Extrafloral Nectary-Bearing Plants Recover Ant Association Benefits Faster and More Effectively after Frost-Fire Events Than Frost
Abstract
1. Introduction
2. Results
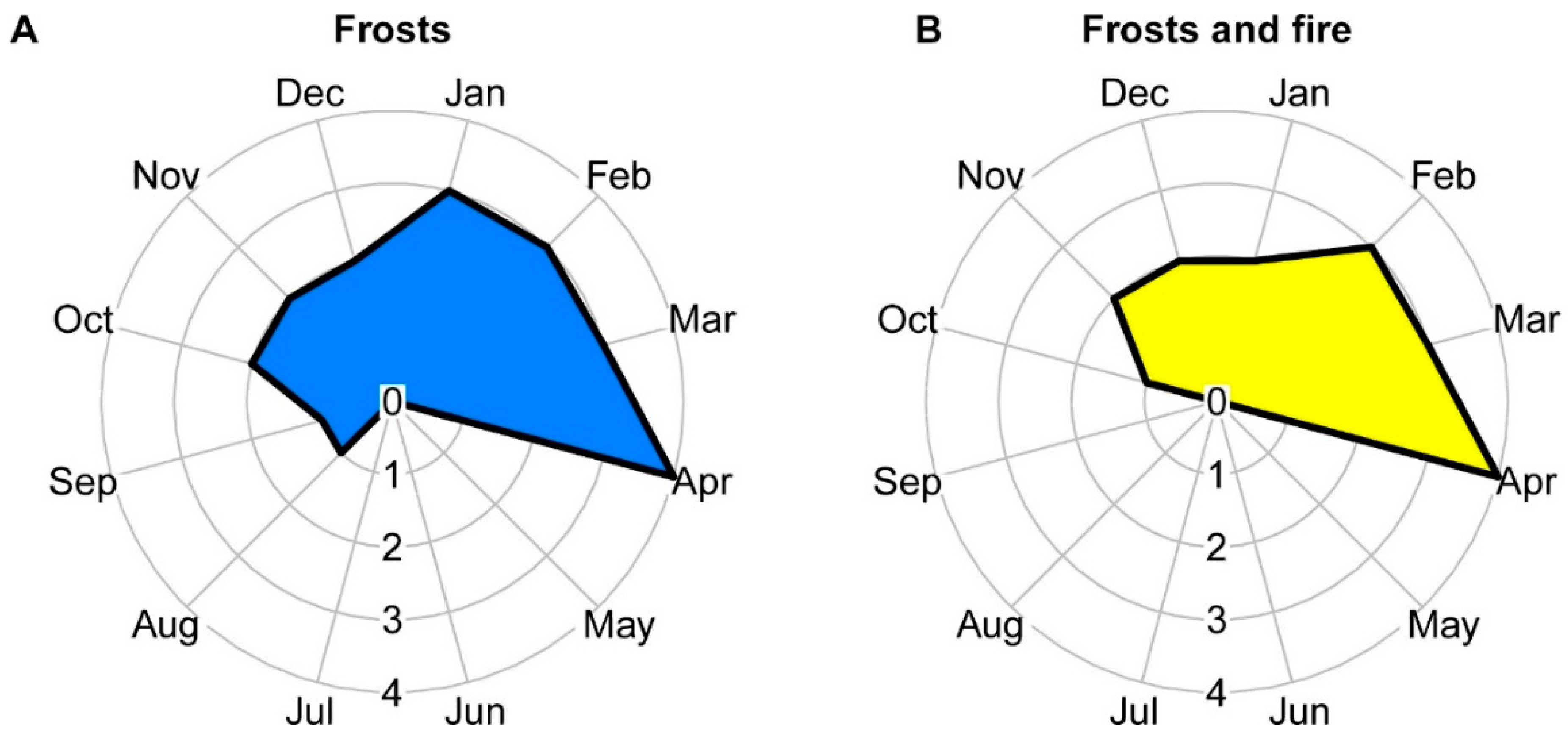
2.1. Regrowth Time
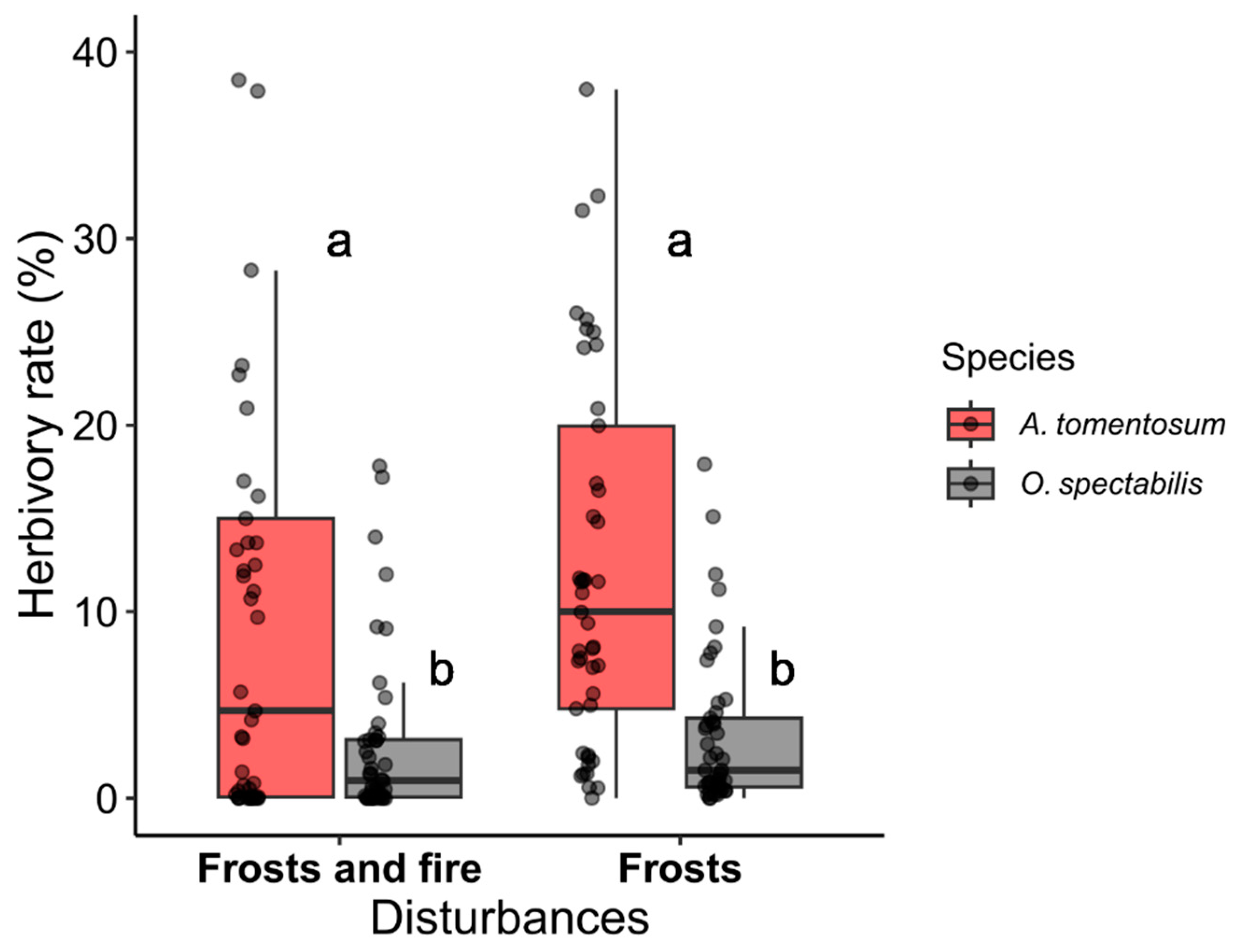
2.2. Herbivory in the Area under the Effects of Frosts and under the Effects of Frosts and Fire
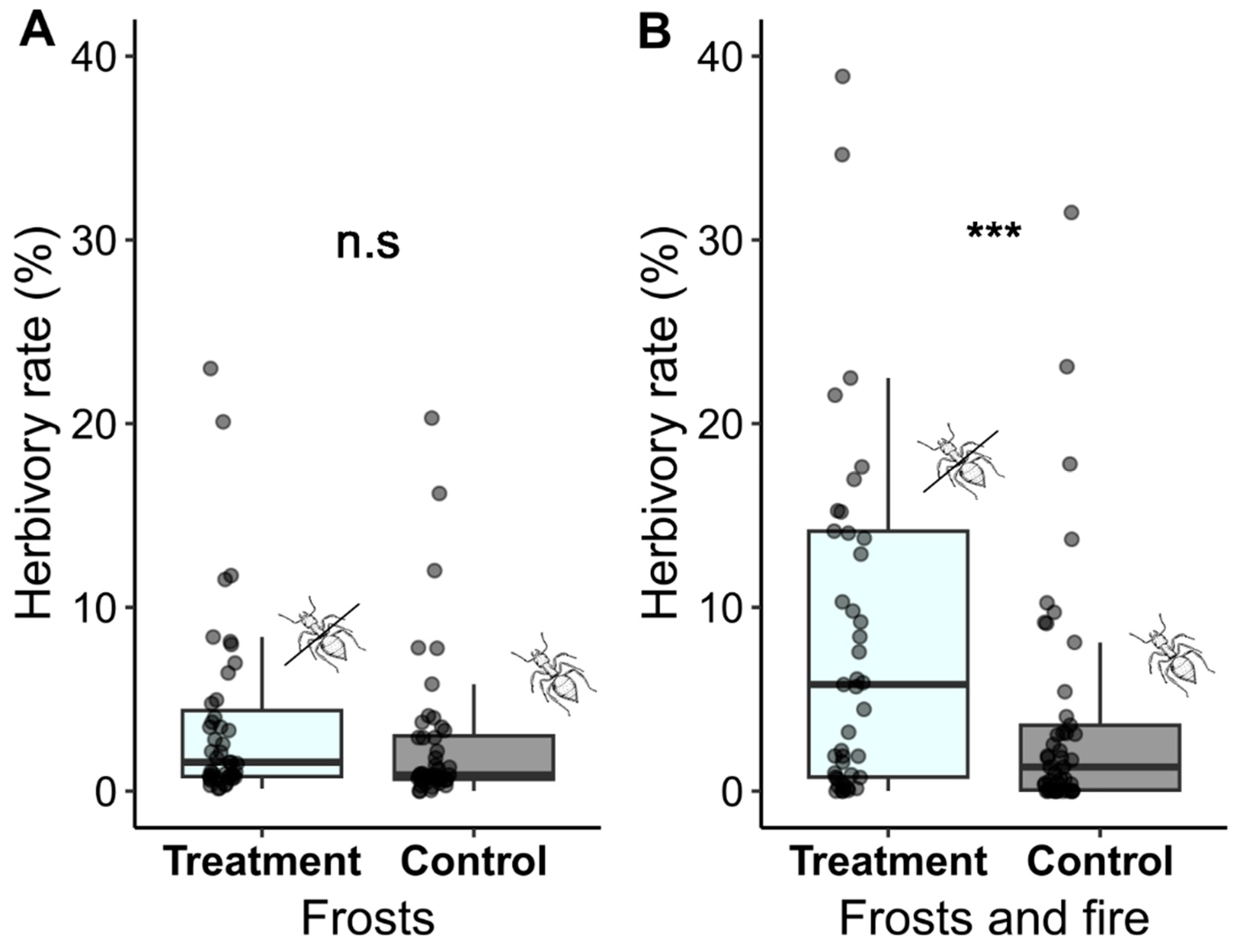
2.3. Effectiveness of Ant Defense
2.4. Effectiveness of Ant Predatory Behavior
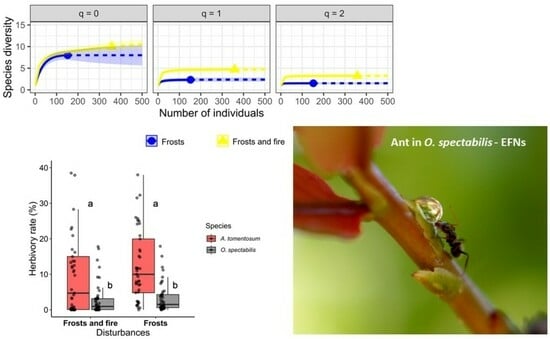
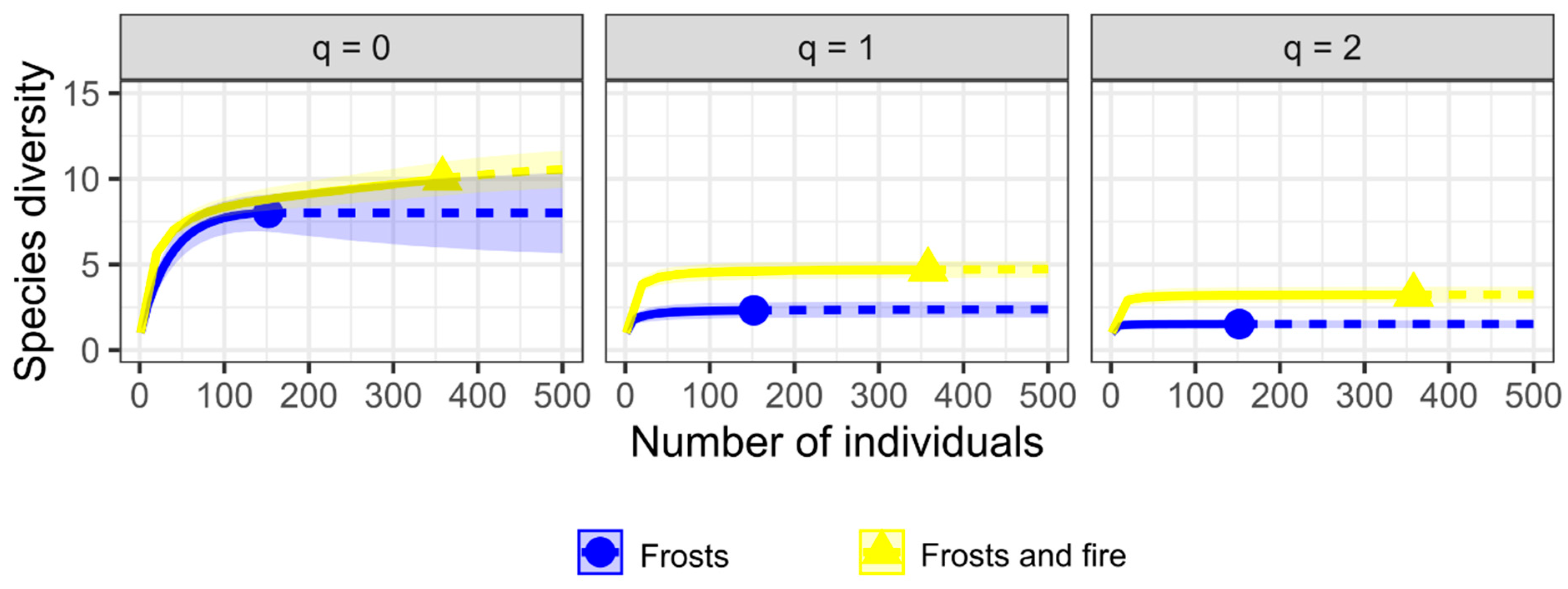
2.5. Ant Diversity
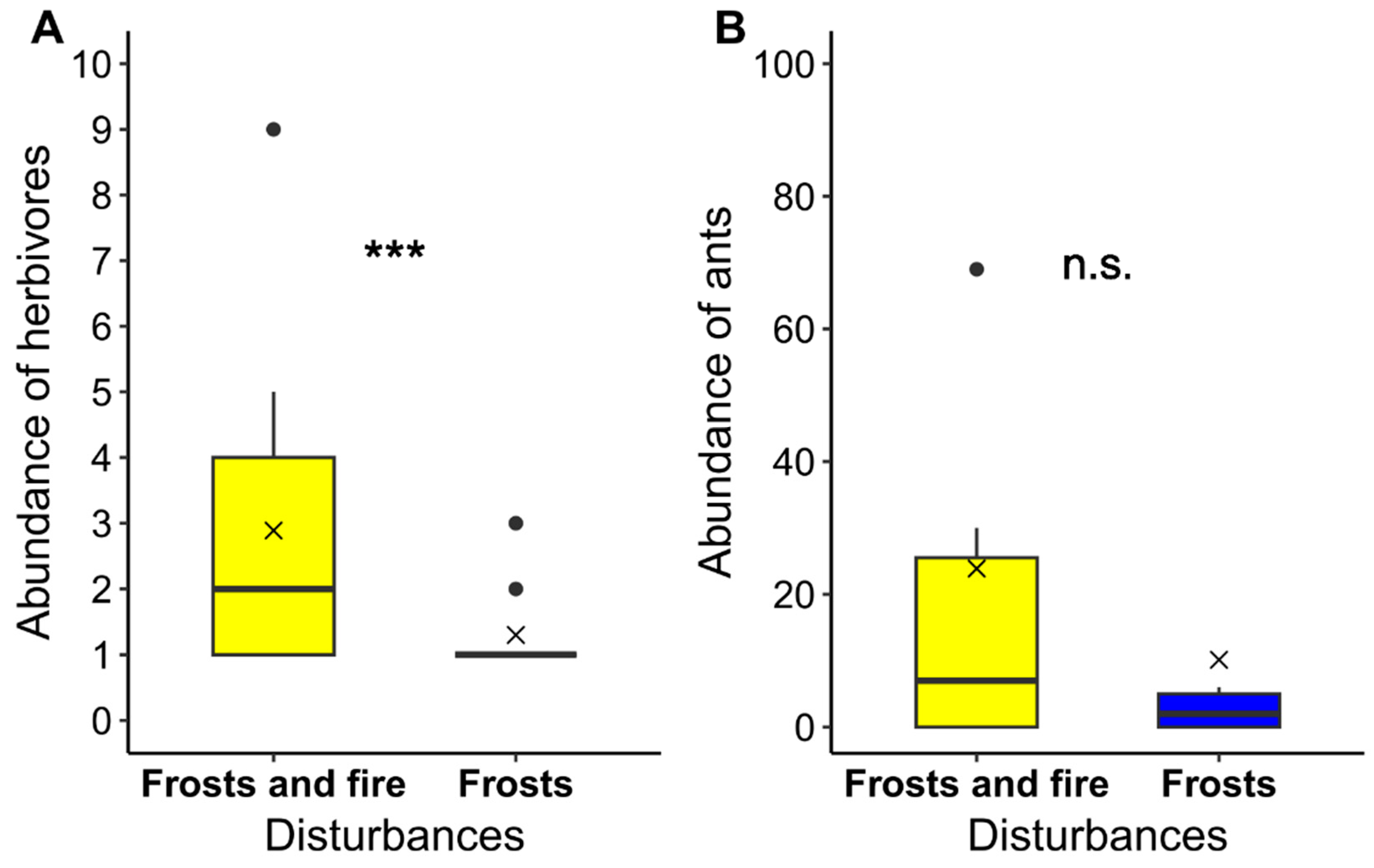
2.6. Abundance of Ants and Herbivores
3. Discussion
3.1. Regrowth Time
3.2. Herbivory Rates
3.3. Effectiveness of Ant Defense
3.4. Effectiveness of Ant Predatory Behavior
4. Methods
4.1. Study Site
4.2. Study System
4.3. Leaf Regrowth Analysis
4.4. Identification of Ant and Herbivore Guilds
4.5. Analysis of Ant Defense Effectiveness in Plants with EFNs
4.6. Analysis of the Effectiveness of Ant Predatory Behavior
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hairston, N.G.; Smith, F.E.; Slobodkin, L.B. Community structure, population control, and competition. Am. Nat. 1960, 94, 421–425. [Google Scholar] [CrossRef]
- Connell, J.H. The Influence of Interspecific Competition and Other Factors on the Distribution of the Barnacle Chthamalus stellatus. Ecology 1961, 42, 710–723. [Google Scholar] [CrossRef]
- Bascompte, J. Mutualism and biodiversity. Curr. Biol. 2019, 29, R467–R470. [Google Scholar] [CrossRef]
- Cosmo, L.G.; Assis, A.P.A.; de Aguiar, M.A.M.; Mathias, M.P.; Valido, A.; Jordano, P.; Thompson, J.N.; Bascompte, J.; Guimarães, P.R. Indirect effects shape species fitness in coevolved mutualistic networks. Nature 2023, 619, 788–792. [Google Scholar] [CrossRef]
- Del-Claro, K.; Torezan-Silingardi, H.M. (Eds.) An Evolutionary Perspective on Plant-Animal Interactions. In Plant–Animal Interactions; Springer: Cham, Switzerland, 2021; pp. 1–15. [Google Scholar]
- Bronstein, J.L. The gift that keeps on giving: Why does biological diversity accumulate around mutualisms? In Plant–Animal Interactions; Del-Claro, K., Torezan-Silingardi, H.M., Eds.; Springer: Cham, Switzerland, 2021; pp. 283–306. [Google Scholar]
- Leal, L.C.; Nogueira, A.; Peixoto, P.E. Which traits optimize plant benefits? Meta-analysis on the effect of partner traits on the outcome of an ant–plant protective mutualism. J. Ecol. 2023, 111, 263–275. [Google Scholar] [CrossRef]
- Vilela, A.A.; Del Claro, V.T.S.; Torezan-Silingardi, H.M.; Del-Claro, K. Climate changes affecting biotic interactions, phenology, and reproductive success in a savanna community over a 10-year period. Arthropod-Plant Interact. 2018, 12, 215–227. [Google Scholar] [CrossRef]
- Calixto, E.S.; Novaes, L.R.; dos Santos, D.F.B.; Lange, D.; Moreira, X.; Del-Claro, K. Climate seasonality drives ant–plant–herbivore interactions via plant phenology in an extrafloral nectary-bearing plant community. J. Ecol. 2020, 109, 639–651. [Google Scholar] [CrossRef]
- Juárez-Juárez, B.; Dáttilo, W.; Moreno, C.E. Synthesis and perspectives on the study of ant-plant interaction networks: A global overview. Ecol. Entomol. 2023, 48, 269–283. [Google Scholar] [CrossRef]
- Del-Claro, K.; Rico-Gray, V.; Torezan-Silingardi, H.M.; Alves-Silva, E.; Fagundes, F.; Lange, D.; Dáttilo, W.; Vilela, A.A.; Aguirre, A.; Rodriguez-Morales, D. Loss and gains in ant–plant interactions mediated by extrafloral nectar: Fidelity, cheats, and lies. Insectes Sociaux 2016, 63, 207–221. [Google Scholar] [CrossRef]
- González-Teuber, M.; Heil, M. Nectar chemistry is tailored for both attraction of mutualists and protection from exploiters. Plant Signal Behav 2009, 4, 809–813. [Google Scholar] [CrossRef]
- Heil, M. Extrafloral nectar at the plant-insect interface: A spotlight on chemical ecology, phenotypic plasticity, and food webs. Annu. Rev. Entomol. 2015, 60, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, E.; Del-Claro, K. Effect of post-fire resprouting on leaf fluctuating asymmetry, extrafloral nectar quality, and ant-plant-herbivore interactions. Naturwissenschaften 2013, 100, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Calixto, E.S.; Lange, D.; Bronstein, J.; Torezan-Silingardi, H.M.; Del-Claro, K. Optimal defense theory in an ant–plant mutualism: Extrafloral nectar as an induced defence is maximized in the most valuable plant structures. J. Ecol. 2020, 109, 167–178. [Google Scholar] [CrossRef]
- Beattie, A.; Hughes, L. Ant-plant interactions. In Plant Animal Interactions: An Evolutionary Approach; Herrera, C.M., Pellmyr, O., Eds.; Blackwell Science: Malden, MA, USA, 2002; pp. 211–236. [Google Scholar]
- Moura, R.F.; Colberg, E.; Alves-Silva, E.; Mendes-Silva, I.; Fagundes, R.; Stefani, V.; Del-Claro, K. Biotic Defenses Against Herbivory. In Plant-Animal Interactions; Del-Claro, K., Torezan-Silingardi, H.M., Eds.; Springer: Cham, Switzerland, 2021; pp. 93–118. [Google Scholar]
- Lach, L.; Hobbs, R.J.; Majer, J.D. Herbivory-induced extrafloral nectar increases native and invasive ant worker survival. Popul. Ecol. 2009, 51, 237–243. [Google Scholar] [CrossRef]
- Byk, J.; Del-Claro, K. Ant–plant interaction in the Neotropical savanna: Direct beneficial effects of extrafloral nectar on ant colony fitness. Popul Ecol. 2011, 53, 327–332. [Google Scholar] [CrossRef]
- Aranda-Rickert, A.; Diez, P.; Marazzi, B. Extrafloral nectar fuels ant life in deserts. AoB Plants 2014, 6, plu068. [Google Scholar] [CrossRef]
- Calixto, E.S.; Lange, D.; Del-Claro, K. Net benefits of a mutualism: Influence of the quality of extrafloral nectar on the colony fitness of a mutualistic ant. Biotropica 2021, 53, 846–856. [Google Scholar] [CrossRef]
- Weber, M.G.; Clement, W.L.; Donoghue, M.J.; Agrawal, A.A. Phylogenetic and experimental tests of interactions among mutualistic plant defense traits in Viburnum (Adoxaceae). Am. Nat. 2012, 180, 450–463. [Google Scholar] [CrossRef]
- Blüthgen, N.; Reifenrath, K. Extrafloral nectaries in an Australian rainforest: Structure and distribution. Aust. J. Bot. 2003, 51, 515–527. [Google Scholar] [CrossRef]
- Díaz-Castelazo, C.; Rico-Gray, V.; Oliveira, P.S.; Cuautle, M. Extrafloral nectary-mediated ant–plant interactions in the coastal vegetation of Veracruz, Mexico: Richness, occurrence, seasonality and ant foraging patterns. Ecoscience 2004, 11, 472–481. [Google Scholar] [CrossRef]
- Machado, S.R.; Morellato, L.P.C.; Sajo, M.G.; Oliveira, O.S. Morphological patterns of extrafloral nectaries in woody plant species of the Brazilian Cerrado. Plant Biol. 2008, 10, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Strassburg, B.B.N.; Brooks, T.; Feltran-Barbieri, R.; Iribarrem, A.; Crouzeilles, R.; Loyola, R.; Latawiec, A.E.; Oliveira Filho, F.J.B.; Scaramuzza, C.A.M.; Scarano, F.R.; et al. Moment of truth for the Cerrado hotspot. Nat. Ecol. Evol. 2017, 1, 99. [Google Scholar] [CrossRef] [PubMed]
- Baronio, G.J.; Souza, C.S.; Maruyama, P.K.; Raizer, J.; Sigrist, M.R.; Aoki, C. Natural fire does not affect the structure and beta diversity of plant-pollinator networks, but diminishes floral-visitor specialization in Cerrado. Flora 2021, 281, 151869. [Google Scholar] [CrossRef]
- Pivello, V.R.; Vieira, I.; Christianini, A.V. Understanding Brazil’s catastrophic fires: Causes, consequences and policy needed to prevent future tragedies. Perspect. Ecol. Conserv. 2021, 19, 233–255. [Google Scholar] [CrossRef]
- Eloy, L.; Schmidt, I.B.; Borges, S.L.; Ferreira, M.C.; Dos Santos, T.A. Seasonal fire management by traditional cattle ranchers prevents the spread of wildfire in the Brazilian Cerrado. Ambio 2018, 48, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.H.S.; Oliveira, M.R.; Fernandes, R.A.M.; Nacagava, V.A.F.; Arguelho, B.A.; Ribeiro, D.B.; Pott, A.; Damasceno, G.A., Jr.; Garcia, L.C. Flowering and fruiting show phenological complementarity in both trees and non-trees in mosaic-burnt floodable savanna. J. Environ. Manag. 2023, 337, 117665. [Google Scholar] [CrossRef] [PubMed]
- Del-Claro, K.; Dirzo, R. Impacts of Anthropocene Defaunation on PlantAnimal Interactions. In Plant–Animal Interactions; Del-Claro, K., Torezan-Silingardi, H.M., Eds.; Springer: Cham, Switzerland, 2021; pp. 333–345. [Google Scholar]
- Ratter, J.A.; Ribeiro, J.F.; Bridgewater, S. The Brazilian Cerrado vegetation and threats to its biodiversity. Ann. Bot. 1997, 80, 223–230. [Google Scholar] [CrossRef]
- Coelho, A.J.P.; Magnago, L.F.S.; Matos, F.A.R.; Mota, N.M.; Diniz, E.S.; Meira-Neto, J.A.A. Effects of anthropogenic disturbances on biodiversity and biomass stock of Cerrado, the Brazilian savanna. Biodivers. Conserv. 2020, 29, 3151–3168. [Google Scholar] [CrossRef]
- Titcomb, G.C.; Amooni, G.; Mantas, J.N.; Young, H.S. The effects of herbivore aggregations at water sources on savanna plants differ across soil and climate gradients. Ecol. Appl. 2021, 31, e02422. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Pean, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. IPCC, 2021: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; p. 2391. [Google Scholar]
- Antonio, A.C.; Scalon, M.C.M.; Rossatto, D.R. The role of bud protection and bark density in frost resistance of savanna trees. Plant Biol. 2019, 22, 55–61. [Google Scholar] [CrossRef]
- Crimp, S.J.; Zheng, B.; Khimashia, N.; David, L.G.; Scott, C.; Mark, H.; Neville, N. Recent changes in southern Australian frost occurrence: Implications for wheat production risk. Crop Pasture Sci. 2016, 67, 801–811. [Google Scholar] [CrossRef]
- Zheng, B.; Chapman, S.C.; Christopher, J.T.; Frederiks, T.M.; Chenu, K. Frost trends and their estimated impact on yield in the Australian wheatbelt. J. Exp. Bot. 2015, 66, 3611–3623. [Google Scholar] [CrossRef]
- Gu, L.; Hanson, P.J.; MacPost, W.; Kaiser, D.P.; Yang, B.; Nemani, R.; Pallardy, S.G.; Meyers, T. The 2007 eastern US spring freeze: Increased cold damage in a warming world? Bioscience 2008, 58, 253–262. [Google Scholar] [CrossRef]
- Inouye, D.W. The ecological and evolutionary significance of frost in the context of climate change. Ecol. Lett. 2000, 3, 457–463. [Google Scholar] [CrossRef]
- Morin, X.; Chuine, I. Will tree species experience increased frost damage due to climate change because of changes in leaf phenology? Can. J. For. Res. 2014, 44, 1555–1565. [Google Scholar] [CrossRef]
- Meier, M.; Fuhrer, J.; Holzkämper, A. Changing risk of spring frost damage in grapevines due to climate change? A case study in the Swiss Rhone Valley. Int. J. Biometeorol. 2018, 62, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Lombardero, M.J.; Castedo-Dorado, F.; Ayres, M.P. Extreme climatic events affect populations of Asian chestnut gall wasps, Dryocosmus kuriphilus, but do not stop the spread. Agric. For. Entomol. 2021, 23, 473–488. [Google Scholar] [CrossRef]
- Vuono, Y.S.; Barbosa, L.M.; Batista, E.A.; Gurgel Filho, O.A. Efeitos biológicos da geada na vegetação do cerrado. Silvic. São Paulo 1982, 16, 545–547. [Google Scholar]
- Childes, S.L.; Walker, B.H. Ecology and dynamics of the woody vegetation on the Kalahari Sands in Hwange National Park, Zimbabwe. Vegetatio 1987, 72, 111–128. [Google Scholar] [CrossRef]
- Bannister, P. Godley review: A touch of frost? Cold hardiness of plants in the southern hemisphere. N. Z. J. Bot. 2007, 45, 1–33. [Google Scholar] [CrossRef]
- Oliveira, O.S.; Freitas, A.V.L. Ant plant herbivore interactions in the neotropical cerrado savanna. Naturwissenschaften 2004, 91, 557–570. [Google Scholar] [CrossRef]
- Price, P.W. The plant vigor hypothesis and herbivore attack. Oikos 1991, 62, 244–251. [Google Scholar] [CrossRef]
- Vieira, E.M.; Andrade, I.; Price, P.W. Fire effects on a Palicourea rigida (Rubiaceae) Gall Midge: A test of the plant vigor hypothesis. Biotropica 1996, 28, 210–217. [Google Scholar] [CrossRef]
- Alves-Silva, E.; Del-Claro, K. Fire triggers the activity of extrafloral nectaries, but ants fail to protect the plant against herbivores in a neotropical savanna. Arthropod-Plant Interact. 2014, 8, 233–240. [Google Scholar] [CrossRef]
- Andrade, J.F.; Batista, J.C.; Pereira, H.S.; Fernandes, G.W.; Santos, J.C. Fire mediated herbivory and plant defense of a neotropical shrub. Arthropod-Plant Interact. 2018, 13, 489–498. [Google Scholar] [CrossRef]
- Banza, P.; Evans, D.M.; Medeiros, R.; Macgregor, C.J.; Belo, A.D.F. Short-term positive effects of wildfire on diurnal insects and pollen transport in a Mediterranean ecosystem. Ecol. Entomol. 2021, 46, 1353–1363. [Google Scholar] [CrossRef]
- Vieira, E.M.; Briani, D.C. Short-term effects of fire on small rodents in the Brazilian Cerrado and their relation with feeding habits. Int. J. Wildland Fire 2013, 22, 1063–1071. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Conner, J.K.; Stinchcombe, J.R. Evolution of plant resistance and tolerance to frost damage. Ecol. Lett. 2004, 7, 1199–1208. [Google Scholar] [CrossRef]
- Marquis, R.J.; Lill, J.T.; Forkner, R.E.; Le Corf, J.; Landosky, J.M.; Whitfeld, J.B. Declines and Resilience of Communities of Leaf Chewing Insects on Missouri Oaks Following Spring Frost and Summer Drought. Front. Ecol. Evol. 2019, 7, 396. [Google Scholar] [CrossRef]
- Lange, D.; Del-Claro, K. Ant-Plant Interaction in a Tropical Savanna: May the Network Structure Vary over Time and Influence on the Outcomes of Associations? PLoS ONE 2014, 9, e105574. [Google Scholar] [CrossRef]
- Fagundes, R.; Lange, D.; Anjos, D.V.; De Lima, F.P.; Nahas, L.; Corro, E.J.; Gomes Silva, P.B.; Del-Claro, K.; Ribeiro, S.P.; Dáttilo, W. Limited effects of fire disturbances on the species diversity and structure of ant-plant interaction networks in Brazilian Cerrado. Acta Oecologica 2018, 93, 65–73. [Google Scholar] [CrossRef]
- Alcolea, M.; Durigan, G.; Christianini, A.V. Prescribed fire enhances seed removal by ants in a Neotropical savanna. Biotropica 2021, 54, 125–134. [Google Scholar] [CrossRef]
- Simon, M.F.; Gether, R.; Queiroz, L.P.; Skema, C.; Pennington, R.T. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proc. Natl. Acad. Sci. USA 2009, 106, 20359–20364. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.F.; Pennington, T. Evidence for Adaptation to Fire Regimes in the Tropical Savannas of the Brazilian Cerrado. Int. J. Plant Sci. 2012, 173, 711–723. [Google Scholar] [CrossRef]
- Hoffman, W.A.; Flake, S.W.; Abreu, R.C.R.; Pilon, N.A.L.; Rossatto, D.R.; Durigan, G. Rare frost events reinforce tropical savanna-forest boundaries. J. Ecol. 2018, 107, 468–477. [Google Scholar] [CrossRef]
- Brando, P.M.; Durigan, G. Changes in cerrado vegetation after disturbance by frost (São Paulo State, Brazil). Plant Ecol. 2005, 175, 205–215. [Google Scholar] [CrossRef]
- Kellomäki, S.; Hänninnen, H.; Kolström, M. Computations on frost damage to scots pine under climatic warming in boreal conditions. Ecol. Appl. 1995, 5, 42–52. [Google Scholar] [CrossRef]
- Leinonen, I. A simulation model for the annual frost hardiness and freeze damage of Scots Pine. Ann. Bot. 1996, 78, 687–693. [Google Scholar] [CrossRef]
- Linkosalo, T.; Carter, T.R.; Hakkinen, R.; Hari, P. Predicting spring phenology and frost damage risk of Betula spp. under climatic warming: A comparison of two models. Tree Physiol. 2000, 20, 1175–1182. [Google Scholar] [CrossRef]
- St. Clair, S.B.; Monson, S.D.; Smith, E.A.; Cahill, D.G.; Calder, W.J. Altered leaf morphology, leaf resource dilution and defense chemistry induction in frost-defoliated aspen (Populus tremuloides). Tree Physiol. 2009, 29, 1259–1268. [Google Scholar] [CrossRef][Green Version]
- Akšić, M.F.; Tosti, T.; Nedić, N.; Marković, N.; Ličina, V.; MilojkovićOpsenica, D.; Tešić, Z. Influence of frost damage on the sugars and sugar alcohol composition in quince (Cydonia oblonga Mill.) floral nectar. Acta Physiol. Plant. 2015, 37, 1701. [Google Scholar] [CrossRef]
- Rosumek, F.B.; Silveira, F.A.O.; Neves, F.S.; Barbosa, N.P.; Diniz, L.; Oki, Y.; Pezzini, F.; Fernandez, W.G.; Cornelissen, T. Ants on plants: A meta-analysis of the role of ants as plant biotic defenses. Oecologia 2009, 160, 537–549. [Google Scholar] [CrossRef]
- Wan, H.Y.; Rhodes, A.C.; St. Clair, S.B. Fire severity alters plant regeneration patterns and defense against herbivores in mixed aspen forests. Oikos 2014, 123, 1479–1488. [Google Scholar] [CrossRef]
- Hood, S.; Sala, A.; Heyerdahl, E.K.; Boutin, M. Low-severity fire increases tree defense against bark beetle attacks. Ecology 2015, 96, 1846–1855. [Google Scholar] [CrossRef]
- Bryant, J.P.; Chapin, F.S., III; Klein, D.R. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 1983, 40, 357–368. [Google Scholar] [CrossRef]
- Del-Claro, K.; Marquis, R.J. Ant Species Identity has a Greater Effect than Fire on the Outcome of an Ant Protection System in Brazilian Cerrado. Biotropica 2015, 47, 459–467. [Google Scholar] [CrossRef]
- Sinclair, B.J.; Ferguson, L.V.; Salehipour-shirazi, G.; MacMillan, H.A. Cross-tolerance and cross-talk in the cold: Relating low temperatures to desiccation and immune stress in insects. Integrat. Comp. Biol. 2017, 53, 545–556. [Google Scholar] [CrossRef]
- Augspurger, C.K. Reconstructing patterns of temperature, phenology, and frost damage over 124 years: Spring damage risk is increasing. Ecology 2013, 94, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.C. Treehoppers (Homoptera: Membracidae) in the Southeast Brazil: Use of host plants. Rev. Bras. Zool. 1995, 12, 595–608. [Google Scholar] [CrossRef]
- Lange, D.; Calixto, E.S.; Rosa, B.B.; Sales, T.A.; Del-Claro, K. Natural history and ecology of foraging of the Camponotus crassus Mayr, 1862 (Hymenoptera: Formicidae). J. Nat. Hist. 2019, 53, 1737–1749. [Google Scholar] [CrossRef]
- Fagundes, R.; Dáttilo, W.; Ribeiro, S.P.; Rico-Gray, V.; Jordano, P.; Del-Claro, K. Differences among ant species in plant protection are related to production of extrafloral nectar and degree of leaf herbivory. Biol. J. Linn. Soc. 2017, 122, 71–83. [Google Scholar] [CrossRef]
- Calixto, E.S.; Lange, D.; Moreira, X.; Del-Claro, K. Plant species specificity of ant–plant mutualistic interactions: Differential predation of termites by Camponotus crassus on five species of extrafloral nectaries plants. Biotropica 2021, 53, 1406–1414. [Google Scholar] [CrossRef]
- Miller, T.E.X. Does having multiple partners weaken the benefits of facultative mutualism? A test with cacti and cactus-tending ants. Oikos 2007, 116, 500–512. [Google Scholar] [CrossRef]
- Frizzo, T.L.M.; Campos, R.I.; Vasconcelos, H.L. Contrasting Effects of Fire on Arboreal and Ground-Dwelling Ant Communities of a Neotropical Savanna. Biotropica 2011, 44, 254–261. [Google Scholar] [CrossRef]
- Sanders, N.J.; Lessard, J.-P.; Fitzpatrick, M.C.; Dunn, R.R. Temperature, but not productivity or geometry, predicts elevational diversity gradients in ants across spatial grains. Glob. Ecol. Biogeogr. 2007, 16, 640–649. [Google Scholar] [CrossRef]
- Hurlbert, A.H.; Ballantyne, F.; Powell, S. Shaking a leg and hot to trot: The effects of body size and temperature on running speed in ants. Ecol. Entomol. 2008, 33, 144–154. [Google Scholar] [CrossRef]
- Dunn, R.R.; Agosti, D.; Andersen, A.N.; Arnan, X.; Bruhl, C.A.; Cerdá, X.; Ellison, A.M.; Fisher, B.L.; Fitzpatrick, M.C.; Gibb, H.; et al. Climatic drivers of hemispheric asymmetry in global patterns of ant species richness. Ecol. Lett. 2009, 12, 324–333. [Google Scholar] [CrossRef]
- Machac, A.; Janda, M.; Dunn, R.R.; Sanders, N.J. Elevational gradients in phylogenetic structure of ant communities reveal the interplay of biotic and abiotic constraints on diversity. Ecography 2011, 34, 364–371. [Google Scholar] [CrossRef]
- Smith, M.A.; Hallwachs, W.; Janzen, D.H. Diversity and phylogenetic community structure of ants along a Costa Rican elevational gradient. Ecography 2014, 37, 720–731. [Google Scholar] [CrossRef]
- Jenkins, C.N.; Sanders, N.J.; Andersen, A.N.; Arnan, X.; Brühl, C.A.; Cerda, X.; Ellison, A.M.; Fisher, B.L.; Fitzpatrick, M.C.; Gotelli, N.J.; et al. Global diversity in light of climate change: The case of ants. Divers. Distrib. 2011, 17, 652–662. [Google Scholar] [CrossRef]
- Arnan, X.; Arcoverde, G.B.; Pie, M.R.; Ribeiro-Neto, J.D.; Leal, I.R. Increased anthropogenic disturbance and aridity reduce phylogenetic and functional diversity of ant communities in Caatinga dry forest. Sci. Total Environ. 2018, 631–632, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Del Toro, I.; Ribbons, R.R.; Pelini, S.L. The little things that run the world revisited: A review of ant-mediated ecosystem services and disservices (Hymenoptera: Formicidae). Myrmecol. News 2012, 17, 133–146. [Google Scholar]
- Parr, C.L.; Bishop, T.R. The response of ants to climate change. Glob. Change Biol. 2022, 28, 3188–3205. [Google Scholar] [CrossRef] [PubMed]
- Yamawo, A.; Tagawa, J.; Hada, Y.; Suzuki, N. Different combinations of multiple defence traits in an extrafloral nectary-bearing plantgrowing under various habitat conditions. J. Ecol. 2014, 102, 238–247. [Google Scholar] [CrossRef]
- Rudgers, J.A.; Strauss, S.Y. A Selection Mosaic in the Facultative Mutualism between Ants and Wild Cotton. Proc. Biol. Sci. 2004, 271, 2481–2488. [Google Scholar] [CrossRef]
- Anjos, D.; Campos, R.; Campos, R.; Ribeiro, S. Monitoring Effect of Fire on Ant Assemblages in Brazilian Rupestrian Grasslands: Contrasting Effects on Ground and Arboreal Fauna. Insects 2017, 8, 64. [Google Scholar] [CrossRef]
- Moura, R.F.; Couto, C.M.V.; Del-Claro, K. Ant nest distribution and richness have opposite effects on a Neotropical plant with extrafloral nectaries. Ecol. Entomol. 2022, 47, 626–635. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Torezan-Silingardi, H.M. Implications of the floral herbivory on Malpighiacea plant fitness: Visual aspect of the flower affects the attractiveness to pollinators. Sociobiology 2013, 60, 323–328. [Google Scholar] [CrossRef]
- Schneider, J.V.; Bissiengou, P.; Amaral, M.C.; Tahir, A.; Fay, M.F.; Thines, M.; Sosef, M.S.; Zizka, G.; Chatrou, L.W. Phylogenetics, ancestral state reconstruction, and a new infrafamilial classification of the pantropical Ochnaceae (Medusagynaceae, Ochnaceae s.str., Quiinaceae) based on five DNA regions. Mol. Phylogenet. Evol. 2014, 78, 199–261. [Google Scholar] [CrossRef]
- Dahlgren, R.M.T. A revised system of classification of the angiosperms. Bot. J. Linn. Soc. 1980, 80, 91–124. [Google Scholar] [CrossRef]
- Oliveira, P.S.; Marquis, R.J. The Cerrados of Brazil: Ecology and Natural History of a Neotropical Savanna; Columbia University Press: New York, NY, USA, 2002; p. 424. [Google Scholar]
- López, L.; Villalba, R. Climate-growth relationships for Aspidosperma tomentosum Mart. in South American tropical dry forests. Ann. For. Sci. 2020, 77, 96. [Google Scholar] [CrossRef]
- Fournier, L.A. Un método cuantitativo para la medición de características fenológicas em árboles. Turrialba 1974, 24, 422–423. [Google Scholar]
- Ribeiro, J.F.; Castro, L.H.R. Método quantitativo para avaliar características fenológicas de arvores. Rev. Bras. De Botânica 1986, 9, 7–11. [Google Scholar]
- Galdiano, M.S.; Calixto, E.S.; Torezan-Silingardi, H.M. Context-dependent outcomes in plant–plant interaction: Impacts of abiotic factors and host plant on a hemiparasitic plant performance. Plant Ecol. 2023, 224, 239–253. [Google Scholar] [CrossRef]
- Baccaro, F.B.; Feitosa, R.M.; Fernández, F.; Fernandes, I.; Souza, J.L.P.; Izzo, T. Guia para os Gêneros de Formigas do Brasil; Editora INPA: Manaus, Brazil, 2015; 388p. [Google Scholar]
- Schmidt, C.A.; Shattuck, S.O. The Higher Classification of the Ant Subfamily Ponerinae (Hymenoptera: Formicidae), with a Review of Ponerine Ecology and Behavior. Zootaxa 2014, 3817, 1–242. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; DM 198; Springer: Berlin/Heidelberg, Germany, 1990; 732p. [Google Scholar]
- Fernandez, F.; Guerrero, R.J.; Sánchez-Restrepo, A.F. Systematics and diversity of Neotropical ants. Rev. Colomb. Entomol. 2021, 47, 1–20. [Google Scholar] [CrossRef]
- ANTWEB. 2021. California Academy of Science. Available online: https://www.antweb.org/ (accessed on 10 June 2022).
- Lemon, J. Plotrix: A package in the red light district of R. R-News 2006, 6, 8–12. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models (Version 0.4.1) [R Package]. Available online: https://cran.r-project.org/package=DHARMa (accessed on 24 August 2023).
- Lenth, R. emmeans: Estimated Marginal Means, Aka Least-Squares Means. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 24 August 2023).
- Therneau, T.M. Survival Analysis Version 3.2-14. Available online: https://github.com/therneau/survival (accessed on 24 August 2023).
- Harrington, D.P.; Fleming, T.R. A Class of Rank Test Procedures for Censored Survival Data. Biometrika 1982, 69, 553–566. [Google Scholar] [CrossRef]
- Kassambara, A.; Kosinski, M. Survminer: Drawing Survival Curves using “ggplot2”. R Package Version 0.4.3. 2018. Available online: https://cran.r-project.org/package=survminer (accessed on 24 August 2023).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; et al. “Vegan: Community Ecology Package”. R Package Version 2.6-4. Available online: http://CRAN.R-project.org/package=vegan (accessed on 24 August 2023).
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porto, G.F.; Pezzonia, J.H.; Del-Claro, K. Extrafloral Nectary-Bearing Plants Recover Ant Association Benefits Faster and More Effectively after Frost-Fire Events Than Frost. Plants 2023, 12, 3592. https://doi.org/10.3390/plants12203592
Porto GF, Pezzonia JH, Del-Claro K. Extrafloral Nectary-Bearing Plants Recover Ant Association Benefits Faster and More Effectively after Frost-Fire Events Than Frost. Plants. 2023; 12(20):3592. https://doi.org/10.3390/plants12203592
Chicago/Turabian StylePorto, Gabriela Fraga, José Henrique Pezzonia, and Kleber Del-Claro. 2023. "Extrafloral Nectary-Bearing Plants Recover Ant Association Benefits Faster and More Effectively after Frost-Fire Events Than Frost" Plants 12, no. 20: 3592. https://doi.org/10.3390/plants12203592
APA StylePorto, G. F., Pezzonia, J. H., & Del-Claro, K. (2023). Extrafloral Nectary-Bearing Plants Recover Ant Association Benefits Faster and More Effectively after Frost-Fire Events Than Frost. Plants, 12(20), 3592. https://doi.org/10.3390/plants12203592