Uncovering the Role of Hormones in Enhancing Antioxidant Defense Systems in Stressed Tomato (Solanum lycopersicum) Plants
Abstract
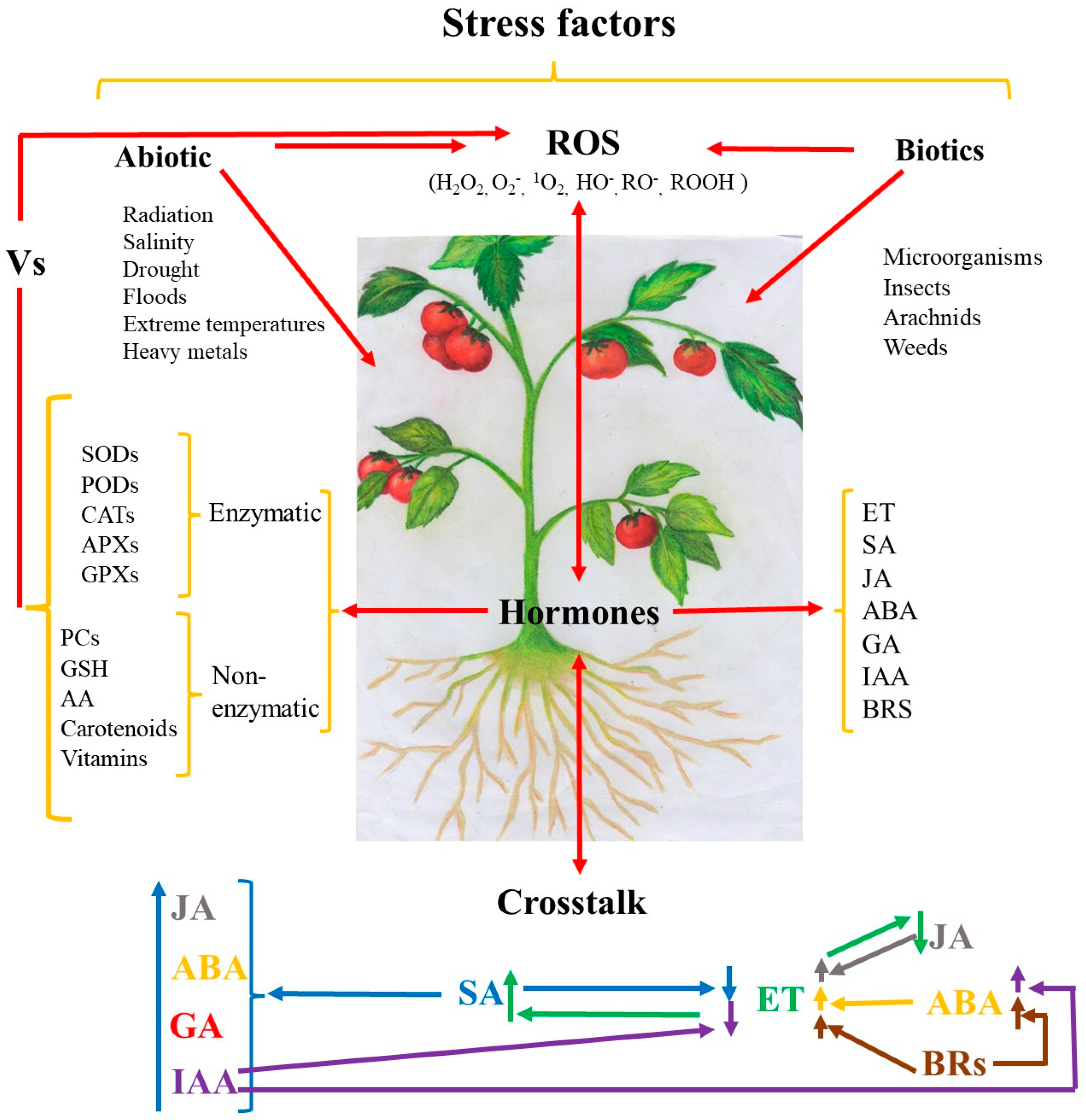
:1. Introduction
2. Enzymatic and Non-Enzymatic Antioxidants in Tomato Plants
2.1. Non-Enzymatic Antioxidant Compounds (NEACs)
2.1.1. Phenolic Compounds (PCs)
2.1.2. Carotenoids
2.1.3. Vitamins
2.1.4. Glutathione (GSH)
2.2. Antioxidant Enzymes (AEs)
2.2.1. Superoxide Dismutases (SODs)
2.2.2. Catalases (CATs)
2.2.3. Ascorbate Peroxidases (APXs)
2.2.4. Glutathione Peroxidases (GPXs)
| Component | Values | Source | Reference |
|---|---|---|---|
| Phenolic acids (mg/100 g DW 1) | 172.19–311.82 | Low 3 | [22,67,68] |
| TFs (mg/100 g DW) | 11.67–35.19 | Low | [22,67,68] |
| Lyc (mg/100 g FW 2) | 18.6–64.98 | High | [27,69] |
| Total carotenoids (mg/100 g FW) | 7.0–19.0 | Medium | [27,68] |
| AA (mg/100 g FW) | 16.32–19.43 | Low | [36,68] |
| Tocopherols (mg/100 g FW) | 0.17–0.62 | Low | [31,68] |
| AC [FRAP (mmol/100 g DW)] | 1.29–2.21 | Medium | [68,70,71] |
| AC [DPPH (mmol/100 g DW)] | 0.85–1.85 | Low | [67,68,70] |
| GSH (mg/100 g FW) | 1.43–1.61 | NR 4 | [72] |
| PODs (U/g FW) | 7.03–19.8 | NR | [73,74] |
| SODs (U/g FW) | 0.35–0.65 | NR | [73] |
| CATs (U/g FW) | 2.08–26.91 | NR | [73,74] |
| APXs (U/g FW) | 10.25–14.05 | NR | [73,74] |
3. Hormones and Their Effects on the Antioxidant System of Tomato Plants
3.1. Ethylene (ET)
| Hormone (C 1) | Variety/Tomato Part | Age | Stress Condition | Results | Values | Reference |
|---|---|---|---|---|---|---|
| ET 2 | Roma and Patio/microshoots | 3 weeks old | NaCl (0–200 mM) | OD increases as ET increases. | EL = 23–80% | [84] |
| MI = 0–73% | ||||||
| ET = 0.043–0.733 µ/Lh | ||||||
| ET 2 | Rio fuego/cells | 4 to 5 days after subculture | NaCl (250 nM) | ROS increases as ET increases | H2DCFDA = 430–643% | [85] |
| ET = 0.199–0.160 nL/g | ||||||
| ET 2 | Rio fuego/roots | 6 weeks old | NaCl (100–250 mM) | ROS increases as ET increases | H2DCFDA = 103–133.4% | [86] |
| EL = 117.90–428.66% | ||||||
| ET = 2.22–4.15 nL/g | ||||||
| ET 2 | Ailsa Craig and OE.MAPK3-5/roots | 6 weeks old | NaCl (100 mM) | AEs and OD were increased in OE.MAPK3-5 tomato plants | POD = 103.58% | [87] |
| SOD = 21.57% | ||||||
| APX = 11.34% | ||||||
| CAT = 48.90% | ||||||
| MDA = 39.02% | ||||||
| H2O2 = −48.6% | ||||||
| ET 2 | Ailsa Craig/roots and leaves | 23-day-old seedlings | NaCl (125 mM) | Lower ET production in S. lycopersicum than in S. chilense produces higher OD | MDA = 36.15–59.67% 4; 36.07–57.86% 5 | [88] |
| ET 2 | Micro-Tom, epi, and Nr/roots | 23 days old | Glomus clarum (10 g) | AEs increase in inoculated tomato plants | Cu/ZnSOD = 513.33% | [91] |
| APX = 106.66% | ||||||
| CAT = 59.46% | ||||||
| ET 2 | WT and Nr/roots | 8 weeks old | PGPB (10−7 CFU/mL) | PGPB increases NEACs in tomato plants | AA = 8.61–54.34% | [92] |
| GSH = 24.28–37.90% | ||||||
| ET 2 | WT and Nr/leaves | 6 to 7 weeks old | Fusaric acid (0.1–1.0 mM) | MDA was higher in Nr tomato plants and increased as FA increased | MDA = 2.17–55.4% | [94] |
| ET 2 | Valouro/fruits | Ripe stage | Cold storage (7 ± 0.5 °C) for 35 days | Some NEACs were reduced as ET increased | ET = 51.74% | [96] |
| AC = −16.2% | ||||||
| AA = −55.18% | ||||||
| PCs = −23.73% | ||||||
| Lyc = 92.0% | ||||||
| ET 2 | Calnegre/fruits | Breaker stage | Cold storage (8 ± 1 °C) for 28 days | After 28 days, ET and lyc increased, while AA was reduced. | ET = 57.35–268.9% | [95] |
| Lyc = 22.8–42.4% | ||||||
| AA = −(54.9–63.4)% | ||||||
| ET 2 | WT and antisense SlACS2/fruits | Mature green stage | Cold storage (4 °C) for 35 days | MDA and EL were less in WT tomato compared to antisense SlACS2 | ET = 564.1% | [14] |
| MDA = −37.3% | ||||||
| EL = −31.7% | ||||||
| ET 3 (0.01%) | Lichun/fruits | Mature green stage | Cold storage (2 ± 1 °C) for up to 3 weeks | Tomato fruit treated with ET presented less OD than untreated and 1-MCP treated | MDA = −(3.3–21.4)% | [97] |
| EL = −(39.4–66.6)% | ||||||
| ET 3 (100 µL/L) | BHN-602/fruits | Mature green stage | ET treatment and storage temperature (20 °C vs. 35 °C for 48 h) | ET treatment and higher temperature of storage increase NEACs | Lyc = 8.8% | [98] |
| Carotenoids = 11.6% | ||||||
| PCs = 5.6% | ||||||
| AC (FRAP) = 13.8% |
3.2. Salicylic Acid (SA)
| Hormone (C 1) | Variety/Tomato Part | Age | Stress Condition | Results | Values | Reference |
|---|---|---|---|---|---|---|
| SA 2 (0.1 mM) | Roma/roots | 7-week-old | NaCl (150–200 mM) | SA application reduces OD | EL = −(32–44%) | [105] |
| SA 2 (0.01 mM) | Super Marmande/roots and leaves | 35 days old | NaCl (100 mM) | SA application reduces OD | MDA = −(43.49–50.14)% 3 and−(23.62–25.88)% 4 | [106] |
| SA 2 (0.1 mM) | Hezuo 903/leaves | 47 days old | NaCl (100 mM) for 14 days | SA application improves the AEs and reduces OD | GSH = 60.1% | [107] |
| H2O2 = −47.2% | ||||||
| TBARS = −53.9% | ||||||
| SOD = 31.6% | ||||||
| CAT = 41.5% | ||||||
| APX = 29.60% | ||||||
| GPX = −25.06% | ||||||
| DHAR = 76.0% | ||||||
| SA 2 (1 mM) | Rio fuego/leaves | 31 days old | NaCl (100 mM) for 7 days | SA application improves AEs | SOD = 46.8% | [108] |
| CAT = 109.9% | ||||||
| APX = 494.9% | ||||||
| GR = 52.9% | ||||||
| AA = 29.5% | ||||||
| GSH = 52.6% | ||||||
| SA 2 (100 mM) | Pusa ruby/leaves | 75 days old | NaCl (250 mM) for 3 days | SA reduces the OD and increases the AEs | EL = −74.6% | [110] |
| SOD = 158.8% | ||||||
| CAT = 137.3% | ||||||
| APX = 166.6% | ||||||
| GR = 172.7% | ||||||
| SA 2 (0.5 and 1.0 mM) | Streenb and Floridat/leaves and roots | 80 days old | Growth under low temperature (10 °C) | SA applied increases AS and reduces OD | POD = 7.5–42.2% 3; 15.8–34.0% 4 | [111] |
| PPO = 14.2–50.1% 3; 18.7–39.8% 4 | ||||||
| AC = 21.4–31.6 % 3; 19.9–28.9% 4 | ||||||
| MDA = −(13.6–33.3%) 3 | ||||||
| EL = −(4.3–12.6%) 3 | ||||||
| SA 2 (200 ppm) | Super strain B/fruits | 3 months old | Growth under changing temperatures (7.8–32.3 °C) | SA increases NEACs | AA = 20.6% | [113] |
| Lyc = 8.4% | ||||||
| SA 2 (1 mM) | Hezuo 903/leaves | 8 days after, with leaves | Heat stress (42 °C for 36 h) | SA reduces OD and improves AS | EL = −27.8% | [112] |
| H2O2 = −22.7% | ||||||
| MDA = −28.1% | ||||||
| SOD = 22.2% | ||||||
| CAT = 100.3% | ||||||
| APX = 32.1% | ||||||
| POD = 61.6% | ||||||
| SA 2 (1 or 2 mM) | Newton/fruits | Mature green stage | Cold storage (1 °C) for 3 weeks | SA reduces OD | EL = −13.94% | [127] |
| MDA = −2.2% | ||||||
| LOX = −(33.6–45.4)% | ||||||
| SA 2 (4-mM foliar-applied plus 1-, 2-, or 4-mM by dipping 5 min) | Baraka/fruits | NP 5 | Cold storage (10 °C) for 40 days | SA application reduces OD and increases AA, without the effect of the concentration used (1, 2, or 4 mM) | EL = −(46.6–48.0%) | [128] |
| AA = 336.6–403.3% | ||||||
| APX = 447.6–455.5% | ||||||
| SA 2 (0.2–1.2 mM) | Samrudhi/fruits | Mature (pink to light red color) | Cold storage /4–5 °C) for 21 days | As increased SA concentration, AA increases but reduces NEACs | AA = 17.9–58.3% | [129] |
| Lyc = −(4.6–32.1)% | ||||||
| β-carotene = −(10.2–42.5)% | ||||||
| SA 2 (0.5–2 mM) | Durinta/fruits | Pink maturity | 5 or 20 °C for 20 days | As increased SA concentration reduces Lyc regardless of the storage temperature | Lyc = −(18.2–21.1)% | [130] |
3.3. Jasmonates
| Hormone (C 1) | Variety/Tomato Part | Age | Stress Condition | Results | Values | Reference |
|---|---|---|---|---|---|---|
| MeJA 2 (1 mM) | Beta/seedlings | 15 days old | Microorganism (Alternaria porri f. sp. Solani) | MeJA application increases NEACs | PCs= 17.1–21.5% | [144] |
| ATH= 12.6–14.1% | ||||||
| JA 2 (0.01–100 nM) | Pusa Ruby/seedlings | 7 days old | Microorganism (Meloidogyne incognita) | MeJA application reduces OD and increases AEs | O2− = −(17.8–30.9)% | [136] |
| SOD = 19.3–43.0% | ||||||
| POD = 15.4–48.6% | ||||||
| CAT = 14.5–52.5% | ||||||
| APX = 1.4–29.5% | ||||||
| DHAR = 18.8–51.9 | ||||||
| GST = −(24.5–35.5)% | ||||||
| GR = 14.5–70.3% | ||||||
| PPO = −(10.9–43.8)% | ||||||
| JA 2 (0.01–100 nM) | Pusa Ruby/seedlings | 7 days old | Microorganism (Meloidogyne incognita) | MeJA application reduces OD and increases NEACs | H2O2 = −(15.2–40.7)% | [135] |
| GSH = 18.8–63.1% | ||||||
| Carotenoids = 25.9–48.7% | ||||||
| TFs = 20.3–56.7% | ||||||
| ATH = 33.3–80.1% | ||||||
| XAN = −(8.8)−94.7% | ||||||
| AA = 7.9–28.9% | ||||||
| Tocopherols = 7.7–21.4% | ||||||
| PCs = 27.5–80.9% | ||||||
| MeJA 2 (0–60 µM) | Rio Grande and Savera/leaves | 50 days old | Seeds dipped into NaCl (5%) for 10 min | MeJA increases AEs | CAT = 6.0–30.2% | [131] |
| PRX = 5.3–25.1% | ||||||
| JA 3 | Castlemart and its JA-deficient mutant/leaves | 45 days old | CdCl2 (5–50 mg/kg soil) | WT tomato showed less OD and higher AEs compared to its JA-deficient mutant | MDA = −26.9% | [9] |
| EL = −27.6% | ||||||
| H2O2 = −21.1% | ||||||
| SOD = 29.5% | ||||||
| POD = 28.9% | ||||||
| CAT = 243.6% | ||||||
| JA 2 (1 nM) | NP 4/leaves | 55 days old | NaCl (200 mM) | JA treatment reduces OD and increases AS | H2O2 = −35.2% | [137] |
| MDA = −22.4% | ||||||
| AA = 40.3% | ||||||
| GSH = 8.6% | ||||||
| TF = 74.3% | ||||||
| SOD = 19.4% | ||||||
| CAT = 27.6% | ||||||
| APX = 20% | ||||||
| GR = 22.4% | ||||||
| MeJA 2 (100 µM) | MicroTom/leaves | 4-leaf stage | Cold stress (4 °C) for 24 h | MeJA increases putrescine and reduces OD | MDA = −41.6% | [138] |
| EL = −19.8% | ||||||
| MeJA 2 (44.8 µL/L) | Carousel/fruits | NP 4 | Cold storage (13 °C) | After 2-weeks of storage (13 °C) MeJA improved NEACs | AA = 50% | [140] |
| PCs = 87.4% | ||||||
| Lyc = 177.8% | ||||||
| β-carotene = 43.3% | ||||||
| MeJA 2 (0.05 mM) | Badun/fruits | Mature green | Cold storage (2 °C) for 28 days | MeJA treatment showed less OD and higher AS than silencing MeJA | MDA = −(39.7–70.3)% | [141] |
| SOD = 39.9–62.0% | ||||||
| POD = 47.7–63.6% | ||||||
| CAT = 36.6–54.6% | ||||||
| APX = 42.6–53.9% | ||||||
| Lyc = 24.1–51.9% |
3.4. Abscisic Acid (ABA)
| Hormone (C 1) | Variety/Tomato Part | Age | Stress Condition | Results | Values | Reference |
|---|---|---|---|---|---|---|
| ABA 2 (50 µM) | LA1698/leaves | Seedlings | NaCl (200 mM) | ABA application reduces OD and increases AS | MDA = −35.6% | [152] |
| H2O2 = −29.6% | ||||||
| SOD = 3.1% | ||||||
| POD = 17.1% | ||||||
| CAT = 3.8% | ||||||
| GR = 138.7% | ||||||
| APX = −20.5% | ||||||
| AA = 40.4% | ||||||
| GSH = 5.8% | ||||||
| ABA 3 | Rheinlands/leaves | 74 days old | NaCl (100 and 250 mM) | Silencing ABA mutants present lower NEACs | Carotenoids= −(60.6–74.6%) | [116] |
| ABA 3 | Ailsa Craig/roots | 13 days old | NaCl (150 mM) | Silencing ABA mutants reduce AEs and increase OD | APX = −33.9% | [150] |
| CAT = −24.2% | ||||||
| MDA = 40.3% | ||||||
| ABA 2 (50 µM) | PKM1/leaves | 7 days after 4-fully-expanded-leaves stage. | Drought (7 days) | ABA application reduces OD and increases AE | H2O2 = −57.0% | [154] |
| SOD = 10.2% | ||||||
| CAT = 233.3% | ||||||
| APX = 26.8% | ||||||
| GR = 6.0% | ||||||
| ABA 2 (150 µM) | Micro-Tom/leaves | 1 month | Drought (6 days) | ABA increases AEs compared to untreated plants | SOD = 6.2% | [155] |
| CAT = 17.8% | ||||||
| APX = 32.0% |
3.5. Gibberellic Acid (GA)
| Hormone (C 1) | Variety/Tomato Part | Age | Stress Condition | Results | Values | Reference |
|---|---|---|---|---|---|---|
| GA 2 (0.4–0.6 mM) | BF1 and UC82B/leaves | 45 days old | NaCl (200 mM) | GA improves AS | APX = 0–9.6% | [161] |
| PPO = 15.1–16.0% | ||||||
| SOD = 32.1–59.2% | ||||||
| TFs = 18.8–100% | ||||||
| PCs = 10.7–19.1% | ||||||
| Carotenoids = 294.4–1980% | ||||||
| GA 2 (100 µM) | NP 4/leaves | 3 weeks old | NaCl (250 mM) | GA application improves AS | GSH = 99.6% | [160] |
| MDA = −13.3% | ||||||
| GA 3 | Micro-Tom and procera mutant/shoots | 30 days old | Drought (7 days) | GA production reduces MDA, but induces H2O2 | MDA = −18.6% | [166] |
| H2O2 = 41.1% | ||||||
| GA 2 (10 µM) | CH/roots | 60 days old | Cd (20 µM) | GA application reduces OD and increases AEs | CAT = 9.3% | [162] |
| GPX = 20.9% | ||||||
| APX = 12.9% | ||||||
| MDA = −38.7% | ||||||
| GA 2 (100 ppm) | Fayrouz, Aziza and N23-48/shoot | 6 weeks old | Temperature of growth when tomato shoots were exposed to 10 and 45 °C | GA increases the AE | CAT = 1.5–13.9% | [163] |
| APX = 9.2–56.7% |
3.6. Auxins
| Hormone (C 1) | Variety/Tomato Part | Age | Stress condition | Results | Values | Reference |
|---|---|---|---|---|---|---|
| IAA 2 (50 µM) | Roots/cv. Navoday | 30 days old | Cd (100 µM) | AS was improved and OD was reduced in IAA treated plants | Carotenoids = 8.1% | [169] |
| APX = 28.0% | ||||||
| GR = 99.4% | ||||||
| AA = 99.3% | ||||||
| GSH = 133.3% | ||||||
| O2− = −32.0% | ||||||
| H2O2 = −27.1% | ||||||
| MDA = 37.1% | ||||||
| EL = 52.6% | ||||||
| IAA, NAA 2 (100 mg/L) | UC82B/leaves | NP 3 | NaCl (200 mM) | IAA increases CAT activity | CAT= 274.1–311.1% | [170] |
| IAA 2 (50 nM) | Five Star F-1 hybrid/leaves | 17 days old after seed germination | Heat shock (38 °C for 4 h) | IAA reduces OD and increases AE | MDA = −38.5% | [171] |
| EL = 20.5% | ||||||
| CAT = 9.6% | ||||||
| POD = 7.7% | ||||||
| SOD = 16.5% | ||||||
| IAA 2 (1 mM) | Pusa ruby/leaves | First fully expandedleaves | Benzoic acid (0.5–1 mM) | IAA reduces OD and increases AE | Carotenoids = 32.4–62.6% | [172] |
| EL = −(21.4–30.4)% | ||||||
| MDA = −(19.7–28.3)% | ||||||
| SOD = 31.7–40.5% | ||||||
| CAT = 66.1–97.3% | ||||||
| APX = 54.1–57.7% | ||||||
| GPX = 45.8–50.7% | ||||||
| IAA 2 (1 mM) | Pusa ruby/leaves | Fully expanded leaves | Vanillic acid (0.5–1 mM) | IAA reduces OD and increases AS | Carotenoids = 13.8–27.3% | [173] |
| MDA = −(9.8–13.0)% | ||||||
| EL = −(31.6–55.3)% | ||||||
| H2O2= −(3.4–18.5)% | ||||||
| SOD = 21.6–28.0% | ||||||
| CAT = 21.4–28.9% | ||||||
| APX = 31.8–34.5% | ||||||
| GPX = 42.0–52.1% | ||||||
| PCs = 23.1–41.0% | ||||||
| ATH = 15.3–34.7% | ||||||
| IAA 2 (0.09 mM) | NP 4/root and shoot | 60 days old | Orobanche ramose L. infection | IAA application improves AS and reduces OD | AC = 131.2% 4; 80.0% 5 | [123] |
| PCs = 48.4% 4; 46.1% 5 | ||||||
| TFs = 115.9% 4; 63.2% 5 | ||||||
| Tocopherols = 40.6% 4; 40.6% 5 | ||||||
| ASA = 21.7% 4; 20.6% 5 | ||||||
| GSH = 27.5% 4; 168.8% 5 | ||||||
| H2O2 = −8.1% 4; −26.2% 5 | ||||||
| MDA = −17.1% 4; −24.5% 5 | ||||||
| CAT = 37.2% 4; 31.1% 5 | ||||||
| POX = 31.0% 4; 33.0% 5 | ||||||
| SOD = 40.1% 4; 26.4% 5 | ||||||
| APX = 30.4% 4; 66.8% 5 | ||||||
| GR = 36.6% 4; 43.2% 5 |
3.7. Brassinosteroids (BRs)
| Hormone (C 1) | Variety/Tomato Part | Age | Stress Condition | Results | Values | Reference |
|---|---|---|---|---|---|---|
| BRs 2 (100 nM) | Hezuo 903/roots | 50 days old | Polychlorinated biphenyls | BRs increases AS and reduces OD | Carotenoids = 4.4–10.5% | [178] |
| H2O2 = ·(13.3–20.9)% | ||||||
| O2−= −(16.5–36.0)% | ||||||
| MDA = −(7.5–8.7)% | ||||||
| SOD = 15.2–30.2% | ||||||
| POD = 64.7–152.8% | ||||||
| CAT = 15.1–20.0% | ||||||
| APX = 35.9–56.6% | ||||||
| GR = 59.0–140% | ||||||
| BRs 2 (10.6 nM) | Amalia/leaves | 21 days old | Temperature (25–40 °C) | BRs increases AEs | SOD = 58.2–81.1% | [180] |
| POD = 12.1–50.5% | ||||||
| CAT = 36.2–84.9% | ||||||
| BRs 2 (0.01–1 mg/L) | 9021/leaves | 55 days old | Temperature (25–40 °C) for 8 days | As increase temperature, the BRs significantly improve the AEs and OD | SOD = 12.9–13.0% | [181] |
| APX = 13.0–35.7% | ||||||
| CAT = 23.4–89.2% | ||||||
| H2O2 = −(26.6–33.8)% | ||||||
| MDA = −(8.4–33.6)% | ||||||
| BRs 2 (100 nM) | Hezuo 903/roots | 50 days-old | Phenanthrene (300 µM) | Foliar application of BRs improves AS and reduces OD | PCs = 5.9% | [179] |
| TF = 10.5% | ||||||
| MDA = −13.3% | ||||||
| AC(DPPH) = 15.6% | ||||||
| BRs 2 (10−8 M) | K-25 and Sarvodya/leaves and fruit | 60 days old and mature fruit | Cd (100 µM) | BRs improves AS (except AA) | SOD = 18.6–27.9% 3 | [182] |
| POX = 26.0–34.6% 3 | ||||||
| CAT = 9.8–14.6% 3 | ||||||
| Lyc = 19.5–22.1% 4 | ||||||
| β-carotene = 8.6–14.8% 4 | ||||||
| AA = −(15.6–19.5)% 4 | ||||||
| BRs 2 (10–7 M) | K-21/leaves | 40 days old | Cr (10 mg/kg soil) | BRs reduces OD and increase AS | H2O2 = −50% | [141] |
| MDA = −49.3% | ||||||
| EL = −28.8% | ||||||
| MG = −30.9% | ||||||
| SOD = 27.3% | ||||||
| CAT = 19.7% | ||||||
| GST = 54.5% | ||||||
| APX = 37.0% | ||||||
| GR = 48.9% | ||||||
| AA = 31.8% | ||||||
| GSH = 17.6% | ||||||
| TF = 60.6% | ||||||
| BRs 2 (1 and 3 µM) | EC-652652 and EC-620419/leaves | 67 days old | Drought | BRs reduce OD and AS | H2O2 = −(16.6–26.1)% | [183] |
| SOD = 8.7–35.5% | ||||||
| Lyc = 4.1–16.0% |
4. Crosstalk among Hormones against Oxidative Damage Caused by Stress Factors
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| ABA | Abscisic acid |
| ABTS | 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid |
| AC | Antioxidant capacity |
| AEs | Antioxidant enzymes |
| APXs | Ascorbate peroxidases |
| ATH | Anthocyanins |
| AS | Antioxidant system |
| ASA | Ascorbate |
| AsA-GSH | Ascorbate-Glutathione |
| BRs | Brassinosteroids |
| CATs | Catalases |
| CHS | Chalcone synthase |
| C4H | Cinnamate 4-hydroxylase |
| DHA | Dehydroascorbate |
| DHAR | Dehydroascorbate reductase |
| DPPH | 2,2-difenil-1-picrilhidrazilo |
| EL | Electrolyte leakage |
| ET | Ethylene |
| ERFs | Ethylene response factors |
| GST | Glutathione-S-transferase |
| G6PDH | Glucose-6-phosphate dehydrogenase |
| FRAP | Ferric reducing antioxidant power |
| GA | Gibberellic acid |
| GPXs | Glutathione peroxidases |
| GR | Glutathione reductase |
| GSH | Glutathione |
| H2DCFDA | 2′,7′-dichlorofluorescein diacetate |
| IAA | Indole-3-acetic acid |
| IBA | Indole-3-butyric acid |
| JA | Jasmonic acid |
| MDA | Malondialdehyde |
| Lyc | Lycopene |
| MDHA | Monodehydroascorbate |
| MeJA | Methyl jasmonic acid |
| MG | Methylglyoxal |
| MI | Membrane injury |
| NAA | Naphthaleneacetic acid |
| NEACs | Non-enzymatic antioxidant compounds |
| Nr | Never ripe |
| OD | Oxidative damage |
| PGPB | Plant growth-promoting bacteria |
| LOX | Lysyl oxidase |
| PAL | Phenylalanine ammonia-lyase |
| PCs | Phenolic compounds |
| POD | guaiacol peroxidase |
| PPO | Polyphenol oxidase |
| PRXs | Peroxidases |
| ROS | Reactive oxygen species |
| RP | Reducing power |
| SA | Salicylic acid |
| SAR | Systemic acquired resistance |
| SKDH | Shikimate dehydrogenase |
| SlMAPK3 | Nitrogen-activated protein kinase |
| SODs | Superoxide dismutases |
| TBARS | Thiobarbituric acid reactive substances |
| TFs | Total flavonoids |
| XAN | Xanthophylls |
References
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and abiotic stresses in plants. Abiotic Biotic Stress Plants 2019, 1–6. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Li, Q.; Ding, W.-Y.; Dong, L.-W.; Deng, M.; Chen, J.H.; Tian, X.; Tian, X.; Hashem, A.; Al-Arjani, F.; et al. Arbuscular mycorrhizal fungi inoculation impacts expression of aquaporins and salt overly sensitive genes and enhances tolerance of salt stress in tomato. Chem. Biol. Technol. Agric. 2023, 10, 5. [Google Scholar] [CrossRef]
- Ramegowda, V.; Senthil-Kumar, M. The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 2015, 176, 47–54. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Wang, J.; Gong, Z.; Zhou, J.-M. Apoplastic ROS signaling in plant immunity. Curr. Opin. Plant Biol. 2017, 38, 92–100. [Google Scholar] [CrossRef]
- Caverzan, A.; Piasecki, C.; Chavarria, G.; Stewart, C.N., Jr.; Vargas, L. Defenses against ROS in crops and weeds: The effects of interference and herbicides. Int. J. Mol. Sci. 2019, 20, 1086. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Li, X.; Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Chen, Z.; Yu, J.-Q. H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J. Exp. Bot. 2014, 65, 4371–4383. [Google Scholar] [CrossRef]
- Zhao, S.; Ma, Q.; Xu, X.; Li, G.; Hao, L. Tomato jasmonic acid-deficient mutant spr2 seedling response to cadmium stress. J. Plant Growth Regul. 2016, 35, 603–610. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, X.; Chai, X.; Xue, D.; Zheng, W.; Shi, Y.; Wang, A. The involvement of jasmonic acid, ethylene, and salicylic acid in the signaling pathway of Clonostachys rosea-induced resistance to gray mold disease in tomato. Phytopathology 2019, 109, 1102–1114. [Google Scholar] [CrossRef]
- Tao, X.; Wu, Q.; Li, J.; Cai, L.; Mao, L.; Luo, Z.; Li, L.; Ying, T. Exogenous methyl jasmonate regulates sucrose metabolism in tomato during postharvest ripening. Postharvest Biol. Technol. 2021, 181, 111639. [Google Scholar] [CrossRef]
- Tao, X.; Wu, Q.; Li, J.; Wang, D.; Nassarawa, S.S.; Ying, T. Ethylene biosynthesis and signal transduction are enhanced during accelerated ripening of postharvest tomato treated with exogenous methyl jasmonate. Sci. Hortic. 2021, 281, 109965. [Google Scholar] [CrossRef]
- Aerts, N.; Mendes, M.P.; Van Wees, S.C.M. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Sheng, J.; Zhao, R.; Wang, Q.; Ma, P.; Shen, L. Ethylene biosynthesis is involved in regulating chilling tolerance and SlCBF1 gene expression in tomato fruit. Postharvest Biol. Technol. 2019, 149, 139–147. [Google Scholar] [CrossRef]
- Shiraz, M.; Sami, F.; Siddiqui, H.; Yusuf, M.; Hayat, S. Interaction of auxin and nitric oxide improved photosynthetic efficiency and antioxidant system of Brassica juncea plants under salt stress. J. Plant Growth Regul. 2020, 40, 2379–2389. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations. FAOSTAT Database. 2017. Available online: https://www.fao.org/faostat/en/#search/tomato (accessed on 3 February 2022).
- Špika, M.J.; Dumičić, G.; Bubola, B.K.; Soldo, B.; Ban, S.G.; Selak, G.V.; Ljubenkov, I.; Mandušić, M.; Žanić, K. Modification of the sensory profile and volatile aroma compounds of tomato fruits by the scion × rootstock interactive effect. Front. Plant Sci. 2021, 11, 616431. [Google Scholar] [CrossRef]
- Galhardo, R.; Ferraz, E.A. Tomatoes and tomato products as dietary sources of antioxidants. Food Rev. Int. 2009, 25, 313–325. [Google Scholar] [CrossRef]
- Fanasca, S.; Colla, G.; Rouphael, Y.; Saccardo, F.; Maiani, G.; Venneria, E.; Azzini, E. Evolution of nutritional value of two tomato genotypes grown in soilless culture as affected by macrocation proportions. HortScience 2006, 41, 1584–1588. [Google Scholar] [CrossRef]
- Luna-Guevara, M.L.; Luna-Guevara, J.J.; Hernández-Carranza, P.; Ruíz-Espinosa, H.; Ochoa-Velasco, C.E. Phenolic compounds: A good choice against chronic degenerative diseases. In Studies in Natural Products Chemistry; Atta-ur-Rahman, F.R.S., Ed.; University of Karachi: Karachi, Pakistan, 2018; Volume 59, pp. 79–108. [Google Scholar] [CrossRef]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.P.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Gómez-Romero, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Metabolite profiling and quantification of phenolic compounds in methanol extracts of tomato fruit. Phytochemistry 2010, 71, 1848–1864. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Pinela, J.; Carvalho, A.M.; Buelga, C.S.; Ferreira, I.C.F.R. Characterization and quantification of phenolic compounds in four tomato (Lycopersicon esculentum L.) farmers’ varieties in Northeastern Portugal homegardens. Plant Foods Hum. Nutr. 2012, 67, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Llorca, M.; Muñoz, P.; Müller, M.; Munné-Bosch, S. Biosynthesis, metabolism and function of auxin, salicylic acid and melatonin in climacteric and non-climacteric fruits. Front. Plant Sci. 2019, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Story, E.N.; Kopec, R.E.; Schwartz, S.J.; Harris, G.K. An update on the health effects of tomato lycopene. Annu. Rev. Food Sci. Technol. 2010, 1, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.-X.; Wei, J.-J.; Zhang, Y.-T.; Song, S.-W.; Su, W.; Sun, G.-W.; Hao, Y.-W.; Liu, H.-C. Supplemental blue and red light promote lycopene synthesis in tomato fruits. J. Integr. Agric. 2019, 18, 590–598. [Google Scholar] [CrossRef]
- Inbaraj, B.; Chen, B.H. Carotenoids in tomato plants. In Tomatoes and Tomato Products; CRC Press: Boca Raton, FL, USA, 2008; pp. 133–164. [Google Scholar]
- Brandt, S.; Pék, Z.; Barna, É.; Lugasi, A.; Helyes, L. Lycopene content and colour of ripening tomatoes as affected by environmental conditions. J. Sci. Food Agric. 2006, 86, 568–572. [Google Scholar] [CrossRef]
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Hajirezaei, M.; Hofius, D.; Sonnewald, U.; Voll, L.M. Specific roles of alpha- and gamma-tocopherol in abiotic stress responses of transgenic tobacco. Plant Physiol. 2007, 143, 1720–1738. [Google Scholar] [CrossRef]
- Raiola, A.; Tenore, G.C.; Barone, A.; Frusciante, L.; Rigano, M.M. Vitamin E content and composition in tomato fruits: Beneficial roles and bio-fortification. Int. J. Mol. Sci. 2015, 16, 29250–29264. [Google Scholar] [CrossRef]
- Štolfa, I.; Žuna, P.T.; Špoljarić, D. Abiotic stress response in plants: The relevance of tocopherols. In Antioxidants and Antioxidant Enzymes in Higher Plants; Hupa, D., Palma, J., Corpa, F., Eds.; Springer: Cham, Switzerland, 2018; pp. 233–252. [Google Scholar]
- Almeida, J.; da Azevedo, M.S.; Spicher, L.; Glauser, G.; Dorp, K.V.; Guyer, L.; del Carranza, A.V.; Asis, R.; de Souza, A.P.; Buckeridge, M.; et al. Down-regulation of tomato PHYTOL KINASE strongly impairs tocopherol biosynthesis and affects prenyllipid metabolism in an organ-specific manner. J. Exp. Bot. 2016, 67, 919–934. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin C content in fruits: Biosynthesis and regulation. Front. Plant Sci. 2019, 9, 2006. [Google Scholar] [CrossRef]
- Valšíková-Frey, M.; Komár, P.; Rehuš, M. The effect of varieties and degree of ripeness on vitamin C content in tomato fruits. Acta Hortic. Regiotect. 2017, 20, 44–48. [Google Scholar] [CrossRef]
- Del Giudice, R.; Raiola, A.; Tenore, G.C.; Frusciante, L.; Barone, A.; Monti, D.M.; Rigano, M.M. Antioxidant bioactive compounds in tomato fruits at different ripening stages and their effects on normal and cancer cells. J. Funct. Foods 2015, 18, 83–94. [Google Scholar] [CrossRef]
- Dorion, S.; Ouellet, J.C.; Rivoal, J. Glutathione metabolism in plants under stress: Beyond reactive oxygen species detoxification. Metabolites 2021, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Ammar, W.B.; Mediouni, C.; Tray, B.; Ghorbel, M.H.; Jemal, F. Glutathione and phytochelatin contents in tomato plants exposed to cadmium. Biol. Plant. 2008, 52, 314–320. [Google Scholar] [CrossRef]
- Kuźniak, E.; Skłodowska, M. Compartment-specific role of the ascorbate–glutathione cycle in the response of tomato leaf cells to Botrytis cinerea infection. J. Exp. Bot. 2005, 56, 921–933. [Google Scholar] [CrossRef]
- Jan, S.; Noman, A.; Kaya, C.; Ashraf, M.; Nasser, A.M.; Ahmad, P. 24-Epibrassinolide alleviates the injurious effects of Cr(VI) toxicity in tomato plants: Insights into growth, physio-biochemical attributes, antioxidant activity and regulation of ascorbate–glutathione and glyoxalase cycles. J. Plant Growth Regul. 2020, 39, 1587–1604. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, R.; Yan, X.; Fan, K. Superoxide dismutase nanozymes: An emerging star for anti-oxidation. J. Mater. Chem. B 2021, 9, 6939–6957. [Google Scholar] [CrossRef]
- Su, W.; Raza, A.; Gao, A.; Jia, Z.; Zhang, Y.; Hussain, M.A.; Mehmood, S.S.; Cheng, Y.; Lv, Y.; Zou, X. Genome-wide analysis and expression profile of superoxide dismutase (SOD) gene family in rapeseed (Brassica napus L.) under different hormones and abiotic stress conditions. Antioxidants 2021, 10, 1182. [Google Scholar] [CrossRef]
- Li, X.; Tsuta, M.; Hayakawa, F.; Nakano, Y.; Kazami, Y.; Ikehata, A. Estimating the sensory qualities of tomatoes using visible and near-infrared spectroscopy and interpretation based on gas chromatography-mass spectrometry metabolomics. Food Chem. 2021, 343, 128470. [Google Scholar] [CrossRef]
- Wang, W.; Xia, M.; Chen, J.; Deng, F.; Yuan, R.; Zhang, X.; Shen, F. Genome-wide analysis of superoxide dismutase gene family in Gossypium raimondii and G. arboretum. Plant Gene 2016, 6, 18–29. [Google Scholar] [CrossRef]
- Feng, K.; Yu, J.; Cheng, Y.; Ruan, M.; Wang, R.; Ye, Q.; Zhou, G.; Li, Z.; Yao, Z.; Yang, Y.; et al. The SOD gene family in tomato: Identification, phylogenetic relationships, and expression patterns. Front. Plant Sci. 2016, 7, 1279. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Yun, L.; Ji, L.; Li, G.; Ji, M.; Shi, Y.; Zheng, X. Catalase (CAT) gene family in wheat (Triticum aestivum L.): Evolution, expression pattern and function analysis. Int. J. Mol. Sci. 2022, 23, 542. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Prieto, M.; Biarnés, X.; Vidossich, P.; Rovira, C. The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, U.K.; Gökçe, Z.N.Ö.; Gökçe, A.F. Drought and salt stress effects on biochemical changes and gene expression of photosystem II and catalase genes in selected onion cultivars. Biologia 2021, 76, 3107–3121. [Google Scholar] [CrossRef]
- Raza, A.; Su, W.; Gao, A.; Mehmood, S.S.; Hussain, M.A.; Nie, W.; Lv, Y.; Zou, X.; Zhang, X. Catalase (CAT) gene family in rapeseed (Brassica napus L. ): Genome-wide analysis, identification, and expression pattern in response to multiple hormones and abiotic stress conditions. Int. J. Mol. Sci. 2021, 22, 4281. [Google Scholar] [CrossRef]
- Kafeel, S.; Hashim, Z.; Fawwad, A.; Nuzhat, N.S. Predisposition of SOD1, GPX1, CAT genetic variants and their haplotypes in cataractogenesis of type 2 diabetes mellitus in Pakistan. Acta Diabetol. 2022, 59, 623–632. [Google Scholar] [CrossRef]
- Kabir, M.H.; Wang, M.H. Functional studies on two catalase genes from tomato (Solanum lycopersicum L.). J. Hortic. Sci. Biotechnol. 2011, 86, 84–90. [Google Scholar] [CrossRef]
- Shoja, H.H.M.; Khezriani, T.; Kolahi, M.; Mohajel, K.E.; Yazdi, M. Morphologic and anatomic response, catalase gene expression in drought varieties of tomato and bioinformatics analyses of microarray studies of the catalase gene. Res. Sq. 2016, 1–23. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Yin, W.; Cui, B.; Yu, X.; Lu, Y.; Hu, Z. Silencing of SlHB2 improves drought, salt stress tolerance, and induces stress-related gene expression in tomato. J. Plant Growth Regul. 2017, 36, 578–589. [Google Scholar] [CrossRef]
- Kuo, E.Y.H.; Cai, M.S.; Lee, T.M. Ascorbate peroxidase 4 plays a role in the tolerance of Chlamydomonas reinhardtii to photo-oxidative stress. Sci. Rep. 2020, 10, 13287. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Shumayla; Chandra, V.P.; Singh, K.; Kumar, S.U. Molecular characterization of ascorbate peroxidase (APX) and APX-related (APX-R) genes in Triticum aestivum L. Genomics 2020, 112, 4208–4223. [Google Scholar] [CrossRef] [PubMed]
- Omoarelojie, L.O.; Kulkarni, M.G.; Finnie, J.F.; Staden, J.V. Biostimulants and the modulation of plant antioxidant systems and properties. In Biostimulants for Crops from Seed Germination to Plant Development; Shubhpriya Gupta, S., Van Staden, J., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 333–363. [Google Scholar] [CrossRef]
- Liu, J.-X.; Feng, K.; Duan, A.Q.; Li, H.; Yang, Q.Q.; Xu, Z.S.; Xiong, A.-S. Isolation, purification and characterization of an ascorbate peroxidase from celery and overexpression of the AgAPX1 gene enhanced ascorbate content and drought tolerance in Arabidopsis. BMC Plant Biol. 2019, 19, 488. [Google Scholar] [CrossRef] [PubMed]
- Ozyigit, I.I.; Filiz, E.; Vatansever, R.; Kurtoglu, K.Y.; Koc, I.; Öztürk, M.X.; Anjum, N.A. Identification and comparative analysis of H2O2-scavenging enzymes (ascorbate peroxidase and glutathione peroxidase) in selected plants employing bioinformatics approaches. Front. Plant Sci. 2016, 7, 301. [Google Scholar] [CrossRef]
- Najami, N.; Janda, T.; Barriah, W.; Kayam, G.; Tal, M.; Guy, M.; Volokita, M. Ascorbate peroxidase gene family in tomato: Its identification and characterization. Mol. Genet. Genom. 2008, 279, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Akbudak, M.A.; Filiz, E.; Vatansever, R.; Kontbay, K. Genome-wide identification and expression profiling of ascorbate peroxidase (APX) and glutathione peroxidase (GPX) genes under drought stress in sorghum (Sorghum bicolor L.). J. Plant Growth Regul. 2018, 37, 925–936. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Bela, K.; Horváth, E.; Rigó, G.; Gallé, Á.; Szabados, L.; Fehér, A.; Csiszár, J. Overexpression of the Arabidopsis glutathione peroxidase-like 5 gene (AtGPXL5) resulted in altered plant development and redox status. Environ. Exp. Bot. 2019, 167, 103849. [Google Scholar] [CrossRef]
- Paiva, A.L.S.; Passaia, G.; Lobo, A.K.M.; Jardim-Messeder, D.; Silveira, J.A.G.; Margis-Pinheiro, M. Mitochondrial glutathione peroxidase (OsGPX3) has a crucial role in rice protection against salt stress. Environ. Exp. Bot. 2019, 158, 12–21. [Google Scholar] [CrossRef]
- Herbette, S.; de Labrouhe, D.T.; Drevet, J.R.; Roeckel-Drevet, P. Transgenic tomatoes showing higher glutathione peroxydase antioxidant activity are more resistant to an abiotic stress but more susceptible to biotic stresses. Plant Sci. 2011, 180, 548–553. [Google Scholar] [CrossRef]
- Karpuz, B.; Çakir, Ö. Effect of proteasome inhibitor MG132 on the expression of oxidative metabolism related genes in tomato. Food Sci. Technol. Camp. 2021, 42, e52420. [Google Scholar] [CrossRef]
- Sharma, N.; Muthamilarasan, M.; Dulani, P.; Prasad, M. Genomic dissection of ROS detoxifying enzyme encoding genes for their role in antioxidative defense mechanism against Tomato leaf curl New Delhi virus infection in tomato. Genomics 2021, 113, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yng, M.H.; ElSohly, M.A.; Khan, I.A. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evid.-Based Complement. Altern. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef] [PubMed]
- Cömert, E.D.; Mogol, B.A.; Gökmen, V. Relationship between color and antioxidant capacity of fruits and vegetables. Curr. Res. Food Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Lee, J.G. Ripening-dependent changes in antioxidants, color attributes, and antioxidant activity of seven tomato (Solanum lycopersicum L.) cultivars. J. Anal. Methods Chem. 2016, 2016, 5498618. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.; Shi, G.; Zhang, X. Selenium delays tomato fruit ripening by inhibiting ethylene biosynthesis and enhancing the antioxidant defense system. Food Chem. 2017, 219, 179–184. [Google Scholar] [CrossRef]
- Yao, G.F.; Wei, Z.-Z.; Li, T.-T.; Tang, J.; Huang, Z.Q.; Yang, F.; Li, Y.-H.; Han, Z.; Hu, F.; Hu, L.-Y.; et al. Modulation of enhanced antioxidant activity by hydrogen sulfide antagonization of ethylene in tomato fruit ripening. J. Agric. Food Chem. 2018, 66, 10380–10387. [Google Scholar] [CrossRef]
- Tao, X.; Wu, Q.; Aalim, H.; Li, L.; Mao, L.; Luo, Z.; Ying, T. Effects of exogenous abscisic acid on bioactive components and antioxidant capacity of postharvest tomato during ripening. Molecules 2020, 25, 1346. [Google Scholar] [CrossRef]
- Basso, A.; Moreira, R.d.F.P.M.; José, H.J. Effect of operational conditions on photocatalytic ethylene degradation applied to control tomato ripening. J. Photochem. Photobiol. A Chem. 2018, 367, 294–301. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Ionica, M.E. Evolution of antioxidant activity and bioactive compounds in tomato (Lycopersicon esculentum Mill.) fruits during growth and ripening. J. Appl. Bot. Food Qual. 2014, 87, 97–103. [Google Scholar] [CrossRef]
- Guo, J.-E. Histone deacetylase gene SlHDT1 regulates tomato fruit ripening by affecting carotenoid accumulation and ethylene biosynthesis. Plant Sci. 2022, 318, 111235. [Google Scholar] [CrossRef]
- Prol, F.V.; López-Gresa, M.P.; Rodrigo, I.; Bellés, J.M.; Lisón, P. Ethylene is involved in symptom development and ribosomal stress of tomato plants upon citrus exocortis viroid infection. Plants 2020, 9, 582. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Li, L.; Chu, Z.; Zhang, H.; Li, H.; Fernie, A.R.; Ouyang, B. Impairment of hormone pathways results in a general disturbance of fruit primary metabolism in tomato. Food Chem. 2019, 274, 170–179. [Google Scholar] [CrossRef]
- Klay, I.; Pirrello, J.; Riahi, L.; Bernadac, A.; Cherif, A.; Bouzayen, M.; Bouzid, S. Ethylene response factor Sl-ERF.B.3 is responsive to abiotic stresses and mediates salt and cold stress response regulation in tomato. Sci. World J. 2014, 2014, 167681. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Zhang, H.; Ma, Q.; Fan, F.; Fu, R.; Ahammed, G.J.; Yu, J.; Shi, K. Role of ethylene biosynthesis and signaling in elevated CO2-induced heat stress response in tomato. Planta 2019, 250, 563–572. [Google Scholar] [CrossRef]
- Pan, Y.; Seymour, G.B.; Lu, C.; Hu, Z.; Chen, X.; Chen, G. An ethylene response factor (ERF5) promoting adaptation to drought and salt tolerance in tomato. Plant Cell Rep. 2012, 31, 349–360. [Google Scholar] [CrossRef]
- Klay, I.; Gouia, S.; Liu, M.; Mila, I.; Khoudi, H.; Bernadac, A.; Bouzayen, M.; Pirrello, J. Ethylene response factors (ERF) are differentially regulated by different abiotic stress types in tomato plants. Plant Sci. 2018, 274, 137–145. [Google Scholar] [CrossRef]
- Shibli, R.A.; Kushad, M.; Yousef, G.G.; Lila, M.A. Physiological and biochemical responses of tomato microshoots to induced salinity stress with associated ethylene accumulation. Plant Growth Regul. 2007, 51, 159–169. [Google Scholar] [CrossRef]
- Poór, P.; Kovács, J.; Szopkó, D.; Tari, I. Ethylene signaling in salt stress- and salicylic acid-induced programmed cell death in tomato suspension cells. Protoplasma 2013, 250, 273–284. [Google Scholar] [CrossRef]
- Poór, P.; Borbély, P.; Kovács, J.; Szepesi, A.; Takács, Z.; Tari, I. Opposite extremes in ethylene/nitric oxide ratio induce cell death in suspension culture and root apices of tomato exposed to salt stress. Acta Biol. Hung. 2014, 65, 428–438. [Google Scholar] [CrossRef]
- Shu, P.; Li, Y.; Li, Z.; Xiang, L.; Sheng, J.; Shen, L. Ferulic acid enhances chilling tolerance in tomato fruit by up-regulating the gene expression of CBF transcriptional pathway in MAPK3-dependent manner. Postharvest Biol. Technol. 2022, 185, 111775. [Google Scholar] [CrossRef]
- Gharbi, E.; Martínez, J.P.; Benahmed, H.; Lepoint, G.; Vanpee, B.; Quinet, M.; Lutts, S. Inhibition of ethylene synthesis reduces salt-tolerance in tomato wild relative species Solanum chilense. J. Plant Physiol. 2017, 210, 24–37. [Google Scholar] [CrossRef]
- Monteiro, C.C.; Carvalho, R.F.; Gratão, P.L.; Carvalho, G.; Tezotto, T.; Medici, L.O.; Peres, L.E.P.; Azevedo, R.A. Biochemical responses of the ethylene-insensitive Never ripe tomato mutant subjected to cadmium and sodium stresses. Environ. Exp. Bot. 2011, 71, 306–320. [Google Scholar] [CrossRef]
- Tian, D.; Peiffer, M.; De Moraes, C.M.; Felton, G.W. Roles of ethylene and jasmonic acid in systemic induced defense in tomato (Solanum lycopersicum) against Helicoverpa zea. Planta 2014, 239, 577–589. [Google Scholar] [CrossRef]
- Fracetto, G.G.M.; Peres, L.E.P.; Mehdy, M.C.; Lambais, M.R. Tomato ethylene mutants exhibit differences in arbuscular mycorrhiza development and levels of plant defense-related transcripts. Symbiosis 2013, 60, 155–167. [Google Scholar] [CrossRef]
- Ibort, P.; Imai, H.; Uemura, M.; Aroca, R. Proteomic analysis reveals that tomato interaction with plant growth promoting bacteria is highly determined by ethylene perception. J. Plant Physiol. 2018, 220, 43–59. [Google Scholar] [CrossRef]
- Gao, Y.; Li, S.-J.; Zhang, S.W.; Feng, T.; Zhang, Z.-Y.; Luo, S.-J.; Mao, H.-Y.; Borkovich, K.A.; Ouyang, S.-Q. SlymiR482e-3p mediates tomato wilt disease by modulating ethylene response pathway. Plant Biotechnol. J. 2021, 19, 17. [Google Scholar] [CrossRef]
- Iqbal, N.; Czékus, Z.; Ördög, A.; Poór, P. Ethylene-dependent effects of fusaric acid on the photosynthetic activity of tomato plants. Photosynthetica 2021, 59, 337–348. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Guillén, F.; Castillo, S.; Zapata, P.J.; Valero, D.; Serrano, M. Effect of ethylene concentration on quality parameters of fresh tomatoes stored using a carbon-heat hybrid ethylene scrubber. Postharvest Biol. Technol. 2009, 51, 206–211. [Google Scholar] [CrossRef]
- Mansourbahmani, S.; Ghareyazie, B.; Zarinnia, V.; Kalatejari, S.; Mohammadi, R.S. Study on the efficiency of ethylene scavengers on the maintenance of postharvest quality of tomato fruit. J. Food Meas. Charact. 2018, 12, 691–701. [Google Scholar] [CrossRef]
- Zhao, D.; Shen, L.; Fan, B.; Yu, M.; Zheng, Y.; Lv, S.; Sheng, J. Ethylene and cold participate in the regulation of LeCBF1 gene expression in postharvest tomato fruits. FEBS Lett. 2009, 583, 3329–3334. [Google Scholar] [CrossRef] [PubMed]
- Loayza, F.E.; Brecht, J.K.; Simonne, A.H.; Plotto, A.; Baldwin, E.A.; Bai, J.; Lon-Kan, E. Synergy between hot water treatment and high temperature ethylene treatment in promoting antioxidants in mature-green tomatoes. Postharvest Biol. Technol. 2020, 170, 111314. [Google Scholar] [CrossRef]
- Cornelia, P.; Adriana, C.; Iulia, V.N. Studies regarding the influence of exogenous salicylic acid treatment on some bioactive compounds of two varieties of cherry tomatoes. Nat. Resour. Sustain. Dev. 2018, 8, 67–75. [Google Scholar] [CrossRef]
- Klessig, D.F.; Choi, H.W.; Dempsey, D.M.A. Systemic acquired resistance and salicylic acid: Past, present, and future. Curr. Rev. 2018, 31, 871–888. [Google Scholar] [CrossRef]
- Baby, J.; Jini, D.; Sujatha, S. Insight into the role of exogenous salicylic acid on plants grown under salt environment. Asian J. Crop Sci. 2010, 2, 226–235. [Google Scholar] [CrossRef]
- Surapu, V.; Ediga, A.; Meriga, B. Salicylic acid alleviates aluminum toxicity in tomato seedlings (Lycopersicum esculentum Mill.) through activation of antioxidant defense system and proline biosynthesis. Adv. Biosci. Biotechnol. 2014, 5, 777–789. [Google Scholar] [CrossRef]
- Falcioni, T.; Ferrio, J.P.; del Cueto, A.I.; Giné, J.; Achón, M.A.; Medina, V. Effect of salicylic acid treatment on tomato plant physiology and tolerance to potato virus X infection. Eur. J. Plant Pathol. 2014, 138, 331–345. [Google Scholar] [CrossRef]
- da Lobato, A.K.S.; Barbosa, M.A.M.; Alsahli, A.A.; Lima, E.J.A.; da Silva, B.R.S.D. Exogenous salicylic acid alleviates the negative impacts on production components, biomass and gas exchange in tomato plants under water deficit improving redox status and anatomical responses. Physiol. Plant. 2020, 172, 869–881. [Google Scholar] [CrossRef]
- Stevens, J.; Senaratna, T.; Sivasithamparam, K. Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): Associated changes in gas exchange, water relations and membrane stabilisation. Plant Growth Regul. 2006, 49, 77–83. [Google Scholar] [CrossRef]
- Manaa, A.; Gharbi, E.; Mimouni, H.; Wasti, S.; Aschi-Smiti, S.; Lutts, S.; Ahmed, H.B. Simultaneous application of salicylic acid and calcium improves salt tolerance in two contrasting tomato (Solanum lycopersicum) cultivars. S. Afr. J. Bot. 2014, 95, 32–39. [Google Scholar] [CrossRef]
- He, Y.; Zhu, Z.J. Exogenous salicylic acid alleviates NaCl toxicity and increases antioxidative enzyme activity in Lycopersicon esculentum. Biol. Plant. 2008, 52, 792–795. [Google Scholar] [CrossRef]
- Tari, I.; Csiszár, J.; Horváth, E.; Poór, P.; Takács, Z.; Szepesi, Á. The alleviation of the adverse effects of salt stress in the tomato plant by salicylic acid shows a time- and organ-specific antioxidant response. Acta Biol. Crac. Ser. Bot. 2015, 57, 21–30. [Google Scholar] [CrossRef]
- Jaime-Pérez, N.; Pineda, B.; García-Sogo, B.; Atares, A.; Athman, A.; Byrt, C.S.; Olías, R.; Asins, M.J.; Gilliham, M.; Moreno, V.; et al. The sodium transporter encoded by the HKT1;2 gene modulates sodium/potassium homeostasis in tomato shoots under salinity. Plant Cell Environ. 2017, 40, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.R.; Ansari, M.W.; Sahoo, R.K.; Wattal, R.K.; Tuteja, N.; Kumar, V.R. Salicylic acid modulates ACS, NHX1, sos1 and HKT1; 2 expression to regulate ethylene overproduction and Na+ ions toxicity that leads to improved physiological status and enhanced salinity stress tolerance in tomato plants cv. Pusa Ruby. Plant Signal. Behav. 2021, 16, 1950888. [Google Scholar] [CrossRef] [PubMed]
- Orabi, S.A.; Dawood, M.; Salman, S. Comparative study between the physiological role of hydrogen peroxide and salicylic acid in alleviating the harmful effect of low temperature on tomato plants grown under sand-ponic culture. Sci. Agric. 2015, 9, 49–59. [Google Scholar] [CrossRef]
- Jahan, M.S.; Wang, Y.; Shu, S.; Zhong, M.; Chen, Z.; Wu, J.; Sun, J.; Guo, S. Exogenous salicylic acid increases the heat tolerance in Tomato (Solanum lycopersicum L) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci. Hortic. 2019, 247, 421–429. [Google Scholar] [CrossRef]
- Ahmed, H.I.; Shabana, A.I.; Elsayed, A.Y. Chilling tolerance enhancement in tomato (Solanum lycopersicum) plant by spraying of some anti-stress compounds. J. Plant Prod. 2016, 7, 185–195. [Google Scholar] [CrossRef]
- Javanmardi, J.; Akbari, N. Salicylic acid at different plant growth stages affects secondary metabolites and phisico-chemical parameters of greenhouse tomato. Adv. Hortic. Sci. 2016, 30, 151–157. [Google Scholar] [CrossRef]
- Kovács, J.; Poór, P.; Szepesi, Á.; Tari, I. Salicylic acid induced cysteine protease activity during programmed cell death in tomato plants. Acta Biol. Hung. 2016, 67, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Poór, P.; Borbély, P.; Czékus, Z.; Takács, Z.; Ördög, A.; Popović, B.; Tari, I. Comparison of changes in water status and photosynthetic parameters in wild type and abscisic acid-deficient sitiens mutant of tomato (Solanum lycopersicum cv. Rheinlands Ruhm) exposed to sublethal and lethal salt stress. J. Plant Physiol. 2019, 232, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Spletzer, M.E.; Enyedi, A.J. Salicylic acid induces resistance to Alternaria solani in hydroponically grown tomato. Phytopathology 1999, 89, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Adss, I.; Hamza, H.; Hafez, E.; Heikal, H. Enhancing tomato fruits post-harvest resistance by salicylic acid and hydrogen peroxide elicitors against rot caused by Alternaria solani. J. Agric. Chem. Biotechnol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Ojha, S.; Chatterjee, N.C. Induction of resistance in tomato plants against Fusarium oxysporum F. SP. Lycopersici mediated through salicylic acid and Trichoderma harzianum. J. Plant Prot. Res. 2012, 52, 220–225. [Google Scholar] [CrossRef]
- Jendoubi, W.; Harbaoui, K.; Hamada, W. Salicylic acid-induced resistance against Fusarium oxysporum f.s.pradicis lycopercisi in hydroponic grown tomato plants. J. New. Sci. 2015, 21, 985–995. [Google Scholar]
- Zehra, A.; Meena, M.; Dubey, M.K.; Aamir, M.; Upadhyay, R.S. Synergistic effects of plant defense elicitors and Trichoderma harzianum on enhanced induction of antioxidant defense system in tomato against Fusarium wilt disease. Bot. Stud. 2017, 58, 44. [Google Scholar] [CrossRef]
- Ibrahim, Y.E. Activities of antioxidants enzymes in salicylic acid treated tomato against Xanthomonas vesicatoria. Afr. J. Microbiol. Res. 2012, 6, 5678–5682. [Google Scholar] [CrossRef]
- Madany, M.M.Y.; Zinta, G.; Abuelsoud, W.; Hozzein, W.N.; Selim, S.; Asard, H.; Elgawad, H.A. Hormonal seed-priming improves tomato resistance against broomrape infection. J. Plant Physiol. 2020, 250, 153184. [Google Scholar] [CrossRef]
- Jiang, N.H.; Zhang, S.H. Effects of combined application of potassium silicate and salicylic acid on the defense response of hydroponically grown tomato plants to Ralstonia solanacearum infection. Sustainability 2021, 13, 3750. [Google Scholar] [CrossRef]
- Nagai, A.; Torres, P.B.; Duarte, L.M.L.; Chaves, A.L.R.; Macedo, A.F.; Floh, E.I.S.; de Oliveira, L.F.; Zuccarelli, R.; dos Santos, D.Y.A.C. Signaling pathway played by salicylic acid, gentisic acid, nitric oxide, polyamines and non-enzymatic antioxidants in compatible and incompatible Solanum-tomato mottle mosaic virus interactions. Plant Sci. 2020, 290, 110274. [Google Scholar] [CrossRef]
- Udalova, Z.V.; Zinovieva, S.V. Effect of salicylic acid on the oxidative and photosynthetic processes in tomato plants at invasion with root-knot nematode meloidogyne incognita (Kofoid Et White, 1919) Chitwood, 1949. Dokl. Biochem. Biophys. 2019, 488, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Mohammadkhani, N. Enhancement of chilling stress tolerance of tomato fruit by postharvest brassinolide treatment. Food Bioprocess Technol. 2014, 7, 909–914. [Google Scholar] [CrossRef]
- Baninaiem, E.; Mirzaaliandastjerdi, A.M.; Rastegar, S.; Abbaszade, K.H. Effect of pre- and postharvest salicylic acid treatment on quality characteristics of tomato during cold storage. Adv. Hortic. Sci. 2016, 30, 183–192. [Google Scholar] [CrossRef]
- Mandal, D.; Hazarika, T.K. Salicylic acid maintained quality and enhanced shelf life of tomato ‘Samrudhi’ at refrigerated storage. Acta Hortic. 2018, 1210, 207–212. [Google Scholar] [CrossRef]
- Ünal, S.; Küçükbasmaci, Ö.A.; Sabir, F.K. Salicylic acid treatments for extending postharvest quality of tomatoes maintained at different storage temperatures. Selcuk. J. Agric. Food Sci. 2021, 35, 35–141. [Google Scholar] [CrossRef]
- Manan, A.; Ayyub, C.M.; Aslam Pervez, M.; Ahmad, R. Methyl jasmonate brings about resistance against salinity stressed tomato plants by altering biochemical and physiological processes. Pak. J. Agric. Sci. 2016, 53, 35–41. [Google Scholar] [CrossRef]
- Singh, A.; Dwivedi, A. Methyl-jasmonate and salicylic acid as potent elicitors for secondary metabolite production in medicinal plants: A review. J. Pharmacogn. Phytochem. 2018, 7, 750–757. [Google Scholar]
- Chen, H.; Jones, A.D.; Howe, G.A. Constitutive activation of the jasmonate signaling pathway enhances the production of secondary metabolites in tomato. FEBS Lett. 2006, 580, 2540–2546. [Google Scholar] [CrossRef]
- Król, P.; Igielski, R.; Pollmann, S.; Kȩpczyńska, E. Priming of seeds with methyl jasmonate induced resistance to hemi-biotroph Fusarium oxysporum f.sp. lycopersici in tomato via 12-oxo-phytodienoic acid, salicylic acid, and flavonol accumulation. J. Plant Physiol. 2015, 179, 122–132. [Google Scholar] [CrossRef]
- Bali, S.; Kaur, P.; Sharma, A.; Ohri, P.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Jasmonic acid-induced tolerance to root-knot nematodes in tomato plants through altered photosynthetic and antioxidative defense mechanisms. Protoplasma 2018, 255, 471–484. [Google Scholar] [CrossRef]
- Bali, S.; Kaur, P.; Jamwal, V.L.; Gandhi, S.G.; Sharma, A.; Ohri, P.; Bhardwaj, R.; Ali, M.A.; Ahmad, P. Seed priming with jasmonic acid counteracts root knot nematode infection in tomato by modulating the activity and expression of antioxidative enzymes. Biomolecules 2020, 10, 98. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, A.M.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Ashraf, M. Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J. Plant Interact. 2018, 13, 64–72. [Google Scholar] [CrossRef]
- Ding, F.; Wang, C.; Xu, N.; Wang, M.; Zhang, S. Jasmonic acid-regulated putrescine biosynthesis attenuates cold-induced oxidative stress in tomato plants. Sci. Hortic. 2021, 288, 110373. [Google Scholar] [CrossRef]
- Abouelsaad, I.; Renault, S. Enhanced oxidative stress in the jasmonic acid-deficient tomato mutant def-1 exposed to NaCl stress. J. Plant Physiol. 2018, 226, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Tzortzakis, N.G.; Economakis, C. Maintaining postharvest quality of the tomato fruit by employing methyl jasmonate and ethanol vapor treatment. J. Food Qual. 2007, 30, 567–580. [Google Scholar] [CrossRef]
- Min, D.; Li, F.; Zhang, X.; Cui, X.; Shu, P.; Dong, L.; Ren, C. SlMYC2 Involved in methyl jasmonate-induced tomato fruit chilling tolerance. J. Agric. Food Chem. 2018, 66, 3110–3117. [Google Scholar] [CrossRef]
- Yu, W.; Yu, M.; Zhao, R.; Sheng, J.; Li, Y.; Shen, L. Ethylene perception is associated with the methyl-jasmonate-mediated immune response against Botrytis cinerea in tomato fruit. J. Agric. Food Chem. 2019, 67, 6725–6735. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, L.; Zhao, R.; Li, R.; Zhang, S.; Yu, W.; Sheng, J.; Shen, L. Melatonin induces disease resistance to Botrytis cinerea in tomato fruit by activating jasmonic acid signaling pathway. J. Agric. Food Chem. 2021, 67, 6116–6124. [Google Scholar] [CrossRef]
- Kȩpczyńska, E.; Król, P. The phytohormone methyl jasmonate as an activator of induced resistance against the necrotroph Alternaria porri f. sp. solani in tomato plants. J. Plant Interact. 2012, 7, 307–315. [Google Scholar] [CrossRef]
- Mou, W.; Li, D.; Luo, Z.; Mao, L.; Ying, T. Transcriptomic analysis reveals possible influences of ABA on secondary metabolism of pigments, flavonoids and antioxidants in tomato fruit during ripening. PLoS ONE 2015, 10, e0129598. [Google Scholar] [CrossRef]
- Maggio, A.; Barbieri, G.; Raimondi, G.; De Pascale, S. Contrasting effects of GA3 treatments on tomato plants exposed to increasing salinity. J. Plant Growth Regul. 2010, 29, 63–72. [Google Scholar] [CrossRef]
- Pompeu, G.B.; Vilhena, M.B.; Gratão, P.L.; Carvalho, R.F.; Rossi, M.L.; Martinelli, A.P.; Azevedo, R.A. Abscisic acid-deficient sit tomato mutant responses to cadmium-induced stress. Protoplasma 2017, 254, 771–783. [Google Scholar] [CrossRef]
- Diao, Q.; Song, Y.; Shi, D.; Qi, H. Interaction of polyamines, abscisic acid, nitric oxide, and hydrogen peroxide under chilling stress in tomato (Lycopersicon esculentum Mill.) Seedlings. Front. Plant Sci. 2017, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, X.; He, Y.; Kou, X.; Xue, Z. Effects of exogenous trehalose on the metabolism of sugar and abscisic acid in tomato seedlings under salt stress. Trans. Tianjin Univ. 2019, 25, 451–471. [Google Scholar] [CrossRef]
- Santos, M.P.; Zandonadi, D.B.; de Sá, A.F.; Costa, E.P.; de Oliveira, C.J.L.; Perez, L.E.P.; Façanha, A.R.; Bressan-Smith, R. Abscisic acid-nitric oxide and auxin interaction modulates salt stress response in tomato roots. Theor. Exp. Plant Physiol. 2020, 32, 301–313. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef]
- Hu, E.; Liu, M.; Zhou, R.; Jiang, F.; Sun, M.; Wen, J.; Zhu, Z.; Wu, Z. Relationship between melatonin and abscisic acid in response to salt stress of tomato. Sci. Hortic. 2021, 285, 110176. [Google Scholar] [CrossRef]
- Zeinali, L.; Heidari, R.; Rahmani, F.; Khara, J. Drought tolerance induced by foliar application of abscisic acid and sulfonamide compounds in tomato. J. Stress Physiol. Biochem. 2014, 10, 326–334. [Google Scholar]
- Ramasamy, S.; Nandagopal, J.G.T.; Balasubramanian, M.; Girija, S. Effect of abscisic acid and selenium foliar sprays on drought mitigation in tomato (Solanum lycopersicum L.). Mater. Today Proc. 2022, 48, 191–195. [Google Scholar] [CrossRef]
- Yan, M.; Yao, Y.; Mou, K.; Dan, Y.; Li, W.; Wang, C.; Liao, W. The involvement of abscisic acid in hydrogen gas-enhanced drought resistance in tomato seedlings. Sci. Hortic. 2022, 292, 110631. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Chen, Z.; Yu, J.-Q. RBOH1-dependent H2O2 production and subsequent activation of MPK1/2 play an important role in acclimation-induced cross-tolerance in tomato. J. Exp. Bot. 2014, 65, 595–607. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, X.; Tang, X.-M.; Xiao, L.; Sun, J.-L.; Yan, X.-F.; Li, D.; Deng, H.-Y.; Ma, X.-R. Comparative transcriptome analysis of tomato (Solanum lycopersicum) in response to exogenous abscisic acid. BMC Genom. 2013, 14, 841. [Google Scholar] [CrossRef] [PubMed]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Camara, M.C.; Vandenberghe, L.P.S.; Rodrigues, C.; de Oliveira, J.; Faulds, C.; Bertrand, E.; Soccol, C.R. Current advances in gibberellic acid (GA3) production, patented technologies and potential applications. Planta 2018, 248, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Halo, B.A.; Khan, A.L.; Waqas, M.; Al-Harrasi, A.; Hussain, J.; Ali, L.; Adnan, M.; Lee, I.-J. Endophytic bacteria (Sphingomonas sp. LK11) and gibberellin can improve Solanum lycopersicum growth and oxidative stress under salinity. J. Plant Interact. 2015, 10, 117–125. [Google Scholar] [CrossRef]
- Esan, A.M.; Olaiya, C.O.; Anifowose, L.O.; Lana, I.O.; Ailenokhuoria, B.V.; Fagbami, O.; Adeyemi, H.R.Y. Effect of plant growth-promoting rhizobacteria and gibberellic acid on salt stress tolerance in tomato genotypes. Afr. Crop Sci. J. 2020, 28, 341–362. [Google Scholar] [CrossRef]
- Khavari-Nejad, R.A.; Najafi, F.; Ranjbari, M. The effects of GA3 application on growth, lipid peroxidation, antioxidant enzymes activities, and sugars levels of cadmium stressed tomato (Lycopersicon esculentum Mill. Cv. CH) plants. Rom. J. Biol.—Plant Biol. 2013, 58, 51–60. [Google Scholar]
- Haroun, S.A.; Gamel, R.M.E.; Bashasha, J.A.; Aldrussi, I.A. Protective role of β-sitosterol or gibberellic acid to Lycopersicum esculentum cultivars under temperature stress. Egypt. J. Bot. 2018, 58, 233–247. [Google Scholar] [CrossRef]
- Omena-Garcia, R.P.; Martins, A.O.; Medeiros, D.B.; Vallarino, J.G.; Ribeiro, D.M.; Fernie, A.R.; Araújo, W.L.; Nunes-Nesi, A. Growth and metabolic adjustments in response to gibberellin deficiency in drought stressed tomato plants. Environ. Exp. Bot. 2019, 159, 95–107. [Google Scholar] [CrossRef]
- Illouz-Eliaz, N.; Nissan, I.; Nir, I.; Ramon, U.; Shohat, H.; Weiss, D. Mutations in the tomato gibberellin receptors suppress xylem proliferation and reduce water loss under water-deficit conditions. J. Exp. Bot. 2020, 71, 3603–3612. [Google Scholar] [CrossRef]
- Gaion, L.A.; Monteiro, C.C.; Cruz, F.J.R.; Rossatto, D.R.; López-Díaz, I.; Carrera, E.; Lima, J.E.; Peres, L.E.P.; Carvalho, R.F. Constitutive gibberellin response in grafted tomato modulates root-to-shoot signaling under drought stress. J. Plant Physiol. 2018, 221, 11–21. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Vega-Celedón, P.; Martínez, C.H.; González, M.; Seeger, M. Biosíntesis de ácido indol-3-acético y promoción del crecimiento de plantas por bacterias. Cultiv. Trop. 2016, 37, 33–39. [Google Scholar] [CrossRef]
- Khan, M.Y.; Prakash, V.; Yadav, V.; Chauhan, D.K.; Prasad, S.M.; Ramawat, N.; Singh, V.P.; Tripathi, D.K.; Sharma, S. Regulation of cadmium toxicity in roots of tomato by indole acetic acid with special emphasis on reactive oxygen species production and their scavenging. Plant Physiol. Biochem. 2019, 142, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Olaiya, C.O.; Anyanwu, G.O. Antistressful action of two auxin analogues on antioxidant enzymes and minerals in tomato (Solanum Lycopersicon). Int. J. Biotechnol. Biochem. 2013, 9, 145–156. [Google Scholar]
- Siddiqui, M.H.; Alamri, S.A.; Al-Khaishany, M.Y.Y.; Al-Qutami, M.A.; Ali, H.M.; Khan, M.N. Sodium nitroprusside and indole acetic acidimprove the tolerance of tomato plants to heat stress by protecting against DNA damage. J. Plant Interact. 2017, 12, 177–186. [Google Scholar] [CrossRef]
- Deen, S.; Singh, N.B. Alleviation of allelopathic stress of benzoic acid by indole acetic acid in Solanum lycopersicum. Sci. Hortic. 2015, 192, 211–217. [Google Scholar] [CrossRef]
- Niharika, K.; Singh, N.B.; Khare, S.; Singh, A.; Yadav, V.; Yadav, R.K. Attenuation of vanillic acid toxicity by foliar application withindole-3-acetic acid in tomato seedlings. Int. J. Veg. Sci. 2021, 28, 211–232. [Google Scholar] [CrossRef]
- Tyburski, J.; Dunajska, K.; Mazurek, P.; Piotrowska, B.; Tretyn, A. Exogenous auxin regulates H2O2 metabolism in roots of tomato (Lycopersicon esculentum Mill.) seedlings affecting the expression and activity of CuZn-superoxide dismutase, catalase, and peroxidase. Acta Physiol. Plant 2009, 31, 249–260. [Google Scholar] [CrossRef]
- Ivanchenko, M.G.; den Os, D.; Monshausen, G.B.; Dubrovsky, J.G.; Bednárová, A.; Krishnan, N. Auxin increases the hydrogen peroxide (H2O2) concentration in tomato (Solanum lycopersicum) root tips while inhibiting root growth. Ann. Bot. 2013, 112, 1107–1116. [Google Scholar] [CrossRef]
- Basit, F.; Liu, J.; An, J.; Chen, M.; He, C.; Zhu, X.; Li, Z.; Hu, J.; Guan, Y. Brassinosteroids as a multidimensional regulator of plant physiological and molecular responses under various environmental stresses. Environ. Sci. Pollut. Res. 2021, 28, 44768–44779. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The physiological and molecular mechanism of brassinosteroid in response to stress: A review. Biol. Res. 2018, 51, 46. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Ruan, Y.P.; Zhou, J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Brassinosteroid alleviates polychlorinated biphenyls-induced oxidative stress by enhancing antioxidant enzymes activity in tomato. Chemosphere 2013, 90, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Zhou, Y.H.; Xia, X.J.; Mao, W.H.; Shi, K.; Yu, J.Q. Brassinosteroid regulates secondary metabolism in tomato towards enhanced tolerance to phenanthrene. Biol. Plant. 2013, 57, 154–158. [Google Scholar] [CrossRef]
- Mazorra, L.M.; Núñez, M.; Hechavarria, M.; Coll, F.; Sánchez-Blanco, M.J. Influence of brassinosteroids on antioxidant enzymes activity in tomato under different temperatures. Biol. Plant 2002, 45, 593–596. [Google Scholar] [CrossRef]
- Ogweno, J.O.; Song, X.S.; Shi, K.; Hu, W.H.; Mao, W.H.; Zhou, Y.H.; Yu, J.Q.; Nogués, S. Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J. Plant Growth Regul. 2008, 27, 49–57. [Google Scholar] [CrossRef]
- Hayat, S.; Hasan, S.A.; Hayat, Q.; Ahmad, A. Brassinosteroids protect Lycopersicon esculentum from cadmium toxicity applied as shotgun approach. Protoplasma 2010, 239, 3–14. [Google Scholar] [CrossRef]
- Jangid, K.K.; Dwivedi, P. Physiological and biochemical changes by nitric oxide and brassinosteroid in tomato (Lycopersicon esculentum Mill.) under drought stress. Acta Physiol. Plant 2017, 39, 73. [Google Scholar] [CrossRef]
- Vardhini, B.V.; Rao, S.S.R. Acceleration of ripening of tomato pericarp discs by brassinosteroids. Phytochemistry 2002, 61, 843–847. [Google Scholar] [CrossRef]
- Ding, Y.; Sheng, J.; Li, S.; Nie, Y.; Zhao, J.; Zhu, Z.; Wang, Z.; Tang, X. The role of gibberellins in the mitigation of chilling injury in cherry tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2015, 101, 88–95. [Google Scholar] [CrossRef]
- Mou, W.; Li, D.; Bu, J.; Jiang, U.; Ullah, Z.; Luo, Z.; Mao, L.; Ying, T. Comprehensive analysis of ABA effects on ethylene biosynthesis and signaling during tomato fruit ripening. PLoS ONE 2016, 11, e0154072. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Liu, L.; Li, S.; Shao, Z.; Meng, F.; Liu, H.; Duan, W.; Liang, D.; Zhu, C.; Xu, T.; et al. Regulation of fruit ripening by the brassinosteroid biosynthetic gene SlCYP90B3 via an ethylene-dependent pathway in tomato. Hortic. Res. 2020, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Diretto, G.; Purgatto, E.; Danoun, S.; Zouine, M.; Li, Z.; Roustan, J.P.; Bouzayen, M.; Giuliano, G.; Chervin, C. Carotenoid accumulation during tomato fruit ripening is modulated by the auxin-ethylene balance. BMC Plant Biol. 2015, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ahammed, G.J.; Li, C.; Bao, X.; Yu, J.; Huang, C.; Yin, H.; Zhou, J. Brassinosteroid ameliorates zinc oxide nanoparticles-induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedling. Front. Plant Sci. 2016, 7, 615. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, Y.; Cheng, Y.; Liu, A.; Chen, S.; Li, X. Abscisic acid and gibberellins act antagonistically to mediate epigallocatechin3gallateretarded seed germination and early seedling growth in tomato. J. Plant Growth Regul. 2020, 39, 1414–1424. [Google Scholar] [CrossRef]
- Heidari, P.; Entazari, M.; Ebrahimi, A.; Ahmadizadeh, M.; Vannozzi, A.; Palumbo, F.; Barcaccia, G. Exogenous EBR ameliorates endogenous hormone contents in tomato species under low-temperature stress. Horticulturae 2021, 7, 84. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Carranza, P.; Avila-Sosa, R.; Vera-López, O.; Navarro-Cruz, A.R.; Ruíz-Espinosa, H.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. Uncovering the Role of Hormones in Enhancing Antioxidant Defense Systems in Stressed Tomato (Solanum lycopersicum) Plants. Plants 2023, 12, 3648. https://doi.org/10.3390/plants12203648
Hernández-Carranza P, Avila-Sosa R, Vera-López O, Navarro-Cruz AR, Ruíz-Espinosa H, Ruiz-López II, Ochoa-Velasco CE. Uncovering the Role of Hormones in Enhancing Antioxidant Defense Systems in Stressed Tomato (Solanum lycopersicum) Plants. Plants. 2023; 12(20):3648. https://doi.org/10.3390/plants12203648
Chicago/Turabian StyleHernández-Carranza, Paola, Raúl Avila-Sosa, Obdulia Vera-López, Addí R. Navarro-Cruz, Héctor Ruíz-Espinosa, Irving I. Ruiz-López, and Carlos E. Ochoa-Velasco. 2023. "Uncovering the Role of Hormones in Enhancing Antioxidant Defense Systems in Stressed Tomato (Solanum lycopersicum) Plants" Plants 12, no. 20: 3648. https://doi.org/10.3390/plants12203648
APA StyleHernández-Carranza, P., Avila-Sosa, R., Vera-López, O., Navarro-Cruz, A. R., Ruíz-Espinosa, H., Ruiz-López, I. I., & Ochoa-Velasco, C. E. (2023). Uncovering the Role of Hormones in Enhancing Antioxidant Defense Systems in Stressed Tomato (Solanum lycopersicum) Plants. Plants, 12(20), 3648. https://doi.org/10.3390/plants12203648








