Insights into Grape Ripe Rot: A Focus on the Colletotrichum gloeosporioides Species Complex and Its Management Strategies
Abstract
1. Introduction
2. Grape Ripe Rot Caused by the Colletotrichum Complex
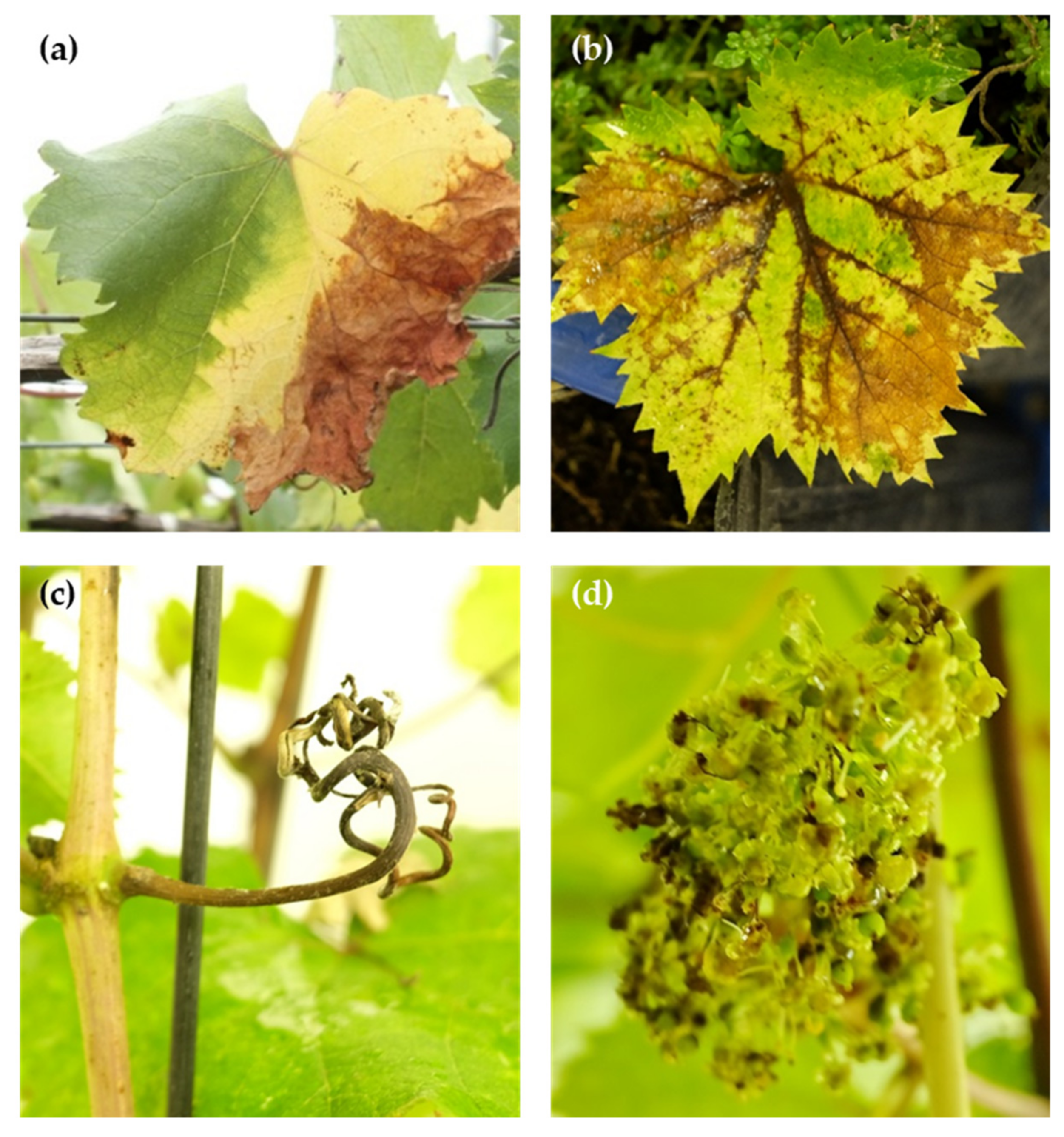
3. Diverse Grapevine Symptoms Caused by the CGSC
4. CGSC Life Cycle and Infection Factors
4.1. Pathogen
4.1.1. Lifestyles and Infection Processes
4.1.2. Overwintering Structures and Primary Inoculum Sources
4.1.3. Secondary Inoculum Sources and Infection Dynamics
4.2. Host: Susceptibility of Berries to CGSC Infection
4.3. Environmental Factors
5. Changes in the Primary Causal Species
6. C. viniferum Emergence in Asia and Other Regions
7. Management of Grape Ripe Rot Caused by the CGSC
7.1. Cultivars with Resistance to Ripe Rot
7.2. Rain Sheltering and Bagging as Adequate Cultivation Techniques
7.3. Sanitation Practice
7.4. Sustaining a Healthy Grape Microbiome
7.5. Alternative Biological and Non-Chemical Measures
7.6. Fungicide Selection
| Target Site/FRAC Code | Active Ingredient | Fungicide Resistance Reported | Use against the Ripe Rot Pathogen | Reference |
|---|---|---|---|---|
| (B1) tubulin polymerization/1 | Benomyl | - | Used in the U.S. Previously used in Australia. Reduction in the mycelial growth and sporulation of the isolates from grape. Colletotrichum gloeosporioides exhibited a higher sensitivity to benomyl than C. acutatum. | [6,69,140] |
| Carbendazim | In the field | Widely used to control grape ripe rot in Korea, India, and China. Reduction in the mycelial growth and sporulation of the isolates from grape. Resistant isolates of C. gloeosporioides (12/18) were found at the site where the fungicides had been used in Korea. Resistant C. gloeosporioides isolates from grapes were prevalent in a study in India. | [127,133,134,140,141] | |
| Thiophanate-methyl | In the field | Used to control grape ripe rot in China. Has a 37% resistance frequency in a study in China. | [133] | |
| (B2) tubulin polymerization/10 | Diethofencarb | In the field | Mixed with carbendazim to control ripe rot in Korea. Resistant isolates of C. gloeosporioides (12/18) were found at the site where the fungicides had been used. | [133,134] |
| (C2) complex II: succinate-dehydrogenase/7 | Benzovindiflupyr | - | Highly effective against C. gloeosporioides isolates from grapevine in vitro. | [135] |
| Fluxapyroxad | - | C. gloeosporioides isolates from grapevine in Japan were insensitive to fluxapyroxad. | [135] | |
| Penthiopyrad | - | Registered for controlling grape ripe rot in Japan. Highly effective against the C. gloeosporioides isolates from grapevine. | [135] | |
| Fluopyram | - | C. gloeosporioides isolates from grapevine in Japan were insensitive to fluopyram. Used in combination with tebuconazole in the grower standard spray program for control of grape ripe rot in the U.S. | [135,137,138] | |
| Boscalid | - | Mild suppression of the grape ripe rot pathogen in vitro. Registered as a mixture with pyraclostrobin for the management of diseases caused by Colletotrichum spp. on grapes in the U.S. C. gloeosporioides isolates from grapevine in Japan were insensitive to boscalid. | [6,19,135] | |
| Pydiflumetofen | - | Registered as a mixture with fludioxonil for management of diseases caused by Colletotrichum spp. on grape in the U.S. | [19] | |
| (C3) complex III: cytochrome bc1 at Qo site/11 | Azoxystrobin | In the field | Inhibition of the grape ripe rot pathogen in vitro. Extensively applied to control grape ripe rot in China. Shows a 97% resistant frequency in C. gloeosporioides in a study in China. Isolated with cross-resistance to pyraclostrobin and other quinone outside inhibitors found in the field. | [6,136] |
| Pyraclostrobin | Isolates from the field cross-resistance to other quinone outside inhibitors | Inhibition of the grape ripe rot pathogen in vitro. Frequently used in preventing grape ripe rot in Taiwan. Registered as a mixture with boscalid for the management of diseases caused by Colletotrichum spp. on grapes in the U.S. Isolated with cross-resistance to azoxystrobin and other quinone outside inhibitors found in the field. | [6,19,100,136,142] | |
| Trifloxystrobin | - | Inhibition of the grape ripe rot pathogen in vitro. | [6] | |
| (D1) methionine biosynthesis (proposed)/9 | Cyprodinil | - | Used in the grower standard spray program for the control of grape ripe rot in the U.S. | [137,138] |
| (E2) MAP/Histidine-Kinase in osmotic signal transduction/12 | Fludioxonil | - | Registered as a mixture with pydiflumetofen for the management of diseases caused by Colletotrichum spp. on grapes in the U.S. | [19] |
| (G1) C14-demethylase in sterol biosynthesis/3 | Prochloraz | Slightly resistance generated in the lab | Frequently used in preventing grape ripe rot in Taiwan. Registered for use in controlling grape ripe rot in China. Effectively inhibited the conidial germination and the mycelial growth of C. viniferum in vitro. The baseline sensitivity of C. gloeosporioides isolates were determined. The isolates from grapes showed a lower sensitivity to prochloraz than the isolates from strawberry. | [80,100,133,142] |
| Difenoconazole | In the field | Intensively used to control grape ripe rot in China. Showed a 65.2% resistant frequency in C. gloeosporioides in a study in China. | [139] | |
| Tebuconazole | Slight resistance generated in the laboratory; in the field | Frequently used in preventing grape ripe rot in Taiwan. Registered for use to control grape ripe rot in China. Used in combination with fluopyram in the grower standard spray program to control grapes’ ripe rot in the U.S. The baseline sensitivity of C. gloeosporioides isolates was determined. The isolates from grapes showed a lower sensitivity to tebuconazole than the isolates from strawberries. Approximately 30% of the C. gloeosporioides showed low-level resistances to tebuconazole in a study in China. | [100,133,137,138,142] | |
| Triadimenol | - | C. acutatum exhibited a higher sensitivity to triadimenol than C. gloeosporioides in Australia. | [6] | |
| (H4) chitin synthase/19 | Polyoxin | - | Registered for the management of diseases caused by Colletotrichum spp. on grape in the U.S. Used to control grape ripe rot in Taiwan. Effectively inhibited the conidial germination of C. viniferum in vitro. | [19,80] |
| (U) cell membrane disruption (proposed)/U12 | Dodine | - | Reduction in the mycelial growth and sporulation of the isolates from grape in vitro. | [140] |
| (M) multisite contact activity/M01 | Oxine-copper | - | Used to control grape ripe rot in Taiwan. Effectively inhibited the conidial germination of C. viniferum in vitro. | [80] |
| (M) multisite contact activity/M03 | Mancozeb | - | Used to control grape ripe rot in Taiwan. Effectively inhibited the conidial germination of C. viniferum in vitro. | [80] |
| Maneb | - | Suppressed the level of ripe rot in the field when applied every two weeks from bloom until near harvest in the U.S. | [69] | |
| Metiram | - | Used to control grape ripe rot in Taiwan. Effectively inhibited the conidial germination of C. viniferum in vitro. | [80] | |
| Thiram | - | Used to control grape ripe rot in Taiwan. Effectively inhibited the conidial germination of C. viniferum in vitro. | [80] | |
| Ziram | - | Registered for the management of diseases caused by Colletotrichum spp. on grapes in the U.S. | [19] | |
| (M) multisite contact activity/M04 | Captan | - | Significant reduction in ripe rot in the field when applied every two weeks from bloom until near harvest in the U.S. Frequently applied in the grower standard spray program in the control of ripe rot of grapes in the U.S. C. acutatum exhibited a higher sensitivity to captan than C. gloeosporioides in Australia. | [6,69,137] |
| Captafol | - | Significant reduction in ripe rot in the field when applied every two weeks from bloom until near harvest in the U.S. | [69] | |
| Folpet | - | Significant reduction in ripe rot in the field when applied every two weeks from bloom until near harvest in the U.S. | [69] | |
| (M) multisite contact activity/M05 | Chlorothalonil | - | Significant reduction in the mycelial growth and sporulation of the C. gloeosporioides isolate from grape in vitro. | [140] |
| (M) multisite contact activity/M07 | Iminoctadine | - | Used to control grape ripe rot in Taiwan. Effectively inhibited the conidial germination and mycelial growth of C. viniferum in vitro. | [80] |
| (M) multisite contact activity/M09 | Dithianon | - | Used to control grape ripe rot in Taiwan. Effectively inhibited the conidial germination of C. viniferum in vitro. | [80] |
7.7. Resistance to Fungicides
8. Integrated Management of Grape Ripe Rot Disease
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OIV Statistics Publications 3. Annual Assessment of World Vine and Wine Sector. Available online: https://www.oiv.int/sites/default/files/documents/OIV_Annual_Assessment_of_the_World_Vine_and_Wine_Sector_in_2021.pdf (accessed on 28 June 2023).
- Wilcox, W.F.; Gubler, W.D.; Uyemoto, J.K. Compendium of Grape Diseases, Disorders, and Pests, 2nd ed.; The American Phytopathological Society: St. Paul, MN, USA, 2015; p. 232. [Google Scholar]
- Crandall, S.G.; Spychalla, J.; Crouch, U.T.; Acevedo, F.E.; Naegele, R.P.; Miles, T.D. Rotting grapes don’t improve with age: Cluster rot disease complexes, management, and future prospects. Plant Dis. 2022, 106, 2013–2025. [Google Scholar] [CrossRef]
- Cosseboom, S.D.; Hu, M. Ontogenic susceptibility of grapevine clusters to ripe rot, caused by the Colletotrichum acutatum and C. gloeosporioides species complexes. Phytopathology 2022, 112, 1956–1964. [Google Scholar] [CrossRef]
- Echeverrigaray, S.; Scariot, F.J.; Fontanella, G.; Favaron, F.; Sella, L.; Santos, M.C.; Schwambach, J.; Pedrotti, C.; Delamare, A.P.L. Colletotrichum species causing grape ripe rot disease in Vitis labrusca and V. vinifera varieties in the highlands of southern Brazil. Plant Pathol. 2020, 69, 1504–1512. [Google Scholar] [CrossRef]
- Greer, L.A.; Harper, J.D.I.; Savocchia, S.; Samuelian, S.K.; Steel, C.C. Ripe rot of south-eastern Australian wine grapes is caused by two species of Colletotrichum: C. acutatum and C. gloeosporioides with differences in infection and fungicide sensitivity. Aust. J. Grape Wine Res. 2011, 17, 123–128. [Google Scholar] [CrossRef]
- Hong, S.K.; Kim, W.G.; Yun, H.K.; Choi, K.J. Morphological variations, genetic diversity and pathogenicity of Colletotrichum species causing grape ripe rot in Korea. Korean Soci. Plant Pathol. 2008, 24, 269–278. [Google Scholar] [CrossRef]
- Lin, C.P.; Wang, C.L.; Tsai, J.N.; Dai, Y.L.; Ann, P.J.; Zhan, Y.M.; Huang, S.Y. Occurence of grape ripe rot in Taiwan and the pathogenicity and phylogenetic relationship of its primary causal agent Colletotrichum viniferum. J. Taiwan Agric. Res. 2022, 71, 135–157. [Google Scholar] [CrossRef]
- Peng, L.J.; Sun, T.; Yang, Y.L.; Cai, L.; Hyde, K.D.; Bahkali, A.H.; Liu, Z.Y. Colletotrichum species on grape in Guizhou and Yunnan provinces, China. Mycoscience 2013, 54, 29–41. [Google Scholar] [CrossRef]
- Yamamoto, J.; Sato, T.; Tomioka, K. Occurrence of ripe rot of grapes (Vitis vinifera L.) caused by Colletotrichum acutatum Simmonds ex Simmonds. Ann. Phytopathol. Soc. Japan 1999, 65, 83–86. [Google Scholar] [CrossRef]
- Cosseboom, S.D.; Hu, M. Predicting ripe rot of grape, caused by Colletotrichum fioriniae, with leaf wetness, temperature, and the crop growth stage. PhytoFrontiers 2022. online ahead of print. [Google Scholar] [CrossRef]
- Oliver, C. Phylogeny, Histological Observation, and In Vitro Fungicide Screening and Field Trials of Multiple Colletotrichum Species, the Causal Agents of Grape Ripe Rot. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2018. [Google Scholar]
- Meunier, M.; Steel, C.C. Effect of Colletotrichum acutatum ripe rot on the composition and sensory attributes of Cabernet Sauvignon grapes and wine. Aust. J. Grape Wine Res. 2009, 15, 223–227. [Google Scholar] [CrossRef]
- Miele, A.; Rizzon, L.A. Physicochemical composition of Cabernet-Sauvignon wine made from grapes affected by grape ripe rot. OENO One 2013, 47, 195. [Google Scholar] [CrossRef]
- Sadoughi, N. Effect of Ripe Rot of Grapes (Colletotrichum spp.) on the Chemical Composition and Off-Flavour Compounds in Grapes and Wine. Ph.D. Thesis, Charles Sturt University, Bathurst, NSW, Australia, 2016. [Google Scholar]
- Whitelaw-Weckert, M.A.; Curtin, S.J.; Huang, R.; Steel, C.C.; Blanchard, C.L.; Roffey, P.E. Phylogenetic relationships and pathogenicity of Colletotrichum acutatum isolates from grape in subtropical Australia. Plant Pathol. 2007, 56, 448–463. [Google Scholar] [CrossRef]
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum—Current status and future directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Dowling, M.; Peres, N.; Villani, S.; Schnabel, G. Managing Colletotrichum on fruit crops: A “complex” challenge. Plant Dis. 2020, 104, 2301–2316. [Google Scholar] [CrossRef]
- Sutton, B.C. The genus Glomerella and its anamorph Colletotrichum. In Colletotrichum: Biology, Pathology and Control; Bailey, J.A., Jeger, M.J., Eds.; CAB International: Oxon, UK, 1992; pp. 1–26. [Google Scholar]
- Sutton, B.C. The Coelomycetes. In Fungi Imperfecti with Pycnidia, Acervuli and Stromata; Commonwealth Mycological Institute: Surrey, UK, 1980; p. 696. [Google Scholar]
- Hyde, K.D.; Cai, L.; McKenzie, E.H.C.; Yang, Y.L.; Zhang, J.Z.; Prihastuti, H. Colletotrichum: A catalogue of confusion. Fungal Divers. 2009, 39, 1–17. [Google Scholar]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.; Crous, P.W. The Colletotrichum acutatum species complex. Stud. Mycol. 2012, 73, 37–113. [Google Scholar] [CrossRef] [PubMed]
- Talhinhas, P.; Baroncelli, R. Colletotrichum species and complexes: Geographic distribution, host range and conservation status. Fungal Divers. 2021, 110, 109–198. [Google Scholar] [CrossRef]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, R.S. Notes on currently accepted species of Colletotrichum. Mycosphere 2016, 7, 1192–1260. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, N.; Weir, B.S.; Hyde, K.D.; Shenoy, B.D. The ApMat marker can resolve Colletotrichum species: A case study with Mangifera indica. Fungal Divers. 2013, 61, 117–138. [Google Scholar] [CrossRef]
- Southworth, E.A. Ripe Rot of Grapes and Apples. J. Mycol. 1891, 6, 164–173. [Google Scholar] [CrossRef]
- Oliver, C. Investigation of Wine Grape Cultivar and Cluster Developmental Stage Susceptibility to Grape Ripe Rot Caused by Two Fungal Species Complexes, Colletotrichum gloeosporioides, and C. acutatum, and the Evaluation of Potential Controls. Master’s Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2016. [Google Scholar]
- Yan, J.Y.; Jayawardena, M.M.R.S.; Goonasekara, I.D.; Wang, Y.; Zhang, W.; Liu, M.; Huang, J.B.; Wang, Z.Y.; Shang, J.J.; Peng, Y.L.; et al. Diverse species of Colletotrichum associated with grapevine anthracnose in China. Fungal Divers. 2015, 71, 233–246. [Google Scholar] [CrossRef]
- Ye, B.; Zhang, J.; Chen, X.; Xiao, W.; Wu, J.; Yu, H.; Zhang, C. Genetic diversity of Colletotrichum spp. causing grape anthracnose in Zhejiang, China. Agronomy 2023, 13, 952. [Google Scholar] [CrossRef]
- Misawa, T.; Kurose, D.; Sato, T. Molecular re-identification of Japanese isolates of the Colletotrichum gloeosporioides species complex associated with grape ripe rot. Ann. Rept. Plant Prot. North Japan 2022, 73, 113–118. [Google Scholar] [CrossRef]
- Kim, J.S.; Hassan, O.; Chang, T. First report of Colletotrichum aenigma causing anthracnose of grape in Korea. Plant Dis. 2021, 105, 2729. [Google Scholar] [CrossRef] [PubMed]
- Yokosawa, S.; Eguchi, N.; Sato, T. Characterization of the Colletotrichum gloeosporioides species complex causing grape ripe rot in Nagano Prefecture, Japan. J. Gen. Plant Pathol. 2020, 86, 163–172. [Google Scholar] [CrossRef]
- Lin, C.P.; Tsai, J.N.; Ann, P.J.; Lu, M.T. Virulence of Colletotrichum spp. from different isolating source in grape orchards was compared on grape. J. Taiwan Agric. Res. 2023, 72, 49–61. [Google Scholar] [CrossRef]
- Batista, D.D.C.; Vieira, W.A.S.; Barbosa, M.A.; Camara, M.P.S. First report of Colletotrichum siamense causing grape ripe rot in Brazil. Plant Dis. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Duan, C.H.; Pan, H.R.; Wang, C.C. Identification, pathogenicity and fungicide sensitivity of Colletotrichum Isolates from five fruit crops in Taiwan. Taiwan Pest Sci. 2018, 5, 91–111. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, X.B.; Jayawardena, R.S.; Yan, J.Y.; Wang, X.D.; Liu, M.; Chen, T.; Liu, X.M.; Wang, J.C.; Chen, Q.X. Identification and characterization of Colletotrichum species causing grape ripe rot in southern China. Mycosphere 2016, 7, 1177–1191. [Google Scholar] [CrossRef]
- Oo, M.M.; Oh, S.K. Identification and characterization of new record of grape ripe rot disease caused by Colletotrichum viniferum in Korea. Mycobiology 2017, 45, 421–425. [Google Scholar] [CrossRef]
- Duan, C.H.; Chen, G.Y. First report of Colletotrichum viniferum causing ripe rot of grape berry in Taiwan. Plant Dis. 2022, 106, 764. [Google Scholar] [CrossRef]
- Soytong, K.; Srinon, W.; Rattanacherdchai, K.; Kanokmedhakul, S.; Kanokmedhakul, K. Application of antagonistic fungi to control anthracnose disease of grape. J. Agric. Sci. Technol. 2005, 1, 33–41. [Google Scholar]
- Zapparata, A.; Da Lio, D.; Sarrocco, S.; Vannacci, G.; Baroncelli, R. First report of Colletotrichum godetiae causing grape (Vitis vinifera) berry rot in Italy. Plant Dis. 2017, 101, 1051–1052. [Google Scholar] [CrossRef]
- Melksham, K.J.; Weckert, M.A.; Steel, C.C. An unusual bunch rot of grapes in sub-tropical regions of Australia caused by Colletotrichum acutatum. Australas. Plant Pathol. 2002, 31, 193–194. [Google Scholar] [CrossRef]
- Shiraishi, M.; Yamada, M.; Mitani, N.; Ueno, T.; Nakaune, R.; Nakano, M. Rapid screening assay for ripe rot resistance in grape cultivars. J. Jpn. Soc. Hort. Sci. 2006, 75, 264–266. [Google Scholar] [CrossRef]
- Chung, P.C.; Wu, H.Y.; Wang, Y.W.; Ariyawansa, H.A.; Hu, H.P.; Hung, T.H.; Tzean, S.S.; Chung, C.L. Diversity and pathogenicity of Colletotrichum species causing strawberry anthracnose in Taiwan and description of a new species, Colletotrichum miaoliense sp. nov. Sci. Rep. 2020, 10, 14664. [Google Scholar] [CrossRef]
- Chung, W.H.; Ishii, H.; Nishimura, K.; Fukaya, M.; Yano, K.; Kajitani, Y. Fungicide sensitivity and phylogenetic relationship of anthracnose fungi isolated from various fruit crops in Japan. Plant Dis. 2006, 90, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.P.; Nogueira Júnior, A.F.; Silva-Junior, G.J.; Ciampi-Guillardi, M.; Amorim, L. Environmental requirements for infection of Colletotrichum acutatum and C. gloeosporioides sensu lato in citrus flowers and prevalence of these pathogens in Brazil. Eur. J. Plant Pathol. 2021, 160, 27–37. [Google Scholar] [CrossRef]
- Ntahimpera, N.; Wilson, L.L.; Ellis, M.A.; Madden, L.V. Comparison of rain effects on splash dispersal of three Colletotrichum species infecting strawberry. Phytopathology 1999, 89, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Salotti, I.; Ji, T.; Rossi, V. Temperature requirements of Colletotrichum spp. belonging to different clades. Front. Plant Sci. 2022, 13, 953760. [Google Scholar] [CrossRef]
- Kummuang, N.; Smith, B.J.; Diehl, S.V.; Graves Jr, C.H. Muscadine grape berry rot diseases in Mississippi: Disease identification and incidence. Plant Dis. 1996, 80, 238–243. [Google Scholar] [CrossRef]
- Santos, R.F.; Ciampi-Guillardi, M.; Amorim, L.; Massola, N.S.; Sposito, M.B. Aetiology of anthracnose on grapevine shoots in Brazil. Plant Pathol. 2018, 67, 692–706. [Google Scholar] [CrossRef]
- Fukaya, M. Position of the secondary infection of grape ripe rot (II): Progress of disease and changes in the number of dispersal conidia on a flower bud. Ann. Phytopath. Soc. Jpn. 1993, 59, 301–302. [Google Scholar]
- Steel, C.; Greer, L.; Samuelian, S.; Savocchia, S. Two species of fungus Colletotrichum responsible for ripe rot of grapes. Wine Vitic. J. 2011, 26, 48–58. [Google Scholar]
- Fan, Y.C.; Guo, F.Y.; Wu, R.H.; Chen, Z.Q.; Li, Z. First report of Colletotrichum gloeosporioides causing anthracnose on grapevine (Vitis vinifera) in Shaanxi province, China. Plant Dis. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Jayawardena, R.S. Mycosphere notes 102–168: Saprotrophic fungi on Vitis in China, Italy, Russia and Thailand. Mycosphere 2018, 9, 1–114. [Google Scholar] [CrossRef]
- Ciofini, A.; Negrini, F.; Baroncelli, R.; Baraldi, E. Management of post-harvest anthracnose: Current approaches and future perspectives. Plants 2022, 11, 1856. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.; Katan, T.; Shabi, E. Characterization of Colletotrichum species responsible for anthracnose diseases of various fruits. Plant Dis. 1998, 82, 596–605. [Google Scholar] [CrossRef]
- Sharma, M.; Kulshrestha, S. Colletotrichum gloeosporioides: An anthracnose causing pathogen of fruits and vegetables. Biosci. Biotechnol. Res. Asia 2015, 12, 115–180. [Google Scholar] [CrossRef]
- Li, Z.; Dos Santos, R.F.; Gao, L.; Chang, P.; Wang, X. Current status and future prospects of grapevine anthracnose caused by Elsinoe ampelina: An important disease in humid grape-growing regions. Mol. Plant Pathol. 2021, 22, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Quimio, T.H.; Quimio, A.J. Notes on Philippine grape and guava anthracnose. Plant Dis. Rep. 1975, 59, 221–224. [Google Scholar]
- Sawant, I.S.; Narkar, S.P.; Shetty, D.S.; Upadhyay, A.; Sawant, S.D. Emergence of Colletotrichum gloeosporioides sensu lato as the dominant pathogen of anthracnose disease of grapes in India as evidenced by cultural, morphological and molecular data. Australas. Plant Pathol. 2012, 41, 493–504. [Google Scholar] [CrossRef]
- Chowdappa, P.; Reddy, G.S.; Kumar, A.; Rao, B.M.; Rawal, R.D. Morphological and molecular characterization of Colletotrichum species causing anthracnose of grape in India. Asian Australas. J. Plant Sci. Biotechnol. 2009, 3, 71–77. [Google Scholar]
- Sawant, I.S.; Narkar, S.P.; Shetty, D.S.; Upadhyay, A.; Sawant, S.D. First report of Colletotrichum capsici causing anthracnose on grapes in Maharashtra, India. New Dis. Rep. 2012, 25, 2. [Google Scholar] [CrossRef]
- Nigar, Q.; Cadle-Davidson, L.; Gadoury, D.M.; Hassan, M.U. First report of Colletotrichum fioriniae causing grapevine anthracnose in New York. Plant Dis. 2022, 107, 223. [Google Scholar] [CrossRef] [PubMed]
- De Silva, D.D.; Crous, P.W.; Ades, P.K.; Hyde, K.D.; Taylor, P.W.J. Life styles of Colletotrichum species and implications for plant biosecurity. Fungal Biol. Rev. 2017, 31, 155–168. [Google Scholar] [CrossRef]
- Daykin, M.E.; Milholland, R.D. Histopathology of ripe rot caused by Colletotrichum gloeosporioides on Muscadine grape. Phytopathology 1984, 74, 1339–1341. [Google Scholar] [CrossRef]
- Leu, L.S.; Chang, C.W. Histological study of Colletotrichum gloeosporioides on grape fruit. Plant Protect. Bull. 1985, 27, 11–18. [Google Scholar]
- Yun, S.C.; Park, E.W. Effects of temperature and wetness period on infection of grape by Colletotrichum gloeosporioides. Korean J. Plant Pathol. 1990, 6, 219–228. [Google Scholar]
- Daykin, M.E. Ripe rot of muscadine grape caused by Colletotrichum gloeosporioides and its control. Phytopathology 1984, 74, 710–714. [Google Scholar] [CrossRef]
- Fukaya, M. Studies on etiology and control of grapevine ripe rot Glomerella cingulata. I: Primary infection of grapevine ripe rot. Bull. Akita Fruit-Tree Exp. Stn. 2001, 27, 24–35. [Google Scholar]
- Ji, T.; Salotti, I.; Dong, C.; Li, M.; Rossi, V. Modeling the effects of the environment and the host plant on the ripe rot of grapes, caused by the Colletotrichum species. Plants 2021, 10, 2288. [Google Scholar] [CrossRef] [PubMed]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier Academia Press: San Diego, CA, USA, 2005; p. 922. [Google Scholar]
- Ji, Y.; Li, X.; Gao, Q.H.; Geng, C.; Duan, K. Colletotrichum species pathogenic to strawberry: Discovery history, global diversity, prevalence in China, and the host range of top two species. Phytopathol. Res. 2022, 4, 42. [Google Scholar] [CrossRef]
- Samuelian, S.K.; Greer, L.A.; Savocchia, S.; Steel, C.C. Application of Cabrio (a.i. pyraclostrobin) at flowering and veraison reduces the severity of bitter rot (Greeneria uvicola) and ripe rot (Colletotrichum acutatum) of grapes. Aust. J. Grape Wine Res. 2014, 20, 292–298. [Google Scholar] [CrossRef]
- Steel, C.C.; Greer, L.A.; Savocchia, S. Grapevine inflorescences are susceptible to the bunch rot pathogens, Greeneria uvicola (bitter rot) and Colletotrichum acutatum (ripe rot). Eur. J. Plant Pathol. 2012, 133, 773–778. [Google Scholar] [CrossRef]
- Engering, A.; Hogerwerf, L.; Slingenbergh, J. Pathogen–Host–Environment interplay and disease emergence. Emerg. Microbes Infect. 2013, 2, 1–7. [Google Scholar] [CrossRef]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef]
- Parker, I.M.; Gilbert, G.S. The evolutionary ecology of novel Plant–Pathogen interactions. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 675–700. [Google Scholar] [CrossRef]
- Sawant, I.S.; Shetty, D.S.; Narkar, S.P.; Ghule, S.; Sawant, S.D. Climate change and shifts in etiology of anthracnose disease of grapevines in India. J. Agrometeorol. 2013, 15, 75–78. [Google Scholar] [CrossRef]
- Duan, C.H.; Chen, G.Y. Molecular identification and fungicide sensitivity of Colletotrichum isolates from grape in Taiwan. J. Plant Med. 2020, 62, 23–32. [Google Scholar] [CrossRef]
- He, L.F.; Li, X.X.; Gao, Y.Y.; Li, B.X.; Mu, W.; Liu, F. Characterization and fungicide sensitivity of Colletotrichum spp. from different hosts in Shandong, China. Plant Dis. 2019, 103, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, J.; Chen, Q.; Liu, Z.; Sun, J.; Yan, Y.; Zhang, H.; Bi, Y. Identification, pathogenicity, and sensitivity to fungicide of Colletotrichum species that causes walnut anthracnose in Beijing. Agronomy 2023, 13, 214. [Google Scholar] [CrossRef]
- Diao, Y.Z.; Zhang, C.; Liu, F.; Wang, W.Z.; Liu, L.; Cai, L.; Liu, X.L. Colletotrichum species causing anthracnose disease of chili in China. Persoonia 2017, 38, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, N.; Sharma, J.; Pokhare, S.S.; Agarrwal, R.; Patil, P.G.; Sirsat, J.D.; Chakranarayan, M.G.; Bicchal, A.; Ukale, A.S.; Marathe, R.A. Characterization of Alternaria and Colletotrichum species associated with pomegranate (Punica granatum L.) in Maharashtra state of India. J. Fungi 2022, 8, 1040. [Google Scholar] [CrossRef]
- Rashid, H.; Ahmed, R.; Chowdhury, S.; Azad, A.K.; Raihan, T.; Haque, M.M.U. First report of Colletotrichum viniferum causing leaf spot of Hopea odorata in Bangladesh. New Dis. Rep. 2020, 42, 19. [Google Scholar] [CrossRef]
- Dou, M.; Hao, Y.; Yang, J.; Yuan, X.; Yin, X.; Jiao, Y.; Zhao, J.; Chen, T.; Wang, Y.; Xu, Y. Genome sequence resource for Colletotrichum viniferum, the cause of grapevine ripe rot in China. Mol. Plant-Microbe Interact. 2022, 35, 90–93. [Google Scholar] [CrossRef]
- Lei, Y.; Yuan, X.J.; Chen, T.; Yuan, Y.; Liu, X.M.; Tang, X.B.; Chen, Q.X. Transcriptome analysis of berries of Spine grape (Vitis davidii Föex) infected by Colletotrichum viniferum during symptom development. Horticulturae 2022, 8, 843. [Google Scholar] [CrossRef]
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.M.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P.; et al. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- He, P.C.; Wang, Y.J.; Wang, G.Y.; Ren, Z.B.; He, C.C. The studies on the Disease–Resistance of Vitis wild species originated in China. Sci. Agric. Sin. 1991, 24, 50–56. [Google Scholar]
- Jang, H.A.; Lee, K.S.; Oo, M.M.; Kwak, T.S.; Yoon, H.Y.; Thinn, K.S.Z.; Kim, M.R.; Kim, D.G.; Lee, J.J.; Lim, G.T.; et al. Analysis of varietal difference and genetic diversity of grapevine cultivarsthrough the leaf inoculation of Colletotrichum spp. J. Agric. Sci. Technol. 2018, 52, 49–60. [Google Scholar] [CrossRef]
- Shiraishi, M.; Koid, M.; Itamura, H.; Yamada, M.; Mitani, N.; Ueno, T.; Nakaune, R.; Nakano, M. Screening for resistance to ripe rot caused by Colletotrichum acutatum in grape germplasm. Vitis 2007, 46, 196–200. [Google Scholar]
- Yamada, M.; Sato, A. Advances in table grape breeding in Japan. Breed. Sci. 2016, 66, 34–45. [Google Scholar] [CrossRef]
- Fu, P.; Tian, Q.; Lai, G.; Li, R.; Song, S.; Lu, J. Cgr1, a ripe rot resistance QTL in Vitis amurensis ‘Shuang Hong’ grapevine. Hortic. Res. 2019, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, H. Review: Research progress in amur grape, Vitis amurensis Rupr. Can. J. Plant Sci. 2013, 93, 565–575. [Google Scholar] [CrossRef]
- Yu, D.; Wei, W.; Fan, Z.; Chen, J.; You, Y.; Huang, W.; Zhan, J. VabHLH137 promotes proanthocyanidin and anthocyanin biosynthesis and enhances resistance to Colletotrichum gloeosporioides in grapevine. Hortic. Res. 2023, 10, uhac261. [Google Scholar] [CrossRef]
- Jang, H.A.; Oo, M.M.; Kim, D.G.; Yoon, H.Y.; Kim, M.R.; Lee, K.S.; Thinn, K.S.Z.; Arif, S.; Geng, J.G.; Min, J.; et al. CC-NBS-LRR, a set of VvCRP markers, can distinguish cultivars with ripe rot resistance to Colletotrichum pathogens in grapevine. Hortic. Environ. Biotechnol. 2020, 61, 915–927. [Google Scholar] [CrossRef]
- Gautam, A.K. Colletotrichum gloeosporioides: Biology, pathogenicity and management in India. J. Plant Physiol. Pathol. 2014, 2, 2. [Google Scholar] [CrossRef]
- Wang, B.; Li, B.H.; Dong, X.L.; Wang, C.X.; Zhang, Z.F. Effects of temperature, wetness duration, and moisture on the conidial germination, infection, and disease incubation period of Glomerella cingulata. Plant Dis. 2015, 99, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Deng, W.; Yang, M.; Wang, H.; Mao, R.; Shao, J.; Fan, J.; Chen, Y.; Fu, Y.; Li, C.; et al. Protecting grapevines from rainfall in rainy conditions reduces disease severity and enhances profitability. Crop. Prot. 2015, 67, 261–268. [Google Scholar] [CrossRef]
- Liu, H.L.; Shen, Y.M.; Chao, C.H.; Huang, T.C.; Wu, S.W.; Hsieh, J.H. Efficacy of fungicide pre-bagging treatments on grape clusters in preventing ripe rot disease of grape. Bull. Taichung Dist. Agric. Res. Ext. Stn. 2016, 131, 19–31. [Google Scholar]
- Huang, R.; Shen, L.; Yu, H.; Jiang, J.; Qin, Y.; Liu, Y.; Zhang, J.; Song, Y. Evaluation of rain-shelter cultivation mode effects on microbial diversity during Cabernet Sauvignon (Vitis vinifera L.) maturation in Jingyang, Shaanxi, China. Food Res. Int. 2022, 156, 111165. [Google Scholar] [CrossRef]
- Meng, J.F.; Ning, P.F.; Xu, T.F.; Zhang, Z.W. Effect of rain-shelter cultivation of Vitis vinifera cv. Cabernet Gernischet on the phenolic profile of berry skins and the incidence of grape diseases. Molecules 2013, 18, 381–397. [Google Scholar] [CrossRef]
- Yu, S.; Li, B.; Guan, T.; Liu, L.; Wang, H.; Liu, C.; Zang, C.; Huang, Y.; Liang, C. A comparison of three types of “vineyard management” and their effects on the structure of Plasmopara viticola populations and epidemic dynamics of grape downy mildew. Plants 2022, 11, 2175. [Google Scholar] [CrossRef]
- Karajeh, M.R. Pre-harvest bagging of grape clusters as a non-chemical physical control measure against certain pests and diseases of grapevines. Org. Agric. 2018, 8, 259–264. [Google Scholar] [CrossRef]
- van Bruggen, A.H.; Gamliel, A.; Finckh, M.R. Plant disease management in organic farming systems. Pest Manag. Sci. 2016, 72, 30–44. [Google Scholar] [CrossRef]
- Leles, N.R.; Genta, W.; Marques, V.V.; Tessmann, D.J.; Roberto, S.R. Management of ripe grape rot on ‘Niagara Rosada’ grapevine. Semi. Ciênc. Agrár. 2022, 43, 2189–2204. [Google Scholar] [CrossRef]
- Billones-Baaijens, R.; Savocchia, S. A review of Botryosphaeriaceae species associated with grapevine trunk diseases in Australia and New Zealand. Australas. Plant Pathol. 2019, 48, 3–18. [Google Scholar] [CrossRef]
- Farrar, J.J.; Baur, M.E.; Elliott, S.F. Adoption of IPM practices in grape, tree fruit, and nut production in the Western United States. J. Integr. Pest Manag. 2016, 7, 8. [Google Scholar] [CrossRef][Green Version]
- Hoffman, L.E.; Wilcox, W.F.; Gadoury, D.M.; Seem, R.C.; Riegel, D.G. Integrated control of grape black rot: Influence of host phenology, inoculum availability, sanitation, and spray timing. Phytopathology 2004, 94, 641–650. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Szabó, M.; Csikász-Krizsics, A.; Dula, T.; Farkas, E.; Roznik, D.; Kozma, P.; Deák, T. Black rot of grapes (Guignardia bidwellii)—A comprehensive overview. Horticulturae 2023, 9, 130. [Google Scholar] [CrossRef]
- Ding, S.; Li, N.; Cao, M.; Huang, Q.; Chen, G.; Xie, S.; Zhang, J.; Cheng, G.; Li, W. Diversity of epiphytic fungi on the surface of Kyoho grape berries during ripening process in summer and winter at Nanning region, Guangxi, China. Fungal Biol. 2019, 123, 283–289. [Google Scholar] [CrossRef]
- Gramaje, D.; Eichmeier, A.; Spetik, M.; Carbone, M.J.; Bujanda, R.; Vallance, J.; Rey, P. Exploring the temporal dynamics of the fungal microbiome in rootstocks, the lesser-known half of the grapevine crop. J. Fungi 2022, 8, 421. [Google Scholar] [CrossRef] [PubMed]
- Kraus, C.; Voegele, R.T.; Fischer, M. Temporal development of the culturable, endophytic fungal community in healthy grapevine branches and occurrence of GTD-associated fungi. Microb. Ecol. 2019, 77, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Howell, K. Community succession of the grapevine fungal microbiome in the annual growth cycle. Environ. Microbiol. 2021, 23, 1842–1857. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Pinho, D.; Sousa, S.; Pinheiro, M.; Egas, C.; Gomes, A.C. Unravelling the diversity of grapevine microbiome. PLoS One 2014, 9, e85622. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Santoni, S.; Weber, A.; This, P.; Peros, J.P. Understanding the phyllosphere microbiome assemblage in grape species (Vitaceae) with amplicon sequence data structures. Sci. Rep. 2019, 9, 14294. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, B.; Korsten, L.; Berg, G. Plant health and sound vibration: Analyzing implications of the microbiome in grape wine leaves. Pathogens 2021, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Broberg, A.; Andreasson, E.; Stenberg, J.A. Biocontrol potential of beneficial fungus Aureobasidium pullulans against Botrytis cinerea and Colletotrichum acutatum. Phytopathology 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Cobos, R.; Ibanez, A.; Diez-Galan, A.; Calvo-Pena, C.; Ghoreshizadeh, S.; Coque, J.J.R. The grapevine microbiome to the rescue: Implications for the biocontrol of trunk diseases. Plants 2022, 11, 840. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Shen, L. Advances and trends in omics technology development. Front. Med. 2022, 9, 911861. [Google Scholar] [CrossRef] [PubMed]
- Furuya, S.; Mochizuki, M.; Aoki, Y.; Kobayashi, H.; Takayanagi, T.; Shimizu, M.; Suzuki, S. Isolation and characterization of Bacillus subtilis KS1 for the biocontrol of grapevine fungal diseases. Biocontrol Sci. Technol. 2011, 21, 705–720. [Google Scholar] [CrossRef]
- Ranade, Y.; Pathak, P.; Chandrashekar, M.; Saha, S. Biological control of Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. by epiphytic bacteria isolated from Vitis vinifera (cv Thompson Seedless) grape berry. Biocontrol Sci. Technol. 2023, 33, 173–189. [Google Scholar] [CrossRef]
- Mochizuki, M.; Yamamoto, S.; Aoki, Y.; Suzuki, S. Isolation and characterisation of Bacillus amyloliquefaciens S13-3 as a biological control agent for anthracnose caused by Colletotrichum gloeosporioides. Biocontrol Sci. Technol. 2012, 22, 697–709. [Google Scholar] [CrossRef]
- Wu, W.S.; Chang, L. Biological control of grape ripe rot and bitter rot. Plant Pathol. Bull. 1993, 2, 20–25. [Google Scholar] [CrossRef]
- Aoki, T.; Aoki, Y.; Ishiai, S.; Otoguro, M.; Suzuki, S. Impact of Bacillus cereus NRKT on grape ripe rot disease through resveratrol synthesis in berry skin. Pest Manag. Sci. 2017, 73, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Du, S.; Ren, Y.; Liu, Y. Biocontrol ability of killer yeasts (Saccharomyces cerevisiae) isolated from wine against Colletotrichum gloeosporioides on grape. J. Basic Microbiol. 2018, 58, 60–67. [Google Scholar] [CrossRef]
- Narkar, S.P.; Shetty, D.S.; Sawant, I.S.; Sawant, S.D. Paradigm shift in the resistance of grape isolates of Colletotrichum gloeosporioides to carbendazim and their biocontrol by Trichoderma harzianum. Indian Phytopath. 2012, 65, 373–377. [Google Scholar]
- Sawant, I.S.; Wadkar, P.N.; Rajguru, Y.R.; Mhaske, N.H.; Salunkhe, V.P.; Sawant, S.D.; Upadhyay, A. Biocontrol potential of two novel grapevine associated Bacillus strains for management of anthracnose disease caused by Colletotrichum gloeosporioides. Biocontrol. Sci. Technol. 2016, 26, 964–979. [Google Scholar] [CrossRef]
- Dong, L.M.; Quyen, N.T.T.; Thao, L.T.T.; Thao, T.T.T.; Quyen, C.T.N.; Thuy, D.T.K. Effect of calcium-alginate and essential oil on Colletotrichum acutatum and the shelf life of the grape. Vietnam. J. Sci. Technol. 2019, 57, 657–664. [Google Scholar] [CrossRef]
- Muñoz, Z.; Moret, A.; Garcés, S. Assessment of chitosan for inhibition of Colletotrichum sp. on tomatoes and grapes. Crop. Prot. 2009, 28, 36–40. [Google Scholar] [CrossRef]
- Liu, H.L.; Chao, C.H.; Shen, Y.M.; Wu, S.W. Control of major gape diseases with phosphorous acid. Bull. Taichung Dist. Agric. Res. Ext. Stn. 2010, 106, 55–64. [Google Scholar] [CrossRef]
- Creasy, G.L.; Creasy, L.L. Grapes, 2nd ed.; CAB International: Oxon, UK, 2018; p. 416. [Google Scholar]
- Chen, D.; Shi, H.J.; Wu, H.M.; Xu, Z.H.; Zhang, C.Q. Resistance of Colletotrichum gloeosporioides causing grape ripe rot to thiophanate-methyl and tebuconazole in Zhejiang. J. Fruit Sci. 2013, 30, 665–668. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Kim, H.Y.; Kim, J.H.; Park, J.H.; Lee, S.B.; Cheong, S.R.; Kim, H.T. Sensitivity of Colletotrichum spp. isolated from grapes in Korea to carbendazim and the mixture of carbendazim plus diethofencarb. Plant Pathol. J. 2010, 26, 49–56. [Google Scholar] [CrossRef][Green Version]
- Ishii, H.; Zhen, F.; Hu, M.; Li, X.; Schnabel, G. Efficacy of SDHI fungicides, including benzovindiflupyr, against Colletotrichum species. Pest Manag. Sci. 2016, 72, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zheng, H.; Zhang, P.; Chen, W.; Zheng, J.; Chen, C.; Cao, A. Molecular and biochemical characterization of Colletotrichum gloeosporioides isolates resistant to azoxystrobin from grape in China. Plant Pathol. 2021, 70, 1300–1309. [Google Scholar] [CrossRef]
- Hu, M.J.; Cosseboom, S. Evaluation of fungicides at different timing for control of ripe rot of grapes, 2018. Plant Dis. Manag. Rep. 2019, 13, PF068. [Google Scholar]
- Nita, M.; Oliver, C.; Melby, D.; Wong, A. Fungicide performance trial for control of Botrytis bunch rot, black rot, and ripe rot of grape in Virginia, 2017. Plant Dis. Manag. Rep. 2018, 12, PF011. [Google Scholar]
- Wang, J.; Shi, D.; Wei, L.; Chen, W.; Ma, W.; Chen, C.; Wang, K. Mutations at sterol 14α-demethylases (CYP51A&B) confer the DMI resistance in Colletotrichum gloeosporioides from grape. Pest Manag. Sci. 2020, 76, 4093–4103. [Google Scholar] [CrossRef] [PubMed]
- López-Zapata, S.P.; Castaño-Zapata, J. In vitro effect of four fungicides on Colletotrichum gloeosporioides causing anthracnosis on the Red Globe grape variety. Rev. Acad. Colomb. Cienc. Ex. Fis. Nat. 2020, 44, 747–758. [Google Scholar] [CrossRef]
- Narkar, S.P.; Sawant, I.S. In vitro evaluation of carbendazim resistant Colletotrichum gloeosporioides isolates of grapes for sensitivity to QoI and DMI fungicides. Indian Phytopath. 2016, 69, 77–81. [Google Scholar]
- Xu, X.F.; Lin, T.; Yuan, S.K.; Dai, D.J.; Shi, H.J.; Zhang, C.Q.; Wang, H.D. Characterization of baseline sensitivity and resistance risk of Colletotrichum gloeosporioides complex isolates from strawberry and grape to two demethylation-inhibitor fungicides, prochloraz and tebuconazole. Australas. Plant Pathol. 2014, 43, 605–613. [Google Scholar] [CrossRef]
- Cortaga, C.Q.; Cordez, B.W.P.; Dacones, L.S.; Balendres, M.A.O.; Dela Cueva, F.M. Mutations associated with fungicide resistance in Colletotrichum species: A review. Phytoparasitica 2023, 51, 569–592. [Google Scholar] [CrossRef]
- Chen, S.; Hu, M.; Schnabel, G.; Yang, D.; Yan, X.; Yuan, H. Paralogous CYP51 genes of Colletotrichum spp. mediate differential sensitivity to sterol demethylation inhibitors. Phytopathology 2020, 110, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.E.; Loeb, G.M.; Cadle-Davidson, L.; Evans, K.J.; Wilcox, W.F. Grape sour rot: A four-way interaction involving the host, yeast, acetic acid bacteria, and insects. Phytopathology 2018, 108, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- André, M.; Lacampagne, S.; Barsacq, A.; Gontier, E.; Petrel, M.; Mercier, L.; Courot, D.; Gény-Denis, L. Physical, anatomical, and biochemical composition of skins cell walls from two grapevine cultivars (Vitis vinifera) of champagne region related to their susceptibility to Botrytis cinerea during ripening. Horticulturae 2021, 7, 413. [Google Scholar] [CrossRef]
- Mundy, D.C. A review of the direct and indirect effects of nitrogen on botrytis bunch rot in wine grapes. N. Z. Plant Prot. 2008, 61, 306–310. [Google Scholar] [CrossRef]

| Colletotrichum Species Complex | Species | Country/ Region | Reference |
|---|---|---|---|
| C. gloeosporioides complex | C. aenigma | China | [30,31] |
| Japan | [32] 2 | ||
| South Korea | [33] 3 | ||
| USA | [4,12] 2 | ||
| C. fructicola | Brazil | [5] | |
| China | [9,31] | ||
| Japan | [34] 2 | ||
| Taiwan | [35] | ||
| USA | [4] 2 | ||
| C. gloeosporioides s.s. | China | [9] | |
| USA | [12] 2 | ||
| Japan | [34] | ||
| C. hebeiense | China | [30] | |
| C. kahawae | Brazil | [5] | |
| USA | [12] 2 | ||
| C. perseae | Japan | [34] | |
| C. siamense | Brazil | [36] | |
| USA | [4] 2 | ||
| C. tropicale | Taiwan | [35,37] | |
| C. viniferum | Brazil | [5] | |
| China | [9,30,38] | ||
| Japan | [32,34] 2 | ||
| South Korea | [39] | ||
| Taiwan | [8,40] | ||
| C. viniferum-like species (Clade V) 1 | Japan | [34] | |
| Other C. gloeosporioides s.l. | Australia | [6,16] | |
| Thailand | [41] 3 | ||
| C. acutatum complex | C. citri | China | [38] |
| C. fioriniae 1 | USA | [4,11,12] | |
| C. godetiae | Italy | [42] | |
| C. limitticola | Brazil | [5] | |
| C. nymphaeae | Brazil | [5] | |
| USA | [12] 2 | ||
| C. pseudoacutatum | China | [31] | |
| Other C. acutatum s.l. | Australia | [6,16,31,43] | |
| Japan | [10,44] | ||
| South Korea | [7] 2 | ||
| C. boninense complex | C. karstii | Brazil | [5] |
| C. orchidearum complex | C. cliviicola (syn. C. cliviae)-like species | China | [38] |
| BCA | Strains/Source | Efficacy against the Ripe Rot Pathogen | Year and Reference |
|---|---|---|---|
| Bacillus sp. | M5/ Grape tissue in Taiwan | Inhibited the growth of C. gloeosporioides in vitro, lowered the ripe rot disease index on detached fruits, and significantly increased the ratio of intact fruits in a Kyoho grape vineyard. | 1993 [124] |
| B. subtilis | KS1/ Grape berry skin of cv. Koshu in Japan. | Suppressed the mycelial growth of C. gloeosporioides in vitro, but did not reduce the bunch rot associated with C. gloeosporioides in a small-scale test in a Koshu vineyard in the 2008 growing season. | 2011 [121] |
| B. amyloliquefaciens | S13-3/ Soil sample from a plum grove in Japan | In vitro inhibition of the mycelial growth of various phytopathogenic fungi, including C. gloeosporioides. Significant reduction in the incidence of ripe rot that is caused by C. gloeosporioides on the grape berries of V. vinifera cv. Semillon in an experimental vineyard. | 2012 [123] |
| B. cereus | NRKT/ Unidentified liana in an experimental vineyard in Japan | Significantly reduced the incidence of ripe rot caused by C. gloeosporioides on the grape berries of V. vinifera cv. Pinot noir in an experimental vineyard. | 2017 [72] |
| Bacillus spp. | B. amyloliquefaciens D747; B. subtilis BV02/ Commercial products | The incidence of grape ripe rot on Vitis labrusca was treated with Bacillus spp., and this was not different from the control in a field experiment conducted in the 2020 season in Brazil. | 2022 [106] |
| B. aerius, B. velezensis, and B. subtilis | B. aerius SB5; B. velezensis SB13; B. subtilis SC15/ Surface of grape berries in India | Significant inhibition of the mycelial growth of C. gloeosporioides. Significant decrease in the infection caused by C. gloeosporioides in a detached leaf assay, a potted plants experiment, and in the field, achieved by using the grape cv. Thompson Seedless. | 2023 [122] |
| Chaetomium, Penicillium, and Trichoderma | Chaetomium cupreum CC, C. globosum CG, Trichoderma harzianum PC01, T. hamatum PC02, and Penicillium chrysogenum KMITL44/ Thailand | The crude extracts from the microbes inhibited the growth of C. gloeosporioides from grape. Applications of the bioproducts in a powder formulation in the field reduced ripe rot disease. | 2005 [41] |
| T. harzianum | NAIMCC-01965/ Plant pathology laboratory of ICAR-National Research Centre for Grapes in India | Could parasitize C. gloeosporioides in vitro and decrease the disease index of the symptoms caused by C. gloeosporioides in the artificially inoculated grape leaves of cv. Thompson Seedless. | 2012 [127] |
| Saccharomyces cerevisiae | GA8/ Isolated from wine | Significant inhibition of the mycelial growth and conidia germination of C. gloeosporioides. Significant control effect against C. gloeosporioides in the artificially inoculated grape berries. | 2018 [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, T.-F.; Shen, Y.-M.; Huang, J.-H.; Tsai, J.-N.; Lu, M.-T.; Lin, C.-P. Insights into Grape Ripe Rot: A Focus on the Colletotrichum gloeosporioides Species Complex and Its Management Strategies. Plants 2023, 12, 2873. https://doi.org/10.3390/plants12152873
Hsieh T-F, Shen Y-M, Huang J-H, Tsai J-N, Lu M-T, Lin C-P. Insights into Grape Ripe Rot: A Focus on the Colletotrichum gloeosporioides Species Complex and Its Management Strategies. Plants. 2023; 12(15):2873. https://doi.org/10.3390/plants12152873
Chicago/Turabian StyleHsieh, Ting-Fang, Yuan-Min Shen, Jin-Hsing Huang, Jyh-Nong Tsai, Ming-Te Lu, and Chu-Ping Lin. 2023. "Insights into Grape Ripe Rot: A Focus on the Colletotrichum gloeosporioides Species Complex and Its Management Strategies" Plants 12, no. 15: 2873. https://doi.org/10.3390/plants12152873
APA StyleHsieh, T.-F., Shen, Y.-M., Huang, J.-H., Tsai, J.-N., Lu, M.-T., & Lin, C.-P. (2023). Insights into Grape Ripe Rot: A Focus on the Colletotrichum gloeosporioides Species Complex and Its Management Strategies. Plants, 12(15), 2873. https://doi.org/10.3390/plants12152873








