G.; Carucci, A.; Bacchetta, G. Phytoremediation of Abandoned Mining Areas Using Native Plant Species: A Sardinian Case Study. Environ. Sci. Eng. 2015, 11, 256–277. [Google Scholar]
- Anawar, H.M.; Freitas, M.C.; Canha, N.; Santa Regina, I. Arsenic, Antimony, and Other Trace Element Contamination in a Mine Tailings Affected Area and Uptake by Tolerant Plant Species. Environ. Geochem. Health 2011, 33, 353–362. [Google Scholar] [CrossRef]
- Cabezudo, B.; Rivera, J.; Cabezudo, E.; Rivera, S.P. Notas taxonómicas y corológicas sobre la flora de Andalucía Occidental. Lagascalia 1980, 9, 223–226. [Google Scholar]
- Turnau, K.; Henriques, F.S.; Anielska, T.; Renker, C.; Buscot, F. Metal Uptake and Detoxification Mechanisms in Erica andevalensis Growing in a Pyrite Mine Tailing. Environ. Exp. Bot. 2007, 61, 117–123. [Google Scholar] [CrossRef]
- Marques, A.P.G.C.; Moreira, H.; Rangel, A.O.; Castro, P.M. Arsenic, Lead and Nickel Accumulation in Rubus ulmifolius Growing in Contaminated Soil in Portugal. J. Hazard. Mater. 2009, 165, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Steingräber, L.F.; Ludolphy, C.; Metz, J.; Kierdorf, H.; Kierdorf, U. Uptake of Lead and Zinc from Soil by Blackberry Plants (Rubus fruticosus L. Agg.) and Translocation from Roots to Leaves. Environ. Adv. 2022, 9, 100313. [Google Scholar] [CrossRef]
- Kharazian, P.; Bacchetta, G.; Cappai, G.; Piredda, M.; Giudici, G. An Integrated Geochemical and Mineralogical Investigation on Soil-Plant System of Pinus halepensis Pioneer Tree Growing on Heavy Metal Polluted Mine Tailing. Plant Biosyst. 2022, 157, 272–285. [Google Scholar] [CrossRef]
- Kharazian, P.; Fernández-Ondoño, E.; Jiménez, M.N.; Sierra Aragón, M.; Aguirre-Arcos, A.; Bacchetta, G.; Cappai, G.; Giudici, G. Pinus halepensis in contaminated mining sites: Study of the transfer of metals in the plant-soil system using the BCR procedure. Toxics 2022, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Disante, K.B.; Fuentes, D.; Cortina, J. Sensitivity to Zinc of Mediterranean Woody Species Is Important for Restoration. Sci. Total Environ. 2010, 408, 2216–2225. [Google Scholar] [CrossRef] [PubMed]
- Pàrraga-Aguado, I.; Querejeta, J.I.; González-Alcaraz, M.N.; Conesa, H.M. Metal(loid) allocation and nutrient retranslocation in Pinus halepensis trees growing on semiarid mine tailings. Sci. Total Environ. 2014, 485–486, 406–414. [Google Scholar] [CrossRef]
- Conesa, H.M.; Párraga-Aguado, I. Effects of a Soil Organic Amendment on Metal Allocation of Trees for the Phytomanagement of Mining-Impacted Soils. Environ. Geochem. Health 2021, 43, 1355–1366. [Google Scholar] [CrossRef]
- Kharazian, P.; Cappai, G.; Boi, M.E.; Porceddu, M.; Piredda, M.; De Giudici, G.; Bacchetta, G. Greenhouse Investigation on the Phytoremediation Potential of Pioneer Tree Pinus halepensis Mill. in Abandoned Mine Site. Int. J. Phytoremediat. 2023, in press. [Google Scholar] [CrossRef]
- Smillie, C. Salicornia spp. as a biomonitor of Cu and Zn in salt marsh sediments. Ecol. Indic. 2015, 56, 70–78. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Trigas, P.; Strid, A.; Dimopoulos, P. Plant Diversity Patterns and Conservation Implications under Climate-Change Scenarios in the Mediterranean: The Case of Crete (Aegean, Greece). Diversity 2020, 12, 270. [Google Scholar] [CrossRef]
- Guardiola, M.; Sáez, L. Are Mediterranean Island Mountains Hotspots of Taxonomic and Phylogenetic Biodiversity? The Case of the Endemic Flora of the Balearic Islands. Plants 2023, 12, 2640. [Google Scholar] [CrossRef]
- Lannuzel, G.; Pouget, L.; Bruy, D.; Hequet, V.; Meyer, S.; Munzinger, J.; Gâteblé, G. Mining Rare Earth Elements: Identifying the Plant Species Most Threatened by Ore Extraction in an Insular Hotspot. Front. Ecol. Evol. 2022, 10, 952439. [Google Scholar] [CrossRef]
- Palliwoda, J.; Priess, J.A. What Do People Value in Urban Green? Linking Characteristics of Urban Green Spaces to Users’ Perceptions of Nature Benefits, Disturbances, and Disservices. Ecol. Soc. 2021, 21, 28. [Google Scholar] [CrossRef]
- Kou, R.; Hunter, R.F.; Cleland, C.; Ellis, G. Physical Environmental Factors Influencing Older Adults’ Park Use: A Qualitative Study. Urban For. Urban Green. 2021, 65, 127353. [Google Scholar] [CrossRef]
- Pardela, Ł.; Lis, A.; Zalewska, K.; Iwankowski, P. How Vegetation Impacts Preference, Mystery and Danger in Fortifications and Parks in Urban Areas. Landsc. Urban Plan. 2022, 228, 104558. [Google Scholar] [CrossRef]
- IUCN. Guidelines for Proctected Area Managment Catrgories; CNPPA with the Assistance of WCMC; IUCN: Gland, Switzerland; Cambridge, UK, 1994. [Google Scholar]
- Muntoni, F.; Balvis, T.; Rizzo, R.; Loru, P. Territorial Planning of Geological Mining Historical and Environmental Park of Sardinia. Geoheritage 2020, 12, 22. [Google Scholar] [CrossRef]
- Stolton, S.; Shadie, P.; Dudley, N. IUCN WCPA Best Practice Guidance on Recognising Protected Areas and Assigning Management Categories and Governance Types. In Best Practice Protected Area Guidelines Series; No. 21; IUCN: Gland, Switzerland, 2013. [Google Scholar]
- Voudouris, P.; Melfos, V.; Mavrogonatos, C.; Photiades, A.; Moraiti, E.; Rieck, B.; Kolitsch, U.; Tarantola, A.; Scheffer, C.; Morin, D.; et al. The Lavrion Mines: A Unique Site of Geological and Mineralogical Heritage. Minerals 2021, 11, 76. [Google Scholar] [CrossRef]
- Fois, M.; Bacchetta, G.; Caria, M.C.; Cogoni, D.; Farris, E.; Fenu, G.; Manca, M.; Pinna, M.S.; Pisanu, S.; Rivieccio, G.; et al. Proposals for Improvement of Annex I of Directive 92/43/EEC: Sardinia. Plant Sociol. 2021, 58, 65–76. [Google Scholar] [CrossRef]
- Porceddu, M.; Santo, A.; Orru, M.; Meloni, F.; Ucchesu, M.; Picciau, R.; Sarigu, M.; Cuena Lombrana, A.; Podda, L.; Sau, S.; et al. Seed Conservation Actions for Thepreservation of Plant Diversity: The Case of the Sardinian Germplasm Bank (BG-SAR). Plant Sociol. 2017, 54, 111–117. [Google Scholar] [CrossRef]
- Shri, M.; Kumar, S.; Chakrabarty, D.; Trivedi, P.K.; Mallick, S.; Misra, P.; Shukla, D.; Mishra, S.; Srivastava, S.; Tripathi, R.; et al. Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol. Environ. Saf. 2009, 272, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Zheng, C.R.; Tu, C.; Shen, Z.G. Chemical Methods and Phytoremediation of Soil Contaminated with Heavy Metals. Chemosphere 2000, 41, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in Soil Using Amendments—A Review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Robinson, B.H.; Anderson, C.W.N.; Dickinson, N.M. Phytoextraction: Where’s the Action? J. Geochem. Explor. 2015, 151, 34–40. [Google Scholar] [CrossRef]
- Mertens, J.; Luyssaert, S.; Verheyen, K. Use and Abuse of Trace Metal Concentrations in Plant Tissue for Biomonitoring and Phytoextraction. Environ. Pollut. 2005, 138, 1–4. [Google Scholar] [CrossRef]
- Neaman, A.; Selles, I.; Martínez, C.E.; Dovletyarova, E.A. Analyzing Soil Metal Toxicity: Spiked or Field-Contaminated Soils? Environ. Toxicol. Chem. 2020, 39, 513–514. [Google Scholar] [CrossRef]
- Santos, E.S.; Abreu, M.M.; Saraiva, J.A. Mutielemental concentration and physiological responses of Lavandula pedunculata growing in soils developed on different mine wastes. Environ. Pollut. 2016, 213, 43–52. [Google Scholar] [CrossRef]

| Family | N. Genera | N. taxa |
|---|---|---|
| Amaranthaceae | 2 | 2 (0) |
| Anacardiaceae | 1 | 1 (0) |
| Asteraceae | 2 | 2 (1) |
| Brassicaceae | 4 | 4 (3) |
| Cistaceae | 1 | 6 (3) |
| Ericaceae | 1 | 2 (1) |
| Euphorbiaceae | 1 | 1 (0) |
| Fabaceae | 1 | 1 (0) |
| Fagaceae | 1 | 1 (0) |
| Juncaceae | 1 | 1 (0) |
| Lamiaceae | 3 | 6 (3) |
| Oleaceae | 2 | 2 (0) |
| Pinaceae | 1 | 1 (0) |
| Poaceae | 1 | 1 (0) |
| Rosaceae | 1 | 1 (0) |
| Scrophulariaceae | 1 | 2 (1) |
| Tamaricaceae | 1 | 2 (1) |
| Thymelaeaceae | 1 | 1 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boi, M.E.; Fois, M.; Podda, L.; Porceddu, M.; Bacchetta, G. Using Mediterranean Native Plants for the Phytoremediation of Mining Sites: An Overview of the Past and Present, and Perspectives for the Future. Plants 2023, 12, 3823. https://doi.org/10.3390/plants12223823
Boi ME, Fois M, Podda L, Porceddu M, Bacchetta G. Using Mediterranean Native Plants for the Phytoremediation of Mining Sites: An Overview of the Past and Present, and Perspectives for the Future. Plants. 2023; 12(22):3823. https://doi.org/10.3390/plants12223823
Chicago/Turabian StyleBoi, Maria Enrica, Mauro Fois, Lina Podda, Marco Porceddu, and Gianluigi Bacchetta. 2023. "Using Mediterranean Native Plants for the Phytoremediation of Mining Sites: An Overview of the Past and Present, and Perspectives for the Future" Plants 12, no. 22: 3823. https://doi.org/10.3390/plants12223823
APA StyleBoi, M. E., Fois, M., Podda, L., Porceddu, M., & Bacchetta, G. (2023). Using Mediterranean Native Plants for the Phytoremediation of Mining Sites: An Overview of the Past and Present, and Perspectives for the Future. Plants, 12(22), 3823. https://doi.org/10.3390/plants12223823











