New Insights in the Detection and Management of Anthracnose Diseases in Strawberries
Abstract
:1. Introduction
2. Anthracnose Diseases in Strawberry
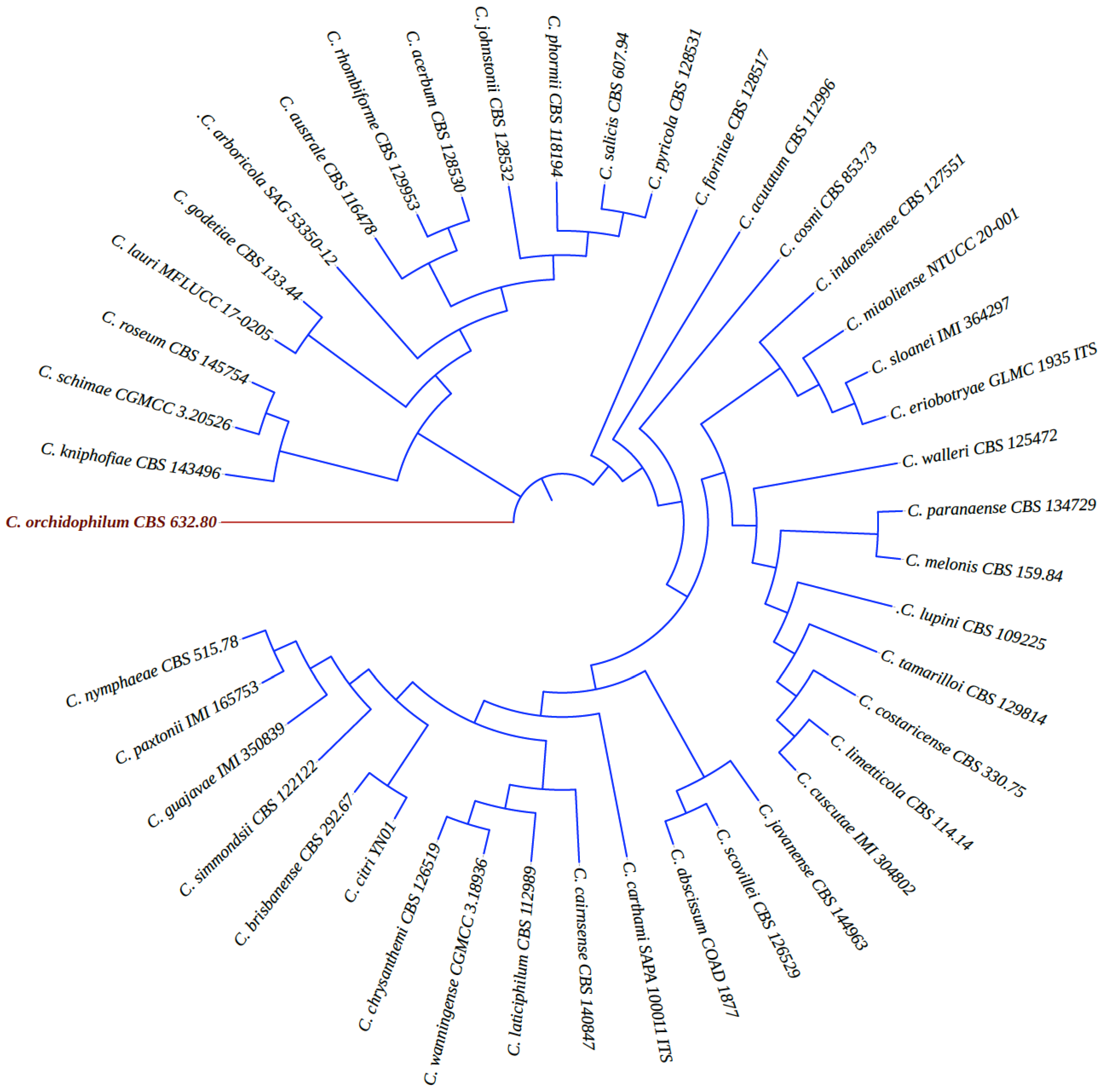
3. Taxonomy of C. acutatum
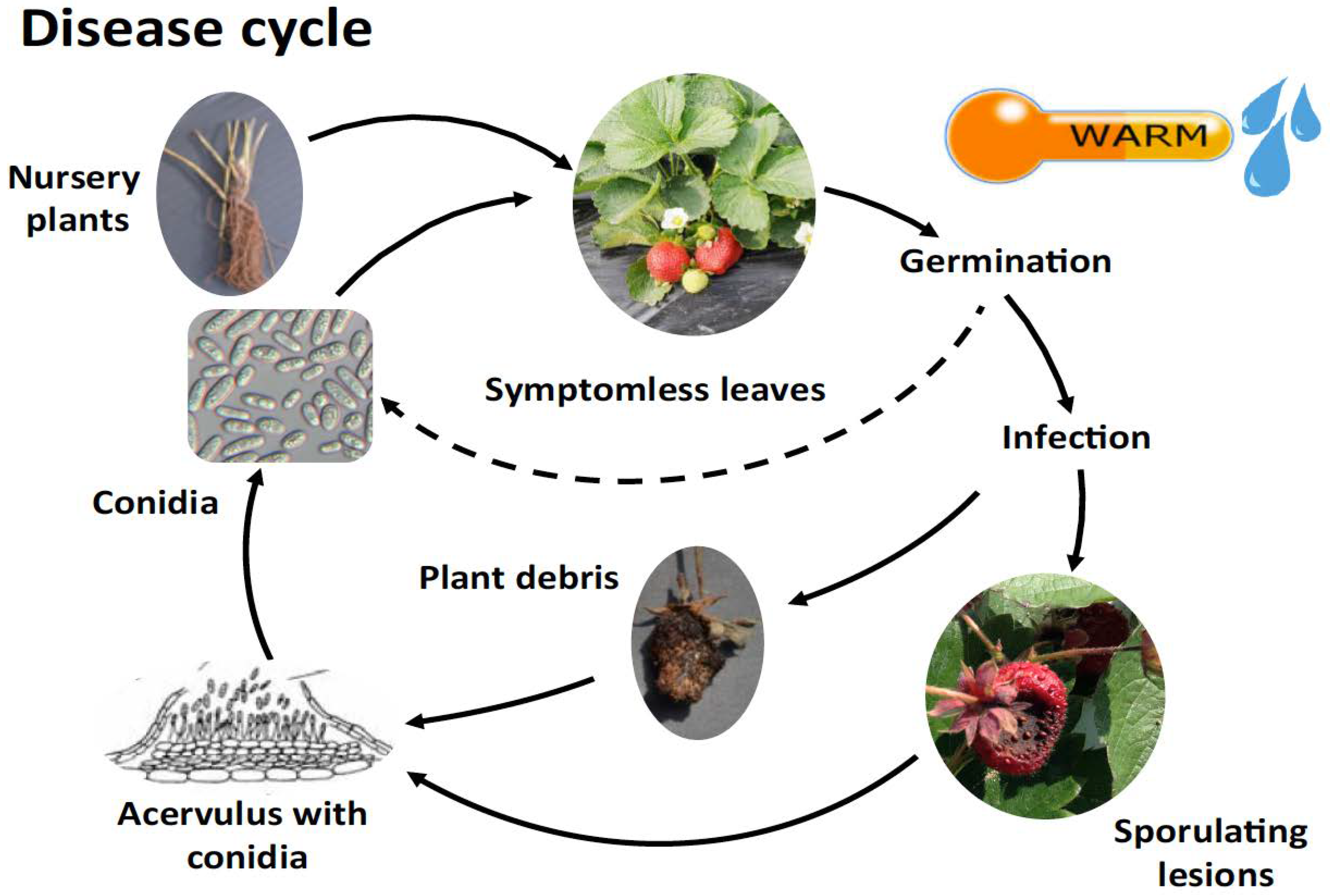
4. Epidemiology and Lifestyle of C. acutatum
5. Detection: Morphological, Molecular, and Remote Sensing
5.1. Remote Sensing of Anthracnose
5.2. Unmanned Aerial Vehicle (UAV) Platform
6. Management: Chemical, Resistance Breeding, Biological and Biorational
7. Anthracnose Diseases Management Challenges
8. Alternative and Sustainable Integrated Pest Management Strategies for Soilborne Diseases
8.1. Overview of Anaerobic Soil Disinfestation (ASD)
8.2. Optimizing Anaerobic Soil Disinfestation (ASD) with Endophytic Bacteria
9. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- FAO; FAOSTAT; Agriculture Organization of the United. FAOSTAT Statistics Database; FAO: Rome, Italy, 2021. [Google Scholar]
- Wu, F.; Guan, Z.; Whidden, A.J. An Overview of the US and Mexico Strawberry Industries. EDIS 2020, 2016, 1–4. [Google Scholar] [CrossRef]
- Samtani, J.B.; Rom, C.R.; Friedrich, H.; Fennimore, S.A.; Finn, C.E.; Petran, A.; Wallace, R.W.; Pritts, M.P.; Fernandez, G.; Chase, C.A. The status and future of the strawberry industry in the United States. HortTechnology 2019, 29, 11–24. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture (USDA). U.S. Strawberry Consumption Continues to Grow. Available online: https://www.ers.usda.gov/data-products/chart-gallery/gallery/chart-detail/?chartId=77884 (accessed on 26 October 2023).
- Brown, M. Florida strawberry production and marketing. In The Strawberry: A Book for Growers; Childers, N.F., Ed.; Dr. Norman N. Childers Publications: Gainesville, FL, USA, 2003; pp. 31–42. [Google Scholar]
- Christman, J.; Samtani, J.B. A Survey of Strawberry Production Practices in Virginia; Virginia Cooperative Extension: Blacksburg, VA, USA, 2019. [Google Scholar]
- Garrido, C.; Carbú, M.; Fernández-Acero, F.J.; González-Rodríguez, V.E.; Cantoral, J.M. New insights in the study of strawberry fungal pathogens. Genes Genomes Genom. 2011, 5, 24–39. [Google Scholar]
- Amil-Ruiz, F.; Garrido-Gala, J.; Gadea, J.; Blanco-Portales, R.; Muñoz-Mérida, A.; Trelles, O.; de Los Santos, B.; Arroyo, F.T.; Aguado-Puig, A.; Romero, F. Partial activation of SA-and JA-defensive pathways in strawberry upon Colletotrichum acutatum interaction. Front. Plant Sci. 2016, 7, 1036. [Google Scholar] [CrossRef]
- Forcelini, B.B.; Peres, N.A. Widespread resistance to QoI fungicides of Colletotrichum acutatum from strawberry nurseries and production fields. Plant Health Prog. 2018, 19, 338–341. [Google Scholar] [CrossRef]
- Dale, A.; Hughes, B.R.; Donnelly, D. The role of micropropagation in producing specific pathogen-tested plants. HortScience 2008, 43, 74–77. [Google Scholar] [CrossRef]
- Forcelini, B.B.; Gonçalves, F.P.; Peres, N.A. Effect of inoculum concentration and interrupted wetness duration on the development of anthracnose fruit rot of strawberry. Plant Dis. 2017, 101, 372–377. [Google Scholar] [CrossRef]
- Poling, E.B. Anthracnose on strawberry: Its etiology, epidemiology, and pathology, together with management strategies for strawberry nurseries: Introduction to the workshop. HortScience 2008, 43, 59–65. [Google Scholar] [CrossRef]
- Miller-Butler, M.A.; Smith, B.J.; Curry, K.J.; Blythe, E.K. Evaluation of detached strawberry leaves for anthracnose disease severity using image analysis and visual ratings. HortScience 2019, 54, 2111–2117. [Google Scholar] [CrossRef]
- Damm, U.; Cannon, P.; Woudenberg, J.; Crous, P. The Colletotrichum acutatum species complex. Stud. Mycol. 2012, 73, 37–113. [Google Scholar] [CrossRef]
- Weir, B.; Johnston, P.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef]
- Howard, C.; Albregts, E. Anthracnose of strawberry fruit caused by Glomerella cingulata in Florida. Plant Dis. 1984, 68, 824–825. [Google Scholar] [CrossRef]
- Liu, F.; Ma, Z.; Hou, L.; Diao, Y.; Wu, W.; Damm, U.; Song, S.; Cai, L. Updating species diversity of Colletotrichum, with a phylogenomic overview. Stud. Mycol. 2022, 101, 1–56. [Google Scholar] [CrossRef]
- Agusti, L.; Bonaterra, A.; Moragrega, C.; Camps, J.; Montesinos, E. Biocontrol of root rot of strawberry caused by Phytophthora cactorum with a combination of two Pseudomonas fluorescens strains. J. Plant Pathol. 2011, 93, 363–372. [Google Scholar]
- Denoyes-Rothan, B.; Guérin, G.; Délye, C.; Smith, B.; Minz, D.; Maymon, M.; Freeman, S. Genetic diversity, and pathogenic variability among isolates of Colletotrichum species from strawberry. Phytopathology 2003, 93, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Mertely, J.; Peres, N. Root Necrosis of Strawberries Caused by Colletotrichum acutatum; UF/IFAS Extension: Gainesville, FL, USA, 2008. [Google Scholar]
- Seijo, T.E.; Chandler, C.K.; Mertely, J.C.; Moyer, C.; Peres, N.A. Resistance of strawberry cultivars and advanced selections to anthracnose and Botrytis fruit rots. Proc. Fla. State Hortic. Soc. 2008, 121, 246–248. [Google Scholar]
- Simmonds, J. A study of the species of Colletotrichum causing ripe fruit rots in Queensland. Qld. J. Agric. Anim. Sci. 1966, 22, 437–459. [Google Scholar]
- Von Arx, J.A. A revision of the fungi classified as Gloeosporium. In A Revision of the Fungi Classified as Gloeosporium; Wiley: Hoboken, NJ, USA, 1970. [Google Scholar]
- Jayawardena, R.; Bhunjun, C.; Hyde, K.; Gentekaki, E.; Itthayakorn, P. Colletotrichum: Lifestyles, biology, morpho-species, species complexes and accepted species. Mycosphere 2021, 12, 519–669. [Google Scholar] [CrossRef]
- Cho, B.-J.; Choi, H.-W.; Kim, D.-H.; Kyu, L.J. Colletotrichum spp. Agents of Anthracnose on Blueberry Leaves in Gangwon Province, Korea. J. For. Environ. Sci. 2021, 37, 154–162. [Google Scholar]
- Talhinhas, P.; Baroncelli, R. Colletotrichum species and complexes: Geographic distribution, host range and conservation status. Fungal Divers. 2021, 110, 109–198. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Phukhamsakda, C.; Jayawardena, R.S.; Jeewon, R.; Promputtha, I.; Hyde, K.D. Investigating species boundaries in Colletotrichum. Fungal Divers. 2021, 107, 107–127. [Google Scholar] [CrossRef]
- Strand, L.L. Integrated Pest Management for Strawberries; UCANR Publications: Davis, CA, USA, 2008; Volume 3351. [Google Scholar]
- Yang, X.; Madden, L.; Wilson, L.; Ellis, M. Effects of surface topography and rain intensity on splash dispersal of Colletotrichum acutatum. Phytopathology 1990, 80, 1115–1120. [Google Scholar] [CrossRef]
- Smith, B.J. Epidemiology and pathology of strawberry anthracnose: A North American perspective. HortScience 2008, 43, 69–73. [Google Scholar] [CrossRef]
- Leandro, L.; Gleason, M.; Nutter, F., Jr.; Wegulo, S.; Dixon, P. Germination and sporulation of Colletotrichum acutatum on symptomless strawberry leaves. Phytopathology 2001, 91, 659–664. [Google Scholar] [CrossRef]
- Prusky, D. Pathogen quiescence in postharvest diseases. Annu. Rev. Phytopathol. 1996, 34, 413–434. [Google Scholar] [CrossRef] [PubMed]
- King, W.; Madden, L.; Ellis, M.; Wilson, L. Effects of temperature on sporulation and latent period of Colletotrichum spp. infecting strawberry fruit. Plant Dis. 1997, 81, 77–84. [Google Scholar] [CrossRef]
- Howard, C.M. Anthracnose of strawberry caused by the Colleiotrichum complex in Florida. Plant Dis. 1992, 76, 976–981. [Google Scholar] [CrossRef]
- Zhang, X. Detection and Management of Colletotrichum Acutatum Sensu Lato on Strawberry. Doctoral Dissertation, Iowa State University, Ames, IA, USA, 2015. [Google Scholar]
- Madden, L.; Wilson, L.; Ellis, M. Field spread of anthracnose fruit rot of strawberry in relation to ground cover and ambient weather conditions. Plant Dis. 1993, 77, 861–866. [Google Scholar] [CrossRef]
- Madden, L.; Yang, X.; Wilson, L. Effects of rain intensity on splash dispersal of Colletotrichum acutatum. Phytopathology 1996, 86, 864–874. [Google Scholar] [CrossRef]
- Ntahimpera, N.; Wilson, L.; Ellis, M.; Madden, L. Comparison of rain effects on splash dispersal of three Colletotrichum species infecting strawberry. Phytopathology 1999, 89, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Agostini, J.; Timmer, L. Population dynamics and survival of strains of Colletotrichum gloeosporioides on citrus in Florida. Phytopathology 1994, 84, 420–425. [Google Scholar] [CrossRef]
- Lilja, A.T.; Parikka, P.K.; Pääskynkivi, E.A.; Hantula, J.I.; Vainio, E.J.; Vartiamäki, H.A.; Lemmetty, A.H.; Vestberg, M.V. Phytophthora cactorum and Colletotrichum acutatum: Survival and Detection. Agric. Conspec. Sci. 2006, 71, 121–128. [Google Scholar]
- MacKenzie, S.; Peres, N.A.; Barquero, M.; Arauz, L.; Timmer, L. Host range and genetic relatedness of Colletotrichum acutatum isolates from fruit crops and leatherleaf fern in Florida. Phytopathology 2009, 99, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zeng, X.; Xiang, F.; Ren, L.; Chen, F.; Gu, Y. Distribution and characteristics of Colletotrichum spp. associated with anthracnose of strawberry in Hubei, China. Plant Dis. 2016, 100, 996–1006. [Google Scholar] [CrossRef]
- Malarczyk, D.; Panek, J.; Frąc, M. Alternative molecular-based diagnostic methods of plant pathogenic fungi affecting berry crops—A Review. Molecules 2019, 24, 1200. [Google Scholar] [CrossRef]
- Aljawasim, B.; Vincelli, P. Evaluation of Polymerase Chain Reaction (PCR)-Based Methods for Rapid, Accurate Detection and Monitoring of Verticillium dahliae in Woody Hosts by Real-Time PCR. Plant Dis. 2015, 99, 866–873. [Google Scholar] [CrossRef]
- Freeman, S.; Rodriguez, R. Differentiation of Colletotrichum species responsible for anthracnose of strawberry by arbitrarily primed PCR. Mycol. Res. 1995, 99, 501–504. [Google Scholar] [CrossRef]
- Fazari, A.; Pellicer-Valero, O.J.; Gómez-Sanchıs, J.; Bernardi, B.; Cubero, S.; Benalia, S.; Zimbalatti, G.; Blasco, J. Application of deep convolutional neural networks for the detection of anthracnose in olives using VIS/NIR hyperspectral images. Comput. Electron. Agric. 2021, 187, 106252. [Google Scholar] [CrossRef]
- Lu, J.; Ehsani, R.; Shi, Y.; Abdulridha, J.; de Castro, A.I.; Xu, Y. Field detection of anthracnose crown rot in strawberry using spectroscopy technology. Comput. Electron. Agric. 2017, 135, 289–299. [Google Scholar] [CrossRef]
- Yeh, Y.-H.; Chung, W.-C.; Liao, J.-Y.; Chung, C.-L.; Kuo, Y.-F.; Lin, T.-T. Strawberry foliar anthracnose assessment by hyperspectral imaging. Comput. Electron. Agric. 2016, 122, 1–9. [Google Scholar] [CrossRef]
- Alijani, Z.; Amini, J.; Ashengroph, M.; Bahramnejad, B.; Mozafari, A.A. Biocontrol of strawberry anthracnose disease caused by Colletotrichum nymphaeae using Bacillus atrophaeus strain DM6120 with multiple mechanisms. Trop. Plant Pathol. 2022, 47, 245–259. [Google Scholar] [CrossRef]
- Veys, C.; Chatziavgerinos, F.; AlSuwaidi, A.; Hibbert, J.; Hansen, M.; Bernotas, G.; Smith, M.; Yin, H.; Rolfe, S.; Grieve, B. Multispectral imaging for presymptomatic analysis of light leaf spot in oilseed rape. Plant Methods 2019, 15, 4. [Google Scholar] [CrossRef]
- Khaliq, A.; Comba, L.; Biglia, A.; Ricauda Aimonino, D.; Chiaberge, M.; Gay, P. Comparison of satellite and UAV-based multispectral imagery for vineyard variability assessment. Remote Sens. 2019, 11, 436. [Google Scholar] [CrossRef]
- Yuan, L.; Yan, P.; Han, W.; Huang, Y.; Wang, B.; Zhang, J.; Zhang, H.; Bao, Z. Detection of anthracnose in tea plants based on hyperspectral imaging. Comput. Electron. Agric. 2019, 167, 105039. [Google Scholar] [CrossRef]
- Canteri, M.H.; Renard, C.M.; Le Bourvellec, C.; Bureau, S. ATR-FTIR spectroscopy to determine cell wall composition: Application on a large diversity of fruits and vegetables. Carbohydr. Polym. 2019, 212, 186–196. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, X.; Zhang, J.; Lan, Y.; Xu, C.; Liang, D. Detection of rice sheath blight using an unmanned aerial system with high-resolution color and multispectral imaging. PLoS ONE 2018, 13, e0187470. [Google Scholar] [CrossRef]
- Liu, C.; Liu, W.; Lu, X.; Ma, F.; Chen, W.; Yang, J.; Zheng, L. Application of multispectral imaging to determine quality attributes and ripeness stage in strawberry fruit. PLoS ONE 2014, 9, e87818. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chung, W.; Liao, J.; Chung, C.; Kuo, Y.; Lin, T. Strawberry anthracnose disease assessment using hyperspectral imaging. In Proceedings of the 6th International Symposium on Machinery and Mechatronics for Agriculture and Biosystems Engineering (ISMAB), Jeonju, Korea, 18–20 June 2012. [Google Scholar]
- Pérez-Roncal, C.; López-Maestresalas, A.; Lopez-Molina, C.; Jarén, C.; Urrestarazu, J.; Santesteban, L.G.; Arazuri, S. Hyperspectral imaging to assess the presence of powdery mildew (Erysiphe necator) in cv. Carignan noir grapevine bunches. Agronomy 2020, 10, 88. [Google Scholar] [CrossRef]
- Jiang, Q.; Wu, G.; Tian, C.; Li, N.; Yang, H.; Bai, Y.; Zhang, B. Hyperspectral imaging for early identification of strawberry leaves diseases with machine learning and spectral fingerprint features. Infrared Phys. Technol. 2021, 118, 103898. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.; Jiang, Q.; Cui, M.; Li, N.; Ou, Y.; Diao, Z.; Zhang, B. Early identification of strawberry leaves disease utilizing hyperspectral imaging combing with spectral features, multiple vegetation indices and textural features. Comput. Electron. Agric. 2023, 204, 107553. [Google Scholar] [CrossRef]
- Liu, C.; Cao, Y.; Wu, E.; Yang, R.; Xu, H.; Qiao, Y. A Discriminative Model for Early Detection of Anthracnose in Strawberry Plants Based on Hyperspectral Imaging Technology. Remote Sens. 2023, 15, 4640. [Google Scholar] [CrossRef]
- Pham, H.; Lim, Y.; Gardi, A.; Sabatini, R.; Pang, E. A novel bistatic lidar system for early-detection of plant diseases from unmanned aircraft. In Proceedings of the 31th Congress of the International Council of the Aeronautical Sciences (ICAS 2018), Belo Horizonte, Brazil, 9–14 September 2018. [Google Scholar]
- Zhang, C.; Kovacs, J.M. The application of small unmanned aerial systems for precision agriculture: A review. Precis. Agric. 2012, 13, 693–712. [Google Scholar] [CrossRef]
- Messina, G.; Peña, J.M.; Vizzari, M.; Modica, G. A comparison of UAV and satellites multispectral imagery in monitoring onion crop. An application in the ‘Cipolla Rossa di Tropea’(Italy). Remote Sens. 2020, 12, 3424. [Google Scholar] [CrossRef]
- Chandel, A.K.; Khot, L.R.; Sallato, B. Apple powdery mildew infestation detection and mapping using high-resolution visible and multispectral aerial imaging technique. Sci. Hortic. 2021, 287, 110228. [Google Scholar] [CrossRef]
- Miller-Butler, M.A. Screening Strawberry Clones for Anthracnose Disease Resistance Using Traditional Techniques and Molecular Markers. Ph.D. Thesis, The University of Southern Mississippi, Hattiesburg, MS, USA, 2016. [Google Scholar]
- Simpson, D.; Berrie, A.; Johnson, A. Hot Water Treatment to Eliminate Colletotrichum acutatum from Strawberry Runner Cuttings. In Proceedings of the V International Strawberry Symposium, Coolum Beach, Australia, 5–10 September 2004; pp. 255–258. [Google Scholar]
- Mertely, J.C.; Peres, N.A. Anthracnose Fruit Rot of Strawberry: PP-207/PP130, rev. 9/2012. EDIS 2012, 2012, 1–4. [Google Scholar] [CrossRef]
- Chechi, A.; Stahlecker, J.; Dowling, M.; Schnabel, G. Diversity in species composition and fungicide resistance profiles in Colletotrichum isolates from apples. Pestic. Biochem. Physiol. 2019, 158, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Sen, S.; Mukherjee, K.; Acharya, K. Postharvest diseases of Indian gooseberry and their management: A review. Int. J. Fruit Sci. 2020, 20, 178–190. [Google Scholar] [CrossRef]
- Melanson, R.A.; Johnson, C.; Schnabel, G.; Ferguson, M.; Desaeger, J.; Schmidt-Jeffris, R.; Burrack, H.J.; Pfeiffer, D.G.; Hale, F.; Jennings, K. 2020 Southeast Regional Strawberry Integrated Pest Management Guide For Plasticulture Production; University of Georgia Cooperative Extension: Athens, GA, USA, 2020. [Google Scholar]
- Rahman, M.; Ballington, J.; Louws, F. Role of foliar hemibiotrophic and fruit resistance in anthracnose-resistant strawberry genotypes for annual hill plasticulture systems. Ann. Appl. Biol. 2013, 163, 102–113. [Google Scholar] [CrossRef]
- Ballington, J.; Shuman, J.; Hokanson, S.; Smith, B.; Giménez, G. Breeding strawberries (Fragaria × ananassa) for resistance to anthracnose caused by Colletotrichum acutatum. In Proceedings of the IV International Strawberry Symposium, Tampere, Finland, 9–14 July 2000; pp. 89–92. [Google Scholar]
- Whitaker, V.; Lee, S.; Osorio, L.; Verma, S.; Roach, J.; Mangandi, J.; Noh, Y.-H.; Gezan, S.; Peres, N. Advances in strawberry breeding at the University of Florida. In Proceedings of the VIII International Strawberry Symposium, Québec, QC, Canada, 13–17 August 2016; pp. 1–6. [Google Scholar]
- Salinas, N.R.; Zurn, J.D.; Mathey, M.; Mookerjee, S.; Denoyes, B.; Perrotte, J.; Potier, A.; Finn, C.E.; Hancock, J.F.; Stewart, P. Validation of molecular markers associated with perpetual flowering in octoploid Fragaria germplasm. Mol. Breed. 2017, 37, 70. [Google Scholar] [CrossRef]
- Adhikari, T.B.; Aryal, R.; Redpath, L.E.; Van den Broeck, L.; Ashrafi, H.; Philbrick, A.N.; Jacobs, R.L.; Sozzani, R.; Louws, F.J. RNA-Seq and Gene Regulatory Network Analyses Uncover Candidate Genes in the Early Defense to Two Hemibiotrophic Colletorichum spp. in Strawberry. Front. Genet. 2022, 12, 805771. [Google Scholar] [CrossRef]
- Ma, L.; Haile, Z.M.; Sabbadini, S.; Mezzetti, B.; Negrini, F.; Baraldi, E. Functional characterization of MANNOSE-BINDING LECTIN 1, a G-type lectin gene family member, in response to fungal pathogens of strawberry. J. Exp. Bot. 2023, 74, 149–161. [Google Scholar] [CrossRef]
- He, T.; Fan, J.; Jiao, G.; Liu, Y.; Zhang, Q.; Luo, N.; Ahmad, B.; Chen, Q.; Wen, Z. Bioinformatics and Expression Analysis of the Chitinase Genes in Strawberry (Fragaria vesca) and Functional Study of FvChi-14. Plants 2023, 12, 1543. [Google Scholar] [CrossRef]
- Shuman, J.L. Anthracnose Fruit Rot Resistance in Strawberry. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2001. [Google Scholar]
- Shi, X.-C.; Wang, S.-Y.; Duan, X.-C.; Wang, Y.-Z.; Liu, F.-Q.; Laborda, P. Biocontrol strategies for the management of Colletotrichum species in postharvest fruits. Crop Prot. 2021, 141, 105454. [Google Scholar] [CrossRef]
- Kim, Y.S.; Balaraju, K.; Jeon, Y. Biological control of apple anthracnose by Paenibacillus polymyxa APEC128, an antagonistic rhizobacterium. Plant Pathol. J. 2016, 32, 251. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.R.; Nesi, C.N.; De Mio, L.L.M. Bacillus spp. and Pseudomonas putida as inhibitors of the Colletotrichum acutatum group and potential to control Glomerella leaf spot. Biol. Control 2014, 72, 30–37. [Google Scholar] [CrossRef]
- Verma, N.; MacDonald, L.; Punja, Z. Inoculum prevalence, host infection and biological control of Colletotrichum acutatum: Causal agent of blueberry anthracnose in British Columbia. Plant Pathol. 2006, 55, 442–450. [Google Scholar] [CrossRef]
- Lopes, M.R.; Klein, M.N.; Ferraz, L.P.; da Silva, A.C.; Kupper, K.C. Saccharomyces cerevisiae: A novel and efficient biological control agent for Colletotrichum acutatum during pre-harvest. Microbiol. Res. 2015, 175, 93–99. [Google Scholar] [CrossRef]
- McInnes, T.; Black, L.; Gatti, J., Jr. Disease-free plants for management of strawberry anthracnose crown rot. Plant Dis. 1992, 76, 260–264. [Google Scholar] [CrossRef]
- Dowling, M.; Peres, N.; Villani, S.; Schnabel, G. Managing Colletotrichum on fruit crops: A “complex” challenge. Plant Dis. 2020, 104, 2301–2316. [Google Scholar] [CrossRef]
- Oldfield, T.L.; Achmon, Y.; Perano, K.M.; Dahlquist-Willard, R.M.; VanderGheynst, J.S.; Stapleton, J.J.; Simmons, C.W.; Holden, N.M. A life cycle assessment of biosolarization as a valorization pathway for tomato pomace utilization in California. J. Clean. Prod. 2017, 141, 146–156. [Google Scholar] [CrossRef]
- Matthiessen, J.N.; Kirkegaard, J.A. Biofumigation and enhanced biodegradation: Opportunity and challenge in soilborne pest and disease management. Crit. Rev. Plant Sci. 2006, 25, 235–265. [Google Scholar] [CrossRef]
- Hansen, Z.; Keinath, A. Increased pepper yields following incorporation of biofumigation cover crops and the effects on soilborne pathogen populations and pepper diseases. Appl. Soil Ecol. 2013, 63, 67–77. [Google Scholar] [CrossRef]
- Morra, M.; Kirkegaard, J. Isothiocyanate release from soil-incorporated Brassica tissues. Soil Biol. Biochem. 2002, 34, 1683–1690. [Google Scholar] [CrossRef]
- Butler, D.M.; Kokalis-Burelle, N.; Muramoto, J.; Shennan, C.; McCollum, T.G.; Rosskopf, E.N. Impact of anaerobic soil disinfestation combined with soil solarization on plant–parasitic nematodes and introduced inoculum of soilborne plant pathogens in raised-bed vegetable production. Crop Prot. 2012, 39, 33–40. [Google Scholar] [CrossRef]
- Shrestha, U.; Augé, R.M.; Butler, D.M. A meta-analysis of the impact of anaerobic soil disinfestation on pest suppression and yield of horticultural crops. Front. Plant Sci. 2016, 7, 1254. [Google Scholar] [CrossRef]
- Molendijk, L.; Bleeker, P.; Lamers, J.; Runia, W. Perspectives of anaerobic soil disinfestation. In Proceedings of the VII International Symposium on Chemical and Non-Chemical Soil and Substrate Disinfestation, Leuven, Belgium, 13–18 September 2009; pp. 277–283. [Google Scholar]
- Shrestha, U.; Dee, M.E.; Piya, S.; Ownley, B.H.; Butler, D.M. Soil inoculation with Trichoderma asperellum, T. harzianum or Streptomyces griseoviridis prior to anaerobic soil disinfestation (ASD) does not increase ASD efficacy against Sclerotium rolfsii germination. Appl. Soil Ecol. 2020, 147, 103383. [Google Scholar] [CrossRef]
- Butler, D.M.; Rosskopf, E.N.; Kokalis-Burelle, N.; Albano, J.P.; Muramoto, J.; Shennan, C. Exploring warm-season cover crops as carbon sources for anaerobic soil disinfestation (ASD). Plant Soil 2012, 355, 149–165. [Google Scholar] [CrossRef]
- Shennan, C.; Muramoto, J.; Koike, S.; Baird, G.; Fennimore, S.; Samtani, J.; Bolda, M.; Dara, S.; Daugovish, O.; Lazarovits, G. Anaerobic soil disinfestation is an alternative to soil fumigation for control of some soilborne pathogens in strawberry production. Plant Pathol. 2018, 67, 51–66. [Google Scholar] [CrossRef]
- Testen, A.L.; Bosques Martinez, M.; Jimenez Madrid, A.; Deblais, L.; Taylor, C.; Paul, P.A.; Miller, S.A. On-farm evaluations of anaerobic soil disinfestation and grafting for management of a widespread soilborne disease complex in protected culture tomato production. Phytopathology 2020, 111, 954–965. [Google Scholar] [CrossRef]
- Hernández-Muñiz, P.; Borrero, C.; Ordóñez-Martín, J.; Pastrana, A.M.; Avilés, M. Optimization of the Use of Industrial Wastes in Anaerobic Soil Disinfestation for the Control of Fusarium Wilt in Strawberry. Plants 2023, 12, 3185. [Google Scholar] [CrossRef]
- Liu, D.; Samtani, J.; Johnson, C.; Zhang, X.; Butler, D.M.; Derr, J. Brewer’s Spent Grain with Yeast Amendment Shows Potential for Anaerobic Soil Disinfestation of Weeds and Pythium irregulare. Agronomy 2023, 13, 2081. [Google Scholar] [CrossRef]
- Song, Z.; Yan, D.; Fang, W.; Zhang, D.; Jin, X.; Li, Y.; Wang, Q.; Wang, G.; Li, Q.; Cao, A. Response of Strawberry Fruit Yield, Soil Chemical and Microbial Properties to Anaerobic Soil Disinfestation with Biochar and Rice Bran. Agriculture 2023, 13, 1466. [Google Scholar] [CrossRef]
- Lamers, J.; Mazzola, M.; Rosskopf, E.; Kokalis-Burelle, N.; Momma, N.; Butler, D.; Shennan, C.; Muramoto, J.; Kobara, Y. Anaerobic soil disinfestation for soil borne disease control in strawberry and vegetable systems: Current knowledge and future directions. In Proceedings of the VIII International Symposium on Chemical and Non-Chemical Soil and Substrate Disinfestation, Turin, Italy, 13–17 July 2014; pp. 165–175. [Google Scholar]
- Liu, D. Evaluation of Anaerobic Soil Disinfestation Using brewers Spent Grain and Yeast Inoculation on Weed Control in Annual Hill Plasticulture Strawberry Production. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, NA, USA, 2021. [Google Scholar]
- Momma, N.; Kobara, Y.; Uematsu, S.; Kita, N.; Shinmura, A. Development of biological soil disinfestations in Japan. Appl. Microbiol. Biotechnol. 2013, 97, 3801–3809. [Google Scholar] [CrossRef]
- Kundan, R.; Pant, G.; Jadon, N.; Agrawal, P. Plant growth promoting rhizobacteria: Mechanism and current prospective. J. Fertil Pestic 2015, 6, 9. [Google Scholar] [CrossRef]
- Jayaprakashvel, M.; Chitra, C.; Mathivanan, N. Metabolites of plant growth-promoting rhizobacteria for the management of soilborne pathogenic fungi in crops. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Springer: Berlin/Heidelberg, Germany, 2019; pp. 293–315. [Google Scholar]
- Aljawasim, B.D.; Khaeim, H.M.; Manshood, M.A. Assessment of arbuscular mycorrhizal fungi (Glomus spp.) as potential biocontrol agents against damping-off disease Rhizoctonia solani on cucumber. J. Crop Prot. 2020, 9, 141–147. [Google Scholar]
- Rahman, M.; Sabir, A.A.; Mukta, J.A.; Khan, M.; Alam, M.; Mohi-Ud-Din, M.; Miah, M.; Rahman, M.; Islam, M.T. Plant probiotic bacteria Bacillus and Paraburkholderia improve growth, yield and content of antioxidants in strawberry fruit. Sci. Rep. 2018, 8, 2504. [Google Scholar] [CrossRef]
- Mei, C.; Amaradasa, B.S.; Chretien, R.L.; Liu, D.; Snead, G.; Samtani, J.B.; Lowman, S. A Potential Application of Endophytic Bacteria in Strawberry Production. Horticulturae 2021, 7, 504. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; Magalhães, K.T.; Lorenzetii, E.R.; Souza, T.P.; Schwan, R.F. A multiphasic approach for the identification of endophytic bacterial in strawberry fruit and their potential for plant growth promotion. Microb. Ecol. 2012, 63, 405–417. [Google Scholar] [CrossRef]
- Baysal, F. Comparative performance of fungicides and biocontrol products in suppression of Rhizoctonia root rot in viburnum. J. Plant Pathol. Microbiol 2018, 9, 1000451. [Google Scholar]
- Rahman, M.; Islam, T.; Jett, L.; Kotcon, J. Probiotic Bacteria, Anaerobic Soil Disinfestation and Mustard Cover Crop Biofumigation Suppress Soilborne Disease and Increase Yield of Strawberry in a Perennial Organic Production System. Plant Dis. 2023, 107, 2490–2499. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljawasim, B.D.; Samtani, J.B.; Rahman, M. New Insights in the Detection and Management of Anthracnose Diseases in Strawberries. Plants 2023, 12, 3704. https://doi.org/10.3390/plants12213704
Aljawasim BD, Samtani JB, Rahman M. New Insights in the Detection and Management of Anthracnose Diseases in Strawberries. Plants. 2023; 12(21):3704. https://doi.org/10.3390/plants12213704
Chicago/Turabian StyleAljawasim, Baker D., Jayesh B. Samtani, and Mahfuzur Rahman. 2023. "New Insights in the Detection and Management of Anthracnose Diseases in Strawberries" Plants 12, no. 21: 3704. https://doi.org/10.3390/plants12213704
APA StyleAljawasim, B. D., Samtani, J. B., & Rahman, M. (2023). New Insights in the Detection and Management of Anthracnose Diseases in Strawberries. Plants, 12(21), 3704. https://doi.org/10.3390/plants12213704









