Polyphenols from Mediterranean Plants: Biological Activities for Skin Photoprotection in Atopic Dermatitis, Psoriasis, and Chronic Urticaria
Abstract
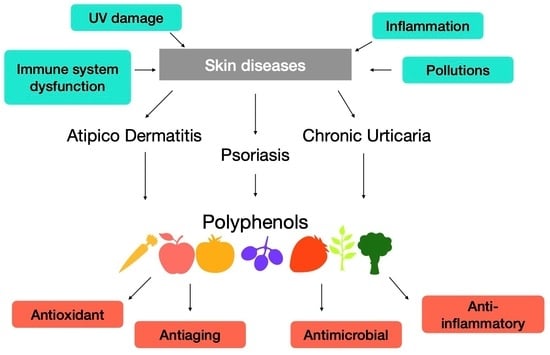
1. Introduction
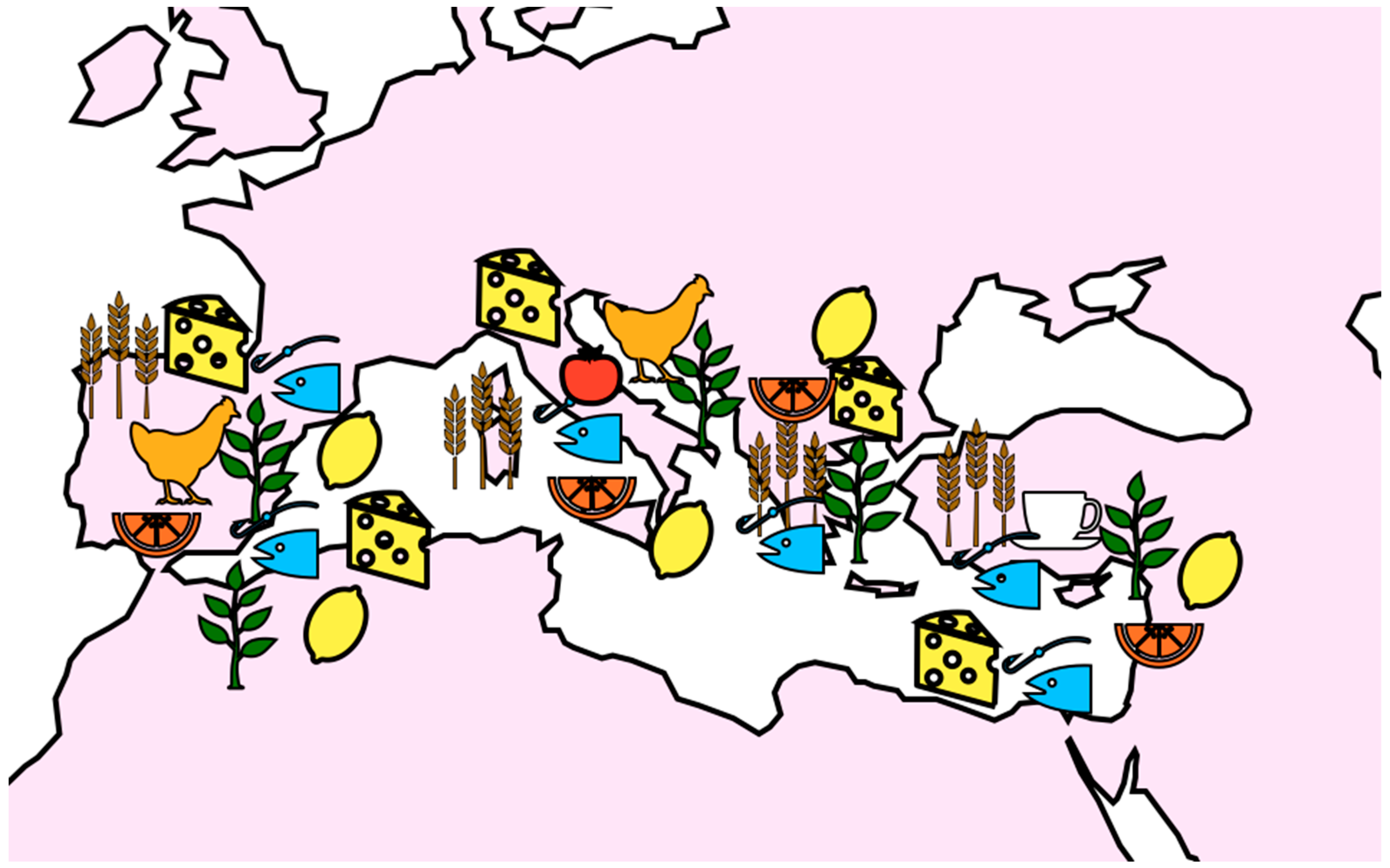
2. Distribution in the Mediterranean Area
2.1. Skin Photoprotection
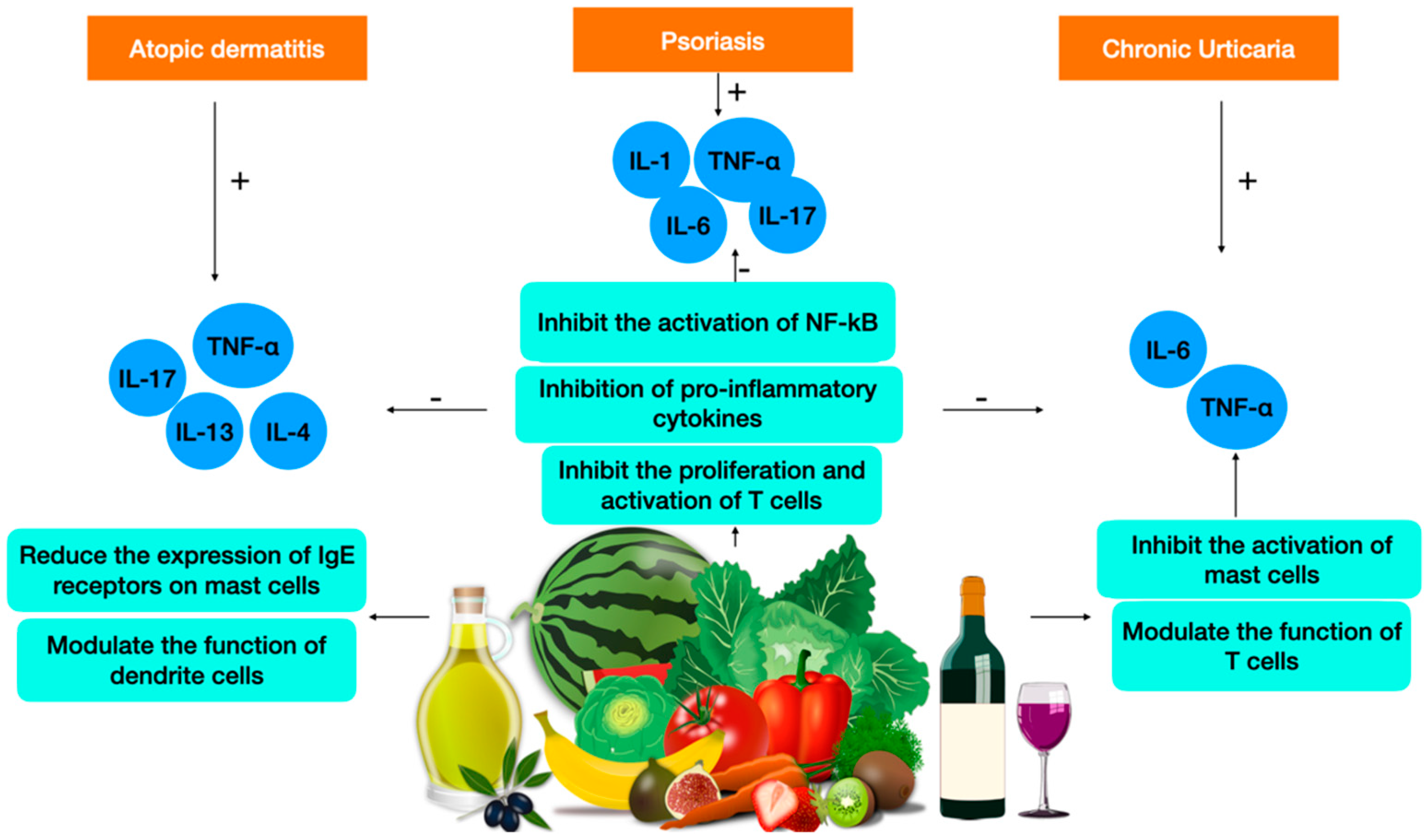
2.2. Atopic Dermatitis
2.3. Psoriasis
2.4. Chronic Urticaria
3. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures; Oxford University Press: Oxford, UK, 2019; Volume 102, pp. 1397–1400. ISBN 1060-3271. [Google Scholar]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Fejér, J.; Kron, I.; Pellizzeri, V.; Pľuchtová, M.; Eliašová, A.; Campone, L.; Gervasi, T.; Bartolomeo, G.; Cicero, N.; Babejová, A.; et al. First Report on Evaluation of Basic Nutritional and Antioxidant Properties of Moringa Oleifera Lam. from Caribbean Island of Saint Lucia. Plants 2019, 8, 537. [Google Scholar] [CrossRef]
- Han, L.; Fu, Q.; Deng, C.; Luo, L.; Xiang, T.; Zhao, H. Immunomodulatory Potential of Flavonoids for the Treatment of Autoimmune Diseases and Tumour. Scand. J. Immunol. 2022, 95, e13106. [Google Scholar] [CrossRef]
- Ku, Y.-S.; Ng, M.-S.; Cheng, S.-S.; Lo, A.W.-Y.; Xiao, Z.; Shin, T.-S.; Chung, G.; Lam, H.-M. Understanding the Composition, Biosynthesis, Accumulation and Transport of Flavonoids in Crops for the Promotion of Crops as Healthy Sources of Flavonoids for Human Consumption. Nutrients 2020, 12, 1717. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Aggarwal, B.B.; Singh, R.B.; Buttar, H.S.; Wilson, D.; De Meester, F. Food Antioxidants and Their Anti-Inflammatory Properties: A Potential Role in Cardiovascular Diseases and Cancer Prevention. Diseases 2016, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Sezer, E.D.; Oktay, L.M.; Karadadaş, E.; Memmedov, H.; Selvi Gunel, N.; Sözmen, E. Assessing Anticancer Potential of Blueberry Flavonoids, Quercetin, Kaempferol, and Gentisic Acid, through Oxidative Stress and Apoptosis Parameters on HCT-116 Cells. J. Med. Food 2019, 22, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus Fruits as a Treasure Trove of Active Natural Metabolites that Potentially Provide Benefits for Human Health. Chem. Cent. J. 2015, 9, 1–14. [Google Scholar] [CrossRef]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual Intake of Anthocyanins and Flavanones and Risk of Cardiovascular Disease in Men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef]
- do Rosario, V.A.; Fitzgerald, Z.; Broyd, S.; Paterson, A.; Roodenrys, S.; Thomas, S.; Bliokas, V.; Potter, J.; Walton, K.; Weston–Green, K. Food Anthocyanins Decrease Concentrations of TNF-α in Older Adults with Mild Cognitive Impairment: A Randomized, Controlled, Double Blind Clinical Trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 950–960. [Google Scholar] [CrossRef]
- Akinwumi, B.C.; Bordun, K.-A.M.; Anderson, H.D. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [PubMed]
- Dull, A.-M.; Moga, M.A.; Dimienescu, O.G.; Sechel, G.; Burtea, V.; Anastasiu, C.V. Therapeutic Approaches of Resveratrol on Endometriosis via Anti-Inflammatory and Anti-Angiogenic Pathways. Molecules 2019, 24, 667. [Google Scholar] [CrossRef]
- Abraham, J.; Johnson, R.W. Consuming a Diet Supplemented with Resveratrol Reduced Infection-Related Neuroinflammation and Deficits in Working Memory in Aged Mice. Rejuvenation Res. 2009, 12, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G. Phenolic Acids and Prevention of Cognitive Decline: Polyphenols with a Neuroprotective Role in Cognitive Disorders and Alzheimer’s Disease. Nutrients 2022, 14, 819. [Google Scholar] [CrossRef]
- Colica, C.; Di Renzo, L.; Aiello, V.; De Lorenzo, A.; Abenavoli, L. Rosmarinic Acid as Potential Anti-Inflammatory Agent. Rev. Recent Clin. Trials 2018, 13, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H. Lignans and Human Health. Crit. Rev. Clin. Lab. Sci. 2007, 44, 483–525. [Google Scholar] [CrossRef]
- Reddy, M.K.; Gupta, S.K.; Jacob, M.R.; Khan, S.I.; Ferreira, D. Antioxidant, Antimalarial and Antimicrobial Activities of Tannin-Rich Fractions, Ellagitannins and Phenolic Acids from Punica Granatum L. Planta Med. 2007, 73, 461–467. [Google Scholar] [CrossRef]
- Al-Harbi, S.A.; Abdulrahman, A.O.; Zamzami, M.A.; Khan, M.I. Urolithins: The Gut Based Polyphenol Metabolites of Ellagitannins in Cancer Prevention, a Review. Front. Nutr. 2021, 8, 647582. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Cicero, N. Development of Food Chemistry, Natural Products, and Nutrition Research: Targeting New Frontiers. Foods 2020, 9, 482. [Google Scholar] [CrossRef]
- Afaq, F.; Katiyar, K.S. Polyphenols: Skin Photoprotection and Inhibition of Photocarcinogenesis. Mini Rev. Med. Chem. 2011, 11, 1200–1215. [Google Scholar] [CrossRef][Green Version]
- Saric, S.; Notay, M.; Sivamani, R.K. Green Tea and Other Tea Polyphenols: Effects on Sebum Production and Acne Vulgaris. Antioxidants 2016, 6, 2. [Google Scholar] [CrossRef]
- Kang, M.C.; Cho, K.; Lee, J.H.; Subedi, L.; Yumnam, S.; Kim, S.Y. Effect of Resveratrol-Enriched Rice on Skin Inflammation and Pruritus in the NC/Nga Mouse Model of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 1428. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin Photoprotection by Natural Polyphenols: Anti-Inflammatory, Antioxidant and DNA Repair Mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Azqueta, A.; Collins, A. Polyphenols and DNA Damage: A Mixed Blessing. Nutrients 2016, 8, 785. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Nosoudi, N.; Karamched, S.; Parasaram, V.; Vyavahare, N. Polyphenol Treatments Increase Elastin and Collagen Deposition by Human Dermal Fibroblasts; Implications to Improve Skin Health. J. Dermatol. Sci. 2021, 102, 94–100. [Google Scholar] [CrossRef]
- Miller, D.L.; Weinstock, M.A. Nonmelanoma Skin Cancer in the United States: Incidence. J. Am. Acad. Dermatol. 1994, 30, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Kligman, L.H.; Akin, F.J.; Kligman, A.M. Sunscreens Prevent Ultraviolet Photocarcinogenesis. J. Am. Acad. Dermatol. 1980, 3, 30–35. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Elmets, C.A. Green Tea Polyphenolic Antioxidants and Skin Photoprotection (Review). Int. J. Oncol. 2001, 18, 1307–1313. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Huang, M.T.; Ho, C.T.; Chang, R.; Ma, W.; Ferraro, T.; Reuhl, K.R.; Yang, C.S.; Conney, A.H. Inhibitory Effect of Green Tea on the Growth of Established Skin Papillomas in Mice. Cancer Res. 1992, 52, 6657–6665. [Google Scholar] [PubMed]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef]
- Righini, S.; Rodriguez, E.J.; Berosich, C.; Grotewold, E.; Casati, P.; Falcone Ferreyra, M.L. Apigenin Produced by Maize Flavone Synthase I and II Protects Plants against UV-B-Induced Damage. Plant Cell Environ. 2019, 42, 495–508. [Google Scholar] [CrossRef]
- Shaik, Y.; Caraffa, A.; Ronconi, G.; Lessiani, G.; Conti, P. Impact of Polyphenols on Mast Cells with Special Emphasis on the Effect of Quercetin and Luteolin. Cent. Eur. J. Immunol. 2018, 43, 476–481. [Google Scholar] [CrossRef]
- Fahlman, B.M.; Krol, E.S. Inhibition of UVA and UVB Radiation-Induced Lipid Oxidation by Quercetin. J. Agric. Food Chem. 2009, 57, 5301–5305. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; Giménez-Bastida, J.A.; González-Sarrías, A.; Espín, J.C. New Insights into the Metabolism of the Flavanones Eriocitrin and Hesperidin: A Comparative Human Pharmacokinetic Study. Antioxidants 2021, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.; Xian, D.; Xiong, X.; Yang, L.; Song, J.; Zhong, J. Proanthocyanidins: Novel Treatment for Psoriasis That Reduces Oxidative Stress and Modulates Th17 and Treg Cells. Redox Rep. 2018, 23, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Saija, A.; Tomaino, A.; Cascio, R.L.; Trombetta, D.; Proteggente, A.; De Pasquale, A.; Uccella, N.; Bonina, F. Ferulic and Caffeic Acids as Potential Protective Agents against Photooxidative Skin Damage. J. Sci. Food Agric. 1999, 79, 476–480. [Google Scholar] [CrossRef]
- Min, S.-Y.; Yan, M.; Kim, S.B.; Ravikumar, S.; Kwon, S.-R.; Vanarsa, K.; Kim, H.-Y.; Davis, L.S.; Mohan, C. Green Tea Epigallocatechin-3-Gallate Suppresses Autoimmune Arthritis through Indoleamine-2, 3-Dioxygenase Expressing Dendritic Cells and the Nuclear Factor, Erythroid 2-like 2 Antioxidant Pathway. J. Inflamm. 2015, 12, 1–15. [Google Scholar] [CrossRef]
- Ahn, S.-C.; Kim, G.-Y.; Kim, J.-H.; Baik, S.-W.; Han, M.-K.; Lee, H.-J.; Moon, D.-O.; Lee, C.-M.; Kang, J.-H.; Kim, B.-H. Epigallocatechin-3-Gallate, Constituent of Green Tea, Suppresses the LPS-Induced Phenotypic and Functional Maturation of Murine Dendritic Cells through Inhibition of Mitogen-Activated Protein Kinases and NF-ΚB. Biochem. Biophys. Res. Commun. 2004, 313, 148–155. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Tomás-Barberán, F.A.; García-Villalba, R. Structural diversity of polyphenols and distribution in foods. In Dietary Polyphenols: Their Metabolism and Health Effects; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 1–29. [Google Scholar]
- Haddad, M.A.; El-Qudah, J.; Abu-Romman, S.; Obeidat, M.; Iommi, C.; Jaradat, D.M. Phenolics in Mediterranean and Middle East Important Fruits. J. AOAC Int. 2020, 103, 930–934. [Google Scholar] [CrossRef]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, L.; Castillo, J.; Quinones, M.; Garcia-Vallve, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of Angiotensin-Converting Enzyme Activity by Flavonoids: Structure-Activity Relationship Studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef] [PubMed]
- Noviello, M.; Caputi, A.F.; Squeo, G.; Paradiso, V.M.; Gambacorta, G.; Caponio, F. Vine Shoots as a Source of Trans-Resveratrol and ε-Viniferin: A Study of 23 Italian Varieties. Foods 2022, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Ilahy, R.; Hdider, C.; Lenucci, M.S.; Tlili, I.; Dalessandro, G. Phytochemical Composition and Antioxidant Activity of High-Lycopene Tomato (Solanum Lycopersicum L.) Cultivars Grown in Southern Italy. Sci. Hortic. 2011, 127, 255–261. [Google Scholar] [CrossRef]
- Papastavropoulou, K.; Oz, E.; Oz, F.; Proestos, C. Polyphenols from Plants: Phytochemical Characterization, Antioxidant Capacity, and Antimicrobial Activity of Some Plants from Different Sites of Greece. Separations 2022, 9, 186. [Google Scholar] [CrossRef]
- Urün, I.; Attar, S.H.; Sönmez, D.A.; Gündeşli, M.A.; Ercişli, S.; Kafkas, N.E.; Bandić, L.M.; Duralija, B. Comparison of Polyphenol, Sugar, Organic Acid, Volatile Compounds, and Antioxidant Capacity of Commercially Grown Strawberry Cultivars in Turkey. Plants 2021, 10, 1654. [Google Scholar] [CrossRef]
- Gülçin, İ.; Bursal, E.; Şehitoğlu, M.H.; Bilsel, M.; Gören, A.C. Polyphenol Contents and Antioxidant Activity of Lyophilized Aqueous Extract of Propolis from Erzurum, Turkey. Food Chem. Toxicol. 2010, 48, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; Darra, N.E.; Kantar, S.E.; Hobaika, Z.; Louka, N.; Maroun, R.G. A Comparative Study of the Phenolic and Technological Maturities of Red Grapes Grown in Lebanon. Antioxidants 2017, 6, 8. [Google Scholar] [CrossRef]
- Menhem, C.; Mattar, J.; Carrillo, C.; Serhan, M. Determination of Polyphenols, Antioxidant Activity, and Antimicrobial Properties of Zhourat Using Different Extraction Conditions. Appl. Food Res. 2021, 1, 100021. [Google Scholar] [CrossRef]
- Gabrielska, J.; Soczyńska-Kordala, M.; Przestalski, S. Antioxidative Effect of Kaempferol and Its Equimolar Mixture with Phenyltin Compounds on UV-Irradiated Liposome Membranes. J. Agric. Food Chem. 2005, 53, 76–83. [Google Scholar] [CrossRef]
- Wen, S.; Zhang, J.; Yang, B.; Elias, P.M.; Man, M.-Q. Role of Resveratrol in Regulating Cutaneous Functions. Evid.-Based Complement. Altern. Med. 2020, 2020, e2416837. [Google Scholar] [CrossRef]
- Zerres, S.; Stahl, W. Carotenoids in Human Skin. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158588. [Google Scholar] [CrossRef]
- OyetakinWhite, P.; Tribout, H.; Baron, E. Protective Mechanisms of Green Tea Polyphenols in Skin. Oxidative Med. Cell. Longev. 2012, 2012, e560682. [Google Scholar] [CrossRef]
- Sim, G.-S.; Lee, B.-C.; Cho, H.S.; Lee, J.W.; Kim, J.-H.; Lee, D.-H.; Kim, J.-H.; Pyo, H.-B.; Moon, D.C.; Oh, K.-W.; et al. Structure Activity Relationship of Antioxidative Property of Flavonoids and Inhibitory Effect on Matrix Metalloproteinase Activity in UVA-Irradiated Human Dermal Fibroblast. Arch. Pharm. Res. 2007, 30, 290–298. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.-W.; Kim, E.K.; Lee, S.-J.; Park, N.-H.; Kim, H.-S.; Kim, H.-K.; Char, K.; Jang, Y.P.; Kim, J.-W. Inhibition Effect of Gynura Procumbens Extract on UV-B-Induced Matrix-Metalloproteinase Expression in Human Dermal Fibroblasts. J. Ethnopharmacol. 2011, 137, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhao, J.; Yang, Z.; Xiong, L.; Li, L.; Gu, Z.; Li, Y. Polyphenolic Sunscreens for Photoprotection. Green Chem. 2022, 24, 3605–3622. [Google Scholar] [CrossRef]
- Elmets, C.A.; Singh, D.; Tubesing, K.; Matsui, M.; Katiyar, S.; Mukhtar, H. Cutaneous Photoprotection from Ultraviolet Injury by Green Tea Polyphenols. J. Am. Acad. Dermatol. 2001, 44, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Song, H.; Hong, S.S.; Boo, Y.C. Marine Alga Ecklonia Cava Extract and Dieckol Attenuate Prostaglandin E2 Production in HaCaT Keratinocytes Exposed to Airborne Particulate Matter. Antioxidants 2019, 8, 190. [Google Scholar] [CrossRef]
- Ferran, M.; Santamaria-Babi, L.F. Pathological Mechanisms of Skin Homing T Cells in Atopic Dermatitis. World Allergy Organ. J. 2010, 3, 44–47. [Google Scholar] [CrossRef]
- Kezic, S.; O’Regan, G.M.; Lutter, R.; Jakasa, I.; Koster, E.S.; Saunders, S.; Caspers, P.; Kemperman, P.M.J.H.; Puppels, G.J.; Sandilands, A.; et al. Filaggrin Loss-of-Function Mutations Are Associated with Enhanced Expression of IL-1 Cytokines in the Stratum Corneum of Patients with Atopic Dermatitis and in a Murine Model of Filaggrin Deficiency. J. Allergy Clin. Immunol. 2012, 129, 1031–1039.e1. [Google Scholar] [CrossRef]
- Atopic Dermatitis: Pathogenetic Mechanisms|Clinical and Experimental Dermatology|Oxford Academic. Available online: https://academic.oup.com/ced/article-abstract/25/7/530/6627852 (accessed on 18 September 2023).
- Howell, M.D.; Kim, B.E.; Gao, P.; Grant, A.V.; Boguniewicz, M.; DeBenedetto, A.; Schneider, L.; Beck, L.A.; Barnes, K.C.; Leung, D.Y.M. Cytokine Modulation of Atopic Dermatitis Filaggrin Skin Expression. J. Allergy Clin. Immunol. 2009, 124, R7–R12. [Google Scholar] [CrossRef] [PubMed]
- Piquero-Casals, J.; Carrascosa, J.M.; Morgado-Carrasco, D.; Narda, M.; Trullas, C.; Granger, C.; Fabbrocini, G. The Role of Photoprotection in Optimizing the Treatment of Atopic Dermatitis. Dermatol. Ther. 2021, 11, 315–325. [Google Scholar] [CrossRef]
- Spergel, J.M.; Paller, A.S. Atopic Dermatitis and the Atopic March. J. Allergy Clin. Immunol. 2003, 112, S118–S127. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Akiyama, H.; Sasai, M.; Taniuchi, S.; Goda, Y.; Toyoda, M.; Kobayashi, Y. Anti-Allergic Effect of Apple Polyphenol on Patients with Atopic Dermatitis: A Pilot Study. Allergol. Int. 2000, 49, 69–73. [Google Scholar] [CrossRef]
- Kundu, J.K.; Shin, Y.K.; Surh, Y.-J. Resveratrol Modulates Phorbol Ester-Induced pro-Inflammatory Signal Transduction Pathways in Mouse Skin in Vivo: NF-ΚB and AP-1 as Prime Targets. Biochem. Pharmacol. 2006, 72, 1506–1515. [Google Scholar] [CrossRef]
- Alesci, A.; Nicosia, N.; Fumia, A.; Giorgianni, F.; Santini, A.; Cicero, N. Resveratrol and Immune Cells: A Link to Improve Human Health. Molecules 2022, 27, 424. [Google Scholar] [CrossRef]
- Sozmen, S.C.; Karaman, M.; Micili, S.C.; Isik, S.; Ayyildiz, Z.A.; Bagriyanik, A.; Uzuner, N.; Karaman, O. Resveratrol Ameliorates 2,4-Dinitrofluorobenzene-Induced Atopic Dermatitis-like Lesions through Effects on the Epithelium. PeerJ 2016, 4, e1889. [Google Scholar] [CrossRef]
- Kim, H.K.; Chang, H.K.; Baek, S.Y.; Chung, J.O.; Rha, C.S.; Kim, S.Y.; Kim, B.J.; Kim, M.N. Treatment of Atopic Dermatitis Associated with Malassezia Sympodialis by Green Tea Extracts Bath Therapy: A Pilot Study. Mycobiology 2012, 40, 124–128. [Google Scholar] [CrossRef]
- Cervi, V.F.; Saccol, C.P.; Sari, M.H.M.; Martins, C.C.; da Rosa, L.S.; Ilha, B.D.; Soares, F.Z.; Luchese, C.; Wilhelm, E.A.; Cruz, L. Pullulan Film Incorporated with Nanocapsules Improves Pomegranate Seed Oil Anti-Inflammatory and Antioxidant Effects in the Treatment of Atopic Dermatitis in Mice. Int. J. Pharm. 2021, 609, 121144. [Google Scholar] [CrossRef]
- Nagano, T.; Nishida, N.; Ito, H. The Inhibitory Effect of a Polyphenol Concentrate from Pomegranate Juice on 2, 4-Dinitrofluorobenzene-Induced Contact Hypersensitivity in Mice. Food Sci. Technol. Res. 2018, 24, 169–175. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Waghule, T.; Gorantla, S.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Psoriasis: Pathological Mechanisms, Current Pharmacological Therapies, and Emerging Drug Delivery Systems. Drug Discov. Today 2020, 25, 2212–2226. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Kikuchi, T.; Fuentes-Duculan, J.; Cardinale, I.; Zaba, L.C.; Haider, A.S.; Bowman, E.P.; Krueger, J.G. Psoriasis Vulgaris Lesions Contain Discrete Populations of Th1 and Th17 T Cells. J. Investig. Dermatol. 2008, 128, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Panda, S. Recent Understanding of the Etiopathogenesis of Psoriasis. Indian J. Paediatr. Dermatol. 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Recent Advances in Understanding Psoriasis—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4850872/ (accessed on 18 September 2023).
- Possible Roles of IL-33 in the Innate-Adaptive Immune Crosstalk of Psoriasis Pathogenesis. Available online: https://www.hindawi.com/journals/mi/2019/7158014/ (accessed on 18 September 2023).
- Meephansan, J.; Tsuda, H.; Komine, M.; Tominaga, S.; Ohtsuki, M. Regulation of IL-33 Expression by IFN-γ and Tumor Necrosis Factor-α in Normal Human Epidermal Keratinocytes. J. Investig. Dermatol. 2012, 132, 2593–2600. [Google Scholar] [CrossRef]
- Meephansan, J.; Komine, M.; Tsuda, H.; Karakawa, M.; Tominaga, S.; Ohtsuki, M. Expression of IL-33 in the Epidermis: The Mechanism of Induction by IL-17. J. Dermatol. Sci. 2013, 71, 107–114. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Wu, L.; Nie, Z.; Mei, Z. Risk of Non-Melanoma Skin Cancer in Patients with Psoriasis: An Updated Evidence from Systematic Review with Meta-Analysis. J. Cancer 2020, 11, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Dickinson, D.; Borke, J.; Walsh, D.S.; Wood, J.; Qin, H.; Winger, J.; Pearl, H.; Schuster, G.; Bollag, W.B. Green Tea Polyphenol Induces Caspase 14 in Epidermal Keratinocytes via MAPK Pathways and Reduces Psoriasiform Lesions in the Flaky Skin Mouse Model. Exp. Dermatol. 2007, 16, 678–684. [Google Scholar] [CrossRef]
- Gugleva, V.; Zasheva, S.; Hristova, M.; Andonova, V. Topical Use of Resveratrol: Technological Aspects. Pharmacia 2020, 67, 89–94. [Google Scholar] [CrossRef]
- Gervasi, T.; Oliveri, F.; Gottuso, V.; Squadrito, M.; Bartolomeo, G.; Cicero, N.; Dugo, G. Nero d’Avola and Perricone Cultivars: Determination of Polyphenols, Flavonoids and Anthocyanins in Grapes and Wines. Nat. Prod. Res. 2016, 30, 2329–2337. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Purzycka-Bohdan, D.; Nedoszytko, B.; Reich, A.; Szczerkowska-Dobosz, A.; Bartosińska, J.; Batycka-Baran, A.; Czajkowski, R.; Dobrucki, I.; Dobrucki, L.; et al. Pathogenesis of Psoriasis in the “Omic” Era. Part III. Metabolic Disorders, Metabolomics, Nutrigenomics in Psoriasis in Psoriasis. Adv. Dermatol. Allergol. 2020, 37, 452–467. [Google Scholar] [CrossRef]
- Reuter, J.; Merfort, I.; Schempp, C.M. Botanicals in Dermatology: An Evidence-Based Review. Am. J. Clin. Dermatol. 2010, 11, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, M.; Giannella, A.; Giannella, J. Use of Resveratrol for the Treatment of Exfoliative Eczema, Acne and Psoriasis. U.S. Patent 09/813,948, 27 December 2001. [Google Scholar]
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. [Google Scholar] [CrossRef]
- Osawa, T. Nephroprotective and Hepatoprotective Effects of Curcuminoids. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Boston, MA, USA, 2007; pp. 407–423. [Google Scholar]
- Zhang, J.; Ma, Y.; Li, W. Curcumin Reduces Inflammation in Mice with the Psoriasis Model by Inhibiting NLRP3 Inflammatory Bodies. Cell. Mol. Biol. 2021, 67, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ortiz, D.; García-Alcocer, G.; Loske, A.M.; Fernández, F.; Becerra-Becerra, E.; Esparza, R.; Gonzalez-Reyna, M.A.; Estevez, M. Green Synthesis and Antiproliferative Activity of Gold Nanoparticles of a Controlled Size and Shape Obtained Using Shock Wave Extracts from Amphipterygium Adstringens. Bioengineering 2023, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-García, R.; Ayiania, M.; Abu-Lail, N.; López-Meza, J.E.; del Río, R.E.; García-Pérez, M.; Ochoa-Zarzosa, A.; García-Pérez, M.-E. Pyrolytic Oils from Amphipterygium Adstringens Bark Inhibit IL-8 Production of IL-17-Stimulated HaCaT Keratinocytes. J. Anal. Appl. Pyrolysis 2020, 145, 104749. [Google Scholar] [CrossRef]
- Kocic, H.; Damiani, G.; Stamenkovic, B.; Tirant, M.; Jovic, A.; Tiodorovic, D.; Peris, K. Dietary Compounds as Potential Modulators of MicroRNA Expression in Psoriasis. Ther. Adv. Chronic Dis. 2019, 10, 2040622319864805. [Google Scholar] [CrossRef]
- Nettis, E.; Foti, C.; Ambrifi, M.; Baiardini, I.; Bianchi, L.; Borghi, A.; Caminati, M.; Canonica, G.W.; Casciaro, M.; Colli, L.; et al. Urticaria: Recommendations from the Italian Society of Allergology, Asthma and Clinical Immunology and the Italian Society of Allergological, Occupational and Environmental Dermatology. Clin. Mol. Allergy 2020, 18, 8. [Google Scholar] [CrossRef]
- Nettis, E.; Distaso, M.; Saitta, S.; Casciaro, M.; Cristani, M.; Saija, A.; Vacca, A.; Gangemi, S.; Minciullo, P.L. Involvement of New Oxidative Stress Markers in Chronic Spontaneous Urticaria. Postep. Dermatol. Alergol. 2017, 34, 448–452. [Google Scholar] [CrossRef]
- Cannavò, S.P.; Riso, G.; Di Salvo, E.; Casciaro, M.; Giuffrida, R.; Minciullo, P.L.; Guarneri, F.; Nettis, E.; Gangemi, S. Oxidative Stress Involvement in Urticaria. J. Biol. Regul. Homeost. Agents 2020, 34, 675–678. [Google Scholar] [CrossRef]
- Activation of Blood Coagulation in Chronic Urticaria: Pathophysiological and Clinical Implications|SpringerLink. Available online: https://link.springer.com/article/10.1007/s11739-009-0333-5 (accessed on 18 September 2023).
- Greaves, M.W.; Tan, K.T. Chronic Urticaria: Recent Advances. Clin. Rev. Allerg. Immunol. 2007, 33, 134–143. [Google Scholar] [CrossRef]
- Grattan, C.E.H.; Wallington, T.B.; Warin, R.P.; Kennedy, C.T.C.; Lbradfield, J.W. A Serological Mediator in Chronic Idiopathic Urticaria—A Clinical, Immunological and Histological Evaluation. Br. J. Dermatol. 1986, 114, 583–590. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Kaplan, A.P. A Role for C5a in Augmenting IgG-Dependent Histamine Release from Basophils in Chronic Urticaria. J. Allergy Clin. Immunol. 2002, 109, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, S.; Kikuchi, Y.; Joseph, K.; Kaplan, A.P. Functional Assessment of Pathogenic IgG Subclasses in Chronic Autoimmune Urticaria. J. Allergy Clin. Immunol. 2005, 115, 815–821. [Google Scholar] [CrossRef] [PubMed]
- In Chronic Idiopathic Urticaria Autoantibodies against FcɛRII/CD23 Induce Histamine Release via Eosinophil Activation—Puccetti—2005—Clinical & Experimental Allergy—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2222.2005.02380.x (accessed on 18 September 2023).
- Colitti, M.; Stefanon, B.; Gabai, G.; Gelain, M.E.; Bonsembiante, F. Oxidative Stress and Nutraceuticals in the Modulation of the Immune Function: Current Knowledge in Animals of Veterinary Interest. Antioxidants 2019, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, C.; Casciaro, M.; Sorbara, E.E.; Calapai, F.; Di Salvo, E.; Pioggia, G.; Navarra, M.; Calapai, G.; Gangemi, S. Nutraceuticals against Oxidative Stress in Autoimmune Disorders. Antioxidants 2021, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, S.; Minciullo, P.L.; Magliacane, D.; Saitta, S.; Loffredo, S.; Saija, A.; Cristani, M.; Marone, G.; Triggiani, M. Oxidative Stress Markers Are Increased in Patients with Mastocytosis. Allergy 2015, 70, 436–442. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Hu, S.; Ge, S.; Jia, M.; Wang, N. Resveratrol Inhibits MRGPRX2-Mediated Mast Cell Activation via Nrf2 Pathway. Int. Immunopharmacol. 2021, 93, 107426. [Google Scholar] [CrossRef]
- Bunselmeyer, B.; Laubach, H.J.; Schiller, M.; Stanke, M.; Luger, T.A.; Brehler, R. Incremental Build-up Food Challenge—A New Diagnostic Approach to Evaluate Pseudoallergic Reactions in Chronic Urticaria: A Pilot Study. Clin. Exp. Allergy 2009, 39, 116–126. [Google Scholar] [CrossRef]
- dos Anjos Oliveira Ferreira, L.; de Paula Barros de Melo, C.; Saito, P.; Iwanaga, C.C.; Nakamura, C.V.; Casagrande, R.; da Conceição Torrado Truiti, M. Nectandra Cuspidata Fraction and the Isolated Polyphenols Protect Fibroblasts and Hairless Mice Skin from UVB-Induced Inflammation and Oxidative Stress. J. Photochem. Photobiol. B Biol. 2020, 205, 111824. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Fattori, V.; Bussmann, A.J.C.; Bottura, C.; Fonseca, M.J.V.; Vignoli, J.A.; Baracat, M.M.; et al. Trans-Chalcone, a Flavonoid Precursor, Inhibits UV-Induced Skin Inflammation and Oxidative Stress in Mice by Targeting NADPH Oxidase and Cytokine Production. Photochem. Photobiol. Sci. 2017, 16, 1162–1173. [Google Scholar] [CrossRef]
- Hossain, S.J.; Tsujiyama, I.; Takasugi, M.; Islam, M.A.; Biswas, R.S.; Aoshima, H. Total Phenolic Content, Antioxidative, Anti-Amylase, Anti-Glucosidase, and Antihistamine Release Activities of Bangladeshi Fruits. Food Sci. Technol. Res. 2008, 14, 261–268. [Google Scholar] [CrossRef]
| Polyphenol Class | Examples | Dietary Sources | Pharmacological Functions | References |
|---|---|---|---|---|
| Flavonoids | Apigenin, Luteolin, Quercetin, Kaempferol | Fruits, vegetables, nuts, and herbs | Anti-inflammatory, antioxidant, anti-cancer, improved cardiovascular health | [7,32,33,34] |
| Flavanones | Hesperetin, Naringenin | Citrus fruits | Anti-inflammatory, antioxidant, improved insulin sensitivity, reduced risk of cardiovascular disease | [10,35] |
| Anthocyanins | Cyanidin, Delphinidin, Pelargonidin | Red, purple, and blue fruits and vegetables | Anti-inflammatory, antioxidant, improved cognitive function, reduced risk of cardiovascular disease | [10,11,36] |
| Stilbenoids | Resveratrol | Grapes, berries, and nuts | Anti-inflammatory, antioxidant, anti-cancer, improved insulin sensitivity, neuroprotective | [12,14,23] |
| Phenolic acids | Caffeic acid, Rosmarinic acid | Fruits, vegetables, and herbs | Anti-inflammatory, antioxidant, reduced risk of chronic diseases | [16,37] |
| Lignans | Secoisolariciresinol, Matairesinol | Flaxseed, sesame seeds, and whole grains | Anti-inflammatory, antioxidant, reduced risk of breast and prostate cancer, improved insulin sensitivity | [17] |
| Tannins | Catechins, Epicatechins | Tea and wine | Anti-inflammatory, antioxidant, potential anti-cancer effects | [18,38,39] |
| Ellagitannins | Punicalagin, Granatin B | Berries and nuts | Anti-inflammatory, antioxidant, potential anti-cancer effects | [18,19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Salvo, E.; Gangemi, S.; Genovese, C.; Cicero, N.; Casciaro, M. Polyphenols from Mediterranean Plants: Biological Activities for Skin Photoprotection in Atopic Dermatitis, Psoriasis, and Chronic Urticaria. Plants 2023, 12, 3579. https://doi.org/10.3390/plants12203579
Di Salvo E, Gangemi S, Genovese C, Cicero N, Casciaro M. Polyphenols from Mediterranean Plants: Biological Activities for Skin Photoprotection in Atopic Dermatitis, Psoriasis, and Chronic Urticaria. Plants. 2023; 12(20):3579. https://doi.org/10.3390/plants12203579
Chicago/Turabian StyleDi Salvo, Eleonora, Sebastiano Gangemi, Claudia Genovese, Nicola Cicero, and Marco Casciaro. 2023. "Polyphenols from Mediterranean Plants: Biological Activities for Skin Photoprotection in Atopic Dermatitis, Psoriasis, and Chronic Urticaria" Plants 12, no. 20: 3579. https://doi.org/10.3390/plants12203579
APA StyleDi Salvo, E., Gangemi, S., Genovese, C., Cicero, N., & Casciaro, M. (2023). Polyphenols from Mediterranean Plants: Biological Activities for Skin Photoprotection in Atopic Dermatitis, Psoriasis, and Chronic Urticaria. Plants, 12(20), 3579. https://doi.org/10.3390/plants12203579