Abstract
Cotton fibers provide an important source of raw materials for the textile industry worldwide. Cotton fiber is a kind of single cell that differentiates from the epidermis of the ovule and provides a perfect research model for the differentiation and elongation of plant cells. Cotton fiber initiation is the first stage throughout the entire developmental process. The number of fiber cell initials on the seed ovule epidermis decides the final fiber yield. Thus, it is of great significance to clarify the mechanism underlying cotton fiber initiation. Fiber cell initiation is controlled by complex and interrelated regulatory networks. Plant phytohormones, transcription factors, sugar signals, small signal molecules, functional genes, non-coding RNAs, and histone modification play important roles during this process. Here, we not only summarize the different kinds of factors involved in fiber cell initiation but also discuss the mechanisms of these factors that act together to regulate cotton fiber initiation. Our aim is to synthesize a systematic and comprehensive review of different factors during fiber initiation that will provide the basics for further illustrating these mechanisms and offer theoretical guidance for improving fiber yield in future molecular breeding work.
1. Introduction
Cotton is an important economic crop worldwide. Cotton has four kinds of cultivated species, including Gossypium Hirsutum, G. Barbadense, G. Arboreum, and G. Herbaceum, of which the first two are allotetraploids (2n = 52) and the latter two are diploids (2n = 26) [1,2]. Fiber, a highly elongated and thickened single cell of the seed trichomes, is the most valuable product from cotton. Cotton fiber development is a very complicated process consisting of four continuous but overlapped stages: cell initiation (3 days before anthesis to 3 days post-anthesis (DPA), −3–3 DPA), cell elongation (3–20 DPA), cell wall thickening (20–45 DPA), and maturation (45–50 DPA) [3]. The number of fiber cell initials determines the cotton fiber yield, whereas cell elongation and cell wall thickening decide the final fiber quality. Upland cotton (G. Hirsutum) with high production and better yield potential is predominantly used as the commercial source of cotton fiber due to its wide adaptability to the environment [4,5]. Sea Island cotton (G. Barbadense) is famous for its long, strong, and fine fiber quality [4,5]. Traditional breeding methods have achieved great success in improving crop traits. However, due to the constraint of narrow genetic background in breeding materials, low efficiency of selection, and other multiple factors, the breeding of important crop varieties has entered a stage of gradual development in recent years. Therefore, accelerating innovation in breeding technology is imperative. An improvement in fiber yield and quality is always the major goal for cotton breeders; to fulfill the requirements of the modern textile industry, traditional breeding methods for cotton fiber yield and quality traits have been explored in different kind of populations, accompanied by cotton industrial development [6]. However, it is a huge challenge to improve fiber yield and quality simultaneously in cotton production.
Cotton fiber yield is essentially determined by the number of fiber cell initials on the seed ovule epidermis. Although cotton fiber initiation has been explored in the past, the molecular mechanism has not yet been well elucidated. Sequencing technology (such as genome sequencing, transcriptomes, and proteome sequencing) in cotton provided new tools with which to uncover the biological mechanisms underlying cotton fiber initiation [1,2]. The identification and characterization of genes contributing to the process are essential for understanding the biological roles and genetic interactions underlying fiber initiation. In addition, functional loci and molecular markers serve as starting points to improve fiber yield through the application of biotechnology, such as gene editing. These new approaches can be used by breeders to develop new varieties that improve cotton fiber yield, which would greatly contribute to cotton production.
Previous studies have shown that transcription factors (TFs) and phytohormones play important roles during cotton fiber development [7,8,9]. In this review, we mainly focus on studies related to cotton fiber initiation. We comprehensively summarize the inheritance and related candidate genes of cotton fiber initiation that have been identified in previous studies. We try to further clarify the regulatory relationships between these candidate genes and aim to provide a complete overview of the regulatory network underlying cotton fiber initiation.
2. Significance of Cotton Fiber Initiation
The initiation of cotton fiber is first visualized as the cell that protrudes from certain protodermal cells in cotton ovules before or on the day of anthesis [10]. The initiation process of fiber is rapid, which makes it hard to observe how protodermal cells differentiate into fiber cells. Biochemical analysis indicates that fiber cell fate determination appears before the formation of visible fiber initials [11]. Cotton fiber is a kind of single-cell trichome that differentiates from the epidermis of the ovule; the final fiber yield is determined by the number of epidermal cells that could potentially develop into fiber cells during the fiber initiation stage. Even though all epidermal cells of the cotton ovule have the potential to develop into fibers, only 25~30% of them ultimately become fibers [3]; this has become the restriction to improving cotton fiber yield. Therefore, it is possible to improve fiber yield by increasing the proportion of epidermal cells that could differentiate into fibers. Moreover, cotton fiber is the longest single cell in plants and could provide the ideal model system for studying diverse aspects of plant cell growth, including cell differentiation and elongation [12]. Thus, molecular investigations of fiber initiation will provide a theoretical basis for improving fiber yield, which is the main objective for breeders in the present and future work. Moreover, they will also enrich investigations of cell fate determination in plants.
Cotton fibers can be classified into two types: spinnable lint fiber and adherent fuzz fiber. Both are unicellular and tubular outgrowths that are indistinguishable in appearance during the early stages of fiber development, suggesting that their differentiation may involve a similar process and share a common pathway of initiation [13]. Lint, which is economically important, initiates on or before the day of anthesis (−1–0 DPA). Lint fibers grow to 2.5–3.5 cm in length, while fuzz fibers initiate on 4–5 DPA and ultimately reach approximately 5 mm in length [14]. Fuzz is found in most cotton varieties and aids the spreading of mature seeds by attaching to the fur of passing animals. However, fuzz is too short to be collected from the seeds through ginning, and any remaining fuzz on the seeds will enhance the cost of seed treatment for the following season [15]. While most cultivars developed in G. Barbadense are fuzzless, almost all cultivars in high-yield G. Hirsutum are fuzzy since fuzzless seeds are usually associated with a lower lint percentage, an important cotton yield component. Moreover, the ultimate capacity of cotton fibers is determined from the number of spinnable lint fibers. Cotton breeders have long aimed to cultivate cotton varieties with high lint percentage and fuzzless seeds to utilize their ginning advantages. To realize this target, it is necessary to uncover the mechanisms underlying the regulation of fiber initiation and to comprehend the biological function and genetic interactions.
3. Progress in Investigation of Cotton Spontaneous Initial Development Mutants
Spontaneous fiber mutants that inhibit the development of fiber initials provide powerful resources for studying the mechanism underlying the early stages of fiber development. Previous studies have described many cotton fiber mutants, including fuzzless, lintless, and fiberless (fuzzless–lintless) mutants. Their qualitative traits have been identified and described as shown in Table 1. The phenotype of representative fiber mutants is shown in Figure 1.
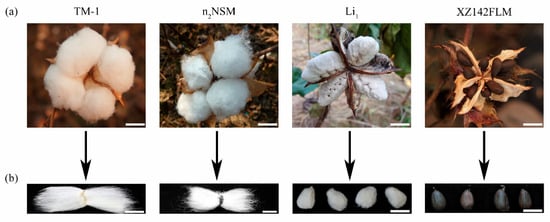
Figure 1.
Cotton fiber phenotypes in cotton fiber in TM-1, n2NSM, Li1, and XZ142FLM. (a) Phenotypes of four representative varieties during the period of boll opening. Among them, white fibers with no black seeds are observed in TM-1, n2NSM, and Li1. Only black seeds are observed in XZ142FLM. Scale bars, 1.0 cm. (b) Examination of seed phenotype after fiber combing. TM-1 seeds have long lint and short fuzz fibers, n2NSM seeds only have long lint, Li1 seeds have short lint and fuzz fibers, and XZ142FLM seeds have no fibers attached. Scale bars, 1.0 cm.

Table 1.
Different kinds of cotton fiber initiation-related mutants.
Table 1.
Different kinds of cotton fiber initiation-related mutants.
| Phenotype | Variety | Genotype | Reference(s) | Resource |
|---|---|---|---|---|
| Fuzzless (no fuzz fibers) | Naked seeds 1 (N1NSM) | N1N1 | [15,16,17,18] | USDA-ARS, College Station, TX, USA |
| Naked seeds 2 (n2NSM) | n2n2 | [16,17,18,19] | USDA-ARS, College Station, TX, USA | |
| Naked seeds 3 (n3) | n3n3 | [20] | USDA-ARS, College Station, TX, USA | |
| Naked tufted seed (n4t) | n4tn4t | [21,22] | USDA-ARS, College Station, TX, USA | |
| GA0149 | FZ | [23] | Institute of Cotton Research, Chinese Academy of Agricultural Sciences, Anyang, China | |
| Lintless (shortened lint fibers <5 mm) | Ligon-lintless 1 (Li1) | Li1Li1 | [24,25,26] | USDA-ARS, College Station, TX, USA |
| Ligon-lintless 2 (Li2) | Li2Li2 | [27,28,29] | USDA-ARS, College Station, TX, USA | |
| Ligon lintless-like mutant (Lix) | LixLix | [30] | Nanjing Agricultural University in China, Nanjing, China | |
| Fiberless (no lint and fuzz fibers) | Xuzhou 142 fiberless mutant (XZ142FLM) | n1n1n2n2li3li3 | [31] | Xuzhou Research Institute of Agricultural Science in China, Xuzhou, China |
| SL1-7-1 | N1N1fl1fl1n3n3 | [32] | USDA-ARS, Stoneville, MS, USA | |
| MD17 | N1N1n2n2n3n3 | [20] | USDA-ARS, Stoneville, MS, USA |
Two of the most well-described fuzzless mutants are the dominant N1N1 and recessive n2n2 loci, both producing naked seeds after ginning [15,16,17,18]. Homozygous N1NSM mutants are completely fuzzless, with a significantly reduced lint percentage [16,17,18]. Genetic mapping of the N1N1 locus found that it is anchored to chromosome A12 and identified as a MYBMIXTA-like (MML) gene GhMML3_A12 (or GhMYB25-like_At) [15]. A recent study further proposed that MML3_D12 (or MYB25-like_Dt) is likely a major contributing locus for the recessive fuzzless trait (n2n2) in G. Barbadense [19]. In another study, another fuzzless seed loci (n3) was found to influence the effects of the N1n1, N2n2, and N3n3 genes in lint percentage [20]. A newly found ethyl methanesulfonate-induced mutant n4t appears to be different from the above two naked seed loci; the mutation was shown to have a less severe negative influence on lint development [21,22]. Recently, a ~6.2 kb insertion larlNDELFZ was found to be related to fuzzless seeds and decreased trichomes in the G. Arboreum fuzzless mutant GA0149 in a genome-wide association study [23]. This insertion was proposed to enhance a dominant-repressor, GaFZ.
Although Ligon lintless-1 (Li1) and Ligon lintless-2 (Li2)are named as lintless mutants they are actually short fiber mutants. Li1 is a dominant and monogenic mutant characterized by its short fibers (~5 mm in length) and the twisted growth of stems, leaves, and flowers in plants [24,25,26], while Li2 exhibits similar fiber length but normal vegetative growth [27,28]. Using map-based cloning strategy, the Li1 locus was identified as a single Gly65Val amino acid replacement in the actin polymerization domain of GhACT_LI1 (Gh_D04G0865) [26]. Recent studies revealed that the small interfering RNA (siRNA)-induced silencing of RanBP1s inhibits cotton fiber elongation in the Li2 mutant [29]. Lix is a lintless loci and produces a plant morphology similarly to that of Li1; it is located on chromosome 4, which is homologous to chromosome 22 [30].
Some fiberless mutants have been described in G. Hirsutum, which include the Xuzhou 142 fiberless line (XZ142FLM) [31], SL1-7-1 [32], and MD17 [20]. However, the genotypes of these lines and their relationships still need to be clearly explained. Among these lines, XZ142FLM is the most studied fiberless mutant; it was originally discovered in a commercial upland cultivar, Xuzhou 142 (XZ142WT). So far, there are three kinds of genotypes for XZ142FLM: a three-locus type (n1n1n2n2li3li3) [31] and two four-locus types (n1n1n2n2li3li3li4li4 and n1n1n2n2li3li3n3n3) [16,32]. The industrially important lint fiber-related gene (Li3) has been isolated and associated with another MML factor GhMML4_D12 via a map-based cloning method [33]. Since some variations have been examined in many undescribed cotton fiber-related mutants [32], we believe that other genes which regulate fuzz or lint fiber initiations, besides these major loci, may exist. Moreover, no fuzzy and lintless phenotype has ever been discovered, indicating that fuzz genesbe epistatic to lint genes [16]. MD17 (N1N1n2n2n3n3) originated from the hybridization between dominant (N1N1N2N2n3n3) and recessive (n1n1n2n2n3n3) naked seed materials, suggesting that the interaction of fuzzless locus (N1N1, n2n2, and n3n3) could also result in the fiberless phenotype [20].
These mutants are significant genetic resources for identifying the genes related to fiber development. The genetic analysis and molecular mapping of these mutants are important steps in isolating these genes, and they may also offer additional clues to their organization and function.
4. Hormonal Control of Fiber Initiation and Development
Phytohormones, including auxin, gibberellin (GA), jasmonic acid (JA), ethylene (Eth), cytokinin (CK), abscisic acid (ABA), brassinosteroid (BR), salicylic acid (SA), and strigolactone (SL), are small endogenous signaling molecules in plants. Some of genes related to these hormones have been reported to regulate fiber development (Table 2).
4.1. Auxin
Auxin and gibberellin have well-elucidated roles in fiber cell initiation. For example, the addition of exogenous IAA and GA3 is indispensable for in vitro culturing of cotton unfertilized ovules [34]. Auxin plays important roles in numerous developmental process such as embryogenesis, root growth, and response to internal and external stimuli [35,36,37]. The accumulation of auxin is mainly from −5 to 10 DPA in the ovule epidermis and fiber cells, reaching its peak at 2–3 DPA [38]. Enhancing auxin levels at the appropriate time and locations during ovule and fiber development could increase cotton fiber yield [38]. Specific expression of auxin biosynthesis-related gene iaaM in cotton epidermal cells using the promoter of the petunia MADS box gene FBP7 (floral binding protein 7) was found to prominently increase the auxin level at the ovule epidermis and significantly enhance the number of lint fibers, which ultimately increased the lint yield by more than 15% [39]. Various studies have indicated that auxin is primarily imported from other tissues in the ovules and transported into fiber cells through PIN-FORMED (GhPIN)-mediated auxin polar transport, rather than in situ synthesis [40,41,42]. Ovule-specific down-regulation of auxin efflux carrier (GhPIN) genes inhibited fiber initiation [40]. Furthermore, ectopic expression of cotton GhPIN1a_Dt, GhPIN6_At, and GhPIN8_At could enhance the length and density of trichomes on Arabidopsis leaves [41,42], implying that PIN proteins could contribute to fiber initiation. In addition to biosynthesis and transportation pathways, the auxin-signaling pathway also plays a role during cotton fiber development. Ectopic expression of cotton auxin response factors GhARF2 and GhARF18 could increase trichome initiations in Arabidopsis. These genes could be key factors in fiber initiation through the regulation of several transcription factors, including MYB and basic helix–loop–helix (bHLH) genes [43]. A recent study indicated that overexpression of GhARF2b using a fiber-specific promoter could increase fiber initiation, whereas the down-regulated expression of GhARF2b resulted in fewer fibers [44]. Compared with GhARF2 and GhARF18, GhIAA16 may have a negative effect during fiber initiation. Compared to the wild-type, GhIAA16 transcripts showed higher expression immediately after flowering in a fiberless mutant [45]. Collectively, these results demonstrate the importance of auxin for fiber initiation.
4.2. GA
GA is a biologically active diterpene hormone that participates in diverse plant developmental processes, including seed germination, root and stem elongation, trichome development, and fruit ripening [46]. Overexpression of the cotton GA 20-oxidase homolog GhGA20ox1 could promote fiber initiation by regulating gibberellin synthesis [47]. DELLA protein is a negative regulatory factor in the GA-signaling pathway; through interplay with TFs or important regulators, it represses binding to target genes or transcriptional activation [48]. Cotton DELLA protein GhGAI1 shows higher expression in XZ142FLM than its wild-type counterpart, implying a negative regulatory role in fiber initiation [49]. The GID1 protein can specifically bind to biologically active GA and further form a complex with DELLA protein. The cotton GID1 homologous gene GhGID1-1 is predominantly expressed in ovules, and it is inhibited by GA, implying a negative role in fiber initiation [50].
4.3. JA
JA also plays crucial roles in regulating plant growth, including growth inhibition, trichome development, and senescence [51]. Cotton ovules grown in medium with 0.001 μM JA exhibited increased fiber cell initiation; however, a higher concentration (2.5 μM) inhibited fiber initiation, implying that the appropriate concentration of JA is important for fiber initiation [52,53]. Real-time quantitative PCR (RT-qPCR) analysis of different fiber mutants indicated that four JA biosynthesis enzymes, the allene oxide cyclase (AOC) genes (GhAOC1-GhAOC4), were expressed at higher levels in −1 DPA ovules in fiberless mutants than in linted–fuzzless and linted–fuzzed lines. The expression of these genes increased under JA treatment. Meanwhile, these AOC genes were predominant expressed in −3–1 DPA ovules, particularly at −1 DPA, indicating their important regulatory role during fiber initiation. Taken together, the appropriate upregulation of AOC expression may be crucial during fiber initiation, while excessive production of AOCs might affect normal fiber development, suggesting a dose-dependent effect on fiber initiation [54]. This is consistent with the effects of different concentrations of JA in vitro ovule cultures [52,53]. The JASMONATE ZIM-DOMAIN (JAZ) protein is one of the crucial inhibitory factors in the JA-signaling pathway. GhJAZ2 shows higher expression during the fiber initiation stage, and upregulated of GhJAZ2 expression in cotton could inhibit fiber initials [53]. We believe that the effect of JA during fiber initiation is regulated in a dose-dependent manner.
4.4. BR
BRs are a kind of steroid hormones in plants and play important roles during cotton fiber development. In cotton, the application of the BR biosynthesis inhibitor, brassinazole (Brz) to floral buds completely blocked fiber differentiation, indicating that BRs play crucial roles during fiber initiation [55]. GhDET2 encodes a steroid 5α-reductase that is highly expressed during the fiber initiation stage. The silencing of cotton GhDET2 inhibited fiber initiation, while upregulation of GhDET2 expression driven by the seed coat-specific promoter pFBP7 enhanced fiber numbers [56]. The components of the BR-signaling pathway have been found to function during fiber development. 14-3-3 proteins play vital roles in the BR-signaling pathway. Suppressing the expression of cotton Gh14-3-3L significantly decreased the initiation of fiber cells, and a further analysis showed that Gh14-3-3L could interact with GhBZR1 to regulate fiber development [57]. Collectively, BRs mainly contribute to fiber initiation.
4.5. Cytokinin
Cytokinin plays a crucial role in plants, including in cell division, apical dominance, and the senescence of plant tissues and organs [58]. Supplementation of cotton ovule culture medium with exogenous cytokinin promotes the growth of ovules which imply that cytokinin plays an important role in ovule development [59]. Cytokinin oxidase/dehydrogenase (CKX) catalyzes the cleavage of the unsaturated side chain of cytokinin N6 and results in the loss of cytokinin activity, which is an important negative regulatory factor in cytokinin metabolism [60]. In conclusion, we suggest that cytokinin is required for fiber cell initiation in cotton.
4.6. ABA
ABA primarily functions in seed dormancy and stress responses [61]. In vitro application of ABA inhibited the initiation of cotton fibers, and this inhibitory effect was highly correlated with ABA levels [34]. Moreover, ABA also shows higher levels during the early stages of fiber formation in XZ142FLM lines [62]. All this evidence indicates that ABA could inhibit fiber cell initiation.
Moreover, there may be crosstalk between different phytohormones in the regulation of cotton fiber initiation. The cotton DELLA protein GhGAI1 inhibits fiber initiations, but adding IAA and BR greatly reduced the expression level of GhGAI1 at 0 DPA [49], indicating that auxin and BR may promote fiber initiation by repressing the DELLA protein GhGAI1, which connects auxin-, GA-, and BR-signaling during cotton fiber initiation (Figure 2).
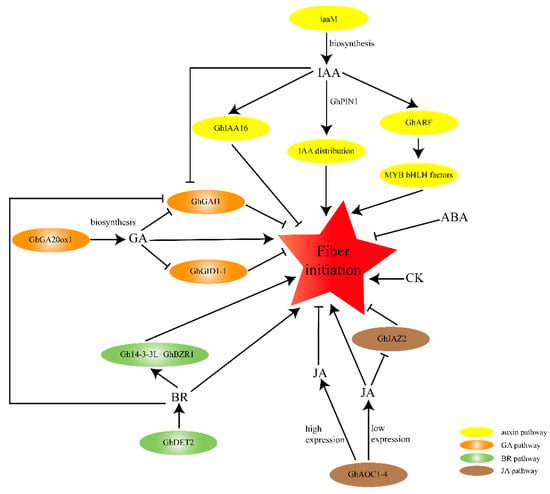
Figure 2.
A schematic model of phytohormones of regulation of cotton fiber initiation by phytohormones. The arrows indicate the promotional effects and bars represent the inhibitory effects. Red five-pointed star represents fiber initiation; Yellow ovals indicate the auxin-signaling pathway; orange ovals represent the GA-signaling pathway; green ovals indicate the BR-signaling pathway; and brown ovals represent the JA-signaling pathway.

Table 2.
Key phytohormone-related genes in regulating cotton fiber initiation.
Table 2.
Key phytohormone-related genes in regulating cotton fiber initiation.
| Gene Type | Gene | Gene Function | References |
|---|---|---|---|
| Auxin-related | iaaM | Positive regulator of fiber initiation | [39] |
| GhPIN3a | Positive regulator of fiber initiation | [40] | |
| GhPIN1a_Dt | Possible positive regulator of fiber initiation | [41,42] | |
| GhPIN6_At | Possible positive regulator of fiber initiation | [41,42] | |
| GhPIN8_At | Possible positive regulator of fiber initiation | [41,42] | |
| GhARF2 | Possible positive regulator of fiber initiation | [43] | |
| GhARF18 | Possible positive regulator of fiber initiation | [43] | |
| GhARF2b | Positive regulator of fiber initiation | [44] | |
| GhIAA16 | Negative regulator of fiber initiation | [45] | |
| GA-related | GhGA20ox1 | Positive regulator of fiber initiation | [47] |
| GhGAI1 | Negative regulator of fiber initiation | [49] | |
| GhGID1-1 | Negative regulator of fiber initiation | [50] | |
| JA-related | GhJAZ2 | Negative regulator of fiber initiation | [53] |
| GhAOC1-4 | Dosage effect on fiber initiation | [54] | |
| BR-related | GhDET2 | Positive regulator of fiber initiation | [56] |
| Gh14-3-3L | Positive regulator of fiber initiation | [57] | |
| CK-related | GhCKXs | Negative regulator of fiber initiation | [60] |
5. Transcription Factors Involved in Fiber Initiation
Transcription factors play important roles in plant growth and development by regulating the transcription rate of downstream genes. In recent years, an increasing number of TFs have been reported to be involved in cotton fiber initiation [8,9,63,64,65] (Table 3).
5.1. MYB Transcription Factors
Over 400 MYB genes are preferentially expressed in at least one stage of fiber development. Suppression of the expression of GhMYB109 leads to a partial (~8%) reduction in fiber initials, indicating that it plays a role in fiber initiation [66]. GhCPC is an R3-type MYB transcription factor; down-regulation of GhCPC expression in cotton leads to a reduction in fiber initiation [67]. Knock down of GhMYB212 expression leads to shorter fibers and a lower lint index [68]. Recent evidence has indicated that genetic variations in MYB5_A12 are associated with fiber initiation in tetraploid cotton [69]. The MIXTA factors are part of subgroup 9 (SBG9) from the R2R3 MYB family. Ten pairs of MIXTA (GhMMLs) homologs were isolated and identified in allotetraploid cotton [2]. The N1N1 loci was identified as a defective allele of the At-subgenome homoeology of GhMYB25-like_At or GhMML3_A12 that generates siRNAs from its 3′ end because the production of the overlapping antisense transcript post-transcriptionally silences both homoeologs of this gene [15]. Recently, we confirmed that the lint fiber factor GhMML4_D12 interacts with the WD40-repeat protein GhWDR in regulating cotton fiber development [33,70]. The combined form between them is similar but different to the MYB-bHLH-WD40 (MBW) complex in its regulation of trichome development, implying that cotton probably evolved a unique and independent process in the regulation of fiber initiation [64]. This finding also improved our understanding of the important function of MML genes in plants and in the regulation of cotton fiber production [64]. It is interesting that GhMML4_D12 is arranged in tandem with GhMML3. These two closely related MIXTA genes regulate fiber initiation in two specialized cell forms (lint and fuzz fibers) [15,33,64]. GhMYB25 (GhMML7)-silencing transgenic lines delay fiber initiation, but their overexpression leads to increased fiber initiation [71]. The reduced expression of GhMYB109 in GhMYB25-silencing lines suggests that GhMYB109 most likely acts downstream of GhMYB25 [71]. Moreover, L1 box protein GbML1 regulates fiber development through interaction with GbMYB25 [72]. GhMYB25-like plays a critical role during fiber initiation, as the knock down expression of GhMYB25-like results in fuzzlessness, with fewer lint fibers on cotton seeds, similarly to the N1NSM mutant [73]. GhMYB25-like seems to be upstream of the other fiber-related MYB transcription factors. The expression of GhMYB25 transcripts was almost abolished in GhMYB25-like-silenced lines, and GhMYB109 also showed significant reduction. The siRNAs produced by GhMML3_A12 in N1NSM plants may be involved in the gene silencing of MYB factors, including GhMYB109, GhMYB25, and GhMML4 [15], which certainly confirms that GhMYB25-like acts upstream of these genes. Collectively, MYB genes, especially MIXTA factors, play important roles during cotton fiber initiation.
5.2. Homeodomain Leucine Zipper (HD-ZIP) Transcription Factors
HD-ZIP factors primarily function in regulating plant growth and development [74]. In cotton, one HD-ZIP transcription factor, GhHD-1, was identified; silencing this gene in cotton delayed the initiation of fibers, while upregulating the expression of GhHD-1 enhanced the number of fiber initials [75]. GhHD-1 transcripts were significantly decreased in GhMYB25-like-silenced lines, implying that GhHD-1 acts downstream of GhMYB25-like [75]. A further analysis in transgenic lines showed that the expression of GhHD-1 was not correlated with GhMYB109 and GhMYB25, indicating that these genes may act in independent pathways to regulate fiber initiation. Cotton PROTODERMAL FACTOR1 gene (GbPDF1)-silenced transgenic lines had delayed fiber initiation [76], and GhHD-1 may be directly involved in the regulation of GbPDF1 through the core cis-element HDZIP2ATATHB2 [75].
5.3. TCP Transcription Factors
TCP proteins belong to a class of plant-specific transcription factors that play important roles in many biological processes in plants. Down-regulation of the expression of the TCP transcription factor GbTCP reduced the lint percentage [77]. GhTCP14 is mainly expressed in fiber cells, especially during the initiation and elongation stages, and overexpression of GhTCP14 in Arabidopsis enhanced the initiation of trichomes and root hairs. A further analysis indicated that GhTCP14 binds to the promoters of PIN2, IAA3, and AUX1, implying that GhTCP14 may act as a key regulator in the auxin-mediated differentiation of cotton fiber cell [78]. Recently, Wang discovered that GhTCP14 is a kind of clock-regulated factor that targets genes crucial for translation and mitochondrial energy production, such as RPL6 and ATP5F1D, indicating that fiber-related TFs are associated with clock-controlled genes [79].
5.4. WRKY Transcription Factors
Plant-specific WRKY proteins regulate various developmental and physiological processes. A recent study showed that GhWRKY16 could contribute to cotton fiber initiation and elongation by directly binding to the promoter of GhMYB25, GhMYB109, GhHOX3, and GhCesA6D-D11. Moreover, GhWRKY16 is phosphorylated via the mitogen-activated protein kinase GhMPK3-1; and phosphorylated GhWRKY16 further promotes the transcription of downstream genes [80].
We found crosstalk between the phytohormone signaling pathways and transcription factors (Figure 3). The JAZ protein GhJAZ2 negatively regulates fiber initiation by interacting with GhMYB25-like [53], which connects JA signaling with the MIXTA factor. Solexa sequencing and Affymetrix gene chip analysis indicated that GbTCP has a positive regulatory effect on JA levels [77]. Moreover, GhTCP14 binds to the promoters of auxin-related genes (PIN2, IAA3, and AUX1) to regulate cotton fiber initiation [78]. These findings demonstrate the relationship between TCP TFs and the JA and the auxin-signaling pathways (Figure 3).
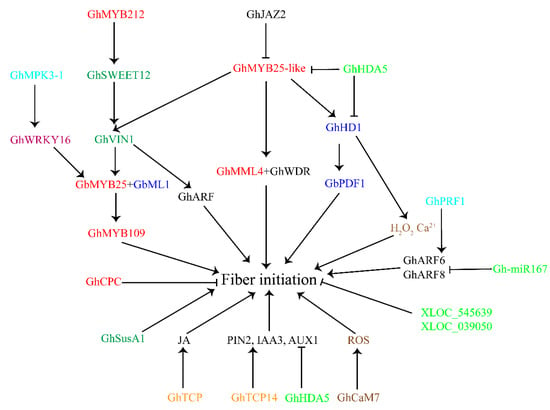
Figure 3.
Diagram summarizing the roles of transcription factors, sugar signaling, small signaling molecules, and non-coding RNAs in cotton fiber initiation. The arrows indicate the promotional effects and bars represent the inhibitory effects. Red font represents MYB transcription factors; dark blue font indicates HD-ZIP transcription factors; purple font represents WRKY transcription factors; orange font indicates TCP transcription factors; dark green font represents sugar-signaling-related genes; brown font indicates small signaling molecules-related genes; light green font represents non-coding RNAs; light blue font indicates other functional genes.

Table 3.
Key transcription factors in regulating cotton fiber initiation.
Table 3.
Key transcription factors in regulating cotton fiber initiation.
| Gene Type | Gene | Gene Function | Reference |
|---|---|---|---|
| MYB | GhMYB109 | Positive regulator of fiber initiation | [66] |
| GhCPC | Negative regulator of fiber initiation | [67] | |
| GhMYB212 | Positive regulator of fiber initiation | [68] | |
| MYB5_A12 | Positive regulator of fiber initiation | [69] | |
| GhMML3_A12(GhMYB25-like) | Positive regulator of fiber initiation | [15,73] | |
| GhMML4_D12 | Positive regulator of fiber initiation | [33,70] | |
| GhMYB25 (GhMML7) | Positive regulator of fiber initiation | [71] | |
| HD-ZIP | GbML1 | Possible positive regulator of fiber initiation | [72] |
| GhHD-1 | Positive regulator of fiber initiation | [75] | |
| GbPDF1 | Positive regulator of fiber initiation | [76] | |
| TCP | GbTCP | Positive regulator of fiber initiation | [77] |
| GhTCP14 | Positive regulator of fiber initiation | [78,79] | |
| WRKY | GhMPK3-1 | Positive regulator of fiber initiation | [80] |
6. Sugar Signaling for Fiber Initiation
Developing cotton fibers are highly active sink cells that require sucrose (Suc) for differentiation, rapid expansion, and cellulose synthesis [81]. Cotton fibers need carbon from Suc, which is unloaded from the phloem of the ovule integument or seed coat. Before use, Suc should be split into either UDP-glucose (UDPG) and fructose (Fru) via sucrose synthase (Sus, EC 2.4.1.13) or hydrolyzed into glucose (Glc) and Fru via invertase (INV, EC 3.2.1.26) [81]. Compared to wild-type, fiber mutants (XZ142FLM and Li1) had increased Sus activity during the fiber cell initiation stage, implying that Sus probably acts as an early indicator for fiber initiation [82]. Sucrose transport proteins (SUTs) are important regulators of carbon allocation in plants [81]. Overexpression of the fungal SUT gene (UmSrt1) in the ovule epidermis and fibers enhanced the content of sugar, leading to an increase in fiber initials in cotton [83]. A vacuolar invertase gene, GhVIN1, shows high expression during the fiber initiation stage. Silencing GhVIN1 resulted in a significant reduction in VIN activity and a completely fiberless phenotype [84]. A further analysis showed that GhMYB25-like might activate the transcription of VIN genes by binding to their promoters. VIN hydrolyzes Suc into glucose and fructose, thus producing hexose signals to activate the expression of GhMYB25 and other MYB-related genes. Recently, co-expression networks generated by a comparative transcriptome analysis between GhVIN1 RNA interference (RNAi) lines and XZ142FLM revealed common differentially expressed genes (DEGs) that regulate cotton fiber initiation, including GhVIN1, GhMYB25-like, GhMYB25, GhMYB109, GhPDF1, and GhHD1 [85]. A further analysis indicated that GhVIN1 and GhMYB25-like may participate in similar pathways that activating several transcription factors and plant hormone-related genes to regulate fiber initiation [85]. In addition, hexose-signaling may control the expression of genes associated with auxin biosynthesis, perception, and signal transduction [63,84]. Moreover, the fiber initiation-related factor GhMYB212 binds the promoter of the sucrose transporter gene GhSWEET12 to activate its expression; GhSWEET12 proteins transport sucrose into fiber cells. In fiber cells, GhVIN1 and GhSus catalyze sucrose to UDP-glucose or other sugars [68]. Collectively, the sugar-signaling pathway works together with MYB transcription factors and auxin-signaling to regulate cotton fiber initiation (Figure 3).
7. Small Signaling Molecules for Fiber Initiation
Ionic calcium (Ca2+) is a ubiquitous intracellular second messenger in plants and Ca2+ signaling plays an important role in the plant developmental processes [86]. Staining of cellular Ca2+ in cotton revealed that fiber cells accumulated more Ca2+ than other ovule cells, suggesting a function of Ca2+ in fiber development [87]. The expression of YC3.60 fluorescent Ca2+ marker in transgenic lines demonstrated the cellular and intracellular distribution of Ca2+ in cotton ovule epidermis and fiber cells [88].
Reactive oxygen species (ROS), including superoxide radical, hydrogen peroxide (H2O2), and hydroxyl radical, were found to regulate cell expansion in plants [89]. Using in vivo and in vitro cultured ovules, it was shown that 30% H2O2 could inhibit the retardation of fiber initials in Xinxiangxiaoji linted–fuzzless mutants (XinFLM) [90]. Pang demonstrated that the accumulation of ROS in ovule epidermal cells is essential for cotton fiber initiation [91]. The fiber-specific accumulation of Ca2+ during the initiation stage is similar to that of ROS in the ovule epidermis, suggesting a possible connection between these two signaling pathways in regulating cotton fiber initiation. The suppression of the calcium sensor GhCaM7 delayed fiber initiation, and a further analysis indicated that GhCaM7 may regulate the accumulation of ROS and serve as a molecular link between Ca2+ and the ROS-signaling pathways during early fiber development [92] (Figure 3). Recent evidence revealed that GaHD1 promotes trichome and cotton fiber initiation through cellular H2O2 and Ca2+ signals [93]. These findings provide evidence that HD-ZIP transcription factors participate in cotton fiber initiation by regulating H2O2 and Ca2+ molecules (Figure 3).
8. Non-Coding RNAs (ncRNAs) and Histone Modification for Fiber Initiation
MicroRNAs (miRNAs) are non-coding small RNAs that are 18–24 nucleotides in length and play important roles during fiber initiation. A comparative miRNA omics analysis isolated seven cotton fiber initiation-related miRNAs in developing ovules and verified, through experiments, that the targets of these miRNAs participate in different metabolic processes and cellular responses, including transcriptional regulation, auxin and GA signal transduction, lignin biosynthesis, and actin bundles [94]. miR828 and miR858 regulate the function of homoeologous MYB2 in both Arabidopsis trichome and cotton fiber development [95]. miR828 preferentially digests the transcripts of GhMYB2_Dt to generate trans-acting siRNAs (ta-siRNAs) [95]. These fiber-related genes can employ the derived siRNAs to target other downstream genes in the coordination of fiber cell development. A differential analysis of microRNAs between XZ142FLM and wild-type plants during fiber initiation identified 26 fiber initiation-related miRNAs, which target transcription factor-coding genes such as MYB, ARF, and LRR [96]. Recently, constitutive expression of the negative auxin-signaling regulatory Gh-miR167 (35S-MIM167) in cotton resulted in irregular fiber formation during the fiber initiation stage. A further analysis showed that, as the expression of ARF6 and ARF8 increased, the appearance of fiber initials on the surface of Gh-miR167 diminution lines was reduced [97], which suggests a coordination between plant hormone and regulatory miRNAs during cotton fiber initiation.
Long non-coding RNAs (lncRNAs) are transcripts with a length of at least 200 bp, which possess no significant coding ability and have been reported to contribute to fiber initiation. Wang found that most lncRNAs showed preferential expression in wild-type cotton ovules during the fiber initiation stage [98]. Specifically, the lncRNA LINC02 had significantly higher transcription levels in wild-type lines compared to fiberless mutants, implying that this gene may contribute to fiber initiation [98]. Silencing two lncRNAs (XLOC_545639 and XLOC_039050) in XZ142FLM via a virus-induced gene-silencing (VIGS) system increased the number of fiber initials on the ovules. This was the first functional identification of lncRNAs involved in fiber initiation, providing a basis for a deeper understanding of lncRNA functions during cotton fiber development [99]. Recently, 3288 lncRNAs were identified via high-throughput sequencing in a novel glabrous mutant ZM24fl, among them, a key lncRNA, MSTRG 2723.1, was isolated, which may activate the transcription of genes involved in pectin metabolism, the GhMYB25-mediating pathway, and the fatty acid metabolism to regulate fiber initiation in the ZM24 cultivar [100]. Taken together, the characterization and expression analysis of ncRNAs will contribute to future investigations of their roles in cotton fiber development.
Histone modification regulates gene expression in eukaryotes. The cotton histone deacetylase 5 gene GhHDA5 is prominently expressed during fiber initiation; downregulated expression of GhHDA5 suppressed fiber initiation and lint yield. Moreover, alterations in ROS homeostasis and increased autophagic cell death were found in 0 DPA ovules of the GhHDA5 RNAi lines [101]. A further analysis indicated that the expression of fiber initiation-related factors GhMYB25-like and GhHD1 was dramatically reduced in GhHDA5 RNAi lines compared to the control [101]. Moreover, the addition of an exogenous histone deacetylation inhibitor (trichostatin A (TSA)) inhibited fiber initiation in in vitro culture of ovules. Further studies revealed that histone deacetylation could regulate some important phytohormone-related genes, thereby activating auxin, GA, and JA signaling pathways, while repressing ABA synthesis and signaling to promote fiber cell initiation [102]. These results indicate that there is crosstalk between histone deacetylation, with key transcription factors, and phytohormone pathways in the regulation of cotton fiber initiation.
9. Other Functional Genes for Fiber Initiation
Arabinogalactan proteins (AGPs) regulate many aspects of development in plants. The suppression of the fasciclin-like AGP GhFLA1 in cotton led to the retardation of fiber initiation and elongation [103]. A further analysis suggested that GhFLA1 may play a role in cotton fiber development by regulating the composition of AGPs and the integrity of the primary cell wall matrix. Sphingolipids are bioactive molecules and crucial components of biomembranes that play roles in various biological processes, including plant growth, developmental regulation, and responses to stimuli. A comparative metabolomics analysis between XZ142FLM, wild-type, and Xinxiangxiaoji lintless–fuzzless mutant (Xinfl) plants revealed that sphingolipids and sterols play important roles during cotton fiber initiation [104]. Moreover, overexpressing of the cotton ceramide synthase gene GhCS1 could inhibited fiber initiation and elongation by promoting the synthesis of ceramides, including dihydroxy long-chain bases and very-long-chain fatty acids [105]. The cytoskeleton-related profilin gene family have undergone strong purifying selection during cotton domestication. The over expression of the fiber-specific profilin gene GhPRF1 could promote fiber cell initials; counter to no fiber cell initiation in RNAi lines, deep studies of the RNAi lines showed that auxin- and JA-signaling genes, together with sugar metabolism genes, were consistent with respect to the reduction in GhPRF1 transcript levels [106], which further connect profilin protein with the phytohormone-signaling pathway and sugar metabolism in regulation of cotton fiber initiation (Figure 3).
10. Conclusions and Future Perspectives
Along with more high-quality genomes in cotton species being completed and the usage of mature gene operation technologies and methods of omics analysis, complicated regulatory networks and metabolic pathways will be revealed to offer new functional genes for cotton breeders in the improvement of crops. Understanding the factors that contribute to fiber initiation helps us to uncover the mechanisms and improve the yield potential of fiber. Here, we need a systematic and comprehensive genetic identification and regulatory analysis of functional genes related to cotton fiber initiation, which will broaden the possibilities for fundamental research in cotton biology.
In recent decades, several important genes associated with cotton fiber initiation have been identified and described. In this review, we summarized the different kinds of factors that contribute to this process and the crosstalk between them. We believe that, in regulating fiber initiation, different factors orchestrate together to form a network. MYB factors, especially the MIXTA-like gene GhMML3 (GhMYB25-like), appear to be the most upstream transcription factors in the regulation of fiber initiation (Figure 3). Phytohormones, including auxin, GA, JA, BR and CK, can increase the amount of fiber initiation by regulating specific transcription factors (Figure 2). Of course, different hormone-signaling pathways and transcription factors can communication with each other. The cotton JAZ protein GhJAZ2 interacts with GhMYB25-like to control fiber initiation, indicating a direct role for JA in fiber initiation. IAA and BR can inhibit the expression of the cotton DELLA protein GhGAI1, which connects the auxin-, BR-, and the GA-signaling pathway (Figure 2). TCP transcription factors (GbTCP and GhTCP14) function during fiber initiation by regulating the level of JA or the transcription level of auxin-related genes (Figure 3)., The expression of cotton homologs associated with MIXTA, MYB5, GL2, and eight genes in the auxin, BR, GA, and ethylene pathways is activated during fiber development but this was inhibited in N1NSM [10], implying a synergistic effect between transcription factors and phytohormones in the regulation of fiber initiation. Moreover, sugar-signaling pathways play important roles in cotton fiber initiation. Knock down of GhVIN1 resulted in fiberless seeds [84], similarly to GhMYB25-like-silenced transgenic lines [73]. Evidence showed that GhMYB25-like may bind to the promoter of GhVIN1 to enhance its transcriptional level [63]. A recent study showed that GhMYB25-like and GhVIN1 may have similar functions during fiber initiation through the regulation of several transcription factors (such as GhMYB25, GhMYB109, GhPDF1, and GhHD1) and phytohormone-related genes (such as GhGA20ox1 and GhDET2) [85]. However, the elaborate crosstalk between these two important genes remains to be further studied. Small signaling molecules also interacts with transcription factors in the regulation of cotton fiber initiation. Newly research has shown that GaHD1 contributes to trichome and fiber initiation by regulating ROS and Ca2+ signals (Figure 3). Moreover, the phytohormone-related regulation pathways also interact with ncRNAs, histone modification genes, and functional genes during cotton fiber initiation (Figure 3). Decreased fiber initials on the surface of Gh-miR167 diminution ovules due to increased expression of ARF6 and ARF8 indicated that ncRNAs could control fiber initiation through the regulation of auxin response factors (Figure 3). Histone deacetylation could affect fiber initiation by controlling phytohormone-related genes (Figure 3). The cotton profilin gene GhPRF1 could contribute to cotton fiber initiation by controlling the expression level of auxin and JA-related genes (Figure 3). Notably, cotton fiber initiation is a complicated process that requires comprehensive alterations in gene expression through developmental and physiological pathways. Among them, transcription factors, especially MIXTA-like genes; phytohormone signals, especially auxin with GA-related genes; and sugar metabolism play dominant roles in regulating cotton fiber initiation. The coordination of various functional genes and signaling pathways integratesmany endogenous and exogenous factors into the process.
With the rapid development of biotechnology, more functional genes and regulation pathways involved in fiber initiation will be identified. Recent technological advances in single-cell sequencing (scRNA-seq) provide new tools for understanding the process of fiber initiation. Wang revealed a fiber-specific circadian-regulated gene expression program during cotton fiber development using scRNA-seq [79]. This research suggests that manipulating the circadian clock in fiber cells could be used to enhance fiber yield. A comparative analysis of the scRNA-seq data between XZ142FLM and wild-type plants further demonstrated the key roles of GhMYB25-like in the regulation of fiber initiation [107]. Future work will discover more functional genes and regulation pathways involved in cotton fiber initiation. We believe that more interactions between different kinds of genes will be found to enrich the network.
The biological breeding technology promoted by molecular biology has broken through the limitations of traditional breeding, making crop breeding more precise and efficient. The application of modern biotechnology in breeding will inevitably accelerate the breeding speed, shorten the breeding period, improve the breeding outcomes and pave the way for cotton variety improvement. We believe these findings and summaries provided in this review, especially those on the important functional genes responsible for fiber initiation, combined with modern molecular biology technology will be helpful in improving the yield in future cotton breeding work.
Author Contributions
Conceptualization, Z.Z. and K.Z. Writing—original draft preparation: Z.Z., K.Z., Y.F., Y.Y., X.C. and L.L. Writing—review and editing: X.C., L.L. and Y.T. Supervision: Y.T. Project management: Y.T. Funding acquisition: Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
The work was financially supported by Natural Science Foundation of China (32200284) and Natural Science Foundation of Jiangsu Province (BK20210877).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paterson, A.H.; Wendel, J.F.; Gundlach, H.; Guo, H.; Jenkins, J.; Jin, D.; Llewellyn, D.; Showmaker, K.C.; Shu, S.; Udall, J.; et al. Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibres. Nature 2012, 492, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Hu, Y.; Jiang, W.; Fang, L.; Guan, X.; Chen, J.; Zhang, J.; Saski, C.A.; Scheffler, B.E.; Stelly, D.M.; et al. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat. Biotechnol. 2015, 33, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Haigler, C.H.; Betancur, L.; Stiff, M.R.; Tuttle, J.R. Cotton fiber: A powerful single-cell model for cell wall and cellulose research. Front. Plant Sci. 2012, 3, 104. [Google Scholar] [CrossRef] [PubMed]
- Avci, U.; Pattathil, S.; Singh, B.; Brown, V.L.; Hahn, M.G.; Haigler, C.H. Cotton fiber cell walls of Gossypium hirsutum and Gossypium barbadense have differences related to loosely-bound xyloglucan. PLoS ONE 2013, 8, e56315. [Google Scholar] [CrossRef]
- Ijaz, B.; Zhao, N.; Kong, J.; Hua, J. Fiber quality improvement in upland cotton (Gossypium hirsutum L.): Quantitative trait loci mapping and marker assisted selection application. Front. Plant Sci. 2019, 10, 1585. [Google Scholar] [CrossRef]
- Yang, Z.; Qanmber, G.; Wang, Z.; Yang, Z.; Li, F. Gossypium genomics: Trends, scope, and utilization for cotton improvement. Trends Plant Sci. 2020, 25, 488–500. [Google Scholar] [CrossRef]
- Xiao, G.; Zhao, P.; Zhang, Y. A pivotal role of hormones in regulating cotton fiber development. Front. Plant Sci. 2019, 10, 87. [Google Scholar] [CrossRef]
- Huang, G.; Huang, J.Q.; Chen, X.Y.; Zhu, Y.X. Recent advances and future perspectives in cotton research. Annu. Rev. Plant Biol. 2021, 72, 437–462. [Google Scholar] [CrossRef]
- Wen, X.; Chen, Z.; Yang, Z.; Wang, M.; Jin, S.; Wang, G.; Zhang, L.; Wang, L.; Li, J.; Saeed, S.; et al. A comprehensive overview of cotton genomics, biotechnology and molecular biological studies. Sci. China Life Sci. 2023, 66, 2214–2256. [Google Scholar] [CrossRef]
- Samuel Yang, S.; Cheung, F.; Lee, J.J.; Ha, M.; Wei, N.E.; Sze, S.H.; Stelly, D.M.; Thaxton, P.; Triplett, B.; Town, C.D.; et al. Accumulation of genome-specific transcripts, transcription factors and phytohormonal regulators during early stages of fiber cell development in allotetraploid cotton. Plant J. 2006, 47, 761–775. [Google Scholar] [CrossRef]
- Comparative proteomic and biochemical analyses reveal different molecular events occurring in the process of fiber initiation between wild-type allotetraploid cotton and its fuzzless-lintless mutant. PLoS ONE 2015, 10, e0117049.
- Yang, C.; Ye, Z. Trichomes as models for studying plant cell differentiation. Cell Mol. Life Sci. 2013, 70, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Machado, A.C.; White, R.G.; Llewellyn, D.J.; Dennis, E.S. Expression profiling identifies genes expressed early during lint fibre initiation in cotton. Plant Cell Physiol. 2006, 47, 107–127. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.L. From fuzz to fiber: Identification of genes involved in cotton fiber elongation. Plant Physiol. 2020, 183, 23–24. [Google Scholar] [CrossRef]
- Wan, Q.; Guan, X.; Yang, N.; Wu, H.; Pan, M.; Liu, B.; Fang, L.; Yang, S.; Hu, Y.; Ye, W.; et al. Small interfering RNAs from bidirectional transcripts of GhMML3_A12 regulate cotton fiber development. New Phytol. 2016, 210, 1298–1310. [Google Scholar] [CrossRef]
- Du, X.M.; Pan, J.J.; Wang, R.H.; Zhang, T.Z.; Shi, Y.Z. Genetic analysis of presence and absence of lint and fuzz in cotton. Plant Breed. 2001, 120, 519–522. [Google Scholar] [CrossRef]
- Turley, R.B.; Kloth, R.H. Identification of a third fuzzless seed locus in upland cotton (Gossypium hirsutum L.). J. Hered. 2002, 93, 359–364. [Google Scholar] [CrossRef]
- Rong, J.; Pierce, G.J.; Waghmare, V.N.; Rogers, C.J.; Desai, A.; Chee, P.W.; May, O.L.; Gannaway, J.R.; Wendel, J.F.; Wilkins, T.A.; et al. Genetic mapping and comparative analysis of seven mutants related to seed fiber development in cotton. Theor. Appl. Genet. 2005, 111, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.H.; Yuan, Y.; Stiller, W.; Jia, Y.; Wang, P.; Pan, Z.; Du, X.; Llewellyn, D.; Wilson, I. Genetic dissection of the fuzzless seed trait in Gossypium barbadense. J. Exp. Bot. 2018, 69, 997–1009. [Google Scholar] [CrossRef]
- Turley, R.B. Registration of MD 17 fiberless upland cotton as a genetic stock. Crop Sci. 2002, 42, 994–995. [Google Scholar] [CrossRef]
- Bechere, E.; Auld, D.L.; Hequet, E. Development of ‘naked-tufted’ seed coat mutants for potential use in cotton production. Euphytica 2009, 167, 333–339. [Google Scholar] [CrossRef]
- Bechere, E.; Turley, R.B.; Auld, D.L.; Zeng, L. A new fuzzless seed locus in an upland cotton (Gossypium hirsutum L.) mutant. Am. J. Plant Sci. 2012, 3, 799–804. [Google Scholar] [CrossRef]
- Wang, X.; Miao, Y.; Cai, Y.; Sun, G.; Jia, Y.; Song, S.; Pan, Z.; Zhang, Y.; Wang, L.; Fu, G.; et al. Large-fragment insertion activates gene GaFZ (Ga08G0121) and associated with fuzz and trichome reduction in cotton (Gossypium arboretum). Plant. Biotechnol. J. 2021, 19, 1110–1124. [Google Scholar] [CrossRef]
- Kohel, R.J. Linkage tests in upland cotton, Gossypium hirsutum L. II. Crop Sci. 1972, 12, 66–69. [Google Scholar] [CrossRef]
- Karaca, M.; Saha, S.; Jenkins, J.N.; Zipf, A.; Kohel, R.; Stelly, D.M. Simple sequence repeat (SSR) markers linked to the Ligon lintless (Li1) mutant in cotton. J. Hered. 2002, 93, 221–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, Y.; Liang, W.; Shen, W.; Feng, H.; Chen, J.; Si, Z.; Hu, Y.; Zhang, T. G65V substitution in actin disturbs polymerization leading to inhibited cell elongation in cotton. Front. Plant Sci. 2019, 10, 1486. [Google Scholar] [CrossRef]
- Narbuth, E.V.; Kohel, R.J. Inheritance and linkage analysis of a new fiber mutant in cotton. J. Hered. 1990, 81, 131–133. [Google Scholar]
- Patel, J.D.; Huang, X.; Lin, L.; Das, S.; Chandnani, R.; Khanal, S.; Adhikari, J.; Shehzad, T.; Guo, H.; Roy-Zokan, E.M.; et al. The Ligon lintless-2 short fiber mutation is located within a terminal deletion of chromosome 18 in cotton. Plant Physiol. 2020, 183, 277–288. [Google Scholar] [CrossRef]
- Naoumkina, M.; Thyssen, G.N.; Fang, D.D.; Florane, C.B.; Li, P. A deletion/duplication in the Ligon lintless-2 locus induces siRNAs that inhibit cotton fiber cell elongation. Plant Physiol. 2022, 190, 1792–1805. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Tong, X.; Liu, F.; Lv, F.; Wang, H.; Zhang, T.; Guo, W. Discovery and identification of a novel Ligon lintless-like mutant (Lix) similar to the Ligon lintless (Li1) in allotetraploid cotton. Theor. Appl. Genet. 2013, 126, 963–970. [Google Scholar] [CrossRef]
- Zhang, T.Z.; Pang, J.J. Genetic analysis of a fuzzless-lintless mutant in Gossypium hirsutum L. Jiangsu J. Agricul. Sci. 1991, 7, 13–16. [Google Scholar]
- Turley, R.B.; Kloth, R.H. The inheritance model for the fiberless trait in upland cotton (Gossypium hirsutum L.) line SL1-7-1: Variation on a theme. Euphytica 2008, 164, 123–132. [Google Scholar] [CrossRef]
- Wu, H.; Tian, Y.; Wan, Q.; Fang, L.; Guan, X.; Chen, J.; Hu, Y.; Ye, W.; Zhang, H.; Guo, W.; et al. Genetics and evolution of MIXTA genes regulating cotton lint fiber development. New Phytol. 2018, 217, 883–895. [Google Scholar] [CrossRef]
- Beasley, C.A.; Ting, I.P. The effects of plant growth substances on in vitro fiber development from fertilized cotton ovules. Amer. J. Bot. 1973, 60, 130–139. [Google Scholar] [CrossRef]
- Wójcik, A.M.; Wójcikowska, B.; Gaj, M.D. Current perspectives on the auxin-mediated genetic network that controls the induction of somatic embryogenesis in plants. Int. J. Mol. Sci. 2020, 21, 1333. [Google Scholar] [CrossRef]
- Dubrovsky, J.G.; Sauer, M.; Mendivil, S.N.; Ivanchenko, M.G.; Friml, J.; Shishkova, S.; Celenza, J.; Benková, E. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc. Natl. Acad. Sci. USA 2008, 105, 8790–8794. [Google Scholar] [CrossRef]
- Shani, E.; Salehin, M.; Zhang, Y.; Sanchez, S.E.; Doherty, C.; Wang, R.; Mangado, C.C.; Song, L.; Tal, I.; Pisanty, O.; et al. Plant stress tolerance requires auxin-sensitive Aux/IAA transcriptional repressors. Curr. Biol. 2017, 27, 437–444. [Google Scholar] [CrossRef]
- Chen, Z.J.; Guan, X.Y. Auxin boost for cotton. Nat. Biotechnol. 2011, 29, 407–409. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, X.; Song, S.; Zeng, Q.; Hou, L.; Li, D.; Zhao, J.; Wei, Y.; Li, X.; Luo, M.; et al. Spatiotemporal manipulation of auxin biosynthesis in cotton ovule epidermal cells enhances fiber yield and quality. Nat. Biotechnol. 2011, 29, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zeng, J.Y.; Long, H.; Xiao, Y.H.; Yan, X.Y.; Pei, Y. Auxin regulates cotton fiber initiation via GhPIN-mediated auxin transport. Plant Cell Physiol. 2017, 58, 385–397. [Google Scholar] [CrossRef]
- Zhang, Y.; He, P.; Yang, Z.; Huang, G.; Wang, L.; Pang, C.; Xiao, H.; Zhao, P.; Yu, J.; Xiao, G. A genome-scale analysis of the PIN gene family reveals its functions in cotton fiber development. Front. Plant Sci. 2017, 8, 461. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, Y.; Zeng, J.; Pei, Y. PIN-formed protein, a door to reveal the mechanism for auxin-triggered initiation of cotton fiber. Plant Signal. Behav. 2017, 12, e1319031. [Google Scholar] [CrossRef]
- Xiao, G.; He, P.; Zhao, P.; Liu, H.; Zhang, L.; Pang, C.; Yu, J. Genome-wide identification of the GhARF gene family reveals that GhARF2 and GhARF18 are involved in cotton fibre cell initiation. J. Exp. Bot. 2018, 69, 4323–4337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, J.; Huang, C.; Zheng, Z.; Liu, X.; Shangguan, X.; Wang, L.; Zhang, Y.; Chen, Z. Characterization of cotton ARF factors and the role of GhARF2b in fiber development. BMC Genom. 2021, 22, 202. [Google Scholar] [CrossRef]
- Han, X.; Xu, X.; Fang, D.D.; Zhang, T.; Guo, W. Cloning and expression analysis of novel Aux/IAA family genes in Gossypium hirsutum. Gene 2012, 503, 83–91. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin localization and transport in plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef]
- Xiao, Y.H.; Li, D.M.; Yin, M.H.; Li, X.B.; Zhang, M.; Wang, Y.J.; Dong, J.; Zhao, J.; Luo, M.; Luo, X.Y.; et al. Gibberellin 20-oxidase promotes initiation and elongation of cotton fibers by regulating gibberellin synthesis. J. Plant Physiol. 2010, 167, 829–837. [Google Scholar] [CrossRef]
- Ito, T.; Okada, K.; Fukazawa, J.; Takahashi, Y. DELLA-dependent and -independent gibberellin signaling. Plant Signal. Behav. 2018, 13, e1445933. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.Y.; Luo, M.; Xiao, Y.H.; Li, X.B.; Tan, K.L.; Hou, L.; Dong, J.; Li, D.M.; Song, S.Q.; Zhao, J.; et al. Brassinosteroids and auxin down-regulate DELLA genes in fiber initiation and elongation of cotton. Agric. Sci. China 2011, 10, 1168–1176. [Google Scholar] [CrossRef]
- Dong, J.; Yin, M.H.; Yang, F.; Zhao, J.; Qin, S.; Hou, L.; Luo, M.; Pei, Y.; Xiao, Y.H. Cloning and expression profile of gibberellin insensitive dwarf GID1 homologous genes from cotton. Acta Agron. 2009, 35, 1822–1830. [Google Scholar] [CrossRef]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef]
- Tan, J.; Tu, L.; Deng, F.; Wu, R.; Zhang, X. Exogenous jasmonic acid inhibits cotton fiber elongation. J. Plant Growth Regul. 2012, 31, 599–605. [Google Scholar] [CrossRef]
- Hu, H.; He, X.; Tu, L.; Zhu, L.; Zhu, S.; Ge, Z.; Zhang, X. GhJAZ2 negatively regulates cotton fiber initiation by interacting with the R2R3-MYB transcription factor GhMYB25-like. Plant J. 2016, 88, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, Y.; Hu, W.; Zhang, X.; Cai, C.; Guo, W. Comparative transcriptomics reveals jasmonic acid-associated metabolism related to cotton fiber initiation. PLoS ONE 2015, 10, e0129854. [Google Scholar] [CrossRef]
- Sun, Y.; Veerabomma, S.; Abdel-Mageed, H.A.; Fokar, M.; Asami, T.; Yoshida, S.; Allen, R.D. Brassinosteroid regulates fiber development on cultured cotton ovules. Plant Cell Physiol. 2005, 46, 1384–1391. [Google Scholar] [CrossRef]
- Luo, M.; Xiao, Y.; Li, X.; Lu, X.; Deng, W.; Li, D.; Hou, L.; Hu, M.; Li, Y.; Pei, Y. GhDET2, a steroid 5α-reductase, plays an important role in cotton fiber cell initiation and elongation. Plant J. 2007, 51, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Z.T.; Li, M.; Wei, X.Z.; Li, X.J.; Li, B.Y.; Li, X.B. Cotton (Gossypium hirsutum) 14-3-3 proteins participate in regulation of fibre initiation and elongation by modulating brassinosteroid signalling. Plant Biotechnol. J. 2015, 13, 269–280. [Google Scholar] [CrossRef]
- Wang, M.; Le Gourrierec, J.; Jiao, F.; Demotes-Mainard, S.; Perez-Garcia, M.D.; Oge, L.; Hamama, L.; Crespel, L.; Bertheloot, J.; Chen, J.; et al. Convergence and divergence of sugar and cytokinin signaling in plant development. Int. J. Mol. Sci. 2021, 22, 1282. [Google Scholar] [CrossRef] [PubMed]
- Beasley, C.A.; Birnbaum, E.H.; Dugger, W.M.; Ting, I.P. A quantitative procedure for estimating cotton fiber growth. Biotech. Histochem. 2009, 49, 85–92. [Google Scholar] [CrossRef]
- Niemann, M.C.E.; Weber, H.; Hluska, T.; Leonte, G.; Anderson, S.M.; Novak, O.; Senes, A.; Werner, T. The cytokinin oxidase/dehydrogenase CKX1 is a membrane-bound protein requiring homooligomerization in the endoplasmic reticulum for its cellular activity. Plant Physiol. 2018, 176, 2024–2039. [Google Scholar] [CrossRef] [PubMed]
- Tuan, P.A.; Kumar, R.; Rehal, P.K.; Toora, P.K.; Ayele, B.T. Molecular mechanisms underlying abscisic acid/gibberellin balance in the control of seed dormancy and germination in cereals. Front. Plant Sci. 2018, 9, 668. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, M.; Qiao, Z.; Yuan, S.; Wang, X.; Hua, S. Effect of phytohormones on fiber initiation of cotton ovule. Acta Physiol. Plant. 2009, 31, 979–986. [Google Scholar] [CrossRef]
- Wang, L.; Kartika, D.; Ruan, Y.L. Looking into hair tonics for cotton fiber initiation. New Phytol. 2021, 229, 1844–1851. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, T. MIXTAs and phytohormones orchestrate cotton fiber development. Curr. Opin. Plant Biol. 2021, 59, 101975. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Z.; Li, F. Updates on molecular mechanisms in the development of branched trichome in Arabidopsis and nonbranched in cotton. Plant Biotechnol. J. 2019, 17, 1706–1722. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Li, Q.; Fan, X.; Yang, W.; Xue, Y. The R2R3 MYB transcription factor GhMYB109 is required for cotton fiber development. Genetics 2008, 180, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhu, Y.; Zhang, T. The R3-MYB gene GhCPC negatively regulates cotton fiber elongation. PLoS ONE 2015, 10, e0116272. [Google Scholar] [CrossRef]
- Sun, W.; Gao, Z.; Wang, J.; Huang, Y.; Chen, Y.; Li, J.; Lv, M.; Wang, J.; Luo, M.; Zuo, K. Cotton fiber elongation requires the transcription factor GhMYB212 to regulate sucrose transportation into expanding fibers. New Phytol. 2019, 222, 864–881. [Google Scholar] [CrossRef]
- Wang, N.; Ma, Q.; Wu, M.; Pei, W.; Song, J.; Jia, B.; Liu, G.; Sun, H.; Zang, X.; Yu, S.; et al. Genetic variation in MYB5_A12 is associated with fibre initiation and elongation in tetraploid cotton. Plant Biotechnol. J. 2021, 19, 1892–1894. [Google Scholar] [CrossRef]
- Tian, Y.; Du, J.; Wu, H.; Guan, X.; Chen, W.; Hu, Y.; Fang, L.; Ding, L.; Li, M.; Yang, D.; et al. The transcription factor MML4_D12 regulates fiber development through interplay with the WD40-repeat protein WDR in cotton. J. Exp. Bot. 2020, 71, 3499–3511. [Google Scholar] [CrossRef]
- Machado, A.; Wu, Y.; Yang, Y.; Llewellyn, D.J.; Dennis, E.S. The MYB transcription factor GhMYB25 regulates early fibre and trichome development. Plant J. 2009, 59, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zuo, K.; Zhang, J.; Liu, X.; Zhang, L.; Sun, X.; Tang, K. An L1 box binding protein, GbML1, interacts with GbMYB25 to control cotton fibre development. J. Exp. Bot. 2010, 61, 3599–3613. [Google Scholar] [CrossRef]
- Walford, S.A.; Wu, Y.; Llewellyn, D.J.; Dennis, E.S. GhMYB25-like: A key factor in early cotton fibre development. Plant J. 2011, 65, 785–797. [Google Scholar] [CrossRef]
- Sharif, R.; Raza, A.; Chen, P.; Li, Y.; El-Ballat, E.M.; Rauf, A.; Hano, C.; El-Esawi, M.A. HD-ZIP gene family: Potential roles in improving plant growth and regulating stress-responsive mechanisms in plants. Genes 2021, 12, 1256. [Google Scholar] [CrossRef] [PubMed]
- Walford, S.A.; Wu, Y.; Llewellyn, D.J.; Dennis, E.S. Epidermal cell differentiation in cotton mediated by the homeodomain leucine zipper gene, GhHD-1. Plant J. 2012, 71, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Tu, L.; Tan, J.; Li, Y.; Nie, Y.; Zhang, X. GbPDF1 is involved in cotton fiber initiation via the core cis-element HDZIP2ATATHB2. Plant Physiol. 2012, 158, 890–904. [Google Scholar] [CrossRef]
- Hao, J.; Tu, L.; Hu, H.; Tan, J.; Deng, F.; Tang, W.; Nie, Y.; Zhang, X. GbTCP, a cotton TCP transcription factor, confers fibre elongation and root hair development by a complex regulating system. J. Exp. Bot. 2012, 63, 6267–6281. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhao, P.M.; Cheng, H.Q.; Han, L.B.; Wu, X.M.; Gao, P.; Wang, H.Y.; Yang, C.L.; Zhong, N.Q.; Zuo, J.R.; et al. The cotton transcription factor TCP14 functions in auxin-mediated epidermal cell differentiation and elongation. Plant Physiol. 2013, 162, 1669–1680. [Google Scholar] [CrossRef]
- Wang, D.; Hu, X.; Ye, H.; Wang, Y.; Yang, Q.; Liang, X.; Wang, Z.; Zhou, Y.; Wen, M.; Yuan, X.; et al. Cell-specific clock-controlled gene expression program regulates rhythmic fiber cell growth in cotton. Genome Biol. 2023, 24, 49. [Google Scholar] [CrossRef]
- Wang, N.N.; Li, Y.; Chen, Y.H.; Lu, R.; Zhou, L.; Wang, Y.; Zheng, Y.; Li, X.B. Phosphorylation of WRKY16 by MPK3-1 is essential for its transcriptional activity during fiber initiation and elongation in cotton (Gossypium hirsutum). Plant Cell 2021, 33, 2736–2752. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Llewellyn, D.J.; Furbank, R.T. The control of single-celled cotton fiber elongation by developmentally reversible gating of plasmodesmata and coordinated expression of sucrose and K+ transporters and expansin. Plant Cell 2001, 13, 47–60. [Google Scholar]
- Ahmed, M.; Akhtar, S.; Fanglu, M.; Hasan, M.M.; Shahid, A.A.; Yanang, X.; Sarwar, M.B.; Rao, A.Q.; Husnain, T.; Wang, X. Sucrose Synthase (SuSy) gene expression: An indicator for cotton fiber initiation and early development. Russ. J. Plant Physiol. 2019, 66, 41–49. [Google Scholar] [CrossRef]
- Ding, X.; Li, X.; Wang, L.; Zeng, J.; Huang, L.; Xiong, L.; Song, S.; Zhao, J.; Hou, L.; Wang, F.; et al. Sucrose enhanced reactive oxygen species generation promotes cotton fibre initiation and secondary cell wall deposition. Plant Biotechnol. J. 2021, 19, 1092–1094. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cook, A.; Patrick, J.W.; Chen, X.Y.; Ruan, Y.L. Silencing the vacuolar invertase gene GhVIN1 blocks cotton fiber initiation from the ovule epidermis, probably by suppressing a cohort of regulatory genes via sugar signaling. Plant J. 2014, 78, 686–696. [Google Scholar] [CrossRef]
- Li, J.; Ruan, Y.L.; Dai, F.; Zhu, S.; Zhang, T. Co-expression networks regulating cotton fiber initiation generated by comparative transcriptome analysis between fiberless XZ142FLM and GhVIN1i. Ind. Crop. Prod. 2023, 194, 116323. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2145. [Google Scholar] [CrossRef] [PubMed]
- Taliercio, E.W.; Boykin, D. Analysis of gene expression in cotton fiber initials. BMC Plant Biol. 2007, 7, 22. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, H.Z.; Hou, L.; Song, S.Q.; Zeng, J.Y.; Pei, Y. In vivo imaging of Ca2+ accumulation during cotton fiber initiation using fluorescent indicator YC3.60. Plant Cell Rep. 2017, 36, 911–918. [Google Scholar] [CrossRef]
- Liszkay, A.; van der Zalm, E.; Schopfer, P. Production of reactive oxygen intermediates (O2˙−, H2O2, and ˙OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 2004, 136, 3114–3123. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, T.; Guo, W. Effect of H2O2 on fiber initiation using fiber retardation initiation mutants in cotton (Gossypium hirsutum). J. Plant Physiol. 2010, 167, 393–399. [Google Scholar] [CrossRef]
- Pang, M.; Sanford, N.; Wilkins, T. ROS accumulation in cotton ovule epidermal cells is necessary for fiber initiation. biorvix. 2013. [Google Scholar] [CrossRef]
- Tang, W.; Tu, L.; Yang, X.; Tan, J.; Deng, F.; Hao, J.; Guo, K.; Lindsey, K.; Zhang, X. The calcium sensor GhCaM7 promotes cotton fiber elongation by modulating reactive oxygen species (ROS) production. New Phytol. 2014, 202, 509–520. [Google Scholar] [CrossRef]
- Ding, M.; Cao, Y.; He, S.; Sun, J.; Dai, H.; Zhang, H.; Sun, C.; Jiang, Y.; Paterson, A.H.; Rong, J. GaHD1, a candidate gene for the Gossypium arboreum SMA-4 mutant, promotes trichome and fiber initiation by cellular H2O2 and Ca2+ signals. Plant Mol. Biol. 2020, 103, 409–423. [Google Scholar] [CrossRef]
- Wang, Z.M.; Xue, W.; Dong, C.J.; Jin, L.G.; Bian, S.M.; Wang, C.; Wu, X.Y.; Liu, J.Y. A comparative miRNAome analysis reveals seven fiber initiation-related and 36 novel miRNAs in developing cotton ovules. Mol. Plant 2012, 5, 889–900. [Google Scholar] [CrossRef]
- Guan, X.; Pang, M.; Nah, G.; Shi, X.; Ye, W.; Stelly, D.M.; Chen, Z.J. miR828 and miR858 regulate homoeologous MYB2 gene functions in Arabidopsis trichome and cotton fibre development. Nat. Commun. 2014, 5, 3050. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Li, C.; Zhang, J.; Li, F.; Ma, L.; Tan, Y.; Wang, Q.; Zhang, B. Differential expression of microRNAs during fiber development between fuzzless-lintless mutant and its wild-type allotetraploid cotton. Sci. Rep. 2017, 7, 3. [Google Scholar] [CrossRef]
- Arora, S.; Singh, A.K.; Chaudhary, B. Coordination of floral and fiber development in cotton (Gossypium) by hormone- and flavonoid-signalling associated regulatory miRNAs. Plant Mol. Biol. 2023, 112, 1–18. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, D.; Tu, L.; Gao, W.; He, Y.; Hu, H.; Wang, P.; Liu, N.; Lindsey, K.; Zhang, X. Long noncoding RNAs and their proposed functions in fibre development of cotton (Gossypium spp.). New Phytol. 2015, 207, 1181–1197. [Google Scholar] [CrossRef]
- Hu, H.; Wang, M.; Ding, Y.; Zhu, S.; Zhao, G.; Tu, L.; Zhang, X. Transcriptomic repertoires depict the initiation of lint and fuzz fibres in cotton (Gossypium hirsutum L.). Plant Biotechnol. J. 2018, 16, 1002–1012. [Google Scholar] [CrossRef]
- Zou, X.; Ali, F.; Jin, S.; Li, F.; Wang, Z. RNA-Seq with a novel glabrous-ZM24fl reveals some key lncRNAs and the associated targets in fiber initiation of cotton. BMC Plant Biol. 2022, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, B.; Singh, S.K.; Rai, K.M.; Singh, S.P.; Sable, A.; Pant, P.; Saxena, G.; Sawant, S.V. Role of GhHDA5 in H3K9 deacetylation and fiber initiation in Gossypium hirsutum. Plant J. 2018, 95, 1069–1083. [Google Scholar] [CrossRef]
- Wei, Z.; Li, Y.; Ali, F.; Wang, Y.; Liu, J.; Yang, Z.; Wang, Z.; Xing, Y.; Li, F. Transcriptomic analysis reveals the key role of histone deacetylation via mediating different phytohormone signalings in fiber initiation of cotton. Cell Biosci. 2022, 12, 107. [Google Scholar] [CrossRef]
- Huang, G.Q.; Gong, S.Y.; Xu, W.L.; Li, W.; Li, P.; Zhang, C.J.; Li, D.D.; Zheng, Y.; Li, F.G.; Li, X.B. A fasciclin-like arabinogalactan protein, GhFLA1, is involved in fiber initiation and elongation of cotton. Plant Physiol. 2013, 161, 1278–1290. [Google Scholar] [CrossRef]
- Wang, Q.; Meng, Q.; Xu, F.; Chen, Q.; Ma, C.; Huang, L.; Li, G.; Luo, M. Comparative metabolomics analysis reveals sterols and sphingolipids play a role in cotton fiber cell initiation. Int. J. Mol. Sci. 2021, 22, 11438. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, Q.; Meng, Q.; Wang, G.; Xu, F.; Chen, Q.; Liu, F.; Hu, Y.; Luo, M. Overexpression of a ceramide synthase gene, GhCS1, inhibits fiber cell initiation and elongation by promoting the synthesis of ceramides containing dihydroxy LCB and VLCFA. Front. Plant Sci. 2022, 13, 1000348. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.K.; Chaudhary, B. Transcriptional loss of domestication-driven cytoskeletal GhPRF1 gene causes defective floral and fiber development in cotton (Gossypium). Plant Mol. Biol. 2021, 107, 519–532. [Google Scholar] [CrossRef]
- Qin, Y.; Sun, M.; Li, W.; Xu, M.; Shao, L.; Liu, Y.; Zhao, G.; Liu, Z.; Xu, Z.; You, J.; et al. Single-cell RNA-seq reveals fate determination control of an individual fibre cell initiation in cotton (Gossypium hirsutum). Plant Biotechnol. J. 2022, 20, 2372–2388. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).