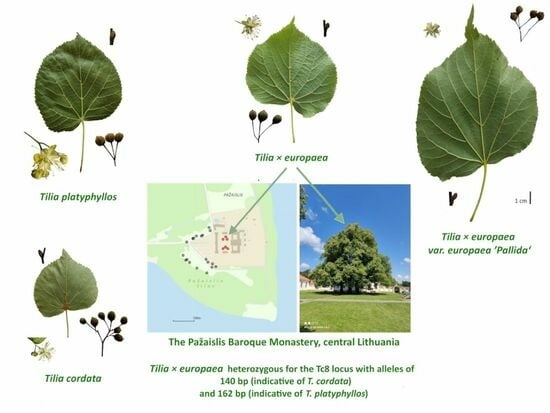
Dendrological Secrets of the Pazaislis Monastery in Central Lithuania: DNA Markers and Morphology Reveal Tilia × europaea L. Hybrids of an Impressive Age
Abstract
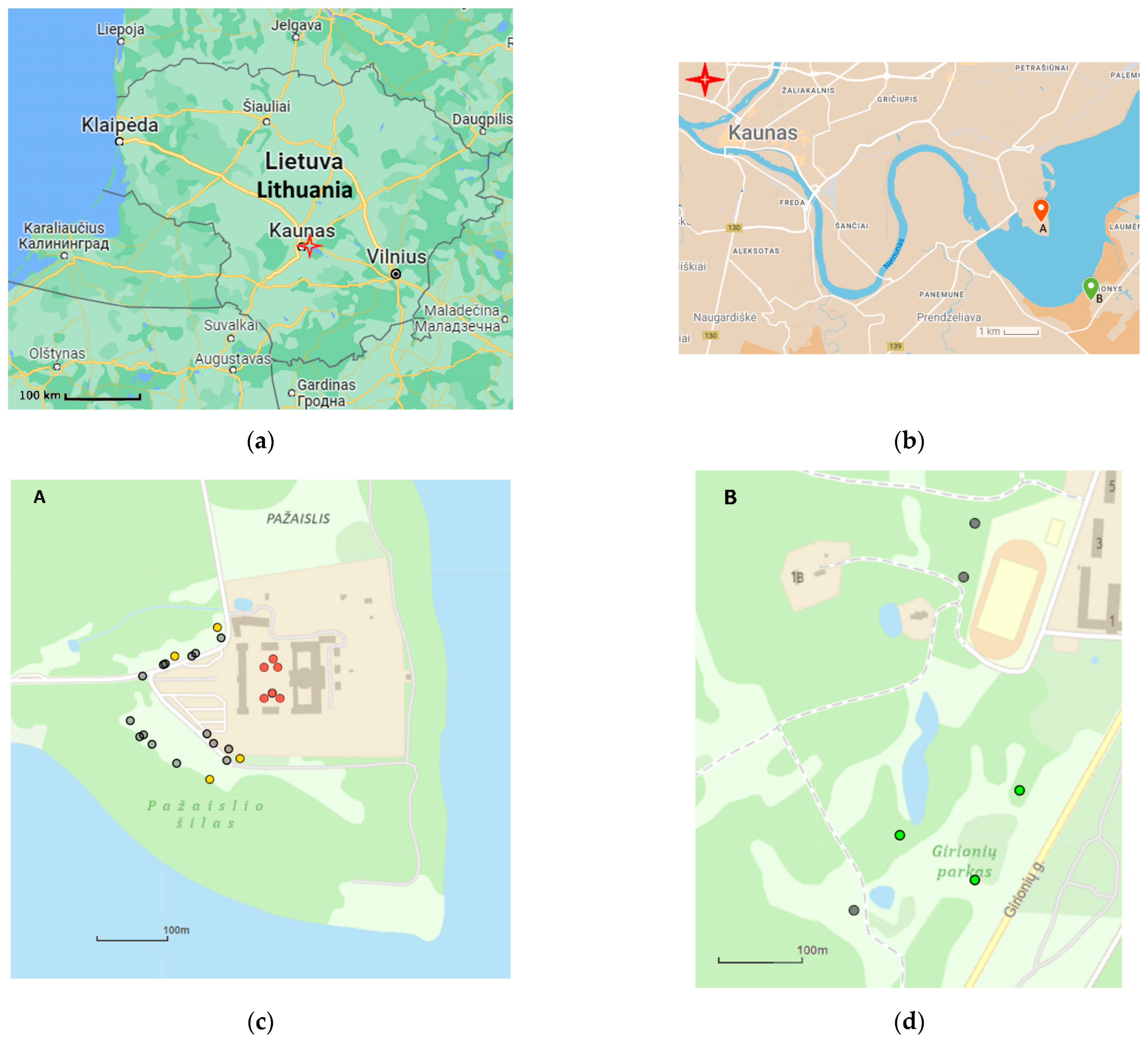
:1. Introduction
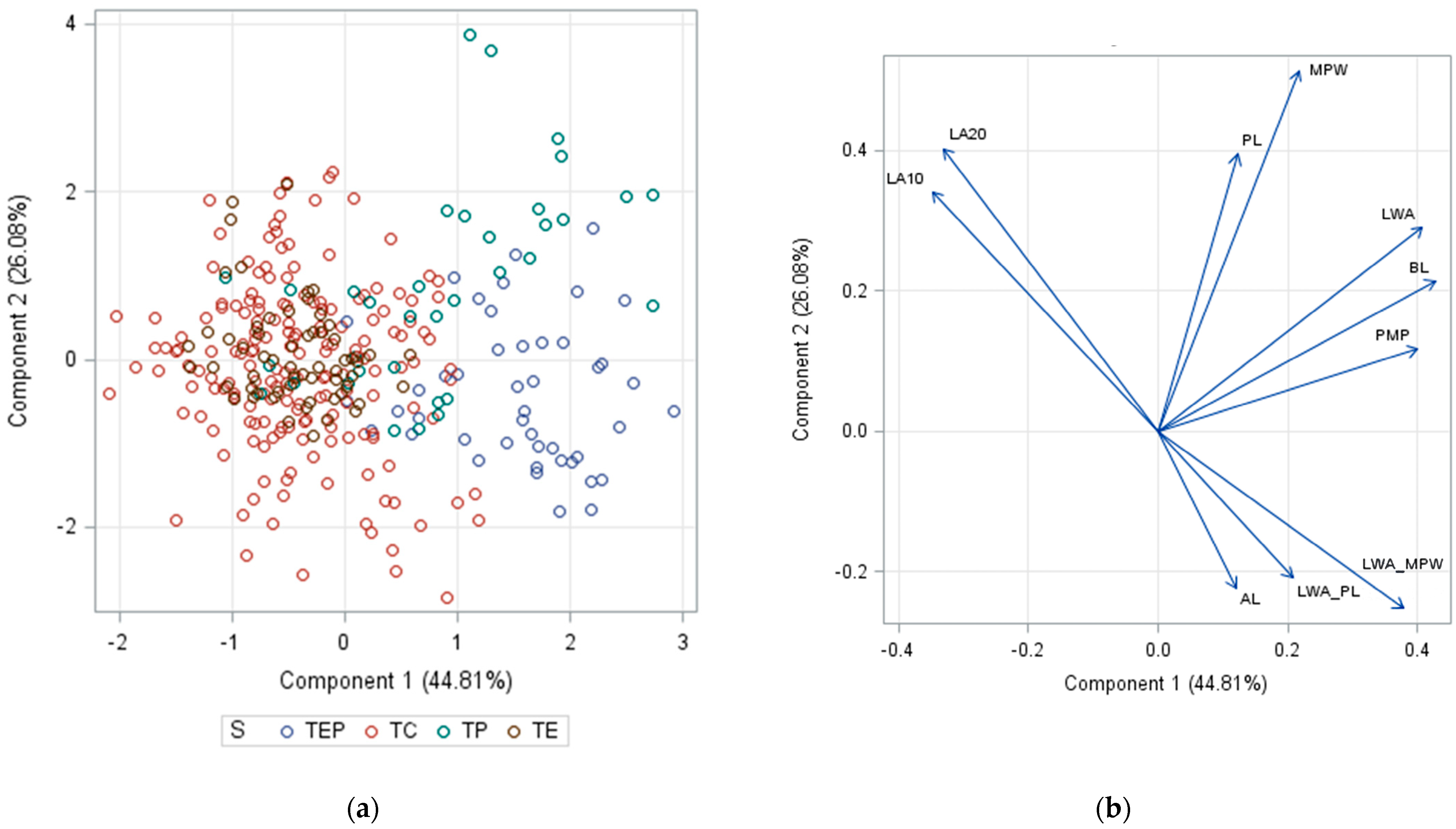
2. Results and Discussion
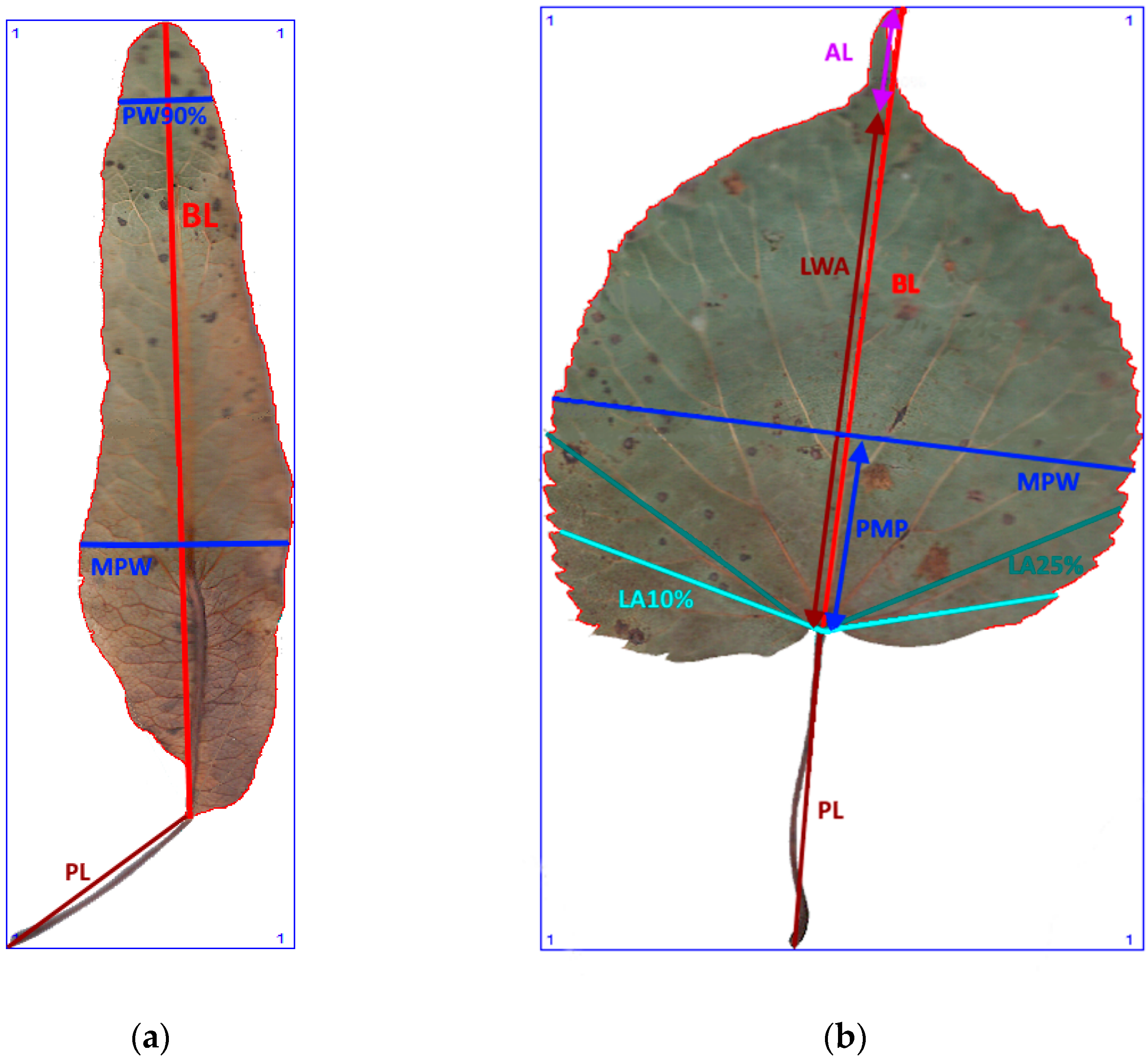
3. Materials and Methods
3.1. DNA Study
3.2. Morphometric Study
3.3. Morphometric Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phuekvilai, P. Relicts, Refugia and Reticulation: A Study of Population History, Hybrids and Phylogeny in the Long-Lived Flowering Tree Genus Tilia. Doctoral Thesis, Newcastle University, Newcastle upon Tyne, UK, 2014. [Google Scholar]
- Vytautas Magnus University. As the Climate Warms up, Lime Trees Are Spreading in Lithuania: Insights from VMU Scientists. Available online: https://www.vdu.lt/lt/siltejant-klimatui-lietuvoje-plinta-liepos-vdu-mokslininku-izvalgos/ (accessed on 28 May 2023). (In Lithuanian).
- Eaton, E.; Caudullo, G.; de Rigo, D. Tilia cordata, Tilia platyphyllos and other limes in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the EU: Luxembourg, 2016; pp. 184–185. [Google Scholar]
- Jensen, J.S.; Canger, S. Lime (Tilia spp.). In Noble Hardwoods Network. Report of the Third Meeting, 13–16 June 1998, Sagadi, Estonia; Turok, J., Jensen, J., Palmberg-Lerche, C., Rusanen, M., Russell, K., de Vries, S., Lipman, E., Eds.; International Plant Genetic Resources Institute: Rome, Italy, 1999; pp. 28–38. ISBN 978-92-9043-403-0. [Google Scholar]
- Bengtsson, R. Variation in Common Lime (Tilia × europaea L.) in Swedish Gardens of the 17th and 18th Centuries. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2005. [Google Scholar]
- De Benedetti, C.; Gerasimenko, N.; Ravazzi, C.; Magri, D. History of Tilia in Europe since the Eemian: Past distribution patterns. Rev. Palaeobot. Palynol. 2022, 307, 104778. [Google Scholar] [CrossRef]
- Pigott, C.D. Lime-Trees and Basswoods: A Biological Monograph of the Genus Tilia; Cambridge University Press: Cambridge, UK, 2012; ISBN 978-0-433-06887-7. [Google Scholar]
- Pigott, C.D. Biological Flora of the British Isles: Tilia platyphyllos. J. Ecol. 2020, 108, 2638–2676. [Google Scholar] [CrossRef]
- Huntley, B.; Birks, H.J.B. Atlas of Past and Present Pollen Maps for Europe, 0–13,000 Years Ago; Cambridge University Press: Cambridge, UK, 1983; 668p. [Google Scholar]
- Pigott, C.D. Tilia cordata Miller. J. Ecol. 1991, 79, 1147–1207. [Google Scholar] [CrossRef]
- Pigott, C.D.; Huntley, J.P. Factors Controlling the Distribution of Tilia cordata at the northern limits of its geographical range III. Nature and causes of seed sterility. New Phytol. 1981, 87, 817–839. [Google Scholar] [CrossRef]
- Danusevičius, D.; Kembrytė, R.; Buchovska, J.; Baliuckas, V.; Kavaliauskas, D. Genetic signature of the natural gene pool of Tilia cordata Mill. in Lithuania: Compound evolutionary and anthropogenic effects. Ecol. Evol. 2021, 11, 6260–6275. [Google Scholar] [CrossRef] [PubMed]
- Stravinskienė, V.; Dičiunaitė, B. Health condition and dendrochronologica lime trees in Kaunas city. Balt. For. 1999, 5, 37–44. [Google Scholar]
- Juknys, R.; Sujetovienė, G.; Žeimavičius, K.; Gustainytė, J. Effects of Climate Warming on Timing of Lime (Tilia Cordata L.) Phenology. In Proceedings of the 8th International Conference on Environmental Engineering, ICEE, Vilnius, Lithuania, 19–20 May 2011; Vilnius Gediminas Technical University, Department of Construction Economics: Vilnius, Lithuania, 2011; Volume 8, p. 139. [Google Scholar]
- Massetti, L.; Petralli, M.; Orlandini, S. The effect of urban morphology on Tilia × europaea flowering. Urban For. Urban Green. 2015, 14, 187–193. [Google Scholar] [CrossRef]
- Boratyńska, K.; Dolatowski, J. Systematyka i geograficzne rozmieszczenie. In Lipy; Instytut Dendrologii PAN: Kórnik, Poland, 1991; pp. 107–119. (In Polish) [Google Scholar]
- Szweykowska, A.; Szweykowski, J. Botanika. Systematyka [Botany. Systematics]; PWN Warszawa: Warsaw, Poland, 2003. (In Polish) [Google Scholar]
- Weryszko-Chmielewska, E.; Sadowska, D.A. The Phenology of flowering and pollen release in four species of linden (Tilia L.). J. Apic. Sci. 2010, 54, 99–108. [Google Scholar]
- Stankevičienė, A. The Monitoring of State of Street Greeneries in Lithuania. In Proceedings of the Smart Bio: ICSB 3rd International Conference, Vytautas Magnus University, Kaunas, Lithuania, 2–4 May 2019; UAB “Reklamos forma”: Panevėžys, Lithuania, 2019; Volume 3, p. 237. [Google Scholar]
- Hansen, O.K.; Thomsen, P.; Rasmussen, C.W. DNA markers provide insight about common lime in historical plantings—An example from the Royal Danish Gardens. Urban For. Urban Green. 2014, 13, 543–552. [Google Scholar] [CrossRef]
- Sjöman, H.; Busse Nielsen, A.; Pauleit, S.; Olsson, M. Habitat studies identifying potential trees for urban paved environments: A case study from Qinling Mt., China. J. Arboric. 2010, 36, 261. [Google Scholar] [CrossRef]
- Phuekvilai, P.; Wolff, K. Genetic diversity in ancient lime trees. In Trees beyond Wood (Colour); Wildtrack Publishing: Yorkshire, UK, 2013; p. 271. [Google Scholar]
- Logan, S.A.; Phuekvilai, P.; Wolff, K. Ancient woodlands in the limelight: Delineation and genetic structure of ancient woodland species Tilia Cordata and Tilia Platyphyllos (Tiliaceae) in the UK. Tree Genet. Genomes 2015, 11, 52. [Google Scholar] [CrossRef]
- Santamour, F.S., Jr.; McArdle, A.J. Checklists of cultivars of linden (Tilia) species. J. Arboric. 1985, 11, 157–164. [Google Scholar] [CrossRef]
- Wicksell, U.; Christensen, K.I. Hybridization among Tilia cordata and T. platyphyllos (Tiliaceae) in Denmark. Nord. J. Bot. 1999, 19, 673–684. [Google Scholar] [CrossRef]
- Fromm, M.; Hattemer, H.H. Inheritance of allozymes and hybridization in two european Tilia species. Heredity 2003, 91, 337–344. [Google Scholar] [CrossRef]
- Ivanov, P.; Loghin, C.; Enescu, C.M. Morphological differentiation between Romanian lime species (Tilia Spp.): A Case Study. In Bulletin of the Transilvania University of Brasov. Series II: Forestry, Wood Industry, Agricultural Food Engineering; Transilvania University Press: Brasov, Romania, 2014; pp. 21–28. [Google Scholar]
- Andrianjara, I.; Bordenave-Jacquemin, M.; Roy, V.; Cabassa, C.; Federici, P.; Carmignac, D.; Marcangeli, Y.; Rouhan, G.; Renard, M.; Nold, F.; et al. Urban tree management: Diversity of Tilia genus in streets and parks of Paris based on morphological and genetic characteristics. Urban For. Urban Green. 2021, 66, 127382. [Google Scholar] [CrossRef]
- Ziaja, M.; Pawłowska, K.A.; Jozefczyk, K.; Pruś, A.; Stefańska, J.; Granica, S. UHPLC-DAD-MS/MS analysis of extracts from linden flowers (Tiliae flos): Differences in the chemical composition between five Tilia species growing in Europe. Ind. Crops Prod. 2020, 154, 112691. [Google Scholar] [CrossRef]
- Liesebach, H.; Sinko, Z. A Contribution to the systematics of the genus Tilia with respect to some hybrids by RAPD analysis. Dendrobiology 2008, 59, 13–22. [Google Scholar]
- Niinemets, Ü. A Review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Solé-Medina, A.; Heer, K.; Opgenoorth, L.; Kaldewey, P.; Danusevicius, D.; Notivol, E.; Robledo-Arnuncio, J.J.; Ramírez-Valiente, J.A. Genetic variation in early fitness traits across european populations of silver birch (Betula pendula). AoB PLANTS 2020, 12, plaa019. [Google Scholar] [CrossRef]
- Pigott, C.D. The Status of Tilia cordata and T. platyphyllos on the Derbyshire limestone. J. Ecol. 1969, 57, 491–504. [Google Scholar] [CrossRef]
- Ramanauskas, V. Dendrologija (Dendrology); Mintis: Vilnius, Lithuania, 1973. (In Lithuanian) [Google Scholar]
- Laurian, L. Planning for street trees and human–nature relations: Lessons from 600 years of street tree planting in Paris. J. Plan. Hist. 2019, 18, 282–310. [Google Scholar] [CrossRef]
- De Langhe, J. Tilia L. (Malvaceae) Vegetative Key to Species in Cultivation. Ghent University Botanical Garden: Ghent, Belgium. Available online: https://www.arboretumwespelaar.be/UserFiles/file/SleutelsPDF/Key_Tilia_JDL.pdf (accessed on 29 August 2023).
- Navasaitis, M.; Ozolinčius, R.; Smaliukas, D.; Balevičienė, J. Lietuvos Dendroflora: Monografija [Dendroflora of Lithuania: Monograph]; Lututė: Kaunas, Lithuania, 2003; ISBN 9955-575-35-2. (In Lithuanian) [Google Scholar]
- Wicklin, R. How to Interpret Graphs in a Principal Component Analysis. Available online: https://blogs.sas.com/content/iml/2019/11/04/interpret-graphs-principal-components.html (accessed on 28 July 2023).
- Wu, Y. Abetter 3D Scatter Plot Macro. Available online: https://blogs.sas.com/content/graphicallyspeaking/2022/07/11/a-better-3d-scatter-plot-macro/ (accessed on 12 April 2023).
| Tilia cordata (TC) | Tilia × europaea (TE) | Tilia × europaea var. europaea ‘Pallida’ (TEP) | Tilia platyphyllos (TP) |
|---|---|---|---|
| A. Upper side of leaf | |||
 |  |  |  |
 |  |  |  |
| B. Lower side of leaf | |||
| B1. Whole leaf | |||
 |  |  |  |
| B2. Base of main veins | |||
 |  |  |  |
| B3. Branches of veins | |||
 |  |  |  |
| B4. Third-row veins | |||
 |  |  |  |
| B5. Leaf margin | |||
 |  |  |  |
| C. Petiole | |||
 |  |  |  |
| D. Twigs | |||
 |  |  |  |
| E. Buds | |||
 |  |  |  |
| F.Bract | |||
| F1. Lower side | |||
 |  |  |  |
| F2. Upper side | |||
 | 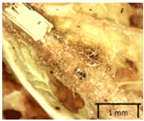 |  |  |
| G. Flowers | |||
 |  |  |  |
 |  |  |  |
| H. Nuts | |||
 |  |  |  |
| No | Species | TC | TE | TEP | TP |
|---|---|---|---|---|---|
| 1 | Nut form, fragility, ribs | Obovoid/spheroid, fragile, blurred | Spheroid/ellipsoid, average hard, average bright | Spheroid/obovoid, fragile, slightly bright | Spheroid/obovoid, hard, bright |
| 2 | Bract: petiole on lower/upper side, Length (cm) | −/−, 6–7 | −/+, 8–9 | −/−, 8–9 | ±/+, 7–8 |
| 3 | Leaf form: Width × length (cm); base | 6.5 × 5.5, cordate | 6.6 × 5.5, cordate | 7 × 7, semicordate | 8 × 7.5, shallowly to deeply cordate |
| 4 | Veins: third row (raised/horizontal or no), secondary (number of pairs) | −, 5–6 | +, 7–8 | −/+, 6–7 | +, 7–9 |
| 5 | Number of bud scales, Size of the outer scale (part from bud) | 2 1/2 | 3 <1/2 | 2–3 ≥1/2 | 3, ≤1/2 |
| 6 | Upper part of leaf hairiness | No | No | No | Yes, at base (rare), veins and petiole (dense) |
| 7 | Lower part of leaf color, hairiness | Pale green; brown stellate hairs in veins axils | Green; light brown stellate hairs in veins axils | Pale green; light brown stellate hairs in base and some veins axils | Green; white, dense fasciculate in axils, and medium density simple hairs in secondary and third row veins, and on petiole |
| 8 | Marginal teeth | Subacute | Subacute | Apiculate/subacute | Apiculate |
| Morphometric Traits *, cm | TC ** | TE | TEP | TP | R2 | F Value | p |
|---|---|---|---|---|---|---|---|
| Min–Max Mean ± SD (n − 1) Turkey Index | |||||||
| Bracts | |||||||
| Total number of measured bracts | 85 | 30 | 20 | 14 | |||
| BL | 2.79–10.10 | 7.24–10.19 | 7.37–10.48 | 5.56–9.72 | 0.26 | 16.55 | <0.0001 |
| 6.85 ± 1.59 | 8.48 ± 0.85 | 8.56 ± 7.37 | 7.98 ± 1.05 | ||||
| b | a | a | a | ||||
| BL_PL | 0.86–27.42 | 0.89–11.97 | 1.82–5.74 | 3.98–28.22 | 0.24 | 15.07 | <0.0001 |
| 5.19 ± 3.94 | 6.59 ± 2.19 | 3.41 ± 1.10 | 11.78 ± 7.18 | ||||
| b | b | c | a | ||||
| PW90 | 0.28–1.93 | 1.04–1.88 | 0.99–2.07 | 0.89–1.70 | 0.18 | 10.47 | <0.0001 |
| 1.14 ± 0.32 | 1.45 ± 0.17 | 1.39 ± 0.30 | 1.30 ± 0.27 | ||||
| b | a | a | ab | ||||
| PL | 0.19–6.86 | 0.74–9.07 | 1.63–4.64 | 0.27–1.40 | 0.17 | 10.23 | <0.0001 |
| 1.76 ± 0.93 | 1.58 ± 1.44 | 2.75 ± 0.86 | 0.87 ± 0.37 | ||||
| b | bc | a | c | ||||
| MPW | |||||||
| Leaves | |||||||
| Total number of measured leaves | 180 | 60 | 45 | 35 | |||
| LWA | 3.24–7.31 | 4.60–6.71 | 5.75–9.42 | 5.49–10.05 | 0.51 | 109.73 | <0.0001 |
| 5.52 ± 0.78 | 5.53 ± 0.43 | 7.31 ± 0.79 | 7.58 ± 1.36 | ||||
| b | b | a | a | ||||
| BL | 4.53–9.15 | 5.45–7.91 | 6.91–10.49 | 6.19–10.81 | 0.46 | 90.84 | <0.0001 |
| 6.81 ± 0.89 | 6.69 ± 0.53 | 8.88 ± 0.87 | 8.34 ± 1.30 | ||||
| c | d | a | b | ||||
| LWA_MPW | 0.71–1.09 | 0.75–0.95 | 0.86–1.26 | 0.83–1.11 | 0.45 | 87.89 | <0.0001 |
| 0.86 ± 0.08 | 0.84 ± 0.05 | 1.03 ± 0.09 | 0.97 ± 0.06 | ||||
| c | c | a | b | ||||
| LA10 | 132–158 | 147–157 | 128–152 | 144–157 | 0.36 | 60.13 | <0.0001 |
| 151.28 ± 4.5 | 152.23 ± 2.27 | 142.24 ± 5.8 | 149.31 ± 3.45 | ||||
| ab | a | c | b | ||||
| AL | 0.43–2.11 | 0.61–1.51 | 0.36–2.59 | 0.23–1.31 | 0.35 | 56.51 | <0.0001 |
| 1.30 ± 0.29 | 1.16 ± 0.18 | 1.57 ± 0.38 | 0.76 ± 0.28 | ||||
| b | c | a | d | ||||
| PMP | 1.05–3.24 | 1.65–3.01 | 1.65–3.75 | 1.32–4.16 | 0.34 | 53.69 | <0.0001 |
| 1.96 ± 0.43 | 2.19 ± 0.31 | 2.85 ± 0.48 | 2.66 ± 0.80 | ||||
| c | b | a | a | ||||
| LA25 | 107–132 | 118–131 | 104–123 | 115–127 | 0.33 | 51.74 | <0.0001 |
| 122.29 ± 5.4 | 124.32 ± 3.12 | 113.42 ± 4.88 | 120.91 ± 3.09 | ||||
| c | a | d | b | ||||
| MPW | 3.85–9.44 | 5.53–7.73 | 5.87–9.57 | 5.86–10.04 | 0.16 | 20.29 | <0.0001 |
| 6.45 ± 1.05 | 6.63 ± 0.52 | 7.12 ± 0.98 | 7.79 ± 1.30 | ||||
| c | cb | b | a | ||||
| LWA_PL | 0.60–2.17 | 0.67–2.12 | 0.77–2.28 | 0.73–2.47 | 0.10 | 12.24 | <0.0001 |
| 1.45 ± 0.27 | 1.57 ± 0.25 | 1.67 ± 0.23 | 1.63 ± 0.32 | ||||
| b | a | a | a | ||||
| PL | 2.24–9.42 | 2.52–8.46 | 3.18–9.58 | 3.32–11.92 | 0.09 | 10.28 | <0.0001 |
| 3.98 ± 1.13 | 3.66 ± 1.06 | 4.45 ± 0.92 | 4.94 ± 1.93 | ||||
| bc | c | ab | a | ||||
| No. | Morphometric Traits | Abbreviation |
|---|---|---|
| 1 | Blade length, cm | BL |
| 2 | Apex length, cm | AL |
| 3 | Blade length without apex, cm | LWA |
| 4 | Maximum blade width, measured perpendicular to blade, cm | MPW |
| 5 | Length to position where maximum blade width, cm | PMP |
| 6 | Blade width perpendicular to blade length at 90% blade length, cm | PW90 |
| 7 | The Blade Lobe Angle at 10% Blade Length, degree | LA10 |
| 8 | The Blade Lobe Angle at 25% Blade Length, degree | LA25 |
| 9 | The Petiole Length, cm | PL |
| Derived variables | ||
| 10 | Ratio (Blade length and petiole length) (1/9) | BL_PL |
| 11 | Ratio (Blade length without apex and Maximum blade width) (3/4) | LWA_MPW |
| 12 | Ratio (Blade length without apex and Petiole Length (3/9) | LWA_PL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurkšienė, G.; Danusevičius, D.; Kembrytė-Ilčiukienė, R.; Baliuckas, V. Dendrological Secrets of the Pazaislis Monastery in Central Lithuania: DNA Markers and Morphology Reveal Tilia × europaea L. Hybrids of an Impressive Age. Plants 2023, 12, 3567. https://doi.org/10.3390/plants12203567
Jurkšienė G, Danusevičius D, Kembrytė-Ilčiukienė R, Baliuckas V. Dendrological Secrets of the Pazaislis Monastery in Central Lithuania: DNA Markers and Morphology Reveal Tilia × europaea L. Hybrids of an Impressive Age. Plants. 2023; 12(20):3567. https://doi.org/10.3390/plants12203567
Chicago/Turabian StyleJurkšienė, Girmantė, Darius Danusevičius, Rūta Kembrytė-Ilčiukienė, and Virgilijus Baliuckas. 2023. "Dendrological Secrets of the Pazaislis Monastery in Central Lithuania: DNA Markers and Morphology Reveal Tilia × europaea L. Hybrids of an Impressive Age" Plants 12, no. 20: 3567. https://doi.org/10.3390/plants12203567
APA StyleJurkšienė, G., Danusevičius, D., Kembrytė-Ilčiukienė, R., & Baliuckas, V. (2023). Dendrological Secrets of the Pazaislis Monastery in Central Lithuania: DNA Markers and Morphology Reveal Tilia × europaea L. Hybrids of an Impressive Age. Plants, 12(20), 3567. https://doi.org/10.3390/plants12203567