Abstract
Climate-change-induced variations in temperature and rainfall patterns are a serious threat across the globe. Flooding is the foremost challenge to agricultural productivity, and it is believed to become more intense under a changing climate. Flooding is a serious form of stress that significantly reduces crop yields, and future climatic anomalies are predicted to make the problem even worse in many areas of the world. To cope with the prevailing flooding stress, plants have developed different morphological and anatomical adaptations in their roots, aerenchyma cells, and leaves. Therefore, researchers are paying more attention to identifying developed and adopted molecular-based plant mechanisms with the objective of obtaining flooding-resistant cultivars. In this review, we discuss the various physiological, anatomical, and morphological adaptations (aerenchyma cells, ROL barriers (redial O2 loss), and adventitious roots) and the phytohormonal regulation in plants under flooding stress. This review comprises ongoing innovations and strategies to mitigate flooding stress, and it also provides new insights into how this knowledge can be used to improve productivity in the scenario of a rapidly changing climate and increasing flood intensity.
1. Introduction
The increasing frequency and intensity of climate change has led to a serious increase in flooding stress [1,2,3]. Temporary and prolonged flooding stress has a significant impact on plant growth and survival, making it imperative for agricultural researchers to identify the involved mechanisms underlying plant adaptation to the aforementioned challenge [4]. Flooding significantly reduces the stomatal conductance, photosynthesis, nitrogen fixation, shoot growth, root development, and nutrient absorption of plants, which results in significant yield losses [5]. Flooding stress also disrupts the gas diffusion between cells and the O2 diffusion in plant tissues, which restricts mitochondrial respiration and O2 exchange, thereby substantially impairing biochemical and physiological processes in plants [6]. The low oxygen availability under flooding activates the anaerobic microbes, which have a negative impact on plant functioning [7,8] and crop productivity [9]. Understanding current developments and foreseeing the upcoming difficulties in plant adaptation to flooding stress is vital in order to cope with the future challenges of food security and the conservation of agriculture under the changing global climate [10,11,12].
Flooding stress causes oxygen deficiency, which can limit root growth and also leads to root death. Further, water and nutrient absorption and transport are impaired under flooding stress, which causes a reduction in growth and field production [13,14]. Flooding stress also decreases the leaf nitrogen content and impairs the leaf’s water potential, CO2 assimilation, and photosynthesis; it also accelerates leaf chlorosis, and senescence may also be observed [13]. Additionally, flooding stress increases reactive oxygen species (ROS) production, which causes oxidative damage and degrades cellular membranes, proteins, and lipids [15]. Water-logging-induced ROS production causes lipid peroxidation, which causes membrane injury, enzyme inactivation, and eventually cell death [16,17].
Plants adopt different physiological, morphological, and biochemical strategies to cope with flooding stress [18,19]. However, the different strategies adopted by plants depend on the alteration of the root structure, the gas exchange mechanism, and metabolic pathways [20,21]. For instance, some plants that are tolerant to low or no oxygen can develop morphological adaptations to compensate for the oxygen deficiency in their roots [22]. Further, plants produce adventitious roots in response to flooding stress, which improves gas transport and nutrient and water uptake and enhances the plant’s survival and productivity [23]. The adventitious roots developed by plants are able to uptake and transport O2, making it available to submerged roots [24]. Plants also change their root architecture, which allows them to withstand flooding stress. For instance, in flooded areas, a shallow root system is more beneficial for O2 uptake, since the upper layer of soil has more O2 compared with lower layers [25]. The maintenance of membrane stability is another important mechanism used by plants to tolerate flooding stress [26]. Plants also activate a vast number of stress response genes, and essential functional proteins are synthesized [27]. For instance, different authors found that plants downregulate photosynthesis-related genes (PsbQ, PsbO, and petF) and light-harvesting chlorophyll protein complex genes (LHCB1, LHCB3, LHCB5, LHCA1, and LHCA4) in response to flooding stress [28,29].
The goal of this review is to present a thorough assessment of the current research addressing plants’ ability to adapt to flooding stress in climatic change scenarios. We focus on the causes, effects, and recent developments in genetic, physiological, and ecological aspects of plants (ongoing breakthroughs) in terms of resisting flooding stress. This review will contribute to the development of sustainable methods to reduce the effects of flooding stress on plants in the era of climate change by synthesizing existing knowledge and emphasizing emerging trends.
2. Causes of Flooding
2.1. Intense Precipitation
Plants face complex physiological and morphological challenges under flooding stress. High rainfall is the main driver of flooding (Figure 1), and an increasing rainfall intensity worsens the arable land’s productivity [30]. Unexpected rainfall significantly impairs the soil quality, soil saturation, root architecture, nutrient intake, and overall crop yield [19,31]. Westra et al. [32] observed a significant increase in flooding stress on crops due to a high intensity of rainfall. Furthermore, climate change has shifted rainfall patterns and contributed to flooding stress, which hinders crop growth [33]. Extreme weather patterns, alterations in land cover, and a rise in sea levels are the most significant sources of intense precipitation and flooding [34]. The temperature rise enables air to hold more moisture, leading to intense precipitation [32]. Heavy rainfall overwhelms the water absorption ability of soil and causes surface water accumulation [35]. The saturated soil possesses decreased O2 availability in the root zone, which leads to flooding stress for crops [36]. Long-term submersion hinders metabolic processes, reduces root respiration and nutrient uptake, and causes asphyxia [31,37]. Additionally, heavy rainfall leads to the loss of fertile topsoil due to soil erosion and thus reduces agricultural productivity [38,39].
2.2. Poor Drainage System
A high water table, over-irrigation, rainfall after irrigation, and inadequate drainage increase flooding stress [5]. The inappropriate engineering of the drainage system hinders water removal from the field, whereas a shallow water table also intensifies the waterlogging conditions Figure 1 [40]. An upward-sloping topography in the land also enhances the flooding situation, where an inefficient irrigation system prevails in low-lying soil positions [5,41]. Thus, the installation of an advanced drainage system can substantially reduce the risk of flooding stress in agricultural fields [42,43].
2.3. Soil Compaction
Water infiltration decreases with soil compaction caused by heavy machinery (tractor wheel), which also causes stress in field crops. This is due to alterations in soil bulk density and soil particles and disturbances in the soil structure caused by soil compaction [5]. For example, puddled rice cultivation leads to poor drainage conditions, which subsequently leads to flooding for the succeeding (wheat) crop [44]. These factors jointly cause severe flooding stress and impair the plants’ performance.
2.4. Soil Type
The clayey soil is very susceptible to water-logging under heavy rainfall and poor irrigation systems [45,46]. Blessitt [45] reported a high water holding capacity in soil clay particles due to the swelling of the particles, which further impedes the water infiltration in the soil profile, thus creating flooding stress to plants [5]. In addition, the natural soil layers restrict the free drainage of water in the deep soil due to the presence of claypan, which reduces infiltration. The depth of the claypan may range from 10 cm to 40 cm [47,48] and these clay particles may be greater than 460 g/kg [49], leading to a perched water table in the upper soil profile during heavy precipitation in the rainy season [50]. These perched tables lead to short-term flooding stress in the area where water readily infiltrates in sandy soil but accumulates above the compacted clay sub-soil [5].
2.5. Snowmelt
Snowmelt also contributes significantly to the development of flooding stress. Snowmelt-induced flooding stress is mainly driven by elevated temperatures. The extent of the relationship between temperature fluctuations, snow accumulation, and melting is critical to the extent of flooding stress [51]. A changing climate exacerbates the snowmelt process and leads to a noteworthy contribution to waterlogging or flooding stress in crops [52]. In this content, agricultural land with a steep slope and no land cover is considered vulnerable to waterlogging and flooding stress [5].
2.6. Over-Irrigation
The excessive application of irrigation can also be an important cause of flooding stress. The excess of water in the root zone causes excessive irrigation, which inhibits gas exchange with the atmosphere and results in an oxygen-deficient environment for the root system [53]. Intensive irrigation exposes the soil to flooding stress, owing to the fact that water seeps through and results in a rise in the soil water table. The intensive method of irrigation exposes soil to waterlogging, and the excessive moisture in the root zone over the field capacity deprives the crops of water and valuable nitrogen. Further, over-irrigation increases the soil salinity, which negatively affects plant growth and development [54].
2.7. Ionic Toxicity
Flooding stress also causes a sharp decrease in soil redox potential, which changes the soil’s chemical profile [55]. Flooding stress changes the availability of mineral substances; it causes a reduction in manganese (Mn4+), iron (Fe3+), and sulfate (SO42−) availability and an increase in potentially toxic elements [56]. For instance, Khabaz-Saberi et al. [57] found that flooding stress in wheat increased the concentrations of shoot aluminum (Al), manganese (Mn), and iron (Fe) 2–10 fold as compared to no flooding stress. In addition, Khabaz-Saberi et al. [58] found that flooding stress in wheat increased the concentrations of Al, Mn, and from 3 to 9 fold, thus resulting in a substantial reduction in aboveground biomass and grain yield. Zeng et al. [59] found that flooding stress in barley increased the toxicity of Fe and Mn, which caused a serious reduction in barley growth and yield.
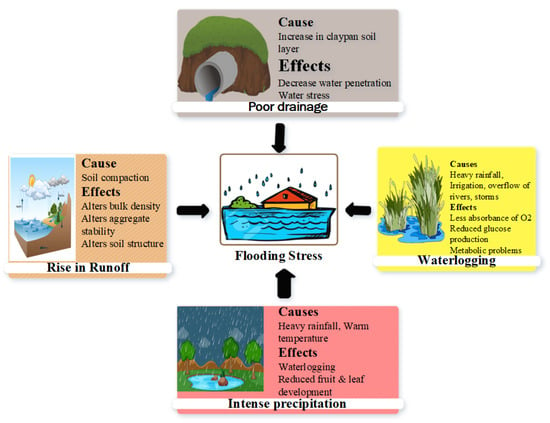
Figure 1.
Heavy rainfall, high temperatures, waterlogging, and poor drainage are important causes of flooding stress.
3. Effects of Flooding Stress on Plants
3.1. Seed Germination
Seed germination is the preliminary step towards plant growth and it is controlled by different hormones and abiotic factors [60,61,62,63]. Among these, O2 availability has prime importance for seed germination [64,65]. Under an optimal oxygen supply, the reserved starch in the seed is converted into sucrose and produces ATP, which facilitates the germination process [66]. ATP production is significantly reduced during low oxygen availability in flooding situations [4]. Interestingly, the flooding tolerance ability also varies amongst plant species [67]. Rice expresses α-amylase activity under low O2 availability that strengthens seeds, allowing them to germinate in flooding conditions [68]. The AMY3 gene is responsible for the α-amylase activity in rice under flooding stress [68]. Further, the calcineurin b-like-interacting protein kinase (CIPK15) aids SnRK1A accumulation, which prompts MYBS1 and AMY3D gene expression to mediate carbohydrate catabolism in flooding stress conditions [68,69]. OsTPP7 is a quantitative trait locus (QTL) responsible for the transcription of the AMY3D gene, and it decomposes starch to provide the ATP necessary for seed germination [4,70]. Although wheat and barley seeds contain high starch content, these cereals are quite sensitive to anoxic (flooding) stress [4,71]. However, this sensitivity can be decreased with the exogenous application of glucose, confirming the role of α-amylase activity towards seed germination in flooding stress conditions [4]. Nevertheless, the molecular-based mechanism responsible for reducing the flooding stress sensitivity still needs to be explored.
3.2. Seedling Establishment
The second most important stage in the plant life cycle is seedling establishment, which is greatly influenced by flooding stress [72,73]. The roots are the preliminary organs that face oxygen deficiency and undergo the various phenotypic alterations that seriously impact plant growth and shoot development during flooding stress [74,75]. Flooding significantly reduces the root length and dry biomass of cereals [76,77]. However, rice is associated with the development of adventitious roots (ARs) to obtain O2 in Arabidopsis [74]. Similarly, Solanum dulcamara continues its life cycle in flooding situations due to the presence of ARs [78]. In Arabidopsis, the HRE2 gene is responsible for the expression of ARs, which helps to modify the root system through RAP2.12 in anoxic stress conditions [74,79]. RAP2.12 helps to reduce root bending and lowers PIN-FORMED2 accumulation and IAA flux from the root tips in the root zone; this ultimately leads to high IAA in the root tips and slanting growth [79].
Agronomically, treatment with calcium peroxide augments seeding establishment by enhancing both the root and shoot length and plant biomass [72]. It provides ATP to germinating seeds by amplifying the α-amylase activity in seeds during flooding conditions [4,72]. Further, the enzymatic (DH, ALDH, and PDC) activity is enhanced in plants facing anoxic respiration [80], whereas exogenously applied calcium peroxide significantly reduces this enzymatic response by enhancing the soluble O2 content in water during flooding stress [72]. Although the oxygen availability in plants under flooding stress is enhanced by calcium peroxide application, it is still necessary to identify the adverse effects of flooding on early stand establishment.
3.3. Reproductive Growth
The crop quality and final yield are largely determined by the successful completion of the plant reproductive stage (Table 1). Under flooding stress, flower and bud initiation and fertilization are affected, which lowers the grain count, size, and weight along with the starch content [81]. Soluble starch synthase is responsible for catalyzing amylopectin, which is downregulated under flooding stress [4]. However, granule-bound starch synthase-I, a key enzyme responsible for amylose synthesis, is upregulated [81]. He et al. [82] found that calcium peroxide treatment enhanced the photosynthetic activity and fruit size of cucumbers during flooding stress conditions. Further, an abundant supply of nitrogen was found to enhance the leaf area, the activity of photosystem II, and the overall maize yield under flooding stress [83].
3.4. Crop Yield
Flooding stress is a serious form of abiotic stress and it can cause yield losses (Table 1) of up to 70% [84]. However, yield losses largely depend on the stage of plant growth (Table 1) and the intensity of flooding stress [54,85,86,87,88,89,90]. For instance, Ploschuk et al. [91] reported that flooding stress at the vegetative growth stages in barley and canola caused more yield losses as compared to flooding stress at the reproductive stage. These authors found that flooding stress reduced the spike and seed production, therefore causing a reduction in productivity. Chen et al. [86] also found a significant decrease in crop yield due to flooding stress in the vegetative stage, while Kaur et al. [5] found that flooding stress at the reproductive stage in soybean caused a yield reduction of up to 20–29%. Likewise, Singh et al. [90] reported a significant decrease in maize and soybean yields due to flooding stress at the vegetative stage as a result of fewer spikes and reduced seed production.

Table 1.
Responses of different plant species to flooding stress.
Table 1.
Responses of different plant species to flooding stress.
| Plant Species | Response | Reference |
|---|---|---|
| Rice | Restricted shoot elongation as well as carbohydrate consumption. | [92] |
| Wheat | The ratio of root/shoot significantly declines. | [26] |
| Maize | Inhibited maize growth, resulting in declines in plant height, ear height, dry weight, leaf area index, and grain yield. | [93] |
| Soybean | Root growth of soybean is significantly suppressed. | [94] |
| Tomato | Flooding stress reduces tomato growth. | [95] |
| Rumex palustris | Inhibition of auxin transport, suppressed ethylene-induced AR formation. | [96] |
| Triarrhena sacchariflora | Activity of anti-oxidative enzymes POD and superoxide dismutase (SOD) in roots increases first and then decreases. | [97] |
| Arabidopsis | Starch content in rosette leaves is reduced with the extension of submerged time during the night, and glucose content declines. | [98] |
Furthermore, flooding water physically disturbs the crop foliage via water flow and abrasion from suspended particles [20]. Under extreme stress, the plant starts wilting, whereas high soil moisture and a humid environment increase the possibility of disease and infection [99]. Scab in wheat (Fusarium graminearum), common smut (Ustilago maydis) in corn, and downy mildew (Sclerophthora macrospora), gray leaf spot (Cercospora zeae-maydis), and sudden death syndrome (Fusarium virguliforme) in soybean are the most commonly reported diseases and infections in plants during flooding stress conditions [5].
3.5. Soil Properties
An ample amount of oxygen in the atmosphere, which easily enters the soil, is necessary for microbial decomposition [5]. Oxygen is a strong electron acceptor and is readily reduced by NO3−, Mn4+, Fe3+, SO2−, and CO2 in anaerobic conditions during flooding (ADD REF). Redox potential (Eh) refers to microbial activity; therefore, it is vital to ensure oxygen availability in the soil composition [100]. Measuring the redox potential (ranges) indicates the presence of NO3− in either oxidized or reduced form, which may lead to nitrogen losses (N2O emission via denitrification). A typical redox potential value varies between −300 and +900 mV, while the Eh of reduced soil falls within the range of −300 mV to +400 mV [101]. Further, the Eh of aerobic (oxic), sub-aerobic, and anaerobic (anoxic) soils in >414, 414–120, and more than 120 mV, respectively [102]. Denitrification starts at 220–280 Eh mV and causes significant nitrogen loss in the environment [103]. The organic matter percentage, electron acceptors, soil pH, and temperature during flooding affect the soil Eh and are considered to control nitrogen losses [104]. In a study, the frequency of O2 reduction increased from anaerobic soil during flooding as the soil Eh decreased by 50 mV each day [105]. Moreover, reduced redox potential was observed with decreased O2 from flooded soil due to anaerobic conditions [106].
Kögel-Knabner et al. [100] reported that the soil pH varied with the soil redox potential in flooded soil. The accumulation of more CO2 from alkaline flooded soil decreased the soil pH, while the pH increased in acidic coil with high proton consumption [107]. Flooding conditions alter the soil pH depending on the amount of OM, the soil type, and the microbial concentration in the soil [108]. Kögel-Knabner et al. [100] observed a variation in Eh of 59 mV, which disturbed the soil pH by one unit [105]. In contrast, another study found that the was no significant effect of flooding on the soil pH [106]. Moreover, the flooding effect helps to control soil chemical reactions, and the temperature of waterlogged soil was observed to be higher than that of non-stressed soil particles [105,106]. Whereas Zurweller et al. [109] could not confirm the temperature and soil Eh variations in flooding stress, recent studies have reported a significant impact of flooding stress on the soil temperature, pH, Eh, and subsequently nitrogen transformation and its availability to crops due to high N losses [105].
Flooding stress reduced the soil nitrogen availability to plants [106,108]. Unger et al. [106] observed a nitrate reduction from 3 to 5 weeks in waterlogged soil, and Kongchum [108] noticed a continuous decline in nitrate concentration with each day of flooding. They further reported 61% less NO3-N from flooded soil compared to normal soil. This significant reduction was due to the large nitrogen losses caused by increased soil runoff, nitrate leaching, and denitrification under flooding stress [5].
Surface runoff and soil erosion must be controlled as they work similarly to enhance nitrogen losses from agricultural fields (ADD REF). More than 5% of applied nitrogen losses are caused by surface runoff (ADD REF). Further, heavy rainfall accounts for 44% of the total nitrogen and 46% of the NO3-N losses from agricultural lands, indicating the role of the precipitation amount and its intensity in the nitrogen losses associated with surface runoff [5]. The conversion of NO3− to any gas representing nitrogen (N) or directly into N molecules is known as the denitrification process, and this process is mainly controlled by various autotrophic and heterotrophic bacteria, called denitrifiers, which are activated under the anoxic conditions that develop during waterlogging or flooding stress.
2NO− 3 → 2NO− 2 → 2NO− → N2O → N2
Clayey soils are highly susceptible to nitrogen losses and therefore more denitrification process takes place in such soils [5]. A perched water table forms in the lower layer of clayey soil with less hydraulic conductivity [50]. Reduced aerobic nitrogen transformation in clayey soil is found due to its poor drainage, and therefore high N2O emissions occur in flooding conditions [108]. Kongchum [108] reported high (1.1 to 2.6%) nitrogen losses from flooded soil as compared to normal soil, where nitrogen was lost by 0.03–0.04% [5]. These results correspond with the findings of Allen et al. [110], who stated a positive correlation between waterlogging and water-filled pore spaces and nitrous oxide (N2O) emissions from flooded soils [110].
Nitrate leaching entails the downward movement of NO3-N and is considered fundamental in the soil environment [111]. Nitrate is highly soluble; therefore, it is highly susceptible to leaching losses [112]. Approximately 10–40% of nitrogen is lost through the leaching process depending on the nitrogenous fertilizer type, application rate, soil characteristics, and temperature and water availability in the soil [113,114]. Advanced studies of nutrient conservation revealed that the microbial population, soil type, and plant root structure can interact to augment nitrogen utilization while lowering its leaching losses [115]. Water percolation and infiltration are significantly lower in clayey soils and represent a positive interaction between soil pores, plant roots, and microbes; thus, less nitrate leaching takes place [116,117]. Moreover, cutting-edge research elucidated the use of emergent technologies including isotopic tracing and molecular biology techniques to understand the flow pathways of leached nitrogen in deep soil horizons [117,118]. Flooding and waterlogging have also received increased attention in the past decade. Modern research has shown high NO3-N leaching in sandy soils owing to their low CEC [119]. Additionally, ongoing research using the modeling and remote sensing approaches is expected to provide a better understanding of the impact of flooding stress on nitrogen losses [120].
4. Adaptations of Plants to Flooding Stress
4.1. Morphological and Anatomical Adaptations
Plants exhibit different morphological and anatomical adaptations to counter the toxic effects of flooding stress (Figure 2). The photosynthesis and respiration rates of crops decrease owing to the low diffusion of O2 and CO2 in the plant body, except in rice, which develops ARs to adapt to anoxic stress. Conversely, other plants adopt specific morphological modifications to avoid a disrupted energy balance due to the low oxygen diffusion in the root respiratory system under flooding stress [12]. This modification includes AR development, elongation of the apical meristem, air film generation in the upper cuticle, and the creation of a barrier to radial oxygen loss (ROL) [121,122,123,124].
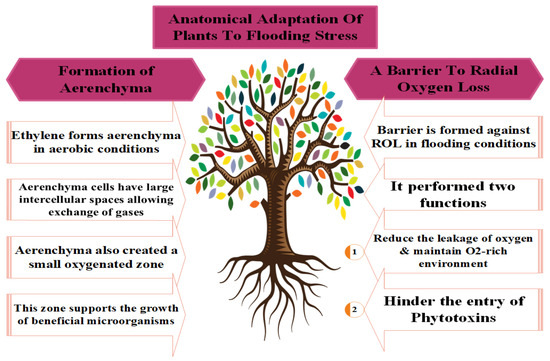
Figure 2.
Different adaptations used by plants to counter the toxic effects of flooding stress.
Amongst these, AR development was most commonly observed by [24]. Meanwhile, ref. [125] identified the locations (internodes of hypocotyl or at stem base) of ARs where the respiration and absorption of nutrients occur in plants during anoxic stress conditions [126]. Newly developed ARs have more aerenchyma cells, which increase O2 intake and diffusion [96]; thus, ARs successfully replace the primary root system under hypoxia conditions and maintain the plant’s normal growth, development, and metabolism [74].
The oxygen-deficient conditions resulting from flooding stress degrade the cortical cell, leading to programmed cell death and producing tissue cavities and aerenchyma cells responsible for root respiration under stress conditions [127]. Yamauchi et al. [122] also noted the continuous physiological metabolic processes of plants under waterlogged conditions. However, a significant amount of radial oxygen leakage (ROL) takes place in the intracellular spaces during oxygen transfer via aerenchyma cells in root respiration (Figure 2) [122]. Further, plants develop a specialized barrier to control ROL, which diverts the O2 molecules in and around the root tips [121].
Low oxygen escape syndrome (LOES) is another key adaptation of plants that occurs through an increased apical meristem during flooding stress. This enlargement of the tender stem allows the plants to escape from the anoxic conditions and acquire contact with air for optimum O2 intake [128]. Ethylene (ET) production under flooding conditions triggers gibberellic acid (GA) production, which enlarged the intermodal distance in rice [128]. Moreover, the formation of a gas film protects plants from the oxygen-deficient conditions that develop during flooding [129]. Pedersen et al. [130] observed that the net photosynthesis rate of waterlogged rice was only 20% after the removal of an artificial gas film. The gas film maintained the respiration and photosynthetic processes by promoting the O2 entry at night and CO2 during the day [128].
4.2. Photosynthetic Adaptation
Flooding stress causes the closure of stomatal conductance, increased stomatal resistance, and reduced CO2 intake, which affects plant photosynthesis [131]. Continuous flooding further reduces enzyme activity, chlorophyll synthesis, and leaf senescence and lowers overall crop productivity [132,133]. Moreover, the loss of pigments (a, b, and carotenoids) and their composition in leaves lowers the photosystem’s efficiency during flooding stress [134]. The expression of the RuBisCo enzyme is mainly controlled by the RuBisCo activase gene and is involved in photosynthesis and photorespiration; it was found to be downregulated in cotton under flooding stress [134]. Sucrose is one of the main products of photosynthesis and its translocation from source to sink was greatly reduced under flooding stress [134]. All these negative changes reduce crop growth and development. Plants undergo a metabolic adaptation involving the secretion of the sucrose synthase enzyme responsible for sucrose breakdown, which is necessary for cellulose biosynthesis to cope with flooding stress [135].
4.3. Respiratory Adaptations
Low oxygen availability during flooding stress causes a significant decline in root respiration [136]. The amount of dissolved O2 in waterlogged soil was observed to be less than 0.05 mmol/m3 compared to the oxygen available (0.23 mol/m3) in cultivated soil [137]. Oxygen (O2) serves as an electron acceptor in the electron transport chain (ETC), and a decrease in the O2 amount reduces ATP generation and leads to a decline in mitochondrial respiration [19,137]. In this context, the glycolysis and ethanol fermentation processes can be alternated as energy sources to ensure continued plant functioning under high water stress [138]. However, the amount of energy produced through the above-mentioned processes is quite low, as only 2 mol of ATP is produced, compared to 36 mol ATP production via the tricarboxylic acid (TCA) cycle [138]. Thus, plants need to amplify the adopted processes during flooding stress to meet the energy demand for normal functioning [12].
Pyruvate fermentation is used as an alternate energy source by plants through lactate dehydrogenase (LDH) or pyruvate decarboxylase (PDC), which convert pyruvate into acetaldehyde to reduce ethanol via the alcoholic dehydrogenase (ADH) enzyme [139,140,141]. Borella et al. [142] also noticed the upregulation of ADH and PDC as an alternate source of energy used for respiration in waterlogging-tolerant crops during stress conditions. Further, many researchers have described a similar mechanism in water-logging-tolerant genotypes of cucumber, cotton, and soybean, allowing them to respire under temporary flooding stress [143,144]. Tougou et al. [145] observed the upregulation of the GmADH2 gene in transgenic soybean cultivars during seed germination under flooding stress conditions. Increased flooding stress tolerance was observed in transgenic Arabidopsis with the overexpression of the kiwifruit PDC1 gene [146]. All these results support the notion of adaptive mechanisms using PDC- and ADH-based genes during flooding stress [12].
PDC’s efficiency to resist flooding stress increased with the production of lactate dehydrogenase (LDH) in Arabidopsis [147]. Further, a reduction in or the absence of LDH promoted the opposite phenotype in Arabidopsis during flooding [147]. The transcript abundances of the ethanol dehydrogenase genes ADH1-1, ADH1-2, and ADH1-3, and the PDC genes PDC1 and PDC2, were downregulated in Petunia plants in which an ET-responsive element-binding factor, PhERF2, was silenced, whereas they were upregulated in PhERF2-overexpressing plants. In contrast, the expression of the LDH gene was upregulated in PhERF2-silenced lines and downregulated in PhERF2-overexpressing lines. This result suggests that the main pathway for NAD+ regeneration in PhERF-overexpressing plants is ethanol fermentation, whereas PhERF2-silenced plants might rely on lactic acid fermentation in response to waterlogging stress [148]. However, glycolysis and ethanol fermentation were observed as a temporary source of energy for root respiration during short-term flooding stress [94]. Meanwhile, alcohol, aldehyde, lactic acid, and anaerobic metabolite production caused cell death during long-term flooding stress [12,94].
4.4. Activation of Antioxidant Defense Systems and Osmolyte Accumulation
Flooding stress induces ROS production, which disrupts ionic homeostasis, membranes, DNA, proteins, and lipids [13,149]. However, plants can overcome flooding by activating antioxidant defense systems and increasing the accumulation of potential osmolytes [150]. Plants activate superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and ascorbate peroxidase (APX), which scavenge the flooding-induced ROS production, thus ensuring plants’ survival under flooding stress [151]. Besides this, plants also activate glutathione (GSH), ascorbic acid (AsA), carotenoids, and tocopherols, which protect membranes and the plant photosynthetic apparatus by scavenging ROS production. Moreover, plants accumulate proline, sugars, soluble proteins, and free amino acids to counter the toxic effects of flooding stress [152].
4.5. Genetic Adaptation to Flood Stress
Plants activate different genes and proteins to counter the toxic effects of flooding stress [27]. For instance, plants downregulate photosynthesis-related genes and light-harvesting chlorophyll protein complex genes in response to flooding stress [29]. In a study, Borrego-Benjumea et al. [28] noted the participation of different genes involved in metabolic pathways (glucose and nitrogen metabolism) at the root level in barley. These authors also found that the downregulation of these genes was involved in ROS detoxification and nitrogen and amino acid metabolism. In another study, Tong et al. [153] found an increase in respiratory burst oxidase homolog (RBOH) expression, which regulates the accumulation of ROS under flooding stress. These authors also found different quantitative trait loci (QTL) for flooding tolerance features linked with the root biomass, chlorophyll content, and germination index.
5. Role of Phytohormones against Flooding Stress
Phytohormones regulate plant functioning to maintain homeostasis and are responsible for normal growth, development, and physiological metabolism under stress (Figure 3) [154,155,156]. The signaling mechanisms created by phytohormones increase the flooding stress tolerance in plants [157,158,159]. The roles of different hormones in inducing flooding tolerance in plants are described below.
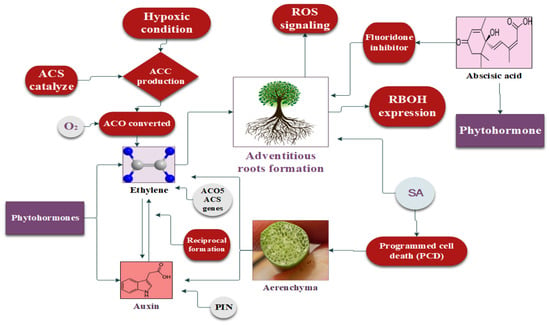
Figure 3.
Regulatory mechanisms of phytohormones including ethylene, auxin, and abscisic acid in plant response to flooding stress.
5.1. Ethylene
Ethylene works as a gaseous hormone in plants, and its diffusion rate is significantly reduced during flooding stress [160]. However, plants have established an adaptive mechanism to accumulate more ethylene to respond to flooding stress. This accumulation is facilitated by the production of 1-aminocyclopropane-1-carboxylic acid (ACC), which serves as the precursor of ethylene production [161]. Under hypoxic conditions, ACC synthase (ACS) catalyzes large quantities of ACC, whereas ACC oxidase (ACO) converts ACC into ethylene in the presence of a small amount of oxygen (O2) [162]. Therefore, the continuous transfer of ACC is necessary, from the hypoxic root system to the lower part of the plant’s aerobic region, for a continuous oxidation reaction to produce ethylene [163]. Asgher [164] reported the activation of the ACO5 and ACS genes during waterlogging in Arabidopsis, resulting in ethylene biosynthesis taking place. AR development is based on the synthesis of ethylene under flooding stress [163]. In [122], the authors observed the expression of the ACO5 and ACS genes in rice developing ARs and aerenchyma under flooding conditions [165]. Sasidharan and Voesenek [166] reported that ethylene (ET) caused cell death during lysogenic aerenchyma, whereas lysosomal aerenchyma cells developed in wheat, maize, and rice during ET accumulation [122,167]. Moreover, ethylene enhances the biosynthesis of IAA in plants facing flooding stress, which further increases the plant’s vegetative growth, which is responsible for its tolerance of and escape from high-water-stress situations [168].
5.2. Gibberellin
Gibberellin (GAs) is essential to control the cell size and number; thus, it plays an important role in the secondary growth and development of plants [169]. Under flooding stress, the GA concentration was found to be increased significantly in waterlogging-tolerant cultivars of soybean compared to stress-sensitive genotypes [170]. Furthermore, the foliar application of GA on peanuts (Arachis hypogaea) under flooding stress improved physiological functioning, vegetative and reproductive growth, yields, and root biomass [171]. Similarly, Hong et al. [172] noticed a significant increase in flood resistance due to a decrease in d malondialdehyde (MDA) content in the leaves and roots of rape plants via the exogenous application of GA. Interestingly, GA biosynthesis inhibitors significantly reduced the intermodal elongation in Oryza sativa (L.) under flooding conditions [124]. Furthermore, Zhang et al. [173] identified a mutation in the signal transduction gene (OsGID1, OsGID2, OsSPY, OsSEC, OsGAMYB). They also found that GA biosynthesis (Os1, OsCPS2, OsKS2, OsKS5, OsKO2, OsKAO, Os13ox, OsGA20ox1, OsGA20ox2, OsGA20ox3, OsGA3ox1, OsGA3ox2) genes are responsible for lowering the intermodal distance in rice, whereas the foliar application of GA augments stem elongation in rice crops during flooding [12]. The upregulation of GA and its involvement in the SK1/2 gene-mediated pathway enhanced the plant height under flooding stress by stem elongation [124,174]. ET-responsive transcription factor (OsEIL1a) activated the SD1 gene, and the SD1 protein stimulated GA (GA4) synthesis, which further enhanced rice stalk growth [163].
5.3. Abscisic Acid
Abscisic acid (ABA) controls stomatal opening by regulating the size of guard cells in plants [173]. ABA helps to initiate the root aerenchyma cells; therefore, it is recognized as an important phytohormone to respond to flooding stress [175]. Flooding stress decreased the ABA proportion in soybeans by 50% compared to normal conditions [176]. The foliar application of 1 µM ABA decreased the cell development of aerenchyma, suggesting that the development of secondary aerenchyma cells is necessary to reduce he negative regulation of ABA [163]. Moreover, the downregulation of ABA content and adventitious root primordia decreased significantly in Solanum dulcamara under flooding stress [177]. Ethylene (ET) built up in the lower portion of the plant stem in waterlogged conditions [56]. As a result, the levels of abscisic acid (ABA) in the adventitious root (AR) primordia as well as the stem primordia decreased significantly [177]. Further, the foliar application of 1 mM ABA reduced AR production during flooding [178]. Conversely, the application of 100 µM of an ABA inhibitor (fluoridone) promoted AR production [179], indicating a negative correlation between ET and ABA in flooding stress conditions. Kim [180] observed a significant decrease in ABA content in soybean after flooding stress, whereas the ABA content was low in waterlogging-resistant cultivars, showing a negative relation between ABA and flooding stress. Meanwhile, significant stem elongation in rice subjected to flooding was observed, with low ABA content, which might have been due to the high ET and GA production under stress [181]. Likewise, ET, along with its precursor (ACC) and inhibitor (1-MCP), was activated simultaneously to control the expression of OsABA8ox1; therefore, ABA was lowered in rice during flooding [182,183].
For kiwifruit (Actinidia deliciosa), the expression of the AdPDC1 gene was upregulated to encode pyruvate decarboxylase in flooding conditions. This highlights the pivotal role of AdPDC1 in mitigating flooding stress [184,185]. In contrast, a decreased root length and seed germination were noticed due to the overexpression of AdPDC1 in Arabidopsis under ABA treatment, showing negative regulation with AdPDC1 in flooding stress [146]. However, ABA accumulates in the aerial parts of the plant during flooding stress and causes stomatal closure under H2O2 production; thus, water transpiration is significantly reduced under flooding stress [186,187].
5.4. Auxin
Auxin (IAA) is one of the most important growth-promoting phytohormones. The production of ethylene (ET) in waterlogging facilitates the transportation of IAA, and, reciprocally, the accumulated auxin stimulates ethylene (ET) biosynthesis [188]. This reciprocal relationship further promotes the transport of IAA to the flooded regions of the plant, where cell division and AR development take place [189]. In a study, AR growth was inhibited by the exogenous application of an auxin transport inhibitor, 1-naphthylphthalamic acid (NPA), on tobacco, cucumber, and tomato plants under flooding stress [190]. Auxin polar transport carrier protein (PIN-FORMED) is responsible for the transportation of auxin within plants. Treatment with NPA reduced the expression of OsPIN2 and AR development by inactivating the PIN protein in rice [191]. Similarly, mutant S. dulcamara lacks PIN expression and, therefore, the transport of auxin was blocked, which caused a significant decrease in the development of adventitious roots (ARs), providing further evidence for the requirement of auxin transport in AR formation [192].
However, certain studies have reported contrasting findings regarding a decrease in IAA levels during stress. For instance, Shimamura [193] observed the development of aerenchyma and ARs after 72 h of waterlogging. Interestingly, physiological tests revealed no noteworthy change in IAA’s endogenous concentration in the hypocotyls compared to controls [194]. This outcome indicates that the presence of accumulated indole-3-acetic acid (IAA) is not a requirement for secondary aerenchyma development in soybean hypocotyls during flooding stress. Moreover, flooding stress can lead to a serious energy deficit by depleting carbohydrates stored in plants. Further, Qi [195] observed a relationship between auxin and sugars, which helped in the commencement and elongation of adventitious roots (ARs) in cucumber during flooding stress. Moreover, photosynthesis supports sugar synthesis in flooding stress by providing energy during the day, which enhances the transportation of auxin and ultimately leads to AR development [196].
5.5. Melatonin
Melatonin (MT) is a plant hormone and acts as an antioxidant that enhances plant growth under stress conditions [197,198,199]. MT is well known for its regulatory effects on plants in abiotic stress conditions [196], although few studies have focused on the effect of MT under flooding stress [200]. Fujita and Hasanuzzaman [201] observed an increase in flooding tolerance in plants owing to an increase in the activity of antioxidant enzymes after MT application. Likewise, MT application enhanced the flooding tolerance in apple seedlings [202]. MT enhanced antioxidant enzyme activity, aerobic respiration, and photosystem II efficiency and prevented chlorosis induced by ROS and MDA content in plants under flooding stress [203,204]. The exogenous application of melatonin upregulated the MT biosynthesis genes, such as MbT5H1, MbAANAT3, and MbASMT9 [205]. A significant increase in the previously mentioned genes’ appearance was noticed in MT-treated seedlings, suggesting the crucial role of melatonin towards flooding stress tolerance [171,206]. Flooding stress negatively impacted photosynthetic ability and increased electrolyte leakage and MDA content in Alfalfa, whereas MT application (100 µM) enhanced stress tolerance in 6-week-old seedlings [207]. The exogenous application of MT not only improved the physiological and biochemical functioning but also elevated the levels of endogenic MT in stressed plants [208,209].
The decreased expression of ET and signaling genes like ACS, ACO, ERF (Table 2) was noticed in MT-treated seedlings [209]. This suggests a negative correlation between MT and ET production; however, no comprehensive results describe the antagonistic crosstalk concerning MT and ET [163]. Gu et al. [204] reported improved anti-oxidative activity to suppress H2O2 and lipid peroxidation with 200 µM MT application, which regulated the aerenchyma cells necessary for the respiration of P. persica during flooding stress. This was due to the induced mRNA concentration of Ca2+ signaling and hypoxia-related ERF VII transcription factor genes [204]. Thus, MT application is suggested to regulate ET homeostasis and flooding stress tolerance [204].
5.6. Brassinosteroids
Brassinosteroids (BRs) are naturally produced steroids that improve plants’ resistance to abiotic and biological stresses and promote growth and development [210]. The application of exogenous 24-epi-brassinolide (EBR) facilitated carbohydrate transfer from the leaves to roots in cucumber under hypoxic stress [211]. This process was accompanied by the activation of glycolytic enzymes in the roots, along with antioxidant enzymes, which led to a reduction in ROS production. BR facilitates the loosening and development of the cucumber hypocotyl and promotes the formation of adventitious roots [212]. This process ultimately improved the oxygen availability within the plant body, thereby enhancing its tolerance to hypoxic stress. An exogenous BR treatment activated the Sub1A gene’s (ethylene-response factor (ERF-VII family)) expression, which increased shoot elongation and biosynthetic genes in rice under flooding conditions [213]. In comparison to low oxygen escape syndrome (LOES), the Sub1A rice genotype exhibited BR biosynthesis genes with higher expression, leading to a rise in endogenic BR levels [214]. This elevated BR level stimulated the expression of the GA catabolism gene (GA2ox7), consequently reducing the concentration of gibberellins (GAs) [215]. Simultaneously, GA-mediated responses under submerged conditions were negatively regulated by the GA signal inhibitory factor (SLR1) protein, a member of the DELLA family. As a result, the rice plants’ growth was preserved. Therefore, in the Sub1A rice genotype, BR acted to restrict shoot extension by inhibiting GA biosynthesis and reducing the effect of GA action [216].

Table 2.
Recently discovered genes and relative phytohormones involved in plant responses to flooding stress.
Table 2.
Recently discovered genes and relative phytohormones involved in plant responses to flooding stress.
| Plant Species | Flooding Type | Gene Name | Function | Reference |
|---|---|---|---|---|
| Arabidopsis | Waterlogging | LSD1, EDS1, and PAD4 | These genes control the formation of Lysigenous aerenchyma by regulating the generation of ethylene and ROS. | [217] |
| Rice | Waterlogging | CIPK15 and SnRK1A | CIPK15 encodes a calcineurin B-like (CBL)-interacting protein kinase that positively regulates the expression of Snf1-related protein kinase 1 (SnRK1A) and functions in rice acclimation to flooding stress by affecting sugar and energy production. | [68] |
| Maize | Waterlogging | Subtol6 | Subtol6 is a major QTL that explains 22% of the phenotypic differences in submergence tolerance within the recombinant inbred lines. | [218] |
| Wheat | Waterlogging | TaERFVII.1 | TaERFVII.1 belongs to the ERF-VII family and functions in the waterlogging tolerance of wheat. The overexpression of TaERFVII.1 increased the survival rate under waterlogging stress | [219] |
| Barley | Waterlogging | HvERF2.11 | The expression of HvERF2.11 can be induced by waterlogging and mediate the waterlogging tolerance of plants through improving some antioxidants’ and ADH enzymes’ activity. | [220] |
| Actinidia deliciosa | Waterlogging | AdPDC1 | AdPDC1 encodes a pyruvate decarboxylase that catalyzes the first step in the ethanolic fermentation pathway, and it may function in kiwifruit’s acclimation to waterlogging stress. | [146] |
| Cucumber | Waterlogging | CsARN6.1 | CsARN6.1 encodes an AAA ATPase; transgenic lines of CsARN6.1 showed increased numbers of ARs by enhancing ATPase activity and further affected waterlogging tolerance. | [126] |
| Mentha arvensis | Waterlogging | MaRAP2-4 | MaRAP2-4 from Mentha arvensis encodes an ERF-I type transcription factor; overexpression of MaRAP2-4 in Arabidopsis enhanced its tolerance to waterlogging and oxidative stress. | [221] |
| Petunia | Waterlogging | PhERF2 | PhERF2 regulates the process of programmed cell death and alcoholic fermentation, which enhances waterlogging tolerance. | [148] |
| Chrysanthemum morifolium | Waterlogging | CmSOS1 | SOS1 encodes a Na+/H+ antiporter and may interact with CmRCD1 to mediate plant tolerance to waterlogging stress. | [222] |
6. Conclusions and Future Prospects
Plants experience a variety of stressful situations during flooding, which depends on the depth and duration of the water. The adaptive characteristics of plants allow them to survive under flooding conditions. These strategies include oxygenating submerged tissues (the positioning of leaves above water to continue carbon fixation; aerenchyma production; and the production of adventitious roots, which act as barriers to prevent radial oxygen loss). To maintain root aeration and prolong water absorption in anaerobic soils, aerenchyma production and the growth of adventitious roots with barriers to radial oxygen loss appear to be the most crucial traits supporting longitudinal oxygen transport. To capture oxygen and continue photosynthesis, a higher proportion of leaves must surpass the water’s surface, which is determined by the length and reorientation of the shoots towards a vertical position. In conditions of low atmospheric evaporative demand, the maintenance of stomata conductance ensures the uptake of CO2 for carbon fixation. Meanwhile, in conditions with a high atmospheric evaporative demand, stomata closure can be helpful in controlling plant water homeostasis, which depends on the equilibrium between water losses by transpiration and water uptake by roots. Future research should combine several flooding regimes to examine how plants react to waterlogging and submersion. This would help us to comprehend the advantages and disadvantages of various combinations of characteristics that confer tolerance in circumstances of fluctuating flooding. Therefore, a better understanding of how plants respond to excess water, in the context of increased flooding in the future, would support breeding programs and lead to better management choices for the cultivation of crops and forage species in flood-prone lands.
Flooding stress is becoming more prevalent as a result of continuously changing climatic conditions, which cause significant damage to plants. These challenges will become more serious in the future due to the increased intensity and frequency of severe weather incidents. Some of the key future challenges for plants related to flooding stress are the following. Plants could become submerged for a long period, which will ultimately lead to a shortage of oxygen and nutrients, resulting in substantial yield reductions. Moreover, precipitation patterns will also be substantially disrupted in the coming years due to climate change. As a result, unexpected rainfall events followed by prolonged dry spells could exacerbate flooding stress for plants, making it difficult for them to cope with changing water levels. Flooding events can cause soil erosion and sediment deposition, which can smother plants and disrupt their root systems. The loss of fertile topsoil and the buildup of toxic substances can further challenge plant growth and survival. Flooding stress can severely impact agricultural productivity, leading to reduced crop yields and food shortages. Addressing these challenges will require a combination of efforts, including research on flood-tolerant plant varieties, sustainable land management practices, ecosystem restoration, and policies aimed at mitigating climate change. By adopting these approaches, we can overcome the more prevailing conditions of flooding stress in the changing environmental conditions.
Author Contributions
Conceptualization, A.A. and A.M. Writing—original draft preparation, A.A. and A.M. Writing—review and editing, H.U.-R., C.L., X.L., J.S., S.N., M.M., M.A. and M.U.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Guangxi Key R&D program (Guike AB21220044) and Guangxi Key R&D program (Guike AB22035057), the Beijing River Water Conservancy Development Foundation (Water Conservancy Youth Talent Development Support Project), and the Guangxi Key Laboratory of Water Engineering Materials and Structures Fund Program.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for supporting this work through large groups under grant number RGP2/227/44.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arnell, N.W.; Lloyd-Hughes, B. The global-scale impacts of climate change on water resources and flooding under new climate and socio-economic scenarios. Clim. Chang. 2014, 122, 127–140. [Google Scholar] [CrossRef]
- Bibi, F.; Rahman, A. An Overview of Climate Change Impacts on Agriculture and their mitigation strategies. Agriculture 2023, 13, 1508. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Yan, H.; Liu, D.L.; Meinke, H.; Hoogenboom, G.; Wang, B.; Peng, B.; Guan, K.; Jaegermeyr, J. Silver lining to a climate crisis in multiple prospects for alleviating crop waterlogging under future climates. Nat. Commun. 2023, 14, 765. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, F.; Meng, Y.; Chandrasekaran, U.; Luo, X.; Yang, W.; Shu, K. Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiol. Biochem. 2020, 148, 228–236. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, G.; Motavalli, P.P.; Nelson, K.A.; Orlowski, J.M.; Golden, B.R. Impacts and management strategies for crop production in waterlogged or flooded soils: A review. Agron. J. 2020, 112, 1475–1501. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Dong, H.; Li, C. Waterlogging stress in cotton: Damage, adaptability, alleviation strategies, and mechanisms. Crop J. 2021, 9, 257–270. [Google Scholar] [CrossRef]
- Rohilla, M.; Singh, N.; Mazumder, A.; Sen, P.; Roy, P.; Chowdhury, D.; Singh, N.K.; Mondal, T.K. Genome-wide association studies using 50 K rice genic SNP chip unveil genetic architecture for anaerobic germination of deep-water rice population of Assam, India. Mol. Genet. Genom. 2020, 295, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Loreti, E.; Perata, P. The many facets of hypoxia in plants. Plants 2020, 9, 745. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Mottaleb, K.A.; Khanal, A.R.; Mohanty, S. Abiotic stress and its impact on production efficiency: The case of rice farming in Bangladesh. Agro-Ecosyst. 2015, 199, 146–153. [Google Scholar] [CrossRef]
- Anderson, R.; Bayer, P.E.; Edwards, D. Climate change and the need for agricultural adaptation. Curr. Opin. Plant Biol. 2020, 56, 197–202. [Google Scholar] [CrossRef]
- Shahzad, A.; Ullah, S.; Dar, A.A.; Sardar, M.F.; Mehmood, T.; Tufail, M.A.; Shakoor, A.; Haris, M. Nexus on climate change: Agriculture and possible solution to cope future climate change stresses. Environ. Sci. Pollut. Res. 2021, 28, 14211–14232. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of waterlogging tolerance in plants: Research progress and prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Jiang, D.; Liu, F.; Dai, T.; Jing, Q.; Cao, W. Effects of salt and waterlogging stresses and their combination on leaf photosynthesis, chloroplast ATP synthesis, and antioxidant capacity in wheat. Plant Sci. 2009, 176, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Pampana, S.; Masoni, A.; Arduini, I. Grain yield of durum wheat as affected by waterlogging at tillering. Cereal Res. Commun. 2016, 44, 706–716. [Google Scholar] [CrossRef]
- Lal, M.; Kumari, A.; Pooja; Sheokand, S. Reactive oxygen species, reactive nitrogen species and oxidative metabolism under waterlogging stress. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Wiley: Hoboken, NJ, USA, 2019; pp. 777–812. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Al Mahmud, J.; Nahar, K.; Anee, T.I.; Inafuku, M.; Oku, H.; Fujita, M. Responses, adaptation, and ROS metabolism in plants exposed to waterlogging stress. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Springer: Berlin/Heidelberg, Germany, 2017; pp. 257–281. [Google Scholar]
- Jia, W.; Ma, M.; Chen, J.; Wu, S. Plant morphological, physiological and anatomical adaption to flooding stress and the underlying molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 1088. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef]
- Jackson, M.; Colmer, T. Response and adaptation by plants to flooding stress. Ann. Bot. 2005, 96, 501–505. [Google Scholar] [CrossRef]
- Kaur, G.; Vikal, Y.; Kaur, L.; Kalia, A.; Mittal, A.; Kaur, D.; Yadav, I. Elucidating the morpho-physiological adaptations and molecular responses under long-term waterlogging stress in maize through gene expression analysis. Plant Sci. 2021, 304, 110823. [Google Scholar] [CrossRef]
- Ayi, Q.; Zeng, B.; Liu, J.; Li, S.; van Bodegom, P.M.; Cornelissen, J.H. Oxygen absorption by adventitious roots promotes the survival of completely submerged terrestrial plants. Ann. Bot. 2016, 118, 675–683. [Google Scholar] [CrossRef]
- Steffens, B.; Steffen-Heins, A.; Sauter, M. Reactive oxygen species mediate growth and death in submerged plants. Front. Plant Sci. 2013, 4, 179. [Google Scholar] [CrossRef] [PubMed]
- Steffens, B.; Rasmussen, A. The physiology of adventitious roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Pais, I.P.; Moreira, R.; Semedo, J.N.; Reboredo, F.H.; Lidon, F.C.; Coutinho, J.; Maçãs, B.; Scotti-Campos, P. Phenotypic Diversity of Seminal Root Traits in Bread Wheat Germplasm from Different Origins. Plants 2022, 11, 2842. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Zhang, J.; Li, X.; Fan, X.; Dong, S.; Liu, P.; Zhao, B. Effects of waterlogging on the yield and growth of summer maize under field conditions. Can. J. Plant Sci. 2014, 94, 23–31. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of plant responses to water stress and related genes: A review. Forests 2022, 13, 324. [Google Scholar] [CrossRef]
- Borrego-Benjumea, A.; Carter, A.; Tucker, J.R.; Yao, Z.; Xu, W.; Badea, A. Genome-wide analysis of gene expression provides new insights into waterlogging responses in barley (Hordeum vulgare L.). Plants 2020, 9, 240. [Google Scholar] [CrossRef]
- Wei, M.; Li, X.; Yang, R.; Li, L.; Wang, Z.; Wang, X.; Sha, A. Novel insights into genetic responses for waterlogging stress in two local wheat cultivars in Yangtze river basin. Front. Genet. 2021, 12, 681680. [Google Scholar] [CrossRef]
- Kundzewicz, Z.W.; Krysanova, V.; Benestad, R.; Hov, Ø.; Piniewski, M.; Otto, I.M. Uncertainty in climate change impacts on water resources. Environ. Sci. Policy 2018, 79, 1–8. [Google Scholar] [CrossRef]
- Setter, T.; Waters, I. Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 2003, 253, 1–34. [Google Scholar] [CrossRef]
- Westra, S.; Fowler, H.J.; Evans, J.P.; Alexander, L.V.; Berg, P.; Johnson, F.; Kendon, E.J.; Lenderink, G.; Roberts, N. Future changes to the intensity and frequency of short-duration extreme rainfall. Rev. Geophys. 2014, 52, 522–555. [Google Scholar] [CrossRef]
- Zargar, U.; Chishti, M.; Ahmad, F.; Rather, M. Does alteration in biodiversity really affect disease outcome?—A debate is brewing. Saudi J. Biol. Sci. 2015, 22, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Pal, S.C.; Chakrabortty, R.; Chowdhuri, I.; Saha, A.; Shit, M. Effects of climate change and sea-level rise on coastal habitat: Vulnerability assessment, adaptation strategies and policy recommendations. J. Environ. Manag. 2023, 330, 117187. [Google Scholar] [CrossRef] [PubMed]
- Milly, P.C.D.; Wetherald, R.T.; Dunne, K.; Delworth, T.L. Increasing risk of great floods in a changing climate. Nature 2002, 415, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Shabala, L.; Barcelo, J.; Poschenrieder, C. Membrane transporters mediating root signalling and adaptive responses to oxygen deprivation and soil flooding. Plant Cell Environ. 2014, 37, 2216–2233. [Google Scholar] [CrossRef]
- Vafaei, A.H.; Kia, M.R.G.; Gougerdchi, V.; Dehghanian, Z.; Lajayer, B.A.; Aftab, T.; Astatkie, T. Insight on abiotic stress management in plants by improving plant nutritional status. In Sustainable Plant Nutrition; Academic Press: Cambridge, MA, USA, 2023; pp. 381–402. [Google Scholar]
- Tang, J.; Xie, Y.; Wu, Y.; Liu, G. Influence of precipitation change and topography characteristics on the development of farmland gully in the black soil region of northeast China. Catena 2023, 224, 106999. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, W.; Cao, W.; Jiao, J.; Yin, Z.; Xu, H. Response of erosion reduction effect of typical soil and water conservation measures in cropland to rainfall and slope gradient changes and their applicable range in the Chinese Mollisols Region, Northeast China. Int. Soil Water Conserv. Res. 2023, 11, 251–262. [Google Scholar] [CrossRef]
- Smith, M.D.; Sikka, A.; Dirwai, T.L.; Mabhaudhi, T. Research and innovation in agricultural water management for a water-secure world. Irrig. Drain. 2023. [Google Scholar] [CrossRef]
- Batey, T. Soil compaction and soil management—A review. Soil Use Manag. 2009, 25, 335–345. [Google Scholar] [CrossRef]
- Blann, K.L.; Anderson, J.L.; Sands, G.R.; Vondracek, B. Effects of agricultural drainage on aquatic ecosystems: A review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 909–1001. [Google Scholar] [CrossRef]
- Kumar, M.; Sharif, M.; Ahmed, S. Flood risk management strategies for national capital territory of Delhi, India. ISH J. Hydraul. Eng. 2019, 25, 248–259. [Google Scholar] [CrossRef]
- Gürsoy, S. Soil compaction due to increased machinery intensity in agricultural production: Its main causes, effects and management. In Technology in Agriculture; Intech Open: London, UK, 2021; pp. 1–18. [Google Scholar]
- Blessitt, J.B. Productivity of Raised Seedbeds for Soybean [Glycine max. (L.) Merr.] Production on Clayey Soils of the Mississippi Delta. Master’s Thesis, Mississippi State University, Mississippi State, MS, USA, 2007. [Google Scholar]
- Han, F.; Kingery, W.; Hargreaves, J.; Walker, T. Effects of land uses on solid-phase distribution of micronutrients in selected vertisols of the Mississippi River Delta. Geoderma 2007, 142, 96–103. [Google Scholar] [CrossRef]
- Deák, J.; Gebhardt, A.; Lewis, H.; Usai, M.R.; Lee, H. Soils disturbed by vegetation clearance and tillage. In Archaeological Soil and Sediment Micromorphology; Wiley: Hoboken, NJ, USA, 2017; pp. 231–264. [Google Scholar]
- Jiang, P.; Anderson, S.; Kitchen, N.; Sadler, E.; Sudduth, K. Landscape and conservation management effects on hydraulic properties of a claypan-soil toposequence. Soil Sci. Soc. Am. J. 2007, 71, 803–811. [Google Scholar] [CrossRef]
- Jamison, V.; Peters, D. Slope length of claypan soil affects runoff. Water Resour. Res. 1967, 3, 471–480. [Google Scholar] [CrossRef]
- Motavalli, P.; Anderson, S.; Pengthamkeerati, P. Surface compaction and poultry litter effects on corn growth, nitrogen availability, and physical properties of a claypan soil. Field Crops Res. 2003, 84, 303–318. [Google Scholar] [CrossRef]
- Barnett, T.P.; Adam, J.C.; Lettenmaier, D.P. Potential impacts of a warming climate on water availability in snow-dominated regions. Nature 2005, 438, 303–309. [Google Scholar] [CrossRef]
- Green, T.R.; Taniguchi, M.; Kooi, H.; Gurdak, J.J.; Allen, D.M.; Hiscock, K.M.; Treidel, H.; Aureli, A. Beneath the surface of global change: Impacts of climate change on groundwater. J. Hydrol. 2011, 405, 532–560. [Google Scholar] [CrossRef]
- Jafari, T.; Kiem, A.S.; Javadi, S.; Nakamura, T.; Nishida, K. Using insights from water isotopes to improve simulation of surface water-groundwater interactions. Sci. Total Environ. 2021, 798, 149253. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Shabala, S.; Meinke, H.; Ahmed, I.; Zhang, Y.; Tian, X.; Zhou, M. The state of the art in modeling waterlogging impacts on plants: What do we know and what do we need to know. Earth’s Future 2020, 8, e2020EF001801. [Google Scholar] [CrossRef]
- Herzog, M.; Striker, G.G.; Colmer, T.D.; Pedersen, O. Mechanisms of waterlogging tolerance in wheat—A review of root and shoot physiology. Plant Cell Environ. 2016, 39, 1068–1086. [Google Scholar] [CrossRef]
- Shabala, S. Physiological and cellular aspects of phytotoxicity tolerance in plants: The role of membrane transporters and implications for crop breeding for waterlogging tolerance. New Phytol. 2011, 190, 289–298. [Google Scholar] [CrossRef]
- Khabaz-Saberi, H.; Setter, T.; Waters, I. Waterlogging induces high to toxic concentrations of iron, aluminum, and manganese in wheat varieties on acidic soil. J. Plant Nutr. 2006, 29, 899–911. [Google Scholar] [CrossRef]
- Khabaz-Saberi, H.; Barker, S.; Rengel, Z. Tolerance to ion toxicities enhances wheat (Triticum aestivum L.) grain yield in waterlogged acidic soils. Plant Soil 2012, 354, 371–381. [Google Scholar] [CrossRef]
- Zeng, F.; Shabala, L.; Zhou, M.; Zhang, G.; Shabala, S. Barley responses to combined waterlogging and salinity stress: Separating effects of oxygen deprivation and elemental toxicity. Front. Plant Sci. 2013, 4, 313. [Google Scholar] [CrossRef] [PubMed]
- Dirk, L.M.; Kumar, S.; Majee, M.; Downie, A.B. PHYTOCHROME INTERACTING FACTOR1 interactions leading to the completion or prolongation of seed germination. Plant Signal. Behav. 2018, 13, e1525999. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Shuai, H.; Luo, X.; Chen, F.; Zhou, W.; Yang, W.; Shu, K. Karrikins: Regulators involved in phytohormone signaling networks during seed germination and seedling development. Front. Plant Sci. 2017, 7, 2021. [Google Scholar] [CrossRef]
- Shu, K.; Liu, X.-d.; Xie, Q.; He, Z.-h. Two faces of one seed: Hormonal regulation of dormancy and germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef]
- Shu, K.; Qi, Y.; Chen, F.; Meng, Y.; Luo, X.; Shuai, H.; Zhou, W.; Ding, J.; Du, J.; Liu, J. Salt stress represses soybean seed germination by negatively regulating GA biosynthesis while positively mediating ABA biosynthesis. Front. Plant Sci. 2017, 8, 1372. [Google Scholar] [CrossRef]
- Christianson, J.A.; Wilson, I.W.; Llewellyn, D.J.; Dennis, E.S. The low-oxygen-induced NAC domain transcription factor ANAC102 affects viability of Arabidopsis seeds following low-oxygen treatment. Plant Physiol. 2009, 149, 1724–1738. [Google Scholar] [CrossRef]
- Park, M.R.; Hasenstein, K.H. Oxygen dependency of germinating Brassica seeds. Life Sci. Space Res. 2016, 8, 30–37. [Google Scholar] [CrossRef]
- Loreti, E.; Alpi, A.; Perata, P. α-Amylase expression under anoxia in rice seedlings: An update. Russ. J. Plant Physiol. 2003, 50, 737–743. [Google Scholar] [CrossRef]
- Zaman, M.; Malik, A.; Kaur, P.; Erskine, W. Waterlogging tolerance of pea at germination. J. Agron. Crop Sci. 2018, 204, 155–164. [Google Scholar] [CrossRef]
- Park, M.; Yim, H.-K.; Park, H.-G.; Lim, J.; Kim, S.-H.; Hwang, Y.-S. Interference with oxidative phosphorylation enhances anoxic expression of rice α-amylase genes through abolishing sugar regulation. JXB 2010, 61, 3235–3244. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-W.; Chen, P.-W.; Lu, C.-A.; Chen, S.; Ho, T.-H.D.; Yu, S.-M. Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding. Sci. Signal. 2009, 2, ra61. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, T.; Pelayo, M.A.F.; Trijatmiko, K.R.; Gabunada, L.F.M.; Alam, R.; Jimenez, R.; Mendioro, M.S.; Slamet-Loedin, I.H.; Sreenivasulu, N.; Bailey-Serres, J. A trehalose-6-phosphate phosphatase enhances anaerobic germination tolerance in rice. Nat. Plants 2015, 1, 15124. [Google Scholar] [CrossRef] [PubMed]
- Perata, P.; Pozueta-Romero, J.; Akazawa, T.; Yamaguchi, J. Effect of anoxia on starch breakdown in rice and wheat seeds. Planta 1992, 188, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Wang, W.; Peng, S.; Nie, L. Seed pelleting with calcium peroxide improves crop establishment of direct-seeded rice under waterlogging conditions. Sci. Rep. 2017, 7, 4878. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, F.; Zhao, S.; Yang, C.; Meng, Y.; Shuai, H.; Luo, X.; Dai, Y.; Yin, H.; Du, J. DA-6 promotes germination and seedling establishment from aged soybean seeds by mediating fatty acid metabolism and glycometabolism. JXB 2019, 70, 101–114. [Google Scholar] [CrossRef]
- Eysholdt-Derzsó, E.; Sauter, M. Hypoxia and the group VII ethylene response transcription factor HRE2 promote adventitious root elongation in Arabidopsis. Plant Biol. 2019, 21, 103–108. [Google Scholar] [CrossRef]
- Panozzo, A.; Dal Cortivo, C.; Ferrari, M.; Vicelli, B.; Varotto, S.; Vamerali, T. Morphological changes and expressions of AOX1A, CYP81D8, and putative PFP genes in a large set of commercial maize hybrids under extreme waterlogging. Front. Plant Sci. 2019, 10, 62. [Google Scholar] [CrossRef]
- Estioko, L.P.; Miro, B.; Baltazar, A.M.; Merca, F.E.; Ismail, A.M.; Johnson, D.E. Differences in responses to flooding by germinating seeds of two contrasting rice cultivars and two species of economically important grass weeds. AoB Plants 2014, 6, plu064. [Google Scholar] [CrossRef]
- Nguyen, T.-N.; Tuan, P.A.; Mukherjee, S.; Son, S.; Ayele, B.T. Hormonal regulation in adventitious roots and during their emergence under waterlogged conditions in wheat. JXB 2018, 69, 4065–4082. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jansen, M.J.; Zhang, Q.; Sergeeva, L.; Ligterink, W.; Mariani, C.; Rieu, I.; Visser, E.J. A disturbed auxin signaling affects adventitious root outgrowth in Solanum dulcamara under complete submergence. J. Plant Physiol. 2018, 224, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Eysholdt-Derzsó, E.; Sauter, M. Root bending is antagonistically affected by hypoxia and ERF-mediated transcription via auxin signaling. Plant Physiol. 2017, 175, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Kennedy, R.A.; Yamasue, Y.; Rumpho, M.E. Genetic and biochemical analysis of anaerobically-induced enzymes during seed germination of Echinochloa crus-galli varieties tolerant and intolerant of anoxia. JXB 2003, 54, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Huang, M.; Huang, X.; Liu, J.; Wang, X.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Effect of post-anthesis waterlogging on biosynthesis and granule size distribution of starch in wheat grains. Plant Physiol. Biochem. 2018, 132, 222–228. [Google Scholar] [CrossRef]
- He, L.; Yu, L.; Li, B.; Du, N.; Guo, S. The effect of exogenous calcium on cucumber fruit quality, photosynthesis, chlorophyll fluorescence, and fast chlorophyll fluorescence during the fruiting period under hypoxic stress. BMC Plant Biol. 2018, 18, 180. [Google Scholar] [CrossRef]
- Wu, W.; Wang, S.; Chen, H.; Song, Y.; Zhang, L.; Peng, C.; Jing, L.; Li, J. Optimal nitrogen regimes compensate for the impacts of seedlings subjected to waterlogging stress in summer maize. PLoS ONE 2018, 13, e0206210. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Lee, S.C.; Brinton, E. Waterproofing crops: Effective flooding survival strategies. Plant Physiol. 2012, 160, 1698–1709. [Google Scholar] [CrossRef]
- Morton, L.W.; Hobbs, J.; Arbuckle, J.G.; Loy, A. Upper Midwest climate variations: Farmer responses to excess water risks. J. Environ. Qual. 2015, 44, 810–822. [Google Scholar] [CrossRef]
- Chen, Y.; Song, J.; Yan, C.; Hong, X. Effects of submergence stress at the vegetative growth stage on hybrid rice growth and grain yield in China. Chilean J. Agric. Res. 2021, 8, 191–201. [Google Scholar] [CrossRef]
- Kaur, G.; Zurweller, B.A.; Nelson, K.A.; Motavalli, P.P.; Dudenhoeffer, C.J. Soil waterlogging and nitrogen fertilizer management effects on corn and soybean yields. Agron. J. 2017, 109, 97–106. [Google Scholar] [CrossRef]
- Abendroth, L.J.; Elmore, R.W.; Boyer, M.J.; Marlay, S.K. Corn Growth and Development; Iowa State University Ames: Ames, IA, USA, 2011. [Google Scholar]
- Luce, G. Unprecedented Rainfall, Flooding and Impact on Wheat and Cover Crops; University of Missouri Extension: Columbia, MO, USA, 2015. [Google Scholar]
- Singh, J.S.; Koushal, S.; Kumar, A.; Vimal, S.R.; Gupta, V.K. Book review: Microbial inoculants in sustainable agricultural productivity-Vol. II: Functional application. Front. Microbiol. 2016, 7, 02105. [Google Scholar] [CrossRef]
- Ploschuk, R.A.; Miralles, D.J.; Colmer, T.D.; Striker, G.G. Waterlogging differentially affects yield and its components in wheat, barley, rapeseed and field pea depending on the timing of occurrence. J. Agron. Crop Sci. 2020, 206, 363–375. [Google Scholar] [CrossRef]
- Haque, E.; Kawaguchi, K.; Komatsu, S. Analysis of proteins in aerenchymatous seminal roots of wheat grown in hypoxic soils under waterlogged conditions (supplementary material). Protein Pept. Lett. 2011, 18, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Nagai, K.; Ashikari, M. Rice growth adapting to deepwater. Curr. Opin. Plant Biol. 2011, 14, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Tamang, B.G.; Magliozzi, J.O.; Maroof, M.S.; Fukao, T. Physiological and transcriptomic characterization of submergence and reoxygenation responses in soybean seedlings. Plant Cell Environ. 2014, 37, 2350–2365. [Google Scholar] [CrossRef]
- Else, M.A.; Janowiak, F.; Atkinson, C.J.; Jackson, M.B. Root signals and stomatal closure in relation to photosynthesis, chlorophyll a fluorescence and adventitious rooting of flooded tomato plants. Ann. Bot. 2009, 103, 313–323. [Google Scholar] [CrossRef]
- Visser, E.J.; Cohen, J.D.; Barendse, G.W.; Blom, C.W.; Voesenek, L.A. An ethylene-mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physiol. 1996, 112, 1687–1692. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Sheng, J.; Jin, S.; Zhou, F.; Hu, Z.; Diao, Y. Transcriptome, physiological and biochemical analysis of Triarrhena sacchariflora in response to flooding stress. BMC Genet. 2019, 20, 88. [Google Scholar] [CrossRef]
- Loreti, E.; Valeri, M.C.; Novi, G.; Perata, P. Gene regulation and survival under hypoxia requires starch availability and metabolism. Plant Physiol. 2018, 176, 1286–1298. [Google Scholar] [CrossRef]
- Urban, D.W.; Roberts, M.J.; Schlenker, W.; Lobell, D.B. The effects of extremely wet planting conditions on maize and soybean yields. Clim. Chang. 2015, 130, 247–260. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Amelung, W.; Cao, Z.; Fiedler, S.; Frenzel, P.; Jahn, R.; Kalbitz, K.; Kölbl, A.; Schloter, M. Biogeochemistry of paddy soils. Geoderma 2010, 157, 1–14. [Google Scholar] [CrossRef]
- Husson, O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: A transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 2013, 362, 389–417. [Google Scholar] [CrossRef]
- Parikh, S.J. Introduction to soil chemistry: Analysis and instrumentation. Soil Sci. Soc. Am. J. 2014, 78, 1828. [Google Scholar] [CrossRef]
- Fageria, N.; Carvalho, G.; Santos, A.; Ferreira, E.; Knupp, A. Chemistry of lowland rice soils and nutrient availability. Commun. Soil Sci. Plant Anal. 2011, 42, 1913–1933. [Google Scholar] [CrossRef]
- Ponnamperuma, F.N. The chemistry of submerged soils. Adv. Agron. 1972, 24, 29–96. [Google Scholar]
- Kaur, G. Use of Nitrogen Fertilizer Sources to Enhance Tolerance and Recovery of Corn Hybrids to Excessive Soil Moisture. Ph.D. Thesis, University of Missouri, Columbia, MO, USA, 2016. [Google Scholar]
- Unger, I.M.; Motavalli, P.P.; Muzika, R.-M. Changes in soil chemical properties with flooding: A field laboratory approach. Agro-Ecosyst. 2009, 131, 105–110. [Google Scholar] [CrossRef]
- Sahrawat, K. Fertility and organic matter in submerged rice soils. Curr. Sci. 2005, 88, 735–739. [Google Scholar]
- Kongchum, M. Effect of Plant residue and Water Management Practices on Soil Redox Chemistry, Methane Emission, and Rice Productivity. Ph.D. Thesis, Louisiana State University, Agricultural & Mechanical College, Baton Rouge, LA, USA, 2005. [Google Scholar]
- Zurweller, B.A.; Motavalli, P.P.; Nelson, K.A.; Dudenhoeffer, C.J. Short-term soil nitrous oxide emissions as affected by enhanced efficiency nitrogen fertilizers and temporarily waterlogged conditions. J. Agric. Sci. 2015, 7, 1. [Google Scholar] [CrossRef][Green Version]
- Allen, D.; Kingston, G.; Rennenberg, H.; Dalal, R.; Schmidt, S. Effect of nitrogen fertilizer management and waterlogging on nitrous oxide emission from subtropical sugarcane soils. Agro-Ecosyst. 2010, 136, 209–217. [Google Scholar] [CrossRef]
- Webb, J.R.; Clough, T.J.; Quayle, W.C. A review of indirect N2O emission factors from artificial agricultural waters. Environ. Res. Lett. 2021, 16, 043005. [Google Scholar] [CrossRef]
- Craswell, E. Fertilizers and nitrate pollution of surface and ground water: An increasingly pervasive global problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar]
- Bamdad, H.; Papari, S.; Lazarovits, G.; Berruti, F. Soil amendments for sustainable agriculture: Microbial organic fertilizers. Soil Use Manag. 2022, 38, 94–120. [Google Scholar] [CrossRef]
- Kacprzak, M.; Malińska, K.; Grosser, A.; Sobik-Szołtysek, J.; Wystalska, K.; Dróżdż, D.; Jasińska, A.; Meers, E. Cycles of carbon, nitrogen and phosphorus in poultry manure management technologies—Environmental aspects. Crit. Rev. Environ. Sci. Technol. 2023, 53, 914–938. [Google Scholar] [CrossRef]
- Mahmud, K.; Panday, D.; Mergoum, A.; Missaoui, A. Nitrogen losses and potential mitigation strategies for a sustainable agroecosystem. Sustain. Sci. 2021, 13, 2400. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, L.; Jin, S.; Guo, Q.; Hou, J. The influence of plants on the migration and transformation of nitrogen in plant-soil systems: A review. J. Soil Sci. Plant Nutr. 2022, 22, 4084–4102. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Silva, L.; Conceição, L.A.; Lidon, F.C.; Patanita, M.; D’Antonio, P.; Fiorentino, C. Digitization of Crop Nitrogen Modelling: A Review. Agronomy 2023, 13, 1964. [Google Scholar] [CrossRef]
- Huang, L.; Fu, C.; Li, T.; Yan, B.; Wu, Y.; Zhang, L.; Ping, W.; Yang, B.; Chen, L. Advances in research on effects of biochar on soil nitrogen and phosphorus. IOP Conf. Ser. Earth Environ. Sci. 2020, 424, 012015. [Google Scholar] [CrossRef]
- Jain, J.; Choudhary, S.; Munoz-Arriola, F.; Khare, D. Remote Sensing and Machine Learning Applications for the Assessment of Urban Water Stress: A Review. In Emerging Technologies for Water Supply, Conservation and Management; Springer: Berlin/Heidelberg, Germany, 2023; pp. 49–64. [Google Scholar]
- Pedersen, O.; Sauter, M.; Colmer, T.D.; Nakazono, M. Regulation of root adaptive anatomical and morphological traits during low soil oxygen. New Phytol. 2021, 229, 42–49. [Google Scholar] [CrossRef]
- Yamauchi, T.; Shiono, K.; Nagano, M.; Fukazawa, A.; Ando, M.; Takamure, I.; Mori, H.; Nishizawa, N.K.; Kawai-Yamada, M.; Tsutsumi, N. Ethylene biosynthesis is promoted by very-long-chain fatty acids during lysigenous aerenchyma formation in rice roots. Plant Physiol. 2015, 169, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, Q.; Ma, X.; Qian, C.; Wang, H.; Ren, N.; Shen, C.; Huang, S.; Xu, X.; Xu, Q. Waterlogging-induced adventitious root formation in cucumber is regulated by ethylene and auxin through reactive oxygen species signalling. Plant Cell Environ. 2019, 42, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Nagai, K.; Furukawa, S.; Song, X.-J.; Kawano, R.; Sakakibara, H.; Wu, J.; Matsumoto, T.; Yoshimura, A.; Kitano, H. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 2009, 460, 1026–1030. [Google Scholar] [CrossRef]
- Xu, P.; Wu, T.; Ali, A.; Wang, J.; Fang, Y.; Qiang, R.; Liu, Y.; Tian, Y.; Liu, S.; Zhang, H. Rice β-Glucosidase 4 (Os1βGlu4) Regulates the Hull Pigmentation via Accumulation of Salicylic Acid. Int. J. Mol. Sci. 2022, 23, 10646. [Google Scholar] [CrossRef]
- Xu, X.; Ji, J.; Xu, Q.; Qi, X.; Weng, Y.; Chen, X. The major-effect quantitative trait locus Cs ARN 6.1 encodes an AAA ATP ase domain-containing protein that is associated with waterlogging stress tolerance by promoting adventitious root formation. TPJ 2018, 93, 917–930. [Google Scholar]
- Evans, D.E. Aerenchyma formation. New Phytol. 2004, 161, 35–49. [Google Scholar] [CrossRef]
- Kuroha, T.; Nagai, K.; Gamuyao, R.; Wang, D.R.; Furuta, T.; Nakamori, M.; Kitaoka, T.; Adachi, K.; Minami, A.; Mori, Y. Ethylene-gibberellin signaling underlies adaptation of rice to periodic flooding. Science 2018, 361, 181–186. [Google Scholar] [CrossRef]
- Winkel, A.; Visser, E.J.; Colmer, T.D.; Brodersen, K.P.; Voesenek, L.A.; Sand-Jensen, K.; Pedersen, O. Leaf gas films, underwater photosynthesis and plant species distributions in a flood gradient. Plant Cell Environ. 2016, 39, 1537–1548. [Google Scholar] [CrossRef]
- Pedersen, O.; Rich, S.M.; Colmer, T.D. Surviving floods: Leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice. TPJ 2009, 58, 147–156. [Google Scholar] [CrossRef]
- Li, C.-X.; Wei, H.; Geng, Y.-H.; Schneider, R. Effects of submergence on photosynthesis and growth of Pterocarya stenoptera (Chinese wingnut) seedlings in the recently-created Three Gorges Reservoir region of China. Wetl. Ecol. Manag. 2010, 18, 485–494. [Google Scholar] [CrossRef]
- Voesenek, L.; Colmer, T.; Pierik, R.; Millenaar, F.; Peeters, A. How plants cope with complete submergence. New Phytol. 2006, 170, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-S.; Yang, C.-Y. Physiological responses and expression profile of NADPH oxidase in rice (Oryza sativa) seedlings under different levels of submergence. Rice 2016, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Anee, T.I.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Bhuiyan, T.F.; Alam, M.U.; Fujita, M.; Hasanuzzaman, M. Oxidative damage and antioxidant defense in Sesamum indicum after different waterlogging durations. Plants 2019, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Kuai, J.; Liu, Z.; Wang, Y.; Meng, Y.; Chen, B.; Zhao, W.; Zhou, Z.; Oosterhuis, D.M. Waterlogging during flowering and boll forming stages affects sucrose metabolism in the leaves subtending the cotton boll and its relationship with boll weight. Plant Sci. 2014, 223, 79–98. [Google Scholar] [CrossRef]
- Loreti, E.; van Veen, H.; Perata, P. Plant responses to flooding stress. Curr. Opin. Plant Biol. 2016, 33, 64–71. [Google Scholar] [CrossRef]
- Limami, A.M.; Diab, H.; Lothier, J. Nitrogen metabolism in plants under low oxygen stress. Planta 2014, 239, 531–541. [Google Scholar] [CrossRef]
- Baxter-Burrell, A.; Yang, Z.; Springer, P.S.; Bailey-Serres, J. RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 2002, 296, 2026–2028. [Google Scholar] [CrossRef]
- Zabalza, A.; Van Dongen, J.T.; Froehlich, A.; Oliver, S.N.; Faix, B.; Gupta, K.J.; Schmalzlin, E.; Igal, M.; Orcaray, L.; Royuela, M. Regulation of respiration and fermentation to control the plant internal oxygen concentration. Plant Physiol. 2009, 149, 1087–1098. [Google Scholar] [CrossRef]
- Zhang, P.; Lyu, D.; Jia, L.; He, J.; Qin, S. Physiological and de novo transcriptome analysis of the fermentation mechanism of Cerasus sachalinensis roots in response to short-term waterlogging. BMC Genom. 2017, 18, 649. [Google Scholar] [CrossRef]
- Caruso, P.; Baldoni, E.; Mattana, M.; Pietro Paolo, D.; Genga, A.; Coraggio, I.; Russo, G.; Picchi, V.; Reforgiato Recupero, G.; Locatelli, F. Ectopic expression of a rice transcription factor, Mybleu, enhances tolerance of transgenic plants of Carrizo citrange to low oxygen stress. Plant Cell Tissue Organ Cult. 2012, 109, 327–339. [Google Scholar] [CrossRef]
- Borella, J.; Becker, R.; Lima, M.C.; Oliveira, D.d.S.C.d.; Braga, E.J.B.; Oliveira, A.C.B.d.; Amarante, L.d. Nitrogen source influences the antioxidative system of soybean plants under hypoxia and re-oxygenation. Sci. Agric. 2019, 76, 51–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, X.; Yang, G.; Li, Z.; Lu, H.; Kong, X.; Eneji, A.E.; Dong, H. Physiological and molecular adjustment of cotton to waterlogging at peak-flowering in relation to growth and yield. Field Crops Res. 2015, 179, 164–172. [Google Scholar] [CrossRef]
- Komatsu, S.; Thibaut, D.; Hiraga, S.; Kato, M.; Chiba, M.; Hashiguchi, A.; Tougou, M.; Shimamura, S.; Yasue, H. Characterization of a novel flooding stress-responsive alcohol dehydrogenase expressed in soybean roots. Plant Mol. Biol. 2011, 77, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Tougou, M.; Hashiguchi, A.; Yukawa, K.; Nanjo, Y.; Hiraga, S.; Nakamura, T.; Nishizawa, K.; Komatsu, S. Responses to flooding stress in soybean seedlings with the alcohol dehydrogenase transgene. Plant Biotechnol. J. 2012, 29, 301–305. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Lu, H.; Kong, X.; Dai, J.; Li, Z.; Dong, H. Growth, lint yield and changes in physiological attributes of cotton under temporal waterlogging. Field Crops Res. 2016, 194, 83–93. [Google Scholar] [CrossRef]
- Dolferus, R.; Wolansky, M.; Carroll, R.; Miyashita, Y.; Ismond, K.; Good, A. Functional analysis of lactate dehydrogenase during hypoxic stress in Arabidopsis. Funct. Plant Biol. 2008, 35, 131–140. [Google Scholar] [CrossRef]
- Yin, D.; Sun, D.; Han, Z.; Ni, D.; Norris, A.; Jiang, C.-Z. PhERF2, an ethylene-responsive element binding factor, plays an essential role in waterlogging tolerance of petunia. Hortic. Res. 2019, 6, 83. [Google Scholar] [CrossRef]
- Pottosin, I.; Zepeda-Jazo, I.; Bose, J.; Shabala, S. An anion conductance, the essential component of the hydroxyl-radical-induced ion current in plant roots. Int. J. Mol. Sci. 2018, 19, 897. [Google Scholar] [CrossRef]
- Li, H.; Cai, J.; Liu, F.; Jiang, D.; Dai, T.; Cao, W. Generation and scavenging of reactive oxygen species in wheat flag leaves under combined shading and waterlogging stress. Funct. Plant Biol. 2011, 39, 71–81. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kulshreshtha, N.; Kumar, A.; Yaduvanshi, N.; Singh, M.; Prasad, K.; Basak, N. Waterlogging effects on elemental composition of wheat genotypes in sodic soils. J. Plant Nutr. 2018, 41, 1252–1262. [Google Scholar] [CrossRef]
- Alizadeh-Vaskasi, F.; Pirdashti, H.; Araei, A.C.; Saadatmand, S. Waterlogging effects on some antioxidant enzymes activities and yield of three wheat promising lines. Acta Agric. Slov. 2018, 111, 621–631. [Google Scholar] [CrossRef]
- Tong, C.; Hill, C.B.; Zhou, G.; Zhang, X.-Q.; Jia, Y.; Li, C. Opportunities for improving waterlogging tolerance in cereal crops—Physiological traits and genetic mechanisms. Plants 2021, 10, 1560. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E.; Bailey-Serres, J.; Brodersen, C.; Buckley, T.N.; Conti, L.; Christmann, A.; Dinneny, J.R.; Grill, E.; Hayes, S.; Heckman, R.W. Burning questions for a warming and changing world: 15 unknowns in plant abiotic stress. Plant Cell 2023, 35, 67–108. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, C.G.; Casalongué, C.A.; Simontacchi, M.; Marquez-Garcia, B.; Foyer, C.H. Interactions between hormone and redox signalling pathways in the control of growth and cross tolerance to stress. Environ. Exp. Bot. 2013, 94, 73–88. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Wu, H.; Chen, H.; Zhang, Y.; Zhang, Y.; Zhu, D.; Xiang, J. Effects of 1-aminocyclopropane-1-carboxylate and paclobutrazol on the endogenous hormones of two contrasting rice varieties under submergence stress. Plant Growth Regul. 2019, 87, 109–121. [Google Scholar] [CrossRef]
- Benschop, J.J.; Bou, J.; Peeters, A.J.; Wagemaker, N.; Guhl, K.; Ward, D.; Hedden, P.; Moritz, T.; Voesenek, L.A. Long-term submergence-induced elongation in Rumex palustris requires abscisic acid-dependent biosynthesis of gibberellin1. Plant Physiol. 2006, 141, 1644–1652. [Google Scholar] [CrossRef]
- Yamauchi, T.; Tanaka, A.; Tsutsumi, N.; Inukai, Y.; Nakazono, M. A role for auxin in ethylene-dependent inducible aerenchyma formation in rice roots. Plants 2020, 9, 610. [Google Scholar] [CrossRef]
- Hartman, S.; Sasidharan, R.; Voesenek, L.A. The role of ethylene in metabolic acclimations to low oxygen. New Phytol. 2021, 229, 64–70. [Google Scholar] [CrossRef]
- Lee, C.; Choi, Y.-E.; Yun, Y.-S. A strategy for promoting astaxanthin accumulation in Haematococcus pluvialis by 1-aminocyclopropane-1-carboxylic acid application. J. Biotechnol. 2016, 236, 120–127. [Google Scholar] [CrossRef]
- Zhang, W.; Wen, C.-K. Preparation of ethylene gas and comparison of ethylene responses induced by ethylene, ACC, and ethephon. Plant Physiol. Biochem. 2010, 48, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Buitrago, S.; Feng, X.; Hu, A.; Zhou, M.; Zhang, W. Ethylene regulates aerenchyma formation in cotton under hypoxia stress by inducing the accumulation of reactive oxygen species. Environ. Exp. Bot. 2022, 197, 104826. [Google Scholar] [CrossRef]
- Asgher, M.; Khan, M.I.R.; Anjum, N.A.; Verma, S.; Vyas, D.; Per, T.S.; Masood, A.; Khan, N.A. Ethylene and polyamines in counteracting heavy metal phytotoxicity: A crosstalk perspective. J. Plant Growth Regul. 2018, 37, 1050–1065. [Google Scholar] [CrossRef]
- Mignolli, F.; Todaro, J.S.; Vidoz, M.L. Internal aeration and respiration of submerged tomato hypocotyls are enhanced by ethylene-mediated aerenchyma formation and hypertrophy. Physiol. Plant. 2020, 169, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, R.; Voesenek, L.A. Ethylene-mediated acclimations to flooding stress. Plant Physiol. 2015, 169, 3–12. [Google Scholar] [CrossRef]
- Yamauchi, T.; Yoshioka, M.; Fukazawa, A.; Mori, H.; Nishizawa, N.K.; Tsutsumi, N.; Yoshioka, H.; Nakazono, M. An NADPH oxidase RBOH functions in rice roots during lysigenous aerenchyma formation under oxygen-deficient conditions. Plant Cell 2017, 29, 775–790. [Google Scholar] [CrossRef]
- Kora, D.; Bhattacharjee, S. Redox gateway associated with adventitious root formation under stress and hormonal signalling in plants. Curr. Sci 2020, 119, 462–472. [Google Scholar] [CrossRef]
- Gomez, M.D.; Barro-Trastoy, D.; Escoms, E.; Saura-Sánchez, M.; Sánchez, I.; Briones-Moreno, A.; Vera-Sirera, F.; Carrera, E.; Ripoll, J.-J.; Yanofsky, M.F. Gibberellins negatively modulate ovule number in plants. Development 2018, 145, dev163865. [Google Scholar] [CrossRef]
- Olorunwa, O.J.; Adhikari, B.; Brazel, S.; Popescu, S.C.; Popescu, G.V.; Shi, A.; Barickman, T.C. Waterlogging during the reproductive growth stage causes physiological and biochemical modifications in the leaves of cowpea (Vigna unguiculata L.) genotypes with contrasting tolerance. Plant Physiol. Biochem. 2022, 190, 133–144. [Google Scholar] [CrossRef]
- Zeng, R.; Chen, T.; Wang, X.; Cao, J.; Li, X.; Xu, X.; Chen, L.; Xia, Q.; Dong, Y.; Huang, L. Physiological and expressional regulation on photosynthesis, starch and sucrose metabolism response to waterlogging stress in peanut. Front. Plant Sci. 2021, 12, 601771. [Google Scholar] [CrossRef]
- Hong, B.; Zhou, B.; Peng, Z.; Yao, M.; Wu, J.; Wu, X.; Guan, C.; Guan, M. Tissue-specific transcriptome and metabolome analysis reveals the response mechanism of Brassica napus to waterlogging stress. Int. J. Mol. Sci. 2023, 24, 6015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, H.; Zhou, M.; Ge, Z.; Zhang, F.; Foyer, C.H.; Yuan, X.; Xie, Y. The coordination of guard-cell autonomous ABA synthesis and DES1 function in situ regulates plant water deficit responses. J. Adv. Res. 2021, 27, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ayano, M.; Kani, T.; Kojima, M.; Sakakibara, H.; Kitaoka, T.; Kuroha, T.; ANGELES-SHIM, R.B.; Kitano, H.; Nagai, K.; Ashikari, M. Gibberellin biosynthesis and signal transduction is essential for internode elongation in deepwater rice. Plant Cell Environ. 2014, 37, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- González-Guzmán, M.; Gómez-Cadenas, A.; Arbona, V. Abscisic acid as an emerging modulator of the responses of plants to low oxygen conditions. Front. Plant Sci. 2021, 12, 661789. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, S.; Yoshioka, T.; Yamamoto, R.; Hiraga, S.; Nakamura, T.; Shimada, S.; Komatsu, S. Role of abscisic acid in flood-induced secondary aerenchyma formation in soybean (Glycine max) hypocotyls. Plant Prod. Sci. 2014, 17, 131–137. [Google Scholar] [CrossRef]
- Dawood, T.; Yang, X.; Visser, E.J.; Te Beek, T.A.; Kensche, P.R.; Cristescu, S.M.; Lee, S.; Floková, K.; Nguyen, D.; Mariani, C. A co-opted hormonal cascade activates dormant adventitious root primordia upon flooding in Solanum dulcamara. Plant Physiol. 2016, 170, 2351–2364. [Google Scholar] [CrossRef]
- Lutts, S.; Hausman, J.-F.; Quinet, M.; Lefèvre, I. Polyamines and their roles in the alleviation of ion toxicities in plants. In Ecophysiology and Responses of Plants under Salt Stress; Springer: Berlin/Heidelberg, Germany, 2013; pp. 315–353. [Google Scholar]
- Finkelsteina, R.R.; Rockb, C.D. Abscisic acid biosynthesis and response. Arab. Book 2002, 11, e0166. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Hwang, S.-J.; Waqas, M.; Khan, A.L.; Lee, J.-H.; Lee, J.-D.; Nguyen, H.T.; Lee, I.-J. Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front. Plant Sci. 2015, 6, 714. [Google Scholar] [CrossRef]
- Fukao, T.; Bailey-Serres, J. Ethylene—A key regulator of submergence responses in rice. Plant Sci. 2008, 175, 43–51. [Google Scholar] [CrossRef]
- Matilla-Vazquez, M.; Matilla, A. Ethylene: Role in plants under environmental stress. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment: Volume 2; Springer: Berlin/Heidelberg, Germany, 2013; pp. 189–222. [Google Scholar]
- Saika, H.; Okamoto, M.; Miyoshi, K.; Kushiro, T.; Shinoda, S.; Jikumaru, Y.; Fujimoto, M.; Arikawa, T.; Takahashi, H.; Ando, M. Ethylene promotes submergence-induced expression of OsABA8ox1, a gene that encodes ABA 8′-hydroxylase in rice. PCP 2007, 48, 287–298. [Google Scholar] [CrossRef]
- Xie, R.; Zheng, L.; Jiao, Y.; Huang, X. Understanding physiological and molecular mechanisms of citrus rootstock seedlings in response to root zone hypoxia by RNA-Seq. Environ. Exp. Bot. 2021, 192, 104647. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Z.; He, J.; Shen, L. iTRAQ-Based Quantitative Proteomics Unveils Protein Dynamics in the Root of Solanum melongena L. under Waterlogging Stress Conditions. Life 2023, 13, 1399. [Google Scholar] [CrossRef] [PubMed]
- Saha, I.; Hasanuzzaman, M.; Dolui, D.; Sikdar, D.; Debnath, S.C.; Adak, M.K. Silver-nanoparticle and abscisic acid modulate sub1A quantitative trait loci functioning towards submergence tolerance in rice (Oryza sativa L.). Environ. Exp. Bot. 2021, 181, 104276. [Google Scholar] [CrossRef]
- Postiglione, A.E.; Muday, G.K. Abscisic acid increases hydrogen peroxide in mitochondria to facilitate stomatal closure. Plant Physiol. 2023, 192, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Gomes, G.; Scortecci, K. Auxin and its role in plant development: Structure, signalling, regulation and response mechanisms. Plant Biol. 2021, 23, 894–904. [Google Scholar] [CrossRef]
- Alaguero-Cordovilla, A.; Sánchez-García, A.B.; Ibáñez, S.; Albacete, A.; Cano, A.; Acosta, M.; Pérez-Pérez, J.M. An auxin-mediated regulatory framework for wound-induced adventitious root formation in tomato shoot explants. Plant Cell Environ. 2021, 44, 1642–1662. [Google Scholar] [CrossRef]
- Li, S.-W.; Xue, L.; Xu, S.; Feng, H.; An, L. Mediators, genes and signaling in adventitious rooting. Bot. Rev. 2009, 75, 230–247. [Google Scholar] [CrossRef]
- Feng, Y.; Bayaer, E.; Qi, Y. Advances in the biological functions of auxin transporters in rice. Agriculture 2022, 12, 989. [Google Scholar] [CrossRef]
- Cui, C.; Wang, Z.; Su, Y.; Wang, T. New insight into the rapid growth of the Mikania micrantha stem based on DIA proteomic and RNA-Seq analysis. J. Proteom. 2021, 236, 104126. [Google Scholar] [CrossRef]
- Shimamura, M. Marchantia polymorpha: Taxonomy, phylogeny and morphology of a model system. PCP 2016, 57, 230–256. [Google Scholar] [CrossRef]
- Cheng, S. Establishing the Biological Role of MPK20 in Primary Cell Wall Formation in Arabidopsis. Bachelor’s Thesis, University of British Columbia, Vancouver, BC, Canada, 2015. [Google Scholar]
- Qi, X.; Li, Q.; Shen, J.; Qian, C.; Xu, X.; Xu, Q.; Chen, X. Sugar enhances waterlogging-induced adventitious root formation in cucumber by promoting auxin transport and signalling. Plant Cell Environ. 2020, 43, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Xie, C.; Zhang, H.; Arnao, M.B.; Ali, M.; Ali, Q.; Muhammad, I.; Shalmani, A.; Nawaz, M.A.; Chen, P. Melatonin and its effects on plant systems. Molecules 2018, 23, 2352. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Hassan, M.U.; Ghareeb, R.Y.; Nawaz, M.; Mahmood, A.; Shah, A.N.; Abdel-Megeed, A.; Abdelsalam, N.R.; Hashem, M.; Alamri, S.; Thabit, M.A. Melatonin: A vital pro-tectant for crops against heat stress: Mechanisms and prospects. Agronomy 2022, 12, 1116. [Google Scholar] [CrossRef]
- Hassan, M.U.; Mahmood, A.; Awan, M.I.; Maqbool, R.; Aamer, M.; Alhaithloul, H.A.; Huang, G.; Skalicky, M.; Brestic, M.; Pandey, S. Melatonin-induced protection against plant abiotic stress: Mechanisms and prospects. Front. Plant Sci. 2022, 13, 902694. [Google Scholar] [CrossRef] [PubMed]
- Subbaiah, C.C.; Sachs, M.M. Molecular and cellular adaptations of maize to flooding stress. Ann. Bot. 2003, 91, 119–127. [Google Scholar] [CrossRef]
- Fujita, M.; Hasanuzzaman, M. Approaches to enhancing antioxidant defense in plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, J.; Tan, D.-X.; Wang, N.; Wang, L.; Shan, D.; Kong, J. Melatonin improves waterlogging tolerance of Malus baccata (Linn.) Borkh. seedlings by maintaining aerobic respiration, photosynthesis and ROS migration. Front. Plant Sci. 2017, 8, 483. [Google Scholar] [CrossRef]
- Javed, J.; Rauf, M.; Arif, M.; Hamayun, M.; Gul, H.; Ud-Din, A.; Ud-Din, J.; Sohail, M.; Rahman, M.M.; Lee, I.-J. Endophytic fungal consortia enhance basal drought-tolerance in Moringa oleifera by upregulating the antioxidant enzyme (APX) through Heat shock factors. Antioxid. Act 2022, 11, 1669. [Google Scholar] [CrossRef]
- Gu, X.; Xue, L.; Lu, L.; Xiao, J.; Song, G.; Xie, M.; Zhang, H. Melatonin enhances the waterlogging tolerance of Prunus persica by modulating antioxidant metabolism and anaerobic respiration. J. Plant Growth Regul. 2021, 40, 2178–2190. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.B.; Sheteiwy, M.S.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-induced water stress tolerance in plants: Recent advances. Antioxidants 2020, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Sun, J.; Guo, S. Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol. 2019, 19, 414. [Google Scholar] [CrossRef] [PubMed]
- Wassie, M.; Zhang, W.; Zhang, Q.; Ji, K.; Chen, L. Effect of heat stress on growth and physiological traits of alfalfa (Medicago sativa L.) and a comprehensive evaluation for heat tolerance. Agronomy 2019, 9, 597. [Google Scholar] [CrossRef]
- Marcińska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Grzesiak, M.T.; Janowiak, F.; Filek, M.; Dziurka, M.; Dziurka, K.; Waligórski, P.; Juzoń, K. Alleviation of osmotic stress effects by exogenous application of salicylic or abscisic acid on wheat seedlings. Int. J. Mol. Sci. 2013, 14, 13171–13193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhou, Y.; Yue, Z.; Chen, X.; Cao, X.; Xu, X.; Xing, Y.; Jiang, B.; Ai, X.; Huang, R. Changes in photosynthesis, chloroplast ultrastructure, and antioxidant metabolism in leaves of sorghum under waterlogging stress. Photosynthetica 2019, 57, 1076–1083. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Xia, X.-J.; Li, X.; Shi, K.; Yu, J.-Q.; Zhou, Y.-H. Role of brassinosteroid in plant adaptation to abiotic stresses and its interplay with other hormones. Curr. Protein Pept. Sci. 2015, 16, 462–473. [Google Scholar] [CrossRef]
- Tyagi, A.; Ali, S.; Park, S.; Bae, H. Exploring the Potential of Multiomics and Other Integrative Approaches for Improving Waterlogging Tolerance in Plants. Plants 2023, 12, 1544. [Google Scholar] [CrossRef]
- Omidian, M.; Roein, Z.; Shiri, M.A. Effect of Foliar Application of 24-Epibrassinolide on Water Use Efficiency and Morpho-Physiological Characteristics of Lilium LA Hybrid under Deficit Irrigation. J. Plant Growth Regul. 2022, 41, 1547–1560. [Google Scholar] [CrossRef]
- Li, Q.-F.; Zhou, Y.; Xiong, M.; Ren, X.-Y.; Han, L.; Wang, J.-D.; Zhang, C.-Q.; Fan, X.-L.; Liu, Q.-Q. Gibberellin recovers seed germination in rice with impaired brassinosteroid signalling. Plant Sci. 2020, 293, 110435. [Google Scholar] [CrossRef]
- Phukan, U.J.; Mishra, S.; Shukla, R.K. Waterlogging and submergence stress: Affects and acclimation. Crit. Rev. Biotechnol. 2016, 36, 956–966. [Google Scholar] [CrossRef]
- Albertos, P.; Wlk, T.; Griffiths, J.; Pimenta Lange, M.J.; Unterholzner, S.J.; Rozhon, W.; Lange, T.; Jones, A.M.; Poppenberger, B. Brassinosteroid-regulated bHLH transcription factor CESTA induces the gibberellin 2-oxidase GA2ox7. Plant Physiol. 2022, 188, 2012–2025. [Google Scholar] [CrossRef] [PubMed]
- Mittal, L.; Tayyeba, S.; Sinha, A.K. Finding a breather for Oryza sativa: Understanding hormone signalling pathways involved in rice plants to submergence stress. Plant Cell Environ. 2022, 45, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Muhlenbock, P.; Plaszczyca, M.; Plaszczyca, M.; Mellerowicz, E.; Karpinski, S. Lysigenous aerenchyma formation in Arabidopsis is controlled by LESION SIMULATING DISEASE1. Plant Cell 2007, 19, 3819–3830. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.T.; Proctor, C.A.; Dou, Y.; Schmitz, A.J.; Phansak, P.; Kruger, G.R.; Zhang, C.; Walia, H. Genetic and molecular characterization of submergence response identifies Subtol6 as a major submergence tolerance locus in maize. PLoS ONE 2015, 10, e0120385. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xu, H.; Rong, W.; Ye, X.; Zhang, Z. Constitutive expression of a stabilized transcription factor group VII ethylene response factor enhances waterlogging tolerance in wheat without penalizing grain yield. Plant Cell Environ. 2019, 42, 1471–1485. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Guo, B.; Shen, H.; Pan, Y.; Hong, Y.; Lv, C.; Xu, R. Overexpression of barley transcription factor HvERF2. 11 in Arabidopsis enhances plant waterlogging tolerance. Int. J. Mol. Sci. 2020, 21, 1982. [Google Scholar] [CrossRef]
- Phukan, U.J.; Jeena, G.S.; Tripathi, V.; Shukla, R.K. Ma RAP 2-4, a waterlogging-responsive ERF from Mentha, regulates bidirectional sugar transporter At SWEET 10 to modulate stress response in Arabidopsis. Plant Biotechnol. J. 2018, 16, 221–233. [Google Scholar] [CrossRef]
- Wang, L.; Gao, J.; Zhang, Z.; Liu, W.; Cheng, P.; Mu, W.; Su, T.; Chen, S.; Chen, F.; Jiang, J. Overexpression of CmSOS1 confers waterlogging tolerance in Chrysanthemum. J. Integr. Plant Biol. 2020, 62, 1059–1064. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).